Abstract
Cancer can be regarded as an invasive organ that exhibits unique plasticity provided by coordinated, cancer cell-stromal cell communication in the tumour microenvironment. Typical stress factors in the tumour niche, such as hypoxia and acidosis, are major drivers and modulators of these events. Recent findings reveal an important role of extracellular vesicles and lipoproteins in cancer cell adaption to exogenous stress. Adaptive mechanisms include stimulation of angiogenesis and increased metastasis. Here, we will discuss the similarities and distinct features of these endogenous nanoparticles and their roles as signalosomes and nutrient sources in cancer. We will focus on the accumulating evidence for a central role of cell-surface heparan sulphate proteoglycans in the uptake of extracellular vesicles and lipoproteins.
This article is part of the discussion meeting issue ‘Extracellular vesicles and the tumour microenvironment’.
Keywords: microvesicles, exosomes, lipoproteins, proteoglycans, hypoxia
1. Introduction
Cancer cells reside within a complex milieu known as the tumour microenvironment where they coexist with stromal cells of diverse origin immersed in an extracellular matrix (ECM). Stromal cells, including endothelial cells, pericytes, fibroblasts and immune cells support malignant progression in multiple ways by e.g. angiogenesis, immunosuppression and ECM remodelling. The recruitment and pro-malignant education of stromal cells involves information exchange by direct cell–cell contact as well as the release of soluble signalling molecules and extracellular vesicles (EVs). The initial driving force of this cross-talk is the oncogenetic profile of malignant cells, but stress factors such as hypoxia and associated acidosis have a key role already at early stages of tumour development. Genetic events together with these extrinsic factors thus shape a hostile microenvironment in which cancer cells either fail to adapt and die, or adapt and become the major drivers of disease progression and treatment resistance.
Hypoxia arises when the rapidly expanding tumour is insufficiently perfused by the existing vasculature. Moreover, intravascular thrombosis causes transient episodes of hypoxia followed by re-oxygenation [1]. How cells respond to hypoxic stress was unravelled by the finding of hypoxia inducible factors (HIFs) that are master regulators of oxygen homeostasis [2,3]. The HIFs exert their function by transcriptional activation of several adaptive mechanisms that drive tumour cell invasiveness and metastasis. Tumour hypoxia is also linked to therapy resistance due to inadequate drug distribution as a consequence of distant or perturbed vasculature and the lack of oxygen-generated free radical species. Accordingly, hundreds of studies have documented how the overexpression of hypoxia-induced proteins associate with worse patient prognosis. Notably, none of these parameters has been routinely implemented as a prognostic or predictive biomarker in clinical oncology. This probably reflects the heterogeneous distribution of hypoxic regions in malignant tumours and the intrinsic challenge to standardize immunohistochemical scoring for clinical routine. Nevertheless, hypoxia-induced proteins, most importantly vascular endothelial growth factor (VEGF), are established targets in the treatment of cancer. However, it has become clear from clinical studies and, more importantly, experience from clinical reality that successful targeting of the tumour microenvironment requires a better understanding of the complex network of adaptive responses in cancer [4]. Here, we will review the functional role of lipoproteins and EVs as nanoparticle structures with metabolic and signalling functions in the tumour microenvironment.
2. Extracellular vesicles and lipoproteins in the spotlight: who's who?
EVs are usually named according to their mode of biogenesis and include exosomes that originate from intracellular multivesicular bodies, microvesicles shed from the plasma membrane, and apoptotic bodies that are released by apoptotic cells. EVs are phospholipid bilayer particles, enriched in cholesterol and ceramides [5], and recently have been established as carriers of various cargo, including proteins, RNAs, lipids and metabolites (reviewed in [6]). Depending on subtype, EVs can be characterized by the enrichment of specific surface proteins, most importantly members of the tetraspanin family, such as CD63, CD81 and CD9. EVs are produced and found in most tissue types and fluids, including tumour, urine, seminal plasma, milk and blood, which is assumed to be their main systemic diffusion method along with rapid transfer by lymphatic vessels [7–11]. As the field of EVs expands, different heterogeneous populations of vesicles have been identified, and in a recent comparative study, novel markers and isolation methods were described [12]. EV subtypes may be secreted through different mechanisms, have distinct uptake mechanisms, and are potentially targeted to recipient cell types to mediate specific biological outputs, as will be discussed further below. However, to date there is no clear experimental distinction between microvesicles and exosomes, or a clear definition of subclasses within the respective type of EV species. Thus, unless stated otherwise, ‘EVs’ is used here as a common term for exosomes and microvesicles.
