Abstract
Cancer cells do not grow as an isolated homogeneous mass; tumours are, in fact, complex and heterogeneous collections of cancer and surrounding stromal cells, collectively termed the tumour microenvironment. The interaction between cancer cells and stromal cells in the tumour microenvironment has emerged as a key concept in the regulation of cancer progression. Understanding the intercellular dialogue in the tumour microenvironment is therefore an important goal. One aspect of this dialogue that has not been appreciated until recently is the role of extracellular vesicles (EVs). EVs are small vesicles released by cells under both normal and pathological conditions; they can transfer biological molecules between cells leading to changes in phenotype. EVs have emerged as important regulators of biological processes and can be dysregulated in diseases such as cancer; rapidly growing interest in their biology and therapeutic potential led to the Royal Society hosting a Scientific Meeting to explore the roles of EVs in the tumour microenvironment. This cross-disciplinary meeting explored examples of how aberrant crosstalk between tumour and stromal cells can promote cancer progression, and how such signalling can be targeted for diagnostic, prognostic and therapeutic benefit. In this review, and the special edition of Philosophical Transactions of the Royal Society B that follows, we will provide an overview of the content and outcomes of this exciting meeting.
This article is part of the discussion meeting issue ‘Extracellular vesicles and the tumour microenvironment’.
Keywords: extracellular vesicles, cancer, tumour microenvironment, exosomes
1. Introduction
Cancer is a nefarious disease that claims millions of victims around the world each year [1]. Improvements in treatment have increased the overall survival rates, particularly for some tumour types but, despite decades of intensive research, some forms of cancer remain frequently refractory to curative therapy [2]. Several factors contribute to this, including: (i) the heterogeneous nature of tumours, both between patients and within the tumour mass itself [3], (ii) the tendency for tumours to evolve and adapt to change in their environment, for example giving them the ability to become resistant to drugs [4], and (iii) the propensity of cancer cells to metastasize to local and distant sites, which is ultimately what normally kills the patient [5]. The need for further research into cancer is therefore greater than ever.
Our opening paragraph paints a rather bleak picture of cancer research and treatment. However, our efforts as a community have yielded countless breakthroughs in our understanding of what drives cancer development and progression. One important concept that has only recently gained broad acceptance is the role of the tumour microenvironment (TME) [6]. Cancer cells do not exist in isolation, but rather they coexist with normal cells in the body. Indeed, a multitude of non-cancer cells can be found throughout the mass of the tumour, collectively termed the tumour microenvironment. In this way tumours are as complex, if not more so, than any healthy organ in the body. Non-tumour cells that reside in the cancer mass include cancer-associated fibroblasts (CAFs) and tumour-associated macrophages (TAMs) (figure 1), as well as other immune cells and endothelial cells embedded in the extracellular matrix (ECM). The interaction between these cells and the tumour are generally accepted to be of crucial importance, but the nature of the interactions and how they may be disrupted remains poorly understood. Cells and non-cellular components of the TME are thought to sustain the tumour and can add to the heterogeneity, adaptability and metastatic ability of cancer [7]. Indeed, they can contribute to most, if not all, aspects of tumour progression. Understanding the tumour microenvironment is therefore of key importance if we are to make genuine breakthroughs in cancer treatment.
Figure 1.
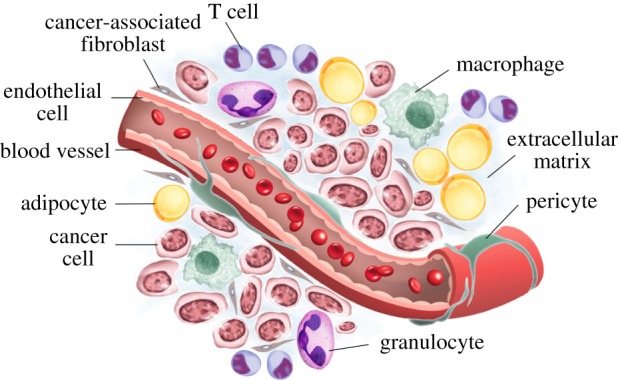
The tumour microenvironment. Schematic illustrating the major cellular and non-cellular components of a typical solid tumour microenvironment.
