Abstract
Objective
We sought to determine the incidence and factors associated with development of diabetes mellitus (DM) in older HIV-infected individuals.
Research design and methods
We analyzed data from people living with HIV (PLWH) ≥50 years of age enrolled in a large urban HIV outpatient clinic in Vancouver, British Columbia. Patients were categorized as having DM if they had random blood sugar ≥11.1 mmol/L, fasting blood sugar ≥7 mmol/L, HbA1C ≥6.5%, antidiabetic medication use during the follow-up period, or medical chart review confirming diagnosis of DM. We estimated the probability of developing DM, adjusting for demographic and clinical factors, using a logistic regression model.
Results
Among 1065 PLWH followed for a median of 13 years (25th and 75th percentile (Q1–Q3): 9-18), the incidence of DM was 1.61/100 person-years follow-up. In the analysis of factors associated with new-onset DM (n=703), 88% were male, 38% had a history of injection drug use, 43% were hepatitis C coinfected, and median body mass index was 24 kg/m2 (Q1–Q3: 21–27). Median age at antiretroviral therapy (ART) initiation was 48 years (Q1–Q3: 43–53) and at DM diagnosis was 55 years (Q1–Q3: 50–61). Patients who started ART in 1997–1999 and had a longer exposure to older ART were at the highest risk of developing DM.
Conclusions
Among PLWH aged ≥50 years, the incidence of DM was 1.39 times higher than men in the general Canadian population of similar age. ART initiated in the early years of the epidemic and exposure to older ART appeared to be the main drivers of the development of DM.
Keywords: hiv, adult diabetes, incidence, aging
Significance of this study.
What is already known about this subject?
It has been shown that people living with HIV have a higher incidence of diabetes mellitus (DM) relative to the general population. Although the usual risk factors for DM are common in this population, the effects of HIV-related factors, including the use of antiretrovirals, are unclear.
What are the new findings?
In this study, we found a high incidence of DM in adults living with HIV and a correlation between advanced HIV infection, the use of older antiretrovirals, and the incidence of DM. Of interest, patients in our cohort who developed DM had normal to low body mass index.
How might these results change the focus of research or clinical practice?
This study highlights the importance of monitoring markers of DM (eg, fasting blood sugar and HbA1C) regularly in all people living with HIV, regardless of their traditional DM risk factors and bodyweight. Detection and treatment of DM in this population may improve patient clinical outcomes.
Introduction
Combination antiretroviral therapy (ART) has dramatically increased the life expectancy of people living with HIV (PLWH)1–3; however, they are now developing comorbidities associated with prolonged survival and aging,4–6 and these may be occurring at earlier ages than in the general population. Many PLWH, particularly those who received older antiretroviral treatments, experienced peripheral fat atrophy, visceral fat accumulation, and metabolic comorbidities, including dyslipidemia and impaired glucose homeostasis, which can lead to increased risk for cardiovascular disease and other related morbidities.7 8
Several studies have reported an increased prevalence and incidence of metabolic disorders such as impaired glucose tolerance and diabetes mellitus (DM) among PLWH.9–12 In a large French cohort of HIV-infected individuals followed for 10 years after ART initiation in 1997–1999, the incidence rate of DM was 1.41 per 100 person-years follow-up.13 A study conducted in the USA estimated the prevalence of DM among HIV-infected adults as 10.3%, approximately 3.8% higher than in the non-HIV-infected population. Their data suggested that DM in this population developed at an earlier age and in the absence of obesity.14
Several factors have been associated with the development of DM in the HIV-infected population. Soon after the introduction of first-generation HIV protease inhibitors (PIs) in 1996, PLWH were found to have a higher incidence of metabolic disorders including DM13 15; however, other ART agents including non-nucleoside reverse transcriptase inhibitors (NNRTIs) and factors like aging, male sex, obesity, non-white ethnicity, family history, and hepatitis C coinfection may also contribute to DM in the HIV population.7 16 17 Only recently, with more HIV-infected patients living longer lives, have we been able to better observe age-related comorbidities, their expression, and outcomes in this population.
The objective of this retrospective study was to determine the incidence of DM in an aging HIV population attending the largest outpatient HIV clinic in British Columbia, Canada, and determine which traditional and HIV-related factors were associated with the development of DM.
