Abstract
Objectives
To systematically review studies comparing extraperitoneal (E-RP) and transperitoneal minimally invasive radical prostatectomy (T-RP).
Methods
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in September 2015. Several databases were searched including Medline and Scopus. Only studies comparing E-RP and T-RP (either laparoscopic or robot-assisted approach) were evaluated. The follow-up of the included patients had to be ≥6 months.
Results
In all, 1256 records were identified after the initial database search. Of these 20 studies (2580 patients) met the inclusion criteria. The hospital stay was significantly lower in the E-RP cohort, with a mean difference of −0.30 days (95% confidence interval [CI] −0.35, −0.24) for the laparoscopic group and 1.09 days (95% CI −1.47, −0.70) for the robotic group (P < 0.001). Early continence rates favoured the E-RP group, although this was statistically significant only in the laparoscopic group (odds ratio [OR] 2.52, 95% CI 1.72, 3.70; P < 0.001). There was no statistically significant difference between the E-RP and T-RP cohorts for 12-month continence rates for both the laparoscopic (OR 1.55, 95% CI 0.89, 2.69; P = 0.12) and robotic groups (OR 3.03, 95% CI 0.54, 16.85; P = 0.21). The overall complication and ileus rates were significantly lower in the E-RP cohort for both the laparoscopic and robotic groups. The symptomatic lymphocele rate favoured the T-RP cohort, although this was statistically significant only in the laparoscopic group (OR 8.69, 95% CI 1.60, 47.17; P = 0.01).
Conclusion
This review suggests that the extraperitoneal approach is associated with a shorter hospital stay, lower overall complication rate, and earlier return to continence when compared to the transperitoneal approach. The transperitoneal approach has a lower lymphocele rate.
Abbreviations: BTR, blood transfusion rate; EBL, estimated blood loss; LOS, length of hospital stay; MD, mean difference; MIRP, minimally invasive radical prostatectomy; OR, odds ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PSM, positive surgical margin; (E-)(T-)RP, (extraperitoneal) (transperitoneal) radical prostatectomy; STROBE, Reporting of Observational Studies in Epidemiology
Keywords: Extraperitoneal, Laparoscopy, Minimally invasive, Robotic, Transperitoneal, Prostatectomy
Introduction
Radical prostatectomy (RP) is the ‘gold standard’ curative surgical treatment option for the management of clinically localised prostate cancer [1]. Since the first description of laparoscopic RP by Schuessler et al. [2], there has been significant evolution in the techniques of minimally invasive RP (MIRP), which include the laparoscopic RP and the robot-assisted laparoscopic RP. There are various techniques for MIRP described in the contemporary literature. Most of these techniques were initially described with conventional laparoscopy and subsequently adopted in robotic surgery. Regardless of the technique used, all of these procedures require either an extraperitoneal or transperitoneal approach. The extraperitoneal approach emulates the open retropubic RP and avoids any access to the peritoneal cavity [3]. In the transperitoneal approach the intraperitoneal space provides more space enabling easier port insertion and robotic docking (in the case of the robot-assisted technique) [3]. Both approaches have advantages and disadvantages, without any clear evidence on the most appropriate approach for MIRP. Thus, the final selection of the approach seems to be more a matter of personal expertise and preference.
The aim of the present study was to systematically review the studies comparing extraperitoneal and transperitoneal approaches with an emphasis on perioperative and immediate outcomes, positive surgical margin (PSM) rate, continence rate, and complications. Additionally, we critically evaluated the methodology and outcome reporting of the existing literature in this field.
Methods
All randomised trials and observational studies comparing extraperitoneal RP (E-RP) and transperitoneal RP (T-RP) were considered.
Search strategy and study selection
The systematic review was performed according to the Cochrane guidelines. Databases searched were Medline, Embase, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and individual urological journals. The search was conducted in August 2015. All studies comparing E-RP and T-RP (both conventional laparoscopy and robotic approach) were evaluated; also references of searched papers were evaluated for potential inclusion.
Comparative outcome endpoints between E-RP and T-RP
-
1.
Perioperative and immediate outcomes: Operative time, estimated blood loss (EBL), blood transfusion rate (BTR), length of hospital stay (LOS), and analgesia requirement.
-
2.
Functional outcome: Continence rates at ≤3 month (early continence) and 12 months.
-
3.
Oncological outcome: overall PSM rate.
-
4.
Complications and mortality: Overall complication, Ileus, lymphocele, bladder neck stenosis, rectal injury, and mortality rates.
Data extraction and statistical analysis
Two reviewers (B.P.R., P.K.) independently identified all studies that appeared to fit the inclusion criteria for full review. Disagreement was resolved by consensus. If data were unavailable or incomplete, the data of the study were included into the review, but not into the meta-analysis. Where possible a meta-analysis was performed for comparable data. If a meta-analysis was not possible the data were presented in a narrative fashion.
A Mantel–Haenszel chi-squared test was used for continuous data and expressed as the mean difference (MD) with 95% CI, and for dichotomous data inverse variance was used and expressed as odds ratio (OR) or risk difference with 95% CI. A P value was considered significant if <0.05. Heterogeneity was analysed using a chi-squared test on N-1 degrees of freedom, with an α of 0.05 used for statistical significance and with the I2 test [1]. I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity. A fixed-effect model was used unless statistically significant high heterogeneity existed between studies. A random-effects model was used if heterogeneity existed.
Quality assessment of studies
An assessment of the methodological quality of the included studies was conducted consistent with the Cochrane handbook for randomised control trials [4]. For quality assessment the selection bias, performance bias, detection bias, attrition bias, and reporting bias were assessed in each of the included studies. For observational studies quality assessment was performed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [5]. Additionally, all studies were evaluated to assess if the quality of the reporting of complications met the Martin criteria for complication reporting [6].