The main lipoprotein classes, HDL, LDL, VLDL and chylomicrons, are lipid particles surrounded by a single phospholipid membrane, and are classically regarded as carriers of lipids. The relative ratio of triglycerides (TGs) and cholesterol as well as associated apolipoproteins (Apo) are the main defining characteristics of lipoprotein subclasses. Chylomicrons are absorbed in the gut from dietary sources while the other subtypes are endogenously produced mostly in the liver. Lipoproteins are transported through blood circulation but are also found in the lymph [13], and cerebrospinal fluid for some types, where they can be locally synthesized [14]. In normal conditions, hepatic VLDL is degraded in the blood by endothelium-bound lipoprotein lipase (LPL), where it transfers TGs to peripheral tissues, hence getting enriched in cholesterol and further losing its ApoE at which stage it becomes LDL. In parallel, VLDL can be processed by cholesteryl ester transfer protein (CETP), losing its ApoB and ApoC and acquiring ApoA apolipoproteins to give rise to smaller, cholesterol-rich HDL particles.
More recent findings point at some important similarities between EVs and lipoproteins: (i) HDL and LDL lipoproteins were shown to carry and deliver miRNAs to target cells [15]; (ii) EV preparations were shown to carry most apolipoproteins classes found on lipoproteins (ApoE, ApoB, ApoC-II, among others) [16]; (iii) the size and density of EVs closely overlap with lipoproteins (figure 1); (iv) accordingly, current EV isolation techniques from blood samples resulted in HDL or LDL co-purification with EV fractions [17,18]. Together, it can be concluded that EV depletion from lipoprotein preparations and vice versa poses a major challenge, and should be carefully considered in biomarker as well as functional studies. To what extent EVs are enzymatically modified by, for example, specific proteases and endothelial LPL during their systemic journey and how this may regulate their function and tissue homing remain to be defined.
Figure 1.
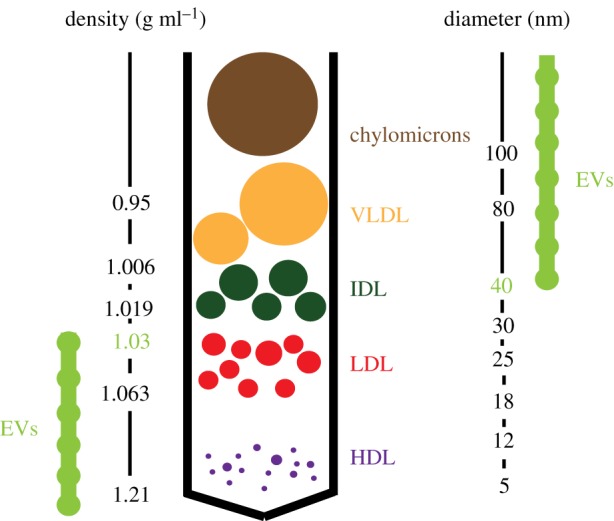
Distribution of lipoproteins versus EVs according to density gradient isolation (left scale) [17,18] and particle size (right scale). The relative size of lipoproteins is shown.
3. Where do they go?
EVs and lipoproteins also share some common biodistribution characteristics. Tumour tissues readily have access to and metabolize circulating lipids ([19–21] and reviewed in [22–24]), and HDL as well as LDL have been shown to transcytose over the endothelial cell barrier [25–27]. Also, in conditions where the endothelial barrier integrity is compromised, i.e. in cardiovascular disease [28] or aggressive tumours, lipoproteins can have direct access deep into tissues. Similarly, in addition to their well-established interactions with endothelial cells and role in angiogenesis stimulation, EVs have been found to carry miRNAs that can increase vascular permeability and thereby facilitate breast cancer metastasis [29,30]. EVs were shown to cross the blood–brain barrier by transcytosis, indicating that in addition to spread by local diffusion they can also travel and penetrate deeply into target tissues. One important difference, however, is the apparent circulating half-life between EVs and lipoproteins; whereas EVs are cleared from the circulation within minutes from injection [31,32], VLDL and HDL circulate for hours and LDL for up to 4 days. Under normal conditions, lipids derived from the selective uptake and hydrolysis of lipoproteins (remnant, LDL and HDL) are readily metabolized within peripheral tissues and stored under the form of intracellular lipid droplets (LDs) primarily in the liver and adipose tissue. EVs on the other hand have been shown to rapidly accumulate in resident macrophages of various organs such as the liver, lungs and spleen up to 48 h after injection and this was dependent on the route of administration [33]. Interestingly, EVs have been shown to accumulate in the tumour tissue of tumour bearing mice [33], and EVs may influence the metastatic potential of primary tumours by colonizing future sites of metastasis in, for example, breast and pancreatic cancer [34–36]. Under pathological conditions of atherosclerosis, oxidized lipoproteins accumulate in macrophages and other subendothelial cells of the vascular wall to induce an inflammatory response and plaque formation. To what extent circulating EVs contribute to these pathological events is an interesting topic of future investigations.