Extracellular vesicles (EVs) are another relatively new concept in cancer biology. EVs were discovered many years ago, but the realization that they are not simply a waste disposal system and that they play important roles in various biological processes only occurred in the last few years [8]. EVs are small lipid-enclosed vesicles that are released by cells into the extracellular space. Several types of EV are produced by cells, and the nomenclature depends on the method of their biogenesis (figure 2). Exosomes are EVs produced when multivesicular bodies (MVBs) fuse with the plasma membrane, releasing the intraluminal vesicles they carry out of the cell [9]. Microvesicles are another class of EV that is formed by outward budding of the plasma membrane [9]. Apoptotic bodies are EVs released by cells undergoing apoptosis [9]. EVs can carry a variety of cargo, including lipids, proteins, coding and non-coding RNA, and even DNA. EVs released by one cell can be taken up by another cell, leading to the transfer of macromolecules from a donor cell to a recipient cell [10]. This can be associated with the regulation of important phenotypic changes in the recipient cell. Indeed, EVs have been shown to have important roles in a range of biological roles [11]. Thus, EVs are emerging as an important part of the molecular dialogue between cells in a complex organism.
Figure 2.
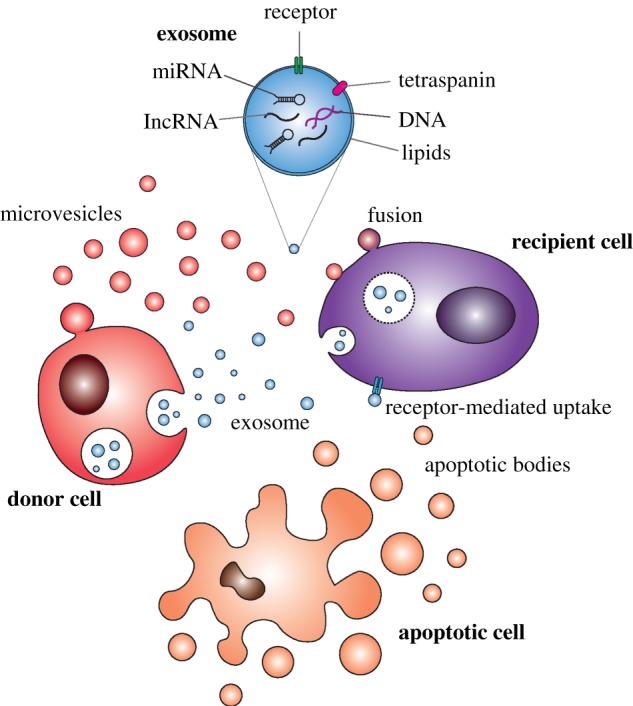
Overview of extracellular vesicle release and uptake. Schematic illustrating the generation of exosomes (blue) from endosomal compartments and plasma membrane–derived microvesicles (pink) from a donor cell. Major mechanisms of uptake by a recipient cell are indicated. The major molecular cargo of exosomes is also illustrated in the cutout, and an apoptotic cell shown to demonstrate the generation of apoptotic bodies.
Given their importance in communication between cells it is reasonable to hypothesize that EVs could mediate crucial interactions between cells in the tumour microenvironment. A number of studies have begun to address this question, and it is indeed emerging that cancer cells and non-malignant cells can exchange EVs. This reciprocal transfer of EVs can play an important role in promoting cancer progression [12]. Therefore, we must try to characterize and understand the roles and mechanisms of EV-mediated communication in the tumour microenvironment, an endeavour which should also yield new therapeutic targets for the treatment for cancer. Given the nascent state of the field and the potential therapeutic rewards, it would seem sensible to bring together researchers in these two fields, EVs and the tumour microenvironment, to discuss the potential synergies and seed new collaborations. For these reasons the Royal Society hosted a Scientific Meeting on the topic of ‘Extracellular Vesicles in the Tumour Microenvironment’. The meeting was held on the 23rd and 24th of January 2017 at the Royal Society's historic headquarters in London. The meeting was well attended, with over 160 scientists at all levels, from undergraduates to group leaders, from clinicians to enthusiastic members of the public. Leading experts from across the globe were invited to present their cutting-edge work in these fields, and a vibrant poster session was held. There were many opportunities for interaction with presenters, including during an active discussion in the final session, in which some of the challenges of working on EVs in the tumour microenvironment were debated. Here we will summarize some of the key messages and try to reflect the excitement of the meeting, as well as introducing the work submitted by some of the speakers to this special edition of Philosophical Transactions of the Royal Society B.