Results
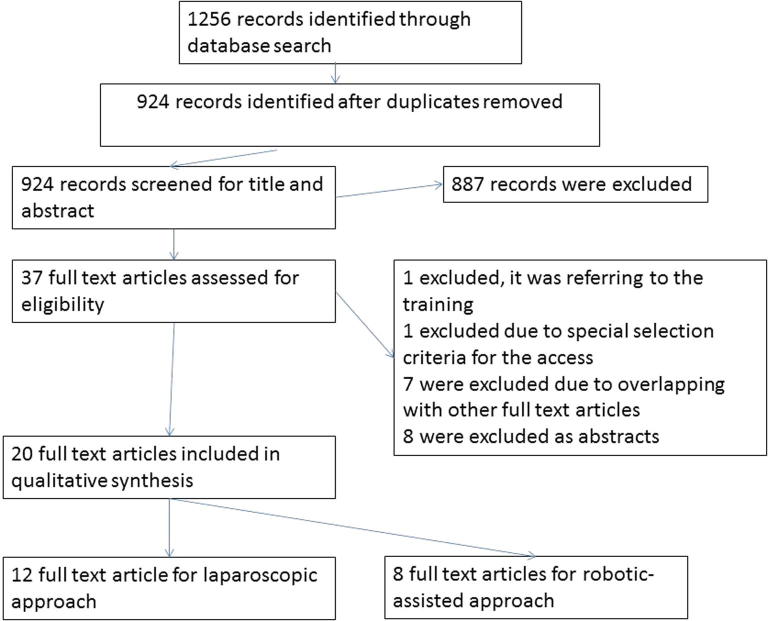
In all, 1256 records were identified after the initial database search. Of these, 20 studies met the inclusion criteria with a total of 2580 patients (E-RP 1380 vs T-RP 1200) (Fig. 1) [3], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. In all, 12 studies compared laparoscopic E-RP and laparoscopic T-RP [8], [9], [10], [13], [14], [15], [17], [20], [21], [22], [23], [24]. Eight studies compared robotic E-RP and robotic T-RP [3], [7], [11], [12], [16], [18], [19], [25]. Only one study was a randomised control trial comparing robotic E-RP and robotic T-RP [3], and 19 were observational comparative studies [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25] (Table 1 [3], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]).
Fig. 1.
PRISMA chart.
Table 1.
Characteristics and summary of immediate outcomes of the included studies.
| Reference | Approach/type of study | E-RP vs T-RP |
|||||
|---|---|---|---|---|---|---|---|
| Number of patients | Mean operative, min | Mean EBL, mL | BTR, % | Mean LOS, days | Mean catheter indwelling, days | ||
| Bivalacqua et al. 2008 [8] | Laparoscopic/unclear | 29 vs 21 | 216 vs 228 | – | 0 vs 0 | 1.9 vs 2.2 | 12 vs 12 |
| Brown et al. 2005 [9] | Laparoscopic/retrospective | 34 vs 122 | 191 vs 197 | – | 0 vs 4 | 1.6 vs 2.1 | – |
| Cathelineau et al. 2004 [10] | Laparoscopic/retrospective | 100 vs 100 | 163 vs 173 | 375 vs 360 | 3 vs 4 | 6.1 vs 5.8 | 6.2 vs 6 |
| Eden et al. 2004 [13] | Laparoscopic/unclear | 100 vs100 | 190.6 vs 338.9 | 201.5 vs 310.5 | 0 vs 2 | 2.6 vs 3.8 | 10.1 vs.11.3 |
| Erdogru et al. 2004 [14] | Laparoscopic/prospective matched pair | 53 vs 53 | 211.8 vs 197.1 | – | 13.2 vs 16.9 | – | 10.6 vs 10.9 |
| Hoznek et al. 2003 [17] | Laparoscopic/retrospective | 20 vs 20 | 169.6 vs 224.2 | 442.1 vs 600.5 | 10 vs 15 | 6.4 vs 6.5 | 4.2 vs 5.3 |
| Phinthusophon et al. 2007 [20] | Laparoscopic/retrospective | 69 vs 56 | 220 vs 350 | 605 vs 883 | 0.32 vs 1.23 | – | 8.9 vs 11.90 |
| Porpiglia et al. 2006 [21] | Laparoscopic/unclear | 80 vs 80 | 133.7 vs 179.4 | 594.34 vs 562.5 | 8.75 vs 11.25 | 8.52 vs 7.6 | 7.21 vs 7.53 |
| Remzi et al. 2005 [22] | Laparoscopic/retrospective | 41 vs 39 | 217 vs 279 | 189 vs 290 | – | 7 vs 7 | 6.1 vs 7.2 |
| Ruiz et al. 2004 [23] | Laparoscopic/unclear | 165 vs 165 | 220 vs 248 | 803 vs 678 | 5.4 vs 1.2 | 6.3 vs 6.7 | 6.6 vs 5.1 |
| Siqueira et al. 2010 [24] | Laparoscopic/retrospective | 40 vs 40 | 267.6 vs 175 | 292.4 vs 177.5 | – | 3 vs 3 | – |
| Gao et al. 2006 [15] | Laparoscopic/unclear | 19 vs 12 | 196 vs 262 | 261 vs 270 | 0 vs 8.3 | 10 vs 12 | 7.2 vs 7.4 |
| Atug et al. 2006 [7] | Robotic/prospective | 40 vs 40 | 229.15 vs 236.23 | 221 vs 250 | – | 1.2 vs 1.1 | – |
| Capello et al. 2007 [3] | Robotic/randomised study | 31 vs 31 | 181 vs 191 | 199 vs 163 | – | – | – |
| Chung et al. 2011 [12] | Robotic/unclear | 155 vs 105 | 150.3 vs 162.1 | 350.8 vs 361.7 | – | 5.1 vs 6.2 | 7.7 vs 7.3 |
| Madi et al. 2007 [19] | Robotic/unclear | 34 vs 21 | 214 vs 241 | 125 vs 150 | – | 1 vs 1 | – |
| Lee et al. 2009 [25] | Robotic/unclear | 30 vs 30 | 206.2 vs 202.1 | 445 vs 361.7 | – | 6.6 vs 6.2 | 7.7 vs 7.3 |
| Jacobs et al. 2011 [18] | Robotic/prospective | 167 vs 48 | 209 vs 310 | 125 vs 188 | – | 1.1 vs 1.5 | – |
| Horstmann et al. 2012 [16] | Robotic/prospective | 103 vs 67 | 192 vs 224 | 276 vs 281 | – | 7.8 vs 8.8 | 6.2 vs 6.5 |
| Anderson et al. 2013 [11] | Robotic/prospective | 70 vs 50 | 236.8 vs 255.7 | 372 vs 342 | 2.9 vs 2 | 1.94 vs 3.54 | – |
Outcomes
Perioperative outcomes
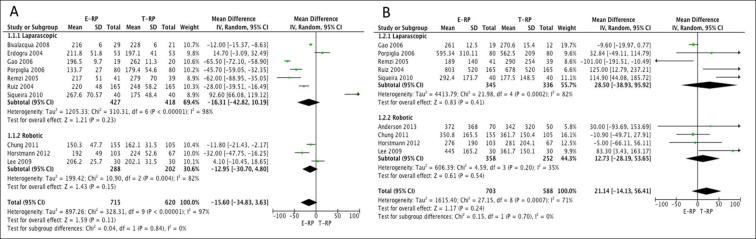
Operative time: All 20 studies reported on operative time [3], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. The mean (SD) operative times ranged between 133.7 (27) and 267.6 (70.57) min for laparoscopic E-RP and between 173 and 350 min for laparoscopic T-RP. The mean (SD) operative times ranged between 150.3 (47.7) and 206.2 (25.7) min for robotic E-RP and between 162.1 (31.5) and 224 (52.6) min for robotic T-RP. In all, 10 studies (seven laparoscopic and three robotic) had data on mean operative time that were suitable for meta-analysis [8], [12], [14], [15], [16], [21], [22], [23], [24], [25]. There was no statistical difference in operating times between the two cohorts for both the laparoscopic (MD –16.31, 95% CI –42.82, 10.19; P = 0.23) and robotic groups (MD −12.95, 95% CI −30.70, 4.80; P = 0.15) (Fig. 2A).