4. Uptake mechanisms: evidence for a key role of heparan sulphate proteoglycans
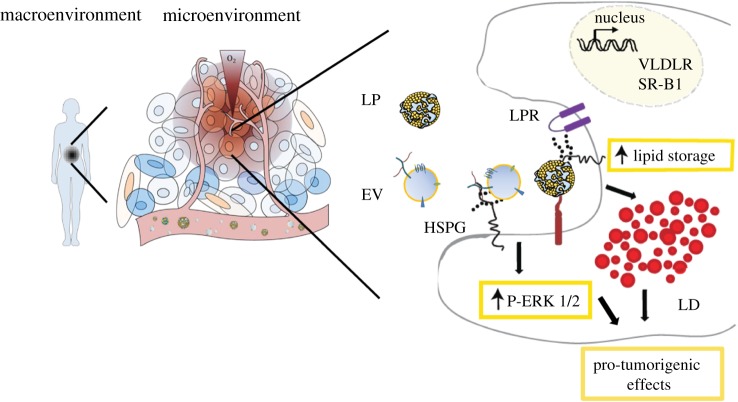
Lipoproteins have been shown to interact with cell-surface heparan sulphate proteoglycans (HSPGs) [37,38] for their clearance through classical, ‘specific’ receptors such as the low-density lipoprotein receptor (LDLR), low density lipoprotein receptor-related protein 1 (LRP1), very low density lipoprotein receptor (VLDLR) and the scavenger receptors (scavenger receptor class B member 1 (SR-B1), platelet glycoprotein 4 (CD36), among others). The glypicans and syndecans are cell-surface HSPGs conjugated with heparan sulphate (HS) chains of repeating hexuronic acid-N-acetylglucosamine disaccharide units sulfated at various positions to yield a highly polyanionic structure that interacts with stretches or patches of basic amino acids present in a wide variety of protein ligands. We and others have established HSPG as a key receptor in macromolecular internalization and intracellular membrane trafficking ([39], and reviewed in [40]). Importantly, HSPG, most notably syndecan-1, has been shown to act as an independent endocytic receptor for remnant, TG-rich lipoproteins in the liver [41]. Several cooperative models are still discussed and seem to be highly tissue and microenvironment dependent. HSPG-bound LPL is another important mediator of lipoprotein binding and processing at the cell surface, partially through ApoE [42,43]. In the atherosclerosis context, the role of HSPG in lipoprotein clearance by macrophages and foam cell formation is firmly established with increased binding and uptake through local particle and HSPG receptor modifications in the hypoxic and acidic plaque microenvironment [44–46]. Interestingly, hypoxia and acidosis may also modulate the release and transfer of EVs in the tumour microenvironment; several reports have shown an increased release of EVs in response to hypoxia [47,48] and oxidative stress [49,50], and acidic pH was shown to stimulate EV release as well as uptake through increased membrane fusion [51].
Depending on their source and target cell, EV internalization may occur via multiple processes such as phagocytosis, macropinocytosis, receptor mediated endocytosis and direct membrane fusion [52,53]. Importantly, as opposed to soluble ligands, EV interaction with target cells most probably depends on cooperative binding of multiple ligands available for high and low affinity interactions. This supposedly polyvalent nature of interactions is important when we try to conceptualize how EVs gain entry into target cells. A recent study found that following EV attachment to the cell membrane, EVs enter cells at specific, active sites, or endocytic hotspots [54]. After endocytosis, EVs were directed to the endoplasmic reticulum and sorted to lysosomes, where if they evade degradation they will deliver their content. Another study found that EVs co-localize with lipid raft markers in recipient tumour and endothelial cells, and it has been suggested that EV uptake is dependent on cholesterol-rich domains of the plasma membrane [55]. The lipoprotein scavenger receptor SR-B1 contributes to the cholesterol balance of the cell by controlling cholesterol efflux and influx between the cells and lipoproteins. In a study by Plebanek and colleagues, EV uptake was blocked by targeting SR-B1, hence disrupting the balance of membrane cholesterol [56]. Moreover, the lipid raft associated protein caveolin-1 was shown to negatively regulate EV uptake through suppression of p44/42 mitogen-activated protein kinases (ERK1/2) signalling [55]. This suggests an interesting link between lipoprotein uptake and the membrane composition of recipient cells with a direct effect on EV endocytosis.