2. New methodology for studying extracellular vesicles in the tumour microenvironment
The field of extracellular vesicles is relatively new and the best methodology for studying them is still being established. Technical challenges such as their small size, heterogeneity and paucity of molecular content make them particularly difficult to study. This difficulty was reflected in several of the discussions through the meeting. However, interesting new findings and methodologies are emerging that will help to push the field forward. For example, Victoria James (Nottingham University, UK) described a method for analysing the nature of vesicular RNA taken up by recipient cells. This will help in deciphering exactly what RNA species are passed between cells in EVs. Using this technique Dr James described how vesicular transfer can occur between prostate cancer cells and bone cells (osteoblasts).
Microscopy is a key area that needs to be developed for improved imaging of EVs. Michiel Pegtel (VU University Medical Center Amsterdam, Netherlands) and James Edgar (University of Cambridge, UK) both presented exquisite images of EVs being released by cells. Dr Pegtel presented a novel fluorescent construct of CD63 (a known EV protein marker), which could be imaged by total internal reflection fluorescence (TIRF) microscopy. Dr Edgar used a combination of techniques including electron microscopy to show that exosomes are tethered to the membrane of MVBs via tetherin, and often remain bound to the plasma membrane after the MVB fuses at the cell surface. Knocking down tetherin increased the release of exosomes into the extracellular space [13]. Tuula Salo (University of Helsinki, Finland) has developed a novel alternative to Matrigel® (a product used to mimic extracellular matrix that is often used in experiments to measure the invasive properties of cancer cells) [14]. Using this material, Myogel, she showed that EVs can induce invasive ability when placed onto recipient cells.
3. Extracellular vesicle heterogeneity
Another important issue in the study of EVs is that of heterogeneity. EVs prepared with most commonly used methodologies are heterogeneous in nature. Any biological effects of the total population of EVs are a result of the overall combined effect of the different subtypes present. However, as results are often conflicting in the literature (and sometimes within studies), it is important to delve deeper into the population of EVs to look for subtype-specific functions. The potential biological roles of different subtypes of EVs within a heterogeneous mix are largely unknown, but insights are beginning to emerge from important studies. Deborah Goberdhan (University of Oxford, UK) presented her exciting results on different subtypes of EVs. She showed that inhibiting a glutamine-sensing transporter affected the mTORC1 signalling pathways and led to the release of EVs with different biomolecular content. These EVs could induce a range of effects in recipient cells which promoted changes in the tumour microenvironment that supported tumour progression [15]. Clotilde Théry (Institut Curie Research Center, France) described her ground-breaking work on characterizing the molecular heterogeneity of EVs. She described the contents and different biological effects of heterogenous EVs released by dendritic cells [16]. She made an excellent analogy between the current field of EVs and the field of immunology a few decades ago. In the field of immunology it was recognized that lymphocytes could be divided into different subtypes; after many years of painstaking research many of these subtypes have been identified and characterized. A similar effort is required in the EV field to identify and study the roles of different vesicular subtypes in the tumour microenvironment and more widely in physiology and pathophysiology.