Fig. 2.
Meta-analysis of operative time (A) and EBL (B).
EBL and BTR: In all, 17 studies reported on EBL. Nine [11], [12], [15], [16], [21], [22], [23], [24] (five laparoscopic and four robotic) of these studies had data that were suitable for meta-analysis. There was no statistical difference in EBL between the two cohorts for both the laparoscopic (MD 28.50, 95% CI −38.93, 95.92; P = 0.41) and robotic groups (MD 12.73, 95% CI −28.19, 53.65; P = 0.54) (Fig. 2B). In all, 10 studies [8], [9], [10], [11], [13], [14], [15], [17], [21], [23] (nine laparoscopic and one robotic) reported on BTR between the two groups. There was no statistical difference in the BTR between the cohorts on meta-analysis for both the laparoscopic (OR 0.92, 95% CI 0.54, 1.55; P = 0.74) and robotic groups (OR 1.44, 95% CI 0.13, 16.34; P = 0.77) (Fig. 3A).
Fig. 3.
Meta-analysis of BTR (A) and LOS (B).
LOS: Data from nine studies [8], [11], [12], [15], [16], [21], [22], [23], [25] (five laparoscopic and four robotic) on mean LOS were considered suitable for meta-analysis. The meta-analysis favoured the E-RP cohort, which had a shorter LOS for both the laparoscopic (MD −0.30, 95% CI −0.35, −0.24; P < 0.001) and robotic groups (MD −1.09, 95% CI −1.47, −0.70; P < 0.001) (Fig. 3B).
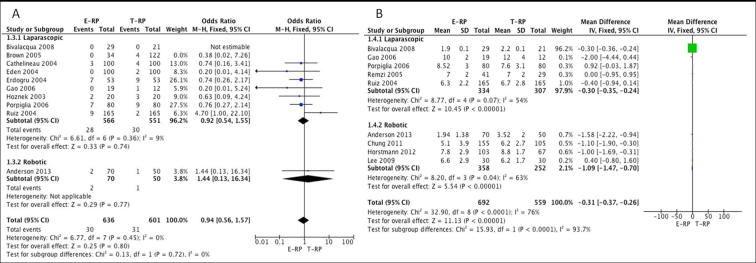
Analgesia requirement: Five studies [8], [12], [14], [21], [22] (four laparoscopic and one robotic) had data on mean analgesia requirements appropriate for meta-analysis. Erdogru et al. [14] reported narcotic and non-narcotic usage separately and hence both the data sets were used for analysis. There was no statistical difference between the two cohorts on meta-analysis for both the laparoscopic (MD −3.33, 95% CI −11.43, 4.77), P = 0.42) and robotic groups (MD 1.20, 95% CI −1.84, 4.24; P = 0.44) (Fig. 4A).
Fig. 4.
Meta-analysis of analgesic usage (A) and early continence (B).
Functional outcome
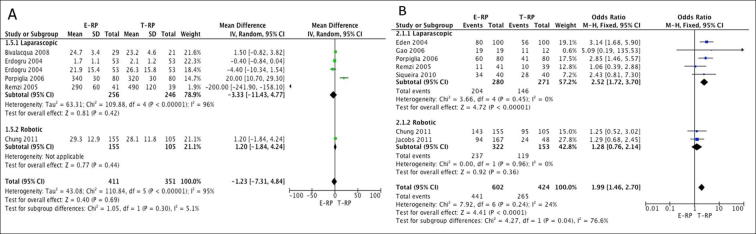
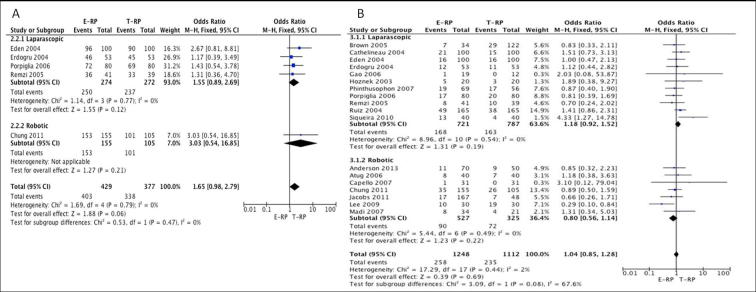
Early continence rates: Seven studies [12], [13], [15], [18], [21], [22], [24] (five laparoscopic and two robotic) had data available that were considered suitable for meta-analysis. The meta-analysis showed the E-RP cohort had better continence rates when compared to the T-RP cohort in the laparoscopic group (OR 2.52, 95% CI 1.72, 3.70; P < 0.001) (Fig. 3B). However, there was no difference in the early continence rates between the two cohorts in the robotic group (OR 1.28, 95% CI 0.76, 2.14; P = 0.36) (Fig. 4B).