How hypoxia regulates EV–cell interaction and EV uptake remains largely unknown. However, leukaemic cells and malignant lung tumours have been shown to crave lipoproteins [57,58], and tumours of various origins were shown to mobilize circulating lipids and lipoproteins for increased proliferation [59,60]. We recently found that increased lipoprotein uptake and storage as LDs in hypoxic and acidic cancer cells were mediated by cell-surface HSPG [61]. Interestingly, EVs also bind to heparin (a HS mimetic) and we found that cancer cell-derived EVs are dependent on HSPG for their uptake [62]. The details of this interaction are currently under investigation, but EVs have been shown to be enriched in specific lipoprotein ligands, such as ApoE that could be potential binding partners of HSPGs [63]. This raises the question to what extent there is competition for cell-surface binding and/or uptake between lipoproteins and EVs. However, preliminary data from our group show that co-incubation of exosome-like EVs with VLDL could actually enhance the uptake of EVs, indicating possible feeding of both particle types into a common, high capacity entry pathway (figure 2). This may occur either as separate particles or through direct co-association. Given that EVs have been shown to carry HS chains [62], one may speculate on a direct EV–lipoprotein interaction or aggregation that could lead to material transfer between the two particle types as well as activation of downstream uptake pathways. In relation to these findings, fibronectin was shown to act as a bridge between the HS on EVs and HS on recipient cells, and a specific antibody against the fibronectin heparin binding domain could block EV–cell interactions [64]. The proteoglycan (PG) expression pattern is known to be altered in different pathological conditions, such as diabetes and cancer. During tumorigenesis, the expression and glycosylation pattern of PGs change in the stroma surrounding cancer cells that may influence tumour growth and neoplastic progression [65]. An increased expression of proteoglycan proteins, including lumican, decorin, versican and biglycan, has been observed in cancer tissues and cells [66]. To what extent this has implications for EV tumour tropism and biodistribution remains to be studied.
Figure 2.
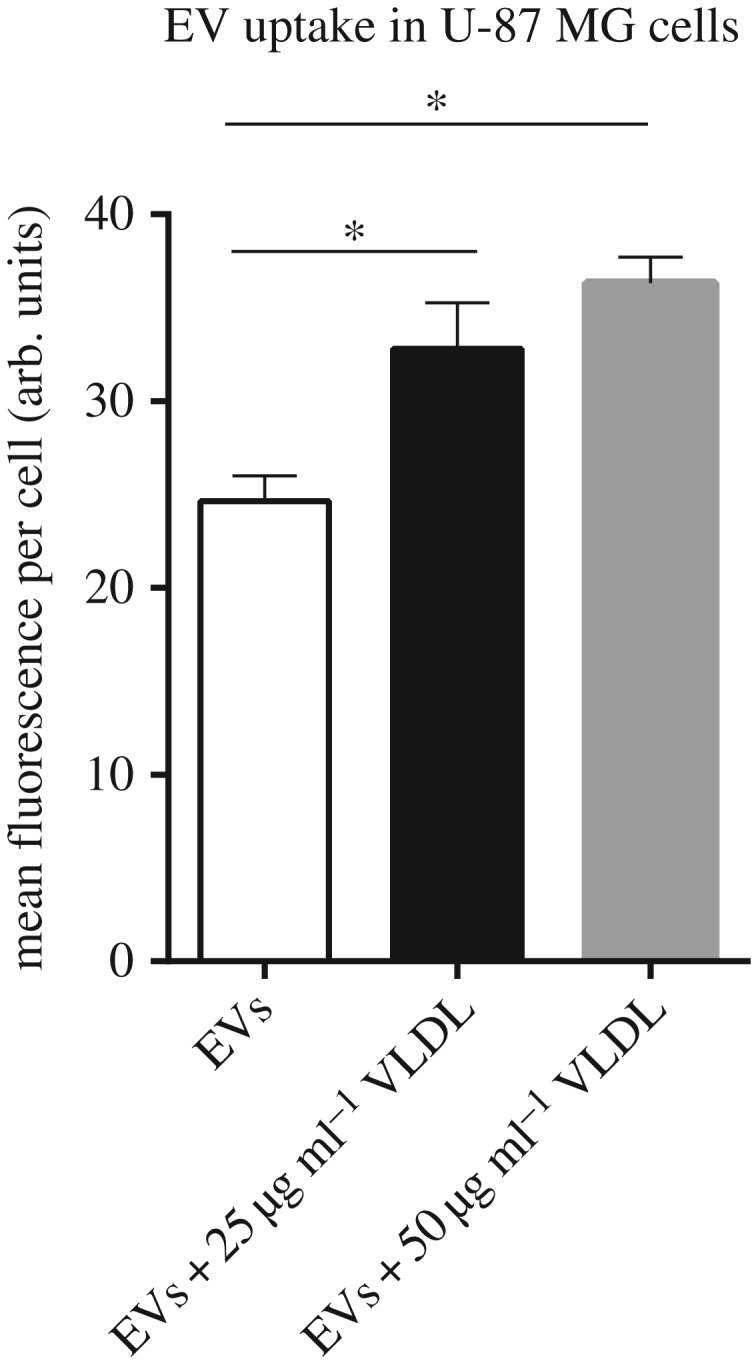
Exosome-like EV uptake in U87-MG glioma cells in the presence or absence of VLDL. EVs were isolated and labelled with the lipophilic fluorophore PKH67 according to a previously published method [54], and uptake (40 µg ml−1) for 1 h in the absence or presence of VLDL at the indicated concentrations was determined by flow cytometry. *p < 0.05.