Research design and methods
Study patients
The British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) distributes ART agents at no cost to all eligible PLWH in the province through its Drug Treatment Program (DTP), as described in detail elsewhere.18 Prescription of ART follows BC-CfE treatment guidelines that are regularly updated and generally remain consistent with international treatment guidelines.19
We examined data from the BC-CfE DTP database for all HIV-positive adults enrolled in an urban outpatient HIV clinic at St. Paul’s Hospital in Vancouver, British Columbia, Canada, up to 31 July 2015. DM criteria, as described below, were obtained from the Outpatient Immunodeficiency Clinic database and medical chart review. Lists of prescribed medications were obtained from a provincial pharmaceutical database. The incidence analysis included individuals who met the following criteria: ≥50 years of age at enrollment in the DTP; ART naive at enrollment, known ART start date, and initiation of ART therapy before 31 July 2015; no diagnosis of DM prior to enrollment; known DM status at ART initiation and at the end of follow-up. Patients who initiated an ART regimen containing an integrase inhibitor or a CCR5 receptor antagonist were excluded as adequate duration of follow-up data was not available for these newer drug classes. The final multivariable analysis excluded patients who started ART before 1997 and who therefore did not have plasma HIV viral load results in the period prior to starting ART.
Outcome measures
Our main outcome, DM diagnosis, followed the criteria of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.20 Patients were categorized as having DM if they had one or more of the following at least once during the follow-up period: fasting blood sugar ≥7 mmol/L, random blood sugar ≥11.1 mmol/L, glycated hemoglobin (HbA1C)≥6.5%, or if they had used one or more antidiabetic medications during the follow-up period (metformin, glyburide, glicazide, liraglutide, exenatide, sitagliptin, saxagliptin, linagliptin, dapagliflozin, canagliflozin, or insulin). Patients who were receiving metformin or liraglutide for purposes other than the treatment of DM, and did not meet any of the laboratory criteria, were excluded from the analysis. The rate of new-onset DM was defined as the number of cases of DM divided by the total number of person-years of follow-up. The follow-up period was defined as the time from initial enrollment in the DTP from 1 January 1997 onward to the date of DM diagnosis or the patients’ last contact with the Outpatient Immunodeficiency Clinic up until 31 July 2015.
Statistical analysis
Bivariable analysis compared characteristics of patients with and without DM diagnosis during follow-up, using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. A logistic regression model was used to identify sociodemographic and clinical characteristics potentially associated with DM, including sex at birth (male or female), self-identified ethnicity (Asian, black, indigenous, Hispanic, or white), history of injection drug use (yes vs no), sexual orientation (heterosexual or homosexual/bisexual), hepatitis C virus (HCV) antibody status (present vs absent), hepatitis B virus (HBV) surface antigen status (present vs absent), history of AIDS-defining illness (yes vs no), calendar year of first ART exposure (1997–1999, 2000–2004, 2005–2009, 2010–2015; intervals based on changes to the BC-CfE ART treatment guidelines),21 main class of first ART regimen (NNRTI, PI, or other), age at HIV diagnosis (≥50 or <50 years), age at ART initiation (≥50 or <50 years), latest weight (kg), latest body mass index (BMI; kg/m2), CD4 nadir (cells/mm3), CD4 count at ART initiation (cells/mm3), proportion of follow-up time receiving ART agents, and proportion of time with plasma viral load ≥500 copies/mL. A backward stepwise selection was used to build the multivariable model, using variables with bivariate association.22 The selection of variables was based on Akaike information criteria and type III P value, which considers the best explanatory model and the best goodness of fit for model selection. Statistical analyses were conducted using SAS software V.9.4.