Continence rates at 12 months: Five studies [12], [13], [14], [21], [22] (four laparoscopic and one robotic) provided data suitable for meta-analysis. There was no statistical difference between the two cohorts for continence rates at 12 months for both the laparoscopic (OR 1.55, 95% CI 0.89, 2.69; P = 0.12) and robotic groups (OR 3.03, 95% CI 0.54, 16.85; P = 0.21) (Fig. 5A).
Fig. 5.
Meta-analysis of 12-month continence (A) and PSM (B).
Oncological outcome
PSM rate: Data from 18 studies [3], [7], [9], [10], [11], [12], [13], [14], [15], [17], [18], [19], [20], [21], [22], [23], [24], [25] (11 laparoscopic and seven robotic) were used to perform a meta-analysis for PSM rate. There was no statistical difference between the two cohorts for PSM rates for both the laparoscopic (OR 1.18, 95% CI 0.92, 1.52; P = 0.19) and robotic groups (OR 0.80, 95% CI 0.56, 1.14; P = 0.22) (Fig. 5B).
Complications
In all, 17 studies reported on complications [3], [7], [8], [9], [10], [11], [12], [13], [14], [16], [17], [19], [20], [21], [22], [23], [24]. Only three studies adopted a complication grading system, which was the Clavien–Dindo grading system in all of them [11], [16], [21]. The assumed grading of the individual complications is shown in Table 2, Table 3.
Table 2.
Estimated Clavien–Dindo grading of reported complications in the studies using the laparoscopic approach.
| Complication | Clavien–Dindo grade | E-RP, n | T-RP, n |
|---|---|---|---|
| Ileus | 2/3A/3B | 1 | 13 |
| Urine leakage | 2/3A/3B | 21 | 33 |
| Bladder neck stenosis | 3B | 4 | 8 |
| Urethral stricture | 3B | 2 | 1 |
| Meatal stricture | 3B | 1 | 0 |
| Rectal injury | 3B | 4 | 8 |
| Surgical intervention for bleeding | 3B | 2 | 6 |
| Lymphocele | 3A/3B | 11 | 0 |
| Epigastric injury | 3B | 2 | 0 |
| Pelvic haematoma | 2/3B | 2 | 4 |
| Scrotal haematoma | 2 | 0 | 1 |
| Rectal haematoma | 2/3B | 1 | 0 |
| Subcutaneous haematoma | 2 | 1 | 6 |
| AUR | 2 | 4 | 7 |
| Nerve paraesthesias | 2 | 1 | 3 |
| Bladder injury | 3B | 1 | 0 |
| Faecal incontinence | 2/3B | 0 | 1 |
| Urethral bleeding | 2 | 1 | 0 |
| Recto-urethral fistula | 3B | 1 | 3 |
| Port site hernia | 3B | 0 | 1 |
| Incisional hernia | 3B | 1 | 0 |
| UTI | 1 | 0 | 10 |
| Urosepsis | 2 | 0 | 1 |
| Unspecified wound complication | 2/3B | 2 | 3 |
| Perineal pain | 2 | 0 | 4 |
| Pulmonary failure requiring intubation | 4A | 0 | 1 |
| Pulmonites | 2 | 0 | 1 |
| C. difficile | 2 | 0 | 1 |
| Acute renal failure | 2/4A | 1 | 0 |
| Myocardial infarction | 2/3A/3B | 1 | 1 |
| Cerebrovascular accident | 3B | 0 | 2 |
| Unspecified medical complication | – | 4 | 4 |
| Death | 5 | 0 | 2 |
| Total, n/N (%) | 69/750 (9.2) | 125/808 (15.5) |
Table 3.
Estimated Clavien–Dindo grading of reported complications in the studies using the robotic approach.
| Complication | Clavien–Dindo grade | E-RP, n | T-RP, n |
|---|---|---|---|
| Ileus | 2/3A/3B | 1 | 11 |
| Arm neuropraxia | 1 | 0 | 1 |
| Thromboembolism | 4 | 2 | 3 |
| Gross haematuria (no surgical intervention) | 2 | 0 | 1 |
| Prolonged drainage | 2 | 1 | 0 |
| Bladder neck stenosis | 3B | 1 | 1 |
| AUR | 1/2 | 1 | 1 |
| Epigastric vessel injury | 3B | 1 | 1 |
| Urine leak | 2/3A/3B | 1 | 3 |
| Rectal injury | 2/3B | 1 | 2 |
| Lymphocele | 2/3A/3B | 14 | 6 |
| Port wound opening | 1 | 1 | 0 |
| Hernia | 3B | 1 | 2 |
| Major haematoma | 3A | 3 | 0 |
| Ureteric injury | 2 | 0 | 1 |
| Death (myocardial infarction) | 5 | 1 | 0 |
| Total, n/N (%) | 29/630 (4.6) | 33/717 (4.6) |
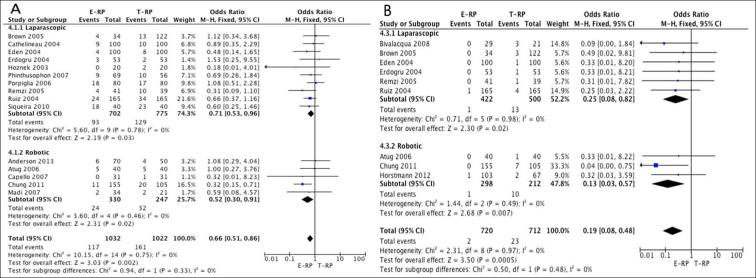
Overall complications: In all, 15 studies [3], [7], [9], [10], [11], [12], [13], [14], [17], [19], [20], [21], [22], [23], [24] (10 laparoscopic and five robotic) had data available suitable for meta-analysis comparing the two cohorts. The meta-analysis favoured the E-RP cohort both in the laparoscopic (OR 0.71, 95% CI 0.53, 0.96; P = 0.03) and robotic groups (OR 0.52, 95% CI 0.30, 0.91; P = 0.02) (Fig. 6A).