5. Functional roles of extracellular vesicles and lipoproteins
The net effect of EVs in recipient cells involves signalling at multiple levels, including EV–cell surface receptor interaction; EV-mediated transfer of genetic material; and transfer of metabolites and cytosolic enzymes. The relevance of these processes in tumour progression has been widely studied, and EV transfer has been implicated in every step of tumour development (reviewed in [67] and 68]) not only on their local environment but also in systemic target niches and organs [69]. EVs have been implicated in the metabolic adaptation that occurs in the tumour microenvironment where different cell populations establish a synergistic relation by exchanging metabolites encapsulated in EVs [70,71]. Cell–cell crosstalk through EVs also includes inhibition of immune surveillance by EV-dependent generation of adenosine that may negatively regulate T cells in the tumour environment [72]. In a recent study by Thomou and colleagues, it was suggested that adipose tissue-derived EV miRNAs regulate gene expression at distant sites such as the liver, thereby interfering with systemic glucose metabolism [73]. Others investigated how liver derived EVs modulate the serum metabolome; ex vivo exposure of serum to EVs from hepatocytes modified the serum metabolite content hypothetically through the enzymatic activity carried by EVs [74]. Together, these studies point at the potential relevance of EVs in metabolic disease, but also during tumour progression as cancer cells are known to rely on reprogramming during adaptation to their microenvironment [75,76]. Accordingly, we demonstrated that hypoxia results in increased glioma cell release of procoagulant EVs that could trigger endothelial cell activation in a paracrine manner [77], and that hypoxia-derived EVs mimic the hypoxic response of glioma tumour cells resulting in enhanced in vivo tumour angiogenesis and growth [78]. Notably, the suggested tumour inhibitory effect of heparin and its derivatives [79] may, at least in part, be related to interference with EV-dependent procoagulant signalling and tumour angiogenesis in the hypoxic tumour niche.
Upon binding, EVs depend on ERK1/2 signalling for their uptake [55] and downstream functional effects [62] through HSPGs. Similarly, in tumour hypoxia and acidosis, increased lipid and lipoprotein uptake along with de novo lipogenesis have been shown to enhance the proliferation of cancer cells from breast, sarcoma and prostate tumours [19], survival and tumour progression of glioma [20], and lung as well as oral cancer metastasis [61,80]. Like EVs, LDL and VLDL trigger a reinforced ERK1/2 signalling response through HSPGs, and this response was further increased in hypoxic conditions [61]. The lipid loaded phenotype observed in several cell types, especially in stressed regions of aggressive tumours, is an emerging hallmark of their adaptive capabilities [81–83]. Interestingly, at least under acidic and hypoxic stress conditions, this adaptive response is dependent on HSPG-mediated recruitment of extracellular lipoproteins [61]. It remains to be investigated how lipoprotein and EV nanoparticles co-regulate downstream signalling events and how this coincides with their intracellular fate following HSPG-dependent endocytosis.
6. Concluding remarks
EVs and lipoproteins exhibit common structural and functional characteristics, which should be taken into account in the interpretation of previous and future data on their respective roles in physiology and in various pathological conditions, most importantly cancer (figure 3). In addition to their resemblance with lipoprotein particles, EVs also share similar characteristics with viral particles, as carriers of RNA and proteins [84]. Indeed, both lipoproteins and EVs can have an important role in viral infection; hepatitis C virus among others uses EVs [85], lipoproteins [86] and the downstream lipid droplets [87] for infection and replication. Hence, EVs and lipoprotein mimetic particles are being developed as drug delivery vehicles due to their stability, half-life and targeting capabilities [88–90]. Interestingly, lipoprotein-like particles have been shown to transfer lipids to EVs intracellularly following SR-B1-mediated uptake [91], further highlighting the possible interplay between the two particle types.
Figure 3.
Schematic summary of HSPG-dependent particle uptake in the tumour microenvironment. Lipoproteins and EVs can potentially interact during circulation in the blood and lymph fluid. In the context of the tumour microenvironment, these particles reach tumour resident cells where they can be internalized through an HSPG-mediated mechanism. This process takes place in the cell membrane domains known as lipid rafts and is associated with ERK1/2 activation. HSPG-mediated lipoprotein uptake can induce lipid droplet formation in recipient cells and this may have implications for the tumorigenic potential of these cells. EVs, extracellular vesicles; HSPG, heparan sulphate proteoglycan; LD, lipid droplet; LPR, lipoprotein receptor; SR-B1, scavenger receptor class B member 1; VLDLR, very low density lipoprotein receptor.
Although the proteome of EVs has been mapped in several studies, limited information is still available on the EV surface proteome. Identifying potential binding ligands for HSPGs and alternative uptake receptors thus remain key challenges. Ongoing investigations should clarify whether heparin and other inhibitors of HSPG function also can block the tumour promoting effects of lipoproteins and EVs in hypoxic tumours in vivo. Further, as hypoxia and acidosis are general characteristics of aggressive tumours, and the stress response to a large degree is universal, a more general role of circulating EVs as dynamic biomarkers of the tumour cell signalling status may be envisioned.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Our work is supported by grants from The Swedish Cancer Fund; the Swedish Research Council; the Swedish Childhood Cancer Foundation; the Gunnar Nilsson, Anna Lisa and Sven Eric Lundgren, and Kamprad Foundations; the Skåne University Hospital donation funds; the Governmental funding of clinical research within the national health services (M.B.); and a donation by Viveca Jeppsson (M.B.).