Results
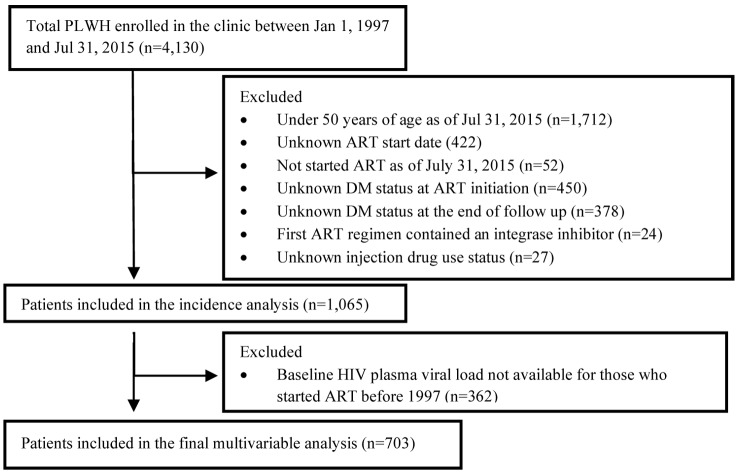
From a total of 4130 PLWH enrolled at the clinic between 1997 and 2015, 1065 met the inclusion criteria for this study, while 703 individuals remained in the final multivariable analysis (figure 1). Among the 1065 PLWH included in the incidence analysis, there were 235 new diagnoses of DM during a median of 13 years of follow-up (quartile 1–quartile 3 (Q1–Q3): 9–18 years), representing an incidence rate of 1.61 new cases per 100 person-years of follow-up. Among the 703 patients included in the bivariable and multivariable analysis, 132 (19%) developed DM (table 1). Among the patients who developed DM, 47 (36%) had HbA1C results on file; their median HbA1C was 5.80%, mean 6.16 (Q1–Q3: 5.3%–6.6%). The cohort was primarily male at birth (89%) and of white ethnicity (73%). Most patients were diagnosed with HIV and started ART at age <50 years (71% and 64%, respectively). HCV coinfection was present in 43%, HBV coinfection in 8%, and 38% had a history of injection drug use. Nearly one-third (31%) had a previous AIDS-defining illness at the end of study follow-up. Of the 703 patients included in the final multivariable analysis, 94 (13%) died and 23 (3%) moved out of the province and were thus lost to follow-up.
Figure 1.
Exclusion criteria and final study sample of people living with HIV (PLWH) enrolled in a large urban HIV clinic in British Columbia, Canada, between 1 January 1997 and 31 July 2015. ART, antiretroviral therapy; DM, diabetes mellitus.
Table 1.
Bivariable analysis of baseline characteristics of 703 study participants aged > 50 years and living with HIV included in the final analysis stratified by new-onset diabetes during follow-up
| New-onset diabetes (n=132) |
No diabetes (n=571) |
P value | |
| Sex at birth; n (%) | |||
| Male | 120 (19) | 502 (81) | 0.368 |
| Female | 12 (15) | 69 (85) | |
| Ethnicity; n (%) | |||
| Asian | 14 (28) | 36 (72) | 0.132 |
| Black | 5 (21) | 19 (79) | 0.793 |
| Indigenous | 20 (25) | 61 (75) | 0.177 |
| Hispanic | 9 (29) | 22 (71) | 0.161 |
| White | 92 (18) | 423 (82) | 0.126 |
| History of IDU; n (%) | |||
| Yes | 53 (20) | 215 (80) | 0.620 |
| No | 79 (18) | 356 (82) | |
| Heterosexual; n (%) | |||
| Yes | 65 (21) | 239 (79) | 0.112 |
| No | 61 (16) | 312 (84) | |
| Homosexual/bisexual; n (%) | |||
| Yes | 59 (17) | 288 (83) | 0.279 |
| No | 67 (20) | 263 (80) | |
| HCV antibody positive; n (%) | |||
| Yes | 64 (21) | 235 (79) | 0.144 |
| No | 68 (17) | 333 (83) | |
| HBV surface antigen positive; n (%) | |||
| Yes | 11 (20) | 43 (80) | 0.720 |
| No | 107 (19) | 462 (81) | |
| AIDS-defining illness at the end of follow-up; n (%) | |||
| Yes | 44 (20) | 177 (80) | 0.604 |
| No | 88 (18) | 394 (82) | |
| Calendar year of first ART; n (%) | |||
| 1997–1999 | 60 (30) | 140 (70) | <0.001 |
| 2000–2004 | 51 (27) | 137 (73) | |
| 2005–2009 | 19 (9) | 182 (91) | |
| 2010–2015 | 2 (2) | 112 (98) | |
| Main class of first ART regimen; n (%) | |||
| NNRTI | 30 (15) | 172 (85) | 0.003 |
| PI | 86 (19) | 374 (81) | |
| Others | 16 (39) | 25 (61) | |
| Age at HIV diagnosis, years; n (%) | |||
| <50 | 93 (19) | 407 (81) | 0.832 |
| ≥50 | 39 (19) | 164 (81) | |
| Age at ART initiation, years; n (%) | |||
| <50 | 88 (20) | 362 (80) | 0.546 |
| ≥50 | 44 (17) | 209 (83) | |
| Latest weight during follow-up (kg); median (Q1–Q3) | 73 (66–86) | 72 (63–84) | 0.122 |
| Latest BMI during follow-up (kg/m2); median (Q1–Q3) | 25 (22–28) | 24 (21–27) | 0.161 |
| Latest hemoglobin during follow-up (g/dL); median (Q1–Q3) | 145 (130–157) | 142 (132–152) | 0.201 |
| CD4 nadir (cells/mm3); median (Q1–Q3) | 80 (21–150) | 130 (40–230) | <0.001 |
| CD4 count at ART initiation (cells/mm3); median (Q1–Q3) | 180 (70–340) | 240 (120–370) | 0.016 |
| Deceased as of 31 July 2015; n(%) | |||
| Yes | 20 (21) | 74 (79) | 0.481 |
| No | 112 (18) | 497 (82) |
Ethnic groups are not mutually exclusive and were unknown in some cases. Other first classes of ART regimen: NRTI only including zidovudine/lamivudine, stavudine/lamivudine, or other NRTI-only regimen (n=33; 80%) and regimens including both an NNRTI and a PI (n=8; 20%).