Fig. 6.
Meta-analysis of overall complications (A) and ileus (B).
Ileus: Data from nine studies [7], [8], [9], [12], [13], [14], [16], [22], [23] (six laparoscopic and three robotic) were pooled for analysis. The meta-analysis favoured the E-RP cohort in both the laparoscopic (OR 0.25, 95% CI 0.08, 0.82; P = 0.02) and robotic groups (OR 0.13, 95% CI 0.03, 0.57; P = 0.007) (Fig. 6B).
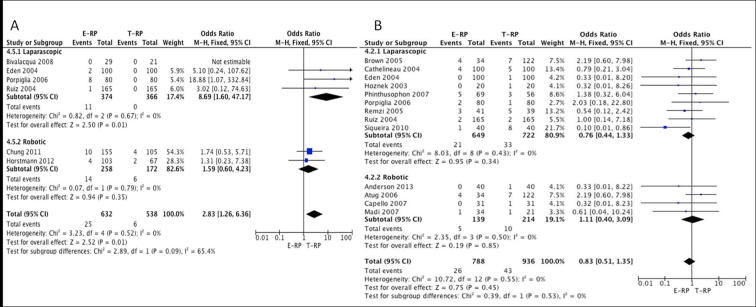
Lymphocele: Six studies [8], [12], [13], [16], [21], [23] (three laparoscopic and three robotic) provided data for the pooled analysis. The meta-analysis favoured the T-RP cohort in the laparoscopic group, which achieved statistical significance (OR 8.69, 95% CI 1.60, 47.17; P = 0.01). In the robotic cohort, although the meta-analysis favoured the T-RP cohort it did not achieve statistical significance (OR 1.59, 95% CI 0.60, 4.23; P = 0.35) (Fig. 7A).
Fig. 7.
Meta-analysis of lymphocele (A) and anastomotic leakage (B).
Anastomotic leak: In all, 13 studies [3], [7], [9], [10], [11], [13], [17], [19], [20], [21], [22], [23], [24] (nine laparoscopic and four robotic) had data available suitable for meta-analysis comparing the two cohorts. The meta-analysis did not show a statistical difference between the two cohorts for urine leakage for both the laparoscopic (OR 0.76, 95% CI 0.44, 1.33; P = 0.34) and robotic groups (OR 1.11, 95% CI 0.40, 3.09; P = 0.85) (Fig. 7B).
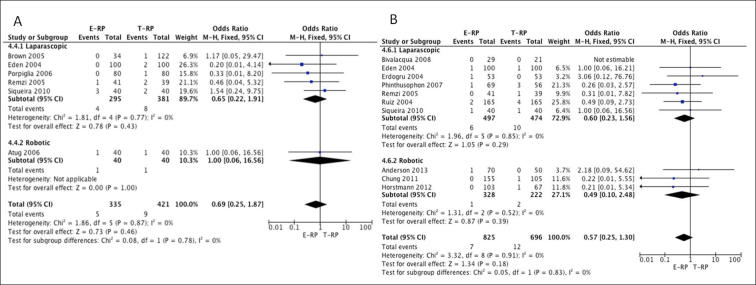
Bladder neck stenosis: Six studies [7], [9], [13], [21], [22], [24] (five laparoscopic and one robotic) had suitable data for meta-analysis. The results did not show a statistical difference between the two cohorts for bladder neck stenosis for both the laparoscopic (OR 0.65, 95% CI 0.22, 1.91; P = 0.43) and robotic groups (OR 1.00, 95% CI 0.06, 16.56; P = 1.0) (Fig. 8A).
Fig. 8.
Meta-analysis of bladder neck stenosis (A) and rectal injury (B).
Rectal injury: In all, 10 studies [8], [11], [12], [13], [14], [16], [20], [22], [23], [24] (seven laparoscopic and three robotic) had comparative data. The meta-analysis did not show a statistical difference between the two cohorts for both the laparoscopic (OR 0.60, 95% CI 0.23, 1.56; P = 0.29) and robotic groups (OR 0.49, 95% CI 0.10, 2.48; P = 0.39) (Fig. 8B).
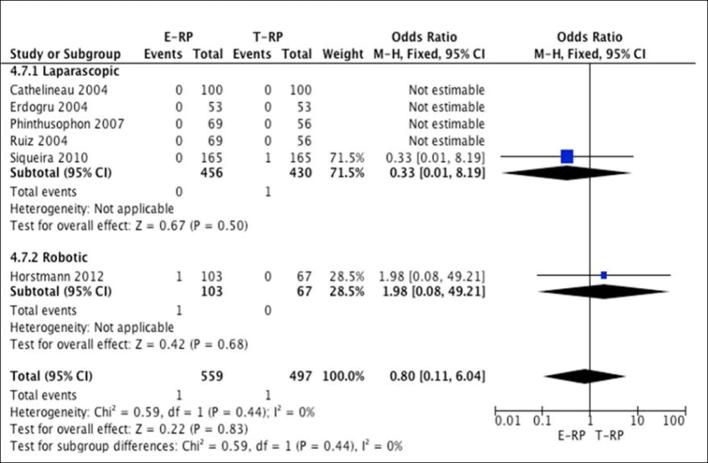
Mortality rates: Six studies [10], [14], [16], [20], [23], [24] (five laparoscopic and one robotic) reported on mortality. The meta-analysis did not show any difference between the two cohorts for both the laparoscopic (OR 0.33, 95% CI 0.01, 8.19; P = 0.5) and robotic groups (OR 1.98, 95% CI 0.08, 49.21; P = 0.68) (Fig. 9).
Fig. 9.
Meta-analysis of mortality.