References
- 1.Brat DJ, Van Meir EG. 2004. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Invest. 84, 397–405. ( 10.1038/labinvest.3700070) [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. 2007. Life with oxygen. Science 318, 62–64. ( 10.1126/science.1147949) [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG Jr, Ratcliffe PJ. 2008. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402. ( 10.1016/j.molcel.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. 2013. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218. ( 10.1200/JCO.2012.46.3653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Record M, Carayon K, Poirot M, Silvente-Poirot S. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 1841, 108–120. ( 10.1016/j.bbalip.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 6.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. 2015. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65, 783–797. ( 10.1093/biosci/biv084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone RM, Bianchini A, Teng K. 1989. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 74, 1844–1851. [PubMed] [Google Scholar]
- 8.Stegmayr B, Ronquist G. 1982. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 10, 253–257. ( 10.1007/BF00255932) [DOI] [PubMed] [Google Scholar]
- 9.Pisitkun T, Shen RF, Knepper MA. 2004. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13 368–13 373. ( 10.1073/pnas.0403453101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. 2007. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978. ( 10.4049/jimmunol.179.3.1969) [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan S, Vannberg FO, Dixon JB. 2016. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 6, 24436 ( 10.1038/srep24436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowal J, et al. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977. ( 10.1073/pnas.1521230113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randolph GJ, Miller NE. 2014. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124, 929–935. ( 10.1172/JCI71610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Eckel RH. 2014. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 25, 8–14. ( 10.1016/j.tem.2013.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michell DL, Vickers KC. 2016. Lipoprotein carriers of microRNAs. Biochim. Biophys. Acta 1861, 2069–2074. ( 10.1016/j.bbalip.2016.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keerthikumar S, et al. 2016. ExoCarta: a web-based compendium of exosomal cargo. J. Mol. Biol. 428, 688–692. ( 10.1016/j.jmb.2015.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. 2014. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles 3, 23262 ( 10.3402/jev.v3.23262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sodar BW, et al. 2016. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 6, 24316 ( 10.1038/srep24316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuemmerle NB, et al. 2011. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 10, 427–436. ( 10.1158/1535-7163.MCT-10-0802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bensaad K, et al. 2014. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 9, 349–365. ( 10.1016/j.celrep.2014.08.056) [DOI] [PubMed] [Google Scholar]
- 21.Guillaumond F, et al. 2015. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA 112, 2473–2478. ( 10.1073/pnas.1421601112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos CR, Schulze A. 2012. Lipid metabolism in cancer. FEBS J. 279, 2610–2623. ( 10.1111/j.1742-4658.2012.08644.x) [DOI] [PubMed] [Google Scholar]
- 23.Kamphorst JJ, Gottlieb E. 2016. Cancer metabolism: friendly neighbours feed tumour cells. Nature 536, 401–402. ( 10.1038/nature19420) [DOI] [PubMed] [Google Scholar]
- 24.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. 2016. Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189 ( 10.1038/oncsis.2015.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Eckardstein A, Rohrer L. 2009. Transendothelial lipoprotein transport and regulation of endothelial permeability and integrity by lipoproteins. Curr. Opin. Lipidol. 20, 197–205. ( 10.1097/MOL.0b013e32832afd63) [DOI] [PubMed] [Google Scholar]
- 26.Bian F, et al. 2014. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br. J. Pharmacol. 171, 2671–2684. ( 10.1111/bph.12616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong SM, et al. 2015. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 108, 268–277. ( 10.1093/cvr/cvv218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabas I, Garcia-Cardeña G, Owens GK. 2015. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209, 13–22. ( 10.1083/jcb.201412052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W. et al. 2014. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25, 501–515. ( 10.1016/j.ccr.2014.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Modica M, Regondi V, Sandri M, Iorio MV, Zanetti A, Tagliabue E, Casalini P, Triulzi T. 2017. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 384, 94–100. ( 10.1016/j.canlet.2016.09.013) [DOI] [PubMed] [Google Scholar]