4. Tumour extracellular vesicles and the immune system
Many studies have shown that EVs play an important role in the immune response [17]. The immune system is implicated in clearing early-stage tumours, and so one of the hallmarks of cancer that progresses is the ability to evade the immune response [7]. As part of this process, cancer cells can release EVs that may subvert cells of the immune system within the tumour microenvironment [12]. Christopher Gregory (MRC Centre for Inflammation Research, The University of Edinburgh, UK) described how apoptotic cells in the tumour microenvironment can activate endothelial cells (which in turn promote angiogenesis) and macrophages, both of which then support the tumour in what Prof Gregory terms the ‘onco-regenerative niche’ [18]. Elke Pogge von Strandmann (University of Cologne, Germany) showed that by activating the retinoic acid–inducible gene I in malignant B cells EVs were released with the ability to trigger natural killer cells to kill the tumour cells [19]. This highlights the complex crosstalk between the tumour and the immune system and how it could potentially be targeted for therapeutic benefit. Muller Fabbri (University of Southern California, USA) described his seminal work on non-canonical functions of miRNAs. He showed that miRNAs do not always act in the classically described way (by silencing gene expression) but can also act as ligands to RNA-binding proteins such as Toll-like receptors (TLRs) [20]. He described how miRNAs released by tumour cells can act as hormones within the tumour microenvironment, binding to TLR8 in TAMs. These TAMs in turn release EVs carrying miRNAs that, when taken up by the tumour cells, induce tumour progression and drug resistance [21].
5. Extracellular vesicles in the tumour microenvironment
Other examples of how EVs modulate crosstalk within the tumour microenvironment were also described. Michael Graner (University of Colorado Denver, USA) showed that glioblastoma cells can release EVs that activate astrocytes, cells that can support neuron function. These activated astrocytes can migrate towards the tumour cells and release various factors that support the growth of the tumour. Ana O'Loghlen (Queen Mary University of London, UK) presented her work on senescent cells. When cells age or accrue damage to their DNA they can become senescent, a state in which the cells are metabolically active but no longer able to divide. Dr O'Loghlen showed here that during senescence the tumour cells secrete EVs that can influence tumour cells and other stromal cells in the tumour microenvironment. Mattias Belting (Lund University, Sweden) has published seminal work on the pathways of EV uptake and the effects of hypoxia on EV function within the tumour microenvironment [22,23]. Here Prof Belting presented his latest work on how the regulation of EV uptake and release can affect tumour aggressiveness and how this could represent an important therapeutic target. Junko Ohyashiki (Tokyo Medical University, Japan) described how hypoxic multiple myeloma can release EVs carrying miRNAs able to induce angiogenesis [24]. Miki De Palma (École polytechnique fédérale de Lausanne) showed how EVs released by cells treated with paclitaxel (a microtubule-stabilizing agent) can affect metastatic colonization in breast cancer. Hector Peinado (Spanish National Cancer Research Centre) described how EVs released by tumour cells can reprogramme stromal cells, including in the lymphatic system. These changes lead to the formation of a pre-metastatic niche, which facilitates the metastatic spread of the tumour to lymph nodes.
6. Extracellular vesicles as biomarkers in the tumour microenvironment
The accessibility of EVs in many different biofluids makes them ideal biomarkers for the diagnosis and prognosis of tumours [25]. Early diagnosis provides one of the best ways of improving survival in cancer, so developing new biomarkers for the disease should be a priority. Stuart Hunt (University of Sheffield, UK) described how EVs released by head and neck cancer cells into the tumour microenvironment could be harnessed as biomarkers in ‘liquid biopsies’ for earlier detection of tumours. Future work is needed to expand on this and test the potential of EVs as biomarkers.
7. Conclusion
These examples of crosstalk between cancer and the tumour microenvironment represent significant examples of an emerging field. Further work is required to fully elucidate the nature and extent of this crosstalk, as well as exploring the therapeutic and diagnostic potential of tapping into this ongoing dialogue in the tumour microenvironment. We hope that this vibrant meeting and this special edition of Philosophical Transactions of the Royal Society B will provide a stimulus to researchers studying the role of EVs in the tumour microenvironment.