Quality assessment of studies and complications reporting
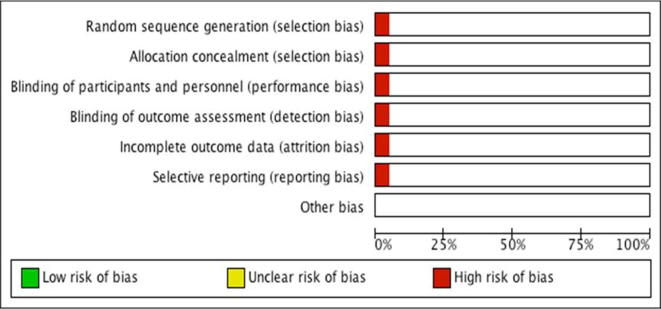
Only one randomised study was identified in the present review, which had a high risk of selection, performance, detection, attrition and reporting biases (Fig. 10) [3]. The remaining 19 studies were observational studies of which five were prospective studies (one matched pair analysis), six retrospective analyses, and eight uncharacterised studies [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. The quality assessment of these studies was done using the STROBE guidelines and is shown in Table 4 [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. Lee et al. [25] was excluded from this assessment as parts of the study were in Korean.
Fig. 10.
Risk of bias chart.
Table 4.
Evaluation of studies using the STROBE guidelines.
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivalacqua et al. 2008 [8] | N | Y | Y | N | P | P | N | P | N | Y | Y | P | Y | P | Y | P | NA | Y | N | P | Y | N |
| Brown et al. 2005 [9] | N | Y | Y | Y | P | P | N | N | N | Y | Y | P | Y | Y | Y | N | NA | Y | N | Y | Y | N |
| Cathelineau et al. 2004 [10] | N | Y | Y | Y | P | P | N | N | N | Y | Y | P | Y | Y | Y | N | NA | N | N | Y | Y | N |
| Eden et al. 2004 [13] | N | Y | Y | P | P | P | N | N | N | Y | N | N | Y | Y | Y | N | Y | Y | N | Y | Y | N |
| Erdogru et al. 2004 [14] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N |
| Hoznek et al. 2003 [17] | N | Y | Y | Y | P | P | P | Y | N | Y | P | P | Y | Y | Y | N | NA | Y | Y | Y | Y | N |
| Phinthusophon et al. 2007 [20] | N | Y | Y | Y | Y | Y | N | P | N | Y | Y | Y | Y | Y | Y | P | NA | P | N | Y | Y | N |
| Porpiglia et al. 2006 [21] | N | Y | Y | P | Y | Y | P | Y | P | Y | P | Y | Y | Y | Y | P | NA | Y | N | Y | Y | N |
| Remzi et al. 2005 [22] | N | Y | Y | Y | Y | Y | P | Y | P | Y | Y | Y | Y | Y | Y | P | NA | Y | N | Y | Y | N |
| Ruiz et al. 2004 [23] | N | Y | Y | P | Y | N | N | N | N | Y | N | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | N |
| Siqueira et al. 2010 [24] | Y | Y | Y | Y | Y | Y | P | P | Y | Y | Y | Y | Y | Y | Y | P | NA | Y | N | Y | Y | N |
| Gao et al. 2006 [15] | N | P | P | P | N | P | Y | Y | N | Y | P | Y | Y | Y | Y | P | NA | Y | N | Y | Y | N |
| Atug et al. 2006 [7] | N | Y | Y | Y | P | N | Y | P | N | Y | Y | Y | Y | Y | Y | N | NA | Y | N | Y | Y | N |
| Chung et al. 2011 [12] | N | Y | Y | Y | P | N | Y | N | P | Y | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | Y | N |
| Madi et al. 2007 [19] | N | Y | Y | P | P | N | P | Y | P | Y | Y | Y | Y | Y | Y | N | NA | P | P | Y | Y | N |
| Jacobs et al. 2011 [18] | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | NA | Y | Y | Y | Y | Y |
| Horstmann et al. 2012 [16] | N | Y | Y | Y | P | Y | Y | Y | Y | P | Y | Y | P | N | Y | Y | NA | Y | Y | Y | Y | N |
| Anderson et al. 2013 [11] | N | Y | Y | Y | Y | P | P | Y | N | Y | Y | Y | Y | N | Y | N | NA | Y | Y | Y | Y | N |
1 – Title and abstract.
Introduction: 2 – Explain the scientific background and rationale for the investigation being reported, 3 – Objectives.
Methods: 4 – Study design, 5 – Setting, 6 – Participants, 7 – Variables, 8 – Data sources/measurement, 9 – Bias, 10 – Study size, 11 – Quantitative variables, 12 – Statistical methods.
Results: 13 – Participants, 14 – Descriptive data, 15 – Outcome data, 16 – Main results, 17 – Other analyses.
Discussion: 18 – Key results, 19 – Limitations, 20 – Interpretation, 21 – Generalisability, 22 – Funding.
Y, yes addressed by study; N, not addressed by study; P, partially addressed by study; NA, not applicable.
Of the 17 studies reporting on complications, the mean (SD, range) number of Martin criteria fulfilled by all the studies was 5.71 (1.57, 2–8) (Table 5) [3], [7], [8], [9], [10], [11], [12], [13], [14], [16], [17], [19], [20], [21], [22], [23], [24]. Only three studies adopted a complication grading system (Clavien–Dindo grading system) [11], [16], [21].
Table 5.
Evaluation of Martin criteria for the studies reporting on complications.