ART, antiretroviral therapy; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; IDU, injection drug use; NNRTI, non-nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; Q1–Q3, 25th and 75th percentile.
As shown in table 1, the frequency of new-onset DM did not differ significantly between patients diagnosed with HIV before the age of 50 years and those diagnosed at the age of 50 years or older (19% vs 19%, respectively; P=0.832), or between those who started ART before or after age 50 (20% vs 17%, respectively; P=0.546). Likewise, there was no association between new-onset DM and having positive HCV antibody or HBV serology, injection drug use, ethnic group, sex at birth, latest weight, or latest BMI. When comparing patients who developed DM with those who did not, those who developed DM had a lower CD4 nadir (80 vs 130 cells/mm3, respectively; P<0.001), lower CD4 count at or before initiation of first ART (180 vs 240 cells/mm3, respectively; P=0.016), and were less likely to have taken an NNRTI-based regimen as their first ART (15% vs 85%; P=0.003). DM was more likely to be diagnosed among patients who started ART in the earlier eras (1997–2004) and less likely among those who started ART more recently (2010–2015; 30% vs 2%; P<0.001).
Patients who developed DM had a shorter time from their HIV diagnosis, were younger at the end of study, spent a greater proportion of follow-up time with plasma viral load ≥500 copies/mL, and had lower CD4 count at the end of follow-up compared with those who did not develop DM (table 2).
Table 2.
Characteristics of the study population (n=703) at the end of the follow-up period (31 July 2015)
| New cases of diabetes (n=132) | No diabetes (n=571) |
P value | |
| Age at diabetes diagnosis or the end of study (years); median (Q1–Q3) | 55 (50–61) | 56 (53–61) | <0.001 |
| CD4 count at the end of follow-up (cells/mm3); median (Q1–Q3) |
395 (260–615) | 530 (350–720) | <0.001 |
| Time from antiretroviral therapy initiation to develop diabetes or the end of study (years); median (Q1–Q3) | 7.9 (5.6–10.6) | 9.3 (5.7–13.5) | 0.005 |
| Time from HIV diagnosis to develop diabetes or the end of study (years); median (Q1–Q3) | 9.0 (6.2–11.6) | 11.8 (7.8–16.2) | <0.001 |
| Proportion of follow-up time with plasma viral load ≥500 copies/mL; median (Q1–Q3) | 16% (6–36) | 7% (2–25) | <0.001 |
Q1–Q3, 25th and 75th percentile.
As shown in table 3, in the multivariable analysis, diagnosis of DM was significantly associated with earlier calendar year of first ART (1997–1999 relative to 2005–2009; adjusted OR (AOR) 48.90; 95% CI (CI) 21.32 to 112.17) and increased length of time on ART (AOR 0.69; 95% CI 0.64 to 0.74), while CD4 nadir and proportion of follow-up time with plasma viral load ≥500 copies/mL were of borderline significance.
Table 3.