- 31.Choi H, Lee DS. 2016. Illuminating the physiology of extracellular vesicles. Stem. Cell Res. Ther. 7, 55 ( 10.1186/s13287-016-0316-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. 2014. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123, 208–216. ( 10.1182/blood-2013-03-489732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiklander OP, et al. 2015. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 ( 10.3402/jev.v4.26316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peinado H, Lavotshkin S, Lyden D. 2011. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin. Cancer Biol. 21, 139–146. ( 10.1016/j.semcancer.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 35.Costa-Silva B, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. ( 10.1038/ncb3169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. 2016. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848. ( 10.1016/j.ccell.2016.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolset SO, Salmivirta M. 1999. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell. Mol. Life Sci. 56, 857–870. ( 10.1007/s000180050031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahley RW, Huang Y. 2007. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J. Clin. Invest. 117, 94–98. ( 10.1172/JCI30889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes BB, et al. 2013. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl Acad. Sci. USA 110, E3138–E3147. ( 10.1073/pnas.1301440110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christianson HC, Belting M. 2014. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix. Biol. 35, 51–55. ( 10.1016/j.matbio.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 41.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. 2009. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119, 3236–3245. ( 10.1172/jci38251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Man FHAF, de Beer F, van der Laarse A, Smelt AHM, Leuven JAG, Havekes LM. 1998. Effect of apolipoprotein E variants on lipolysis of very low density lipoproteins by heparan sulphate proteoglycan-bound lipoprotein lipase. Atherosclerosis 136, 255–262. ( 10.1016/S0021-9150(97)00218-9) [DOI] [PubMed] [Google Scholar]
- 43.Libeu CP, et al. 2001. New insights into the heparan sulfate proteoglycan-binding activity of apolipoprotein E. J. Biol. Chem. 276, 39 138–39 144. ( 10.1074/jbc.M104746200) [DOI] [PubMed] [Google Scholar]
- 44.Crucet M, Wust SJA, Spielmann P, Luscher TF, Wenger RH, Matter CM. 2013. Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis 229, 110–117. ( 10.1016/j.atherosclerosis.2013.04.034) [DOI] [PubMed] [Google Scholar]
- 45.Oorni K, Rajamaki K, Nguyen SD, Lahdesmaki K, Plihtari R, Lee-Rueckert M, Kovanen PT. 2015. Acidification of the intimal fluid: the perfect storm for atherogenesis. J. Lipid Res. 56, 203–214. ( 10.1194/jlr.R050252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wada Y, et al. 2002. Lipid accumulation in smooth muscle cells under LDL loading is independent of LDL receptor pathway and enhanced by hypoxic conditions. Arterioscler. Thromb. Vasc. Biol. 22, 1712–1719. ( 10.1161/01.ATV.0000033834.57737.9B) [DOI] [PubMed] [Google Scholar]
- 47.King HW, Michael MZ, Gleadle JM. 2012. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 12, 421 ( 10.1186/1471-2407-12-421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. 2014. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl Acad. Sci. USA 111, E3234–E3242. ( 10.1073/pnas.1410041111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. 2010. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 5, e15353 ( 10.1371/journal.pone.0015353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. 2011. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 6, e16899 ( 10.1371/journal.pone.0016899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parolini I, et al. 2009. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34 211–34 222. ( 10.1074/jbc.M109.041152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. 2010. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687. ( 10.1111/j.1600-0854.2010.01041.x) [DOI] [PubMed] [Google Scholar]
- 53.Mulcahy LA, Pink RC, Carter DR. 2014. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 ( 10.3402/jev.v3.24641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heusermann W, et al. 2016. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 213, 173–184. ( 10.1083/jcb.201506084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M. 2013. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17 713–17 724. ( 10.1074/jbc.M112.445403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plebanek MP, Mutharasan RK, Volpert O, Matov A, Gatlin JC, Thaxton CS. 2015. Nanoparticle targeting and cholesterol flux through scavenger receptor type B-1 inhibits cellular exosome uptake. Sci. Rep. 5, 15724 ( 10.1038/srep15724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho YK, Smith RG, Brown MS, Goldstein JL. 1978. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood 52, 1099–1114. [PubMed] [Google Scholar]
- 58.Vitols S, Peterson C, Larsson O, Holm P, Aberg B. 1992. Elevated uptake of low density lipoproteins by human lung cancer tissue in vivo. Cancer Res. 52, 6244–6247. [PubMed] [Google Scholar]
- 59.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. 2009. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 69, 5259–5266. ( 10.1158/0008-5472.CAN-08-3444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Li L, Lian J, Schauer S, Vesely PW, Kratky D, Hoefler G, Lehner R. 2016. Tumor-induced hyperlipidemia contributes to tumor growth. Cell Rep. 15, 336–348. ( 10.1016/j.celrep.2016.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menard JA, et al. 2016. Metastasis stimulation by hypoxia and acidosis-induced extracellular lipid uptake is mediated by proteoglycan-dependent endocytosis. Cancer Res. 76, 4828–4840. ( 10.1158/0008-5472.CAN-15-2831) [DOI] [PubMed] [Google Scholar]