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | TC, n |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bivalacqua et al. 2008 [8] | N | N | N | P | N | N | Y | N | Y | N | 2 |
| Brown et al. 2005 [9] | Y | N | Y | Y | N | Y | Y | N | Y | Y | 5 |
| Cathelineau et al. 2004 [10] | Y | P | N | Y | Y | Y | Y | N | Y | N | 6 |
| Eden et al. 2004 [13] | N | P | Y | P | N | Y | Y | N | Y | N | 4 |
| Erdogru et al. 2004 [14] | Y | Y | Y | Y | Y | Y | Y | N | Y | N | 8 |
| Hoznek et al. 2003 [17] | Y | N | N | Y | N | Y | Y | N | Y | Y | 6 |
| Phinthusophon et al. 2007 [20] | Y | Y | Y | Y | Y | Y | Y | N | N | N | 7 |
| Porpiglia et al. 2006 [21] | N | Y | Y | Y | N | Y | Y | Y | Y | N | 7 |
| Remzi et al. 2005 [22] | Y | Y | Y | Y | N | Y | Y | N | Y | N | 7 |
| Ruiz et al. 2004 [23] | N | Y | Y | Y | Y | Y | Y | N | Y | N | 7 |
| Siqueira et al. 2010 [24] | Y | Y | Y | N | Y | Y | Y | N | Y | N | 7 |
| Atug et al. 2006 [7] | Y | N | N | Y | N | Y | Y | N | Y | N | 5 |
| Capello et al. 2007 [3] | Y | N | N | N | N | Y | Y | N | N | N | 3 |
| Chung et al. 2011 [12] | N | Y | Y | Y | N | Y | Y | N | Y | N | 6 |
| Madi et al. 2007 [19] | N | Y | N | Y | N | Y | Y | N | Y | N | 5 |
| Horstmann et al. 2012 [16] | Y | N | N | Y | Y | N | Y | Y | Y | N | 6 |
| Anderson et al. 2013 [11] | Y | P | P | Y | N | Y | Y | Y | Y | N | 6 |
| Mean (SD) TC, n | 5.71 (1.57) |
1 – Method of accruing data defined – Prospective or retrospective accrual of data are indicated.
2 – Duration of follow-up indicated – Report clarifies the time period of postoperative accrual of complications such as 30 days or same hospitalisation.
3 – Outpatient information included – Study indicates that complications first identified following discharge are included in the analysis.
4 – Definition of complications provided – Article defines at least one complication with specific inclusion criteria.
5 – Mortality rate and causes of death listed – The number of patients who died in the postoperative period of study are recorded together with cause of death.
6 – Morbidity rate and total complications indicated – The number of patients with any complication and the total numbers of complications are recorded.
7 – Procedure-specific complications included.
8 – Severity grade utilised – Any grading system designed to clarify severity of complications including major and minor is reported.
9 – LOS data – Median or mean LOS indicated in the study.
10 – Risk factors included in the analysis – Evidence of risk stratification and method used indicated by study.
Y, yes study addressed criteria completely; N, no study did not address criteria; P, study partially addressed criteria; TC, total number of Martin criteria met by each study.
Discussion
The results of the present meta-analysis of 2580 patients comparing extraperitoneal and transperitoneal approaches in MIRP showed interesting results. The LOS in the E-RP cohort was significantly lesser than that of the T-RP cohort for both the laparoscopic and robotic groups. There was no significant difference in the operative time, EBL, BTR, and analgesic requirements between the two cohorts.
Early continence rates favoured the E-RP cohort in both the laparoscopic and robotic approaches. Nevertheless, statistical significance was achieved only in the laparoscopic group. The above observation may be attributed to protracted bladder dysfunction due to increased bladder manipulation in the transperitoneal approach [13]. However, these results have to be treated with caution in view of potential confounding factors, such as the proportion of patients undergoing a nerve-sparing approach in each cohort, which in itself could influence continence rates [26]. Furthermore, the 12-month continence rate between the two approaches was not statistically significantly different, presumably as any factors that may have influenced early continence will probably have subsided.
The meta-analysis of studies reporting on overall complication rates showed lower complication rates in the E-RP cohort for both the laparoscopic and robotic groups. The specific complication of ileus had a significantly higher rate in the T-RP cohort for both the laparoscopic and robotic groups. Factors such as bowel manipulation, bowel irritation by urine and blood with the transperitoneal approach would probably contribute to this trend. Furthermore, the consequent quicker resumption of normal bowel activity could be a plausible explanation for the shorter LOS in the E-RP cohort as well. The symptomatic lymphocele rate was higher in the T-RP cohort, although statistical significance was achieved only in the laparoscopic group. In a transperitoneal approach, accumulated lymph fluid can be absorbed into the peritoneum; hence these patients are less likely to develop a symptomatic lymphocele, which probably explains this trend. There has been a suggestion by some authors that an extraperitoneal approach places increased stress on the vesico-urethral anastomosis [7], [27]. The latter suggestion was not confirmed in terms of increased rates of urine leak requiring longer periods of catheterisation or drain insertion as evidenced from the present review. Amongst the 20 studies included in the present review only three adopted the Clavien–Dindo grading system for complications and hence a meta-analysis using Clavien–Dindo grading was not performed [11], [16], [21]. The latter observation probably represents a paucity of studies with standardised complication reporting, and also represents a limitation of the present analysis.
Both the E-RP and T-RP cohorts had equivalent results for PSM rates in both the laparoscopic and robotic groups. The latter observation practically dictate that neither of the access approaches has an oncological advantage over the other.
Clearly both approaches have advantages and disadvantages. The extraperitoneal approach has various advantages such as: a less steep Trendelenburg position reducing anaesthetic adverse effects, decreased intra-abdominal complications, and confinement of blood and urine in the extraperitoneal space [28], [29]. These factors may lead to fewer overall complications as evidenced in the present review. Furthermore, the present review suggests that E-RP is associated with a shorter LOS and earlier return of continence. On the contrary, the extraperitoneal approach has been related to increased symptomatic lymphocele rates. Techniques such as fenestration of the peritoneum have been suggested to address this issue [30]. Furthermore, with a restricted cranial boundary, the extraperitoneal approach is not an ideal approach when an extended pelvic lymphadenectomy is anticipated. During recent years, there has been an increased trend towards adopting the transperitoneal approach worldwide, particularly in robotic surgery [7], [27]. The likely explanation for this trend is the marginally more challenging extraperitoneal access and more limited space associated with an extraperitoneal approach making the procedure potentially more difficult. The authors advocate that surgeons proficient in the transperitoneal approach are unlikely to have a significant learning curve before being able to perform the extraperitoneal approach competently. Nevertheless, it is imperative that urologists specialising in MIRP surgery are familiar with both approaches, therefore reaping benefits of either approach when indicated.