Univariable and multivariable logistic regression model of clinical characteristics associated with incidence of diabetes (n=703)
| OR (95% CI) |
Adjusted OR (95% CI) |
|
| Sex at birth | ||
| Female | 0.73 (0.38 to 1.39) | 0.53 (0.23 to 1.22) |
| Male (ref) | 1.00 | 1.00 |
| Calendar year of first antiretroviral therapy | ||
| 1997–1999 | 4.11 (2.34 to 7.19) | 48.90 (21.32 to 112.17) |
| 2000–2004 | 3.57 (2.01 to 6.32) | 7.22 (3.7 to 14.1) |
| 2005–2009 (ref) | 1.00 | 1.00 |
| 2010–2015 | 0.17 (0.04 to 0.75) | 0.05 (0.01 to 0.24) |
| CD4 nadir (per 100 cells/mm3 increase) | 0.64 (0.53 to 0.78) | 0.79 (0.61 to 1.02) |
| Time from antiretroviral therapy initiation to either the date of diabetes diagnosis or the end of study (per year increase) | 0.94 (0.90 to 0.98) | 0.69 (0.64 to 0.74) |
| Proportion of follow-up time with plasma viral load ≥500 copies/mL (per 10% increase) | 1.12 (1.04 to 1.22) | 0.93 (0.83 to 1.03) |
| Proportion of follow-up time on antiretroviral therapy agents (per 10% increase) | ||
| Stavudine | 1.39 (1.27 to 1.53) | Not selected |
| Zidovudine | 1.19 (1.08 to 1.31) | |
| Lopinavir | 1.19 (1.11 to 1.28) | |
| Indinavir | 1.44 (1.20 to 1.73) | |
| Saquinavir | 1.17 (0.94 to 1.46) | |
| Nelfinavir | 1.63 (1.19 to 2.24) |
Patients who developed DM had different exposures to particular ART agents, measured as the mean proportion of time during follow-up that the individual was on the medication, compared with those who did not develop DM (table 4). Patients who developed DM had a greater proportion of follow-up time exposed to stavudine, zidovudine, didanosine, indinavir, nelfinavir, and lopinavir, and a smaller proportion of time exposed to atazanavir, tenofovir disoproxil fumarate, lamivudine or emtricitabine, and darunavir. However, the mean proportion of follow-up time on NNRTIs did not differ between those who developed DM and those who did not (23.3% vs 28.3%; P=0.31; not shown).
Table 4.
Proportion of time (%) on antiretroviral agents during follow-up (n=703)
| Antiretroviral agents | Proportion of follow-up time (%); no diabetes (n=571) | Proportion of follow-up time (%); with diabetes (n=132) | P value |
| Mean (SD) | Mean (SD) | ||
| Nucleoside reverse transcriptase inhibitors | |||
| Stavudine | 7.2 (14.6) | 21.1 (26.4) | <0.001 |
| Zidovudine | 5.0 (14.5) | 10.9 (22.8) | 0.011 |
| Didanosine | 3.6 (11.0) | 11.5 (21.7) | <0.001 |
| Lamivudine or emtricitabine | 82.5 (23.1) | 78.8 (23.2) | 0.027 |
| Abacavir | 23.1 (32.7) | 16.3 (26.6) | 0.084 |
| Tenofovir disoproxil fumarate | 51.5 (38.1) | 38.4 (33.4) | <0.001 |
| Protease inhibitors | |||
| Indinavir | 2.1 (7.5) | 6.4 (15.0) | <0.001 |
| Saquinavir | 1.3 (6.5) | 2.3 (9.6) | 0.079 |
| Nelfinavir | 0.6 (3.5) | 2.8 (11.4) | 0.033 |
| Lopinavir | 9.3 (21.1) | 21.1 (29.2) | <0.001 |
| Atazanavir | 38.1 (39.0) | 28.4 (33.5) | 0.021 |
| Darunavir | 3.5 (13.2) | 1.6 (9.1) | 0.007 |
Conclusions
In this cohort of 1065 of PLWH, aged 50 years and older followed for a median of 13 years, the incidence of DM was 1.61 cases per 100 person-years, 1.39 times higher than the rate among men in the general Canadian population of similar age (1.16 cases per 100 person-years among men, and 0.99 cases per 100 person-years for men and women combined).23 ART initiation in earlier eras (1997–2004), earlier calendar year of first ART, and increased length of time on ART were strongly associated with the incidence of DM in this population. Greater proportion of follow-up time with plasma viral load ≥500 copies/mL and lower CD4 count at the end of follow-up were associated with higher incidence of DM in the univariable analysis, but not in the adjusted model.
Recent studies have reported a higher incidence of DM in PLWH receiving combination ART compared with their HIV-negative counterparts.14 17 The incidence of DM among HIV patients in the Multicenter AIDS Cohort Study was 4.7 cases per 100 person-years of follow-up,24 whereas in the APROCO study it was 1.4 cases per 100 person-years of follow-up.13 These differences in DM incidence could be related to sample size and the demographic composition of the different cohorts. In our study, the incidence of new-onset DM peaked in those who initiated ART in 1997–1999 when patients were treated with older ART agents. In 2000, the incidence of DM started to drop dramatically with the introduction of new ART regimens, and only two (1.5%) new DM cases were identified between 2010 and 2015.