Acknowledgements
We thank the Royal Society for selecting this exciting topic for a Scientific Meeting. We thank those supporting and organizing the meeting, and in particular Reisha Simmonds for her invaluable help. We would like to thank all the speakers for presenting their work and contributing to this special edition and Helen Eaton for her editorial assistance. Finally, we would like to thank Stuart Hunt, Aled Clayton and Andrew Devitt for their help in organizing and running the meeting.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to writing the manuscript and/or preparing the figures. All authors gave approval for submission.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. 2016. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Biomarkers Prev. 25, 16–27. ( 10.1158/1055-9965.EPI-15-0578) [DOI] [PubMed] [Google Scholar]
- 2.Pierotti MA. 2017. The molecular understanding of cancer: from the unspeakable illness to a curable disease. Ecancermedicalscience 11, 747 ( 10.3332/ecancer.2017.747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alizadeh AA, et al. 2015. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 21, 846–853. ( 10.1038/nm.3915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. 2014. Drug resistance in cancer: an overview. Cancers 6, 1769–1792. ( 10.3390/cancers6031769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leber MF, Efferth T. 2009. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 34, 881–895. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. 2015. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 13, 45 ( 10.1186/s12916-015-0278-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 8.Théry C. 2011. Exosomes: secreted vesicles and intercellular communications. F1000 Biol. Rep. 3, 15 ( 10.3410/B3-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Raposo G, Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. ( 10.1146/annurev-cellbio-101512-122326) [DOI] [PubMed] [Google Scholar]
- 10.Mulcahy LA, Pink RC, Carter DR. 2014. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 ( 10.3402/jev.v3.24641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yáñez-Mó M, et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 ( 10.3402/jev.v4.27066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webber J, Yeung V, Clayton A. 2015. Extracellular vesicles as modulators of the cancer microenvironment. Semin. Cell Dev. Biol. 40, 27–34. ( 10.1016/j.semcdb.2015.01.013) [DOI] [PubMed] [Google Scholar]
- 13.Edgar JR, Manna PT, Nishimura S, Banting G, Robinson MS. 2016. Tetherin is an exosomal tether. Elife 5, e17180 ( 10.7554/eLife.17180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salo T, et al. 2015. A novel human leiomyoma tissue derived matrix for cell culture studies. BMC Cancer 15, 981 ( 10.1186/s12885-015-1944-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan SJ, et al. 2016. PAT4 levels control amino-acid sensitivity of rapamycin-resistant mTORC1 from the Golgi and affect clinical outcome in colorectal cancer. Oncogene 35, 3004–3015. ( 10.1038/onc.2015.363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowal J, et al. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977. ( 10.1073/pnas.1521230113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins PD, Dorronsoro A, Booker CN. 2016. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest. 126, 1173–1180. ( 10.1172/JCI81131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory CD, Ford CA, Voss JJ. 2016. Microenvironmental effects of cell death in malignant disease. Adv. Exp. Med. Biol. 930, 51–88. ( 10.1007/978-3-319-39406-0_3) [DOI] [PubMed] [Google Scholar]
- 19.Daßler-Plenker J, et al. 2016. RIG-I activation induces the release of extracellular vesicles with antitumor activity. Oncoimmunology 5, e1219827 ( 10.1080/2162402X.2016.1219827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbri M, et al. 2012. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl Acad. Sci. USA 109, E2110–E2116. ( 10.1073/pnas.1209414109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challagundla KB, et al. 2015. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J. Natl. Cancer Inst. 107, djv135 ( 10.1093/jnci/djv135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Mörgelin M, Belting M. 2013. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288, 17 713–17 724. ( 10.1074/jbc.M112.445403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucharzewska P, et al. 2013. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl Acad. Sci. USA 110, 7312–7317. ( 10.1073/pnas.1220998110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohyashiki JH, Umezu T, Ohyashiki K. 2016. Exosomes promote bone marrow angiogenesis in hematologic neoplasia: the role of hypoxia. Curr. Opin Hematol. 23, 268–273. ( 10.1097/MOH.0000000000000235) [DOI] [PubMed] [Google Scholar]
- 25.Properzi F, Logozzi M, Fais S. 2013. Exosomes: the future of biomarkers in medicine. Biomark Med. 7, 769–778. ( 10.2217/bmm.13.63) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