- 62.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. 2013. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl Acad. Sci. USA 110, 17 380–17 385. ( 10.1073/pnas.1304266110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haraszti RA, et al. 2016. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 5, 32570 ( 10.3402/jev.v5.32570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. 2016. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J. Biol. Chem. 291, 1652–1663. ( 10.1074/jbc.M115.686295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coulson-Thomas YM, Gesteira TF, Norton AL, Kao WW, Nader HB, Coulson-Thomas VJ. 2015. The role of proteoglycans in the reactive stroma on tumor growth and progression. Histol. Histopathol. 30, 33–41. ( 10.14670/HH-30.33) [DOI] [PubMed] [Google Scholar]
- 66.Pan S, Brentnall TA, Chen R. 2016. Glycoproteins and glycoproteomics in pancreatic cancer. World. J. Gastroenterol. 22, 9288–9299. ( 10.3748/wjg.v22.i42.9288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whiteside TL. 2016. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 74, 103–141. ( 10.1016/bs.acc.2015.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Syn N, Wang LZ, Sethi G, Thiery JP, Goh BC. 2016. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 37, 606–617. ( 10.1016/j.tips.2016.04.006) [DOI] [PubMed] [Google Scholar]
- 69.Hoshino A, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. ( 10.1038/nature15756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao H, et al. 2016. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 5, e10250 ( 10.7554/eLife.10250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Achreja A, Zhao H, Yang L, Yun TH, Marini J, Nagrath D. 2017. Exo-MFA - A 13C metabolic flux analysis framework to dissect tumor microenvironment-secreted exosome contributions towards cancer cell metabolism. Metab. Eng. 43, 156–172. ( 10.1016/j.ymben.2017.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. 2011. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 187, 676–683. ( 10.4049/jimmunol.1003884) [DOI] [PubMed] [Google Scholar]
- 73.Thomou T, et al. 2017. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455. ( 10.1038/nature21365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royo F, et al. 2017. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci. Rep. 7, 42798 ( 10.1038/srep42798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu D, et al. 2017. Chronic inflammation confers to the metabolic reprogramming associated with tumorigenesis of colorectal cancer. Cancer Biol. Ther. 18, 237–244. ( 10.1080/15384047.2017.1294292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 77.Svensson KJ, et al. 2011. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl Acad. Sci. USA 108, 13 147–13 152. ( 10.1073/pnas.1104261108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kucharzewska P, et al. 2013. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl Acad. Sci. USA 110, 7312–7317. ( 10.1073/pnas.1220998110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belting M. 2014. Glycosaminoglycans in cancer treatment. Thromb. Res. 133, S95–101. ( 10.1016/S0049-3848(14)50016-3) [DOI] [PubMed] [Google Scholar]
- 80.Pascual G, et al. 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. ( 10.1038/nature20791) [DOI] [PubMed] [Google Scholar]
- 81.Baenke F, Peck B, Miess H, Schulze A. 2013. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 6, 1353–1363. ( 10.1242/dmm.011338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abramczyk H, Surmacki J, Kopec M, Olejnik AK, Lubecka-Pietruszewska K, Fabianowska-Majewska K. 2015. The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 140, 2224–2235. ( 10.1039/c4an01875c) [DOI] [PubMed] [Google Scholar]
- 83.Koizume S, Miyagi Y. 2016. Lipid droplets: a key cellular organelle associated with cancer cell survival under normoxia and hypoxia. Int. J. Mol. Sci. 17, 1430 ( 10.3390/ijms17091430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nolte-'t Hoen E, Cremer T, Gallo RC, Margolis LB. 2016. Extracellular vesicles and viruses: are they close relatives? Proc. Natl Acad. Sci. USA 113, 9155–9161. ( 10.1073/pnas.1605146113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chahar HS, Bao X, Casola A. 2015. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 7, 3204–3225. ( 10.3390/v7062770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vercauteren K, Mesalam AA, Leroux-Roels G, Meuleman P. 2014. Impact of lipids and lipoproteins on hepatitis C virus infection and virus neutralization. World J. Gastroenterol. 20, 15 975–15 991. ( 10.3748/wjg.v20.i43.15975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogawa K, Hishiki T, Shimizu Y, Funami K, Sugiyama K, Miyanari Y, Shimotohno K. 2009. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85, 217–228. ( 10.2183/pjab.85.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thaxton CS, Rink JS, Naha PC, Cormode DP. 2016. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv. Drug Deliv. Rev. 106, 116–131. ( 10.1016/j.addr.2016.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang GL, Liu Y, Huang HL. 2016. Synthetic lipoproteins as carriers for drug delivery. Curr. Med. Chem. 23, 4601–4608. ( 10.2174/0929867323666161024150151) [DOI] [PubMed] [Google Scholar]
- 90.Di Rocco G, Baldari S, Toietta G. 2016. Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem. Cells Int. 2016, 5029619 ( 10.1155/2016/5029619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Angeloni NL, McMahon KM, Swaminathan S, Plebanek MP, Osman I, Volpert OV, Thaxton CS. 2016. Pathways for modulating exosome lipids identified by high-density lipoprotein-like nanoparticle binding to scavenger receptor type B-1. Sci. Rep. 6, 22915 ( 10.1038/srep22915) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.