Most new DM diagnoses occurred in an era when PLWH were exposed to first-generation ART agents with significant metabolic toxicities. This is consistent with previous studies and might be related to the use of older NRTIs (eg, zidovudine, stavudine, and didanosine), which have a role in the development of insulin resistance.13 25–29 The increased lipolysis observed in patients receiving older NRTIs may lead to increased circulating free fatty acids, which may promote insulin resistance in the liver and skeletal muscle.30 On the other hand, clinical evidence for a direct effect of thymidine analogue NRTIs on insulin resistance is also emerging.31
Furthermore, we observed an association between the use of older PIs (eg, lopinavir, nelfinavir, and indinavir) and the risk of developing DM in this population. Similar findings were reported in previous studies.24 26 32 Several mechanisms have been postulated to explain how PIs may lead to insulin resistance, one of which is the effect of PIs on the glucose transporter GLUT4 and the reduction of insulin sensitivity.33 It has been proposed that acute inhibition of the high capacity GLUT4 transporter contributes to the early stages of PI-associated insulin resistance by decreasing peripheral glucose disposal.34
Previous studies suggested that ongoing inflammation due to chronic HIV infection may contribute to the pathogenesis of DM.13 Although in our study there was an association between DM and indicators of more advanced HIV infection (low CD4 cell count nadir and having a plasma viral load for > 500 copies/mL for a greater proportion of follow-up time) in the univariable model, these were only marginally significant in the multivariable model.
Although HCV coinfection has been described as a risk factor for the development of DM,35 we found that HCV coinfection, defined as ever having a positive HCV antibody test, was not associated with the development of DM in our HIV-positive cohort. These findings are consistent with other studies14 and add support to the hypothesis that HCV infection affects the risk of DM in a complex manner. Liver fibrosis and cirrhosis, as well as other traditional risk factors, have been linked to the development of DM in HCV-infected patients.36 37
Our study did not demonstrate a significant association between obesity (high BMI) and the incidence of new-onset DM in PLWH. Of note, the most recent BMI of patients in this study was 24 kg/m2 (median), lower than the BMI noted in other HIV cohorts (median 26 kg/m2).24 In part this could be related to the fact that many of the patients in our cohort received older ART medications, which can cause lipoatrophy, and they also exhibited relatively advanced HIV infection (as indicated by low CD4 nadir and history of AIDS-defining illness), which is generally associated with low weight. The lower BMI in our study population (only 24% and 8% meeting criteria for overweight and obesity, respectively) differs from previous observations of a high prevalence of obesity that has been described among ART-exposed HIV-infected adults in the USA and Canada. In that cohort, after 3 years of ART, 18% of adults who were overweight at initiation of therapy had become obese, and 22% of those with a normal BMI at initiation had become overweight.38 The relatively low BMI in our study population may reflect the lower proportion of individuals in the general population who are overweight or obese (31% and 15%, respectively) within British Columbia, relative to Canada as a whole (33% and 20%, respectively).39 Lower BMI in this study may also be associated with the relatively high proportion of individuals who inject drugs (38%) as injection drug use has previously been found to be associated with lower BMI.40
The strengths of this study are that it was conducted in a large cohort of older PLWH and used data from multiple sources to ensure as complete and accurate data collection as possible. However, certain limitations should be noted. First, the clinical data were limited and there was no information on some traditional risk factors for DM, such as family history. Likewise, while BMI was available, measures of visceral adiposity, such as waist circumference, were not collected. Fat redistribution among PLWH receiving ART can increase visceral adiposity; however, increased visceral adiposity remains associated with increased BMI and obesity within this population.41 42 Second, BMI and other clinical data were gathered in the course of routine clinical care, but not in a standardized fashion. In addition, this study did not exclude patients who may have developed latent autoimmune diabetes as an adult; as patients on insulin were included, this diagnosis cannot be excluded. While HbA1C can underestimate the diagnosis of DM in PLWH if they have anemia, due to short red blood cell life span, in this cohort the mean hemoglobin was in the normal range at 139.8 g/dL. It is unlikely many DM cases were overlooked since multiple other criteria (fasting glucose and pharmacological history) were used to define DM in addition to HbA1C; indeed, only one-third of those diagnosed with DM had a HbA1C result on file. Lastly, the retrospective nature of this study did not allow us to match cases to a control group.
There are important clinical implications from the findings of this study. Healthcare providers should follow existing DM and HIV treatment guidelines,43 which recommend that fasting blood sugar and HbA1C be obtained prior to and after starting ART. Although the ART agents currently in use are less likely to cause metabolic toxicities than the older ones, the incidence of DM is relatively high in the general population and even higher in patients infected with HIV. These findings also support recommendations for early initiation of ART, given that patients with better immune and virological control had a lower incidence of DM.
In conclusion, the incidence of DM in this cohort of older HIV-positive patients was higher than in the general population in Canada. Longer exposure to ART agents and the use of older drugs were associated with a higher incidence of DM in our study. On the other hand, obesity, HCV coinfection, and older age were not associated with the incidence of DM in this cohort. The incidence of DM is likely to decline in PLWH who initiated ART more recently with the use of newer ART agents.
Footnotes
Contributors: Study conception and design: FS, MH, CMP, VDL and SAG. Data acquisition: SC. Data analysis: MY, JC and VDL. Data interpretation: FS, MH, CMP, MY, GPB, VDL, JSGM and SAG. Manuscript draft: FS. Critical revision for important intellectual content: FS, MH, CMP, MY, JC, SC, GPB, VDL, JSGM and SAG. Approval of final version and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Competing interests: JSGM has received funding for clinical trials that have been paid to the institution from: the BC Ministry of Health for the STOP HIV/AIDS Initiative, NIDA-NIH (R01DA036307) for STOP HIV/AIDS in IDUs, McGill University for CTN 222 and Gladstone Institute of Virology-UCSF for Diflunisal in HIV. JSGM also reports grants from Janssen, Merck, Abbvie, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, outside the submitted work; he has served on the advisory boards for Teva, Gilead Sciences, and InnaVirVax. SAG reports personal fees from Gilead Sciences and ViiV Healthcare, outside the submitted work. MH reports participation in clinical trials with AbbVie, Amgen Canada and Gilead Sciences Canada, and received grants and personal fees from Gilead Sciences Canada, Merck Canada, and ViiV Healthcare, outside the submitted work. CMP reports grants from ViiV Healthcare Canada, outside the submitted work. GPB reports personal fees from Lily, Boehringer Ingelheim, Merck, AstraZeneca, Novo Nordisk, Janssen, Amgen, and Sanofi, outside the submitted work.
Ethics approval: University of British Columbia/Providence Health Care Research Ethics Board (H05-50123).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are housed at the British Columbia Centre for Excellence in HIV/AIDS Drug Treatment Program. Requests for data access may be made to the British Columbia Centre for Excellence in HIV/AIDS Data Committee under the Director of Operations, Irene Day, iday@cfenet.ubc.ca.
References
- 1. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS 2009;4:194–9. 10.1097/COH.0b013e328329fc8d [DOI] [PubMed] [Google Scholar]
- 3. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008;372:293–9. 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV 2015;2:e288–98. 10.1016/S2352-3018(15)00077-6 [DOI] [PubMed] [Google Scholar]
- 5. Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015;60:627–38. 10.1093/cid/ciu869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014;59:1787–97. 10.1093/cid/ciu701 [DOI] [PubMed] [Google Scholar]
- 7. Kalra S, Kalra B, Agrawal N, et al. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr 2011;3:2 10.1186/1758-5996-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis 2012;205(Suppl 3):S383–90. 10.1093/infdis/jis205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34. 10.1097/QAD.0b013e32832bd7af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Florescu D, Kotler DP. Insulin resistance, glucose intolerance and diabetes mellitus in HIV-infected patients. Antivir Ther 2007;12:149–62. [DOI] [PubMed] [Google Scholar]
- 11. Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009;50:499–505. 10.1097/QAI.0b013e31819c291b [DOI] [PubMed] [Google Scholar]
- 12. Traoré Y, Bensghir R, Ihbibane F, et al. Diabetes and human immunodeficiency virus infection: Epidemiological, therapeutic aspects and patient experience. Presse Med 2016;45(Pt 1):e139–43. 10.1016/j.lpm.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 13. Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012;26:303–14. 10.1097/QAD.0b013e32834e8776 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care 2017;5:e000304 10.1136/bmjdrc-2016-000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levitt NS, Peer N, Steyn K, et al. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res Clin Pract 2016;119:41–7. 10.1016/j.diabres.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 16. Glümer C, Carstensen B, Sandbaek A, et al. A Danish diabetes risk score for targeted screening: the Inter99 study. Diabetes Care 2004;27:727–33. 10.2337/diacare.27.3.727 [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen LD, Mathiesen ER, Kronborg G, et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLoS One 2012;7:e44575 10.1371/journal.pone.0044575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Low-Beer S, Chan K, Yip B, et al. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr 2000;23:295–301. 10.1097/00042560-200004010-00003 [DOI] [PubMed] [Google Scholar]
- 19. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of hiv infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA 2016;316:191–210. 10.1001/jama.2016.8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldenberg R, Punthakee Z. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 2013;37(Suppl 1):S8–11. 10.1016/j.jcjd.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 21. Vella S, Schwartländer B, Sow SP, et al. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS 2012;26:1231–41. 10.1097/QAD.0b013e32835521a3 [DOI] [PubMed] [Google Scholar]
- 22. Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr 2010;55:460–5. 10.1097/QAI.0b013e3181f2ac87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Public Health Agency of Canada. Diabetes in Canada: facts and figures from a public health perspective. Ottawa: Public Health Agency of Canada, 2011. [Google Scholar]
- 24. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–84. 10.1001/archinte.165.10.1179 [DOI] [PubMed] [Google Scholar]
- 25. De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in hiv-infected patients: The data collection on adverse events of anti-hiv drugs (D:A:D) study. Diabetes Care 2008;31:1224–9. 10.2337/dc07-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–9. 10.1086/518619 [DOI] [PubMed] [Google Scholar]
- 27. Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and insulin resistance in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr 2008;49:369–76. 10.1097/QAI.0b013e318189a780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brambilla AM, Novati R, Calori G, et al. Stavudine or indinavir-containing regimens are associated with an increased risk of diabetes mellitus in HIV-infected individuals. AIDS 2003;17:1993–5. 10.1097/00002030-200309050-00022 [DOI] [PubMed] [Google Scholar]
- 29. Lo YC, Chen MY, Sheng WH, et al. Risk factors for incident diabetes mellitus among HIV-infected patients receiving combination antiretroviral therapy in Taiwan: a case-control study. HIV Med 2009;10:302–9. 10.1111/j.1468-1293.2008.00687.x [DOI] [PubMed] [Google Scholar]
- 30. van der Valk M, Bisschop PH, Romijn JA, et al. Lipodystrophy in HIV-1-positive patients is associated with insulin resistance in multiple metabolic pathways. AIDS 2001;15:2093–100. 10.1097/00002030-200111090-00004 [DOI] [PubMed] [Google Scholar]
- 31. Blümer RM, van Vonderen MG, Sutinen J, et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS 2008;22:227–36. 10.1097/QAD.0b013e3282f33557 [DOI] [PubMed] [Google Scholar]
- 32. Justman JE, Benning L, Danoff A, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J Acquir Immune Defic Syndr 2003;32:298–302. 10.1097/00126334-200303010-00009 [DOI] [PubMed] [Google Scholar]
- 33. Murata H, Hruz PW, Mueckler M. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS 2002;16:859–63. 10.1097/00002030-200204120-00005 [DOI] [PubMed] [Google Scholar]
- 34. Murata H, Hruz PW, Mueckler M. Investigating the cellular targets of HIV protease inhibitors: implications for metabolic disorders and improvements in drug therapy. Curr Drug Targets Infect Disord 2002;2:1–8. 10.2174/1568005024605882 [DOI] [PubMed] [Google Scholar]
- 35. Gastaldi G, Goossens N, Clément S, et al. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: A review. J Adv Res 2017;8:149–59. 10.1016/j.jare.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon C, Gulick RM, Hoover DR, et al. Case-control study of diabetes mellitus in HIV-infected patients. J Acquir Immune Defic Syndr 2004;37:1464–9. 10.1097/01.qai.0000137373.26438.18 [DOI] [PubMed] [Google Scholar]
- 37. Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003;38:50–6. 10.1053/jhep.2003.50291 [DOI] [PubMed] [Google Scholar]
- 38. Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016;32:50–8. 10.1089/aid.2015.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Statistics Canada. Health indicator profile, age standardized rates, annual estimates, by sex, Canada, provinces and territories, occasional. Ottawa: Statistics Canada, 2015. [Google Scholar]
- 40. Quach LA, Wanke CA, Schmid CH, et al. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend 2008;95:30–6. 10.1016/j.drugalcdep.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huis In ’t Veld D, Pengpid S, Colebunders R, et al. BOdy mass index and waist circumference in patients with HIV in South Africa and associated socio-demographic, health related and psychosocial factors. AIDS Behav 2017. (Epub ahead of print: 27 Feb 2017). 10.1007/s10461-017-1737-2 [DOI] [PubMed] [Google Scholar]
- 42. Erlandson KM, Zhang L, Lake JE, et al. Changes in weight and weight distribution across the lifespan among HIV-infected and -uninfected men and women. Medicine 2016;95:e5399 10.1097/MD.0000000000005399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Diabetes Association. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin Diabetes 2017;35:5–26. 10.2337/cd16-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]