The present review further highlights a significant paucity in good quality studies that are available to advise future practice, which has been its chief limitation. The present review identified only one randomised study, which was deemed to have significant bias (Fig. 6). Furthermore, the quality of the observational studies in the present review was also poor when evaluated using the STROBE guidelines, with eight uncharacterised studies (Table 4). Thus, careful interpretation of the present results should be considered. It should be noted that the overall heterogeneity of the pooled results was low with an I2 ranging between 0% and 24% in the case of BTR, continence, and complications (Fig. 3, Fig. 4, Fig. 6, Fig. 7, Fig. 8, Fig. 9). The latter observation dictates that these results could be considered as accurate and further strengthens any results related to the above parameters. On the other hand, a high level of overall heterogeneity was calculated for operative time, EBL, LOS, and use of analgesics, with an I2 value ranging between 71% and 97% (Figs. Fig. 2, Fig. 3, Fig. 4). It should be noted that the I2 can be misleading as the magnitude and directions of the effects may influence its value and the P value from the chi-squared test or the CI may be related to the strength of evidence of heterogeneity. When the P value of the chi-squared and the CI are considered for the operative time, EBL, LOS, and use of analgesics, the results of the pooled analysis can be considered as accurate.
Another issue addressed in the present review is the lack of standardised surgical outcome and complication reporting that limited the number of studies suitable for meta-analysis. Systematic reviews play an important role in scrutinising and collating available evidence and thus advising future practice. The lack of standardised reporting makes it impossible to meaningfully evaluate all of the available data. In terms of complication reporting, there are a number of grading systems available with the Clavien–Dindo system being the most popular [6]. In the present review, only three studies adopted this system [11], [16], [21]. In an attempt to evaluate more accurately the reported complications, the authors followed the 10 criteria set by the Martin criteria. In the present review the mean number of Martin criteria fulfilled per study was a mere 5.71 [6].
Dahm et al. [31] in a recent review article highlighted some of the issues with performing high quality surgical research. A number of issues, such as equipoise and blinding, have been a significant impediment in the performance of surgical trials. Nevertheless, equipoise is unlikely to be a significant issue when comparing the extraperitoneal and transperitoneal approaches due to both approaches having a reasonable well-proven safety track record. Hence, recruitment failure is unlikely. With regard to blinding, clearly surgeon blinding is not possible. However, patient blinding would be possible in this clinical scenario. Furthermore, the role of prospective, collaborative research in surgical research cannot be emphasised more. The Idea, Development, Exploration, Assessment, Long-term study (IDEAL) collaboration framework advised a staged approach [32]. The latter study recommended the initiation of any surgical research with prospective collaborative studies in order to evaluate feasibility of future randomised control trials, a strategy ideal for comparing trans- and extraperitoneal approaches in MIRP.
Conclusions
The present review suggests that the extraperitoneal approach is associated with a shorter LOS, lower overall complication rate, and earlier return to continence when compared to the transperitoneal approach. Transperitoneal approaches are associated with lower rates of lymphocele formation. The present review also highlights the paucity of high-quality evidence available in this area and poor outcome and complication reporting in the literature. There is a need for consensus amongst the academic and clinical urological community to form standardised outcomes to which all reporting literature should adhere.
Acknowledgments
None.
Compliance with ethical standards
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors. This was a systematic review and therefore informed consent is not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Laparoscopy/Robotics
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Heidenreich A., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Schuessler W.W., Schulam P.G., Clayman R.V., Kavoussi L.R. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 3.Capello S.A., Boczko J., Patel H.R., Joseph J.V. Randomized comparison of extraperitoneal and transperitoneal access for robot-assisted radical prostatectomy. J Endourol. 2007;21:1199–1202. doi: 10.1089/end.2007.9906. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org. Accessed July 2017.
- 5.Rai B.P., Shelley M., Coles B., Biyani C.S., El-Mokadem I., Nabi G. Surgical management for upper urinary tract transitional cell carcinoma. Cochrane Database Syst Rev. 2011;4 doi: 10.1002/14651858.CD007349.pub2. CD007349. [DOI] [PubMed] [Google Scholar]
- 6.Mitropoulos D., Artibani W., Graefen M., Remzi M., Rouprêt M., Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU Guidelines Panel Assessment and Recommendations. Eur Urol. 2012;61:341–349. doi: 10.1016/j.eururo.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Atug F., Castle E.P., Woods M., Srivastav S.K., Thomas R., Davis R. Transperitoneal versus extraperitoneal robotic-assisted radical prostatectomy: is one better than the other? Urology. 2006;68:1077–1081. doi: 10.1016/j.urology.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Bivalacqua T.J., Schaeffer E.M., Alphs H., Mettee L., Wagner A.A., Su L.M. Intraperitoneal effects of extraperitoneal laparoscopic radical prostatectomy. Urology. 2008;72:273–277. doi: 10.1016/j.urology.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 9.Brown J.A., Rodin D., Lee B., Dahl D.M. Transperitoneal versus extraperitoneal approach to laparoscopic radical prostatectomy: an assessment of 156 cases. Urology. 2005;65:320–324. doi: 10.1016/j.urology.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Cathelineau X., Cahill D., Widmer H., Rozet F., Baumert H., Vallancien G. Transperitoneal or extraperitoneal approach for laparoscopic radical prostatectomy: a false debate over a real challenge. J Urol. 2004;171:714–716. doi: 10.1097/01.ju.0000103885.71434.02. [DOI] [PubMed] [Google Scholar]
- 11.Anderson C., Ayres B., Issa R., Perry M., Liatsikos E., Stolzenburg J.U. Extraperitoneal robot-assisted radical prostatectomy: comparison with transperitoneal technique. World J Clin Urol. 2013;2:3–9. [Google Scholar]
- 12.Chung J.S., Kim W.T., Ham W.S., Yu H.S., Chae Y., Chung S.H. Comparison of oncological results, functional outcomes, and complications for transperitoneal versus extraperitoneal robot-assisted radical prostatectomy: a single surgeon's experience. J Endourol. 2011;25:787–792. doi: 10.1089/end.2010.0222. [DOI] [PubMed] [Google Scholar]
- 13.Eden C.G., King D., Kooiman G.G., Adams T.H., Sullivan M.E., Vass J.A. Transperitoneal or extraperitoneal laparoscopic radical prostatectomy: does the approach matter? J Urol. 2004;172:2218–2223. doi: 10.1097/01.ju.0000144640.26182.41. [DOI] [PubMed] [Google Scholar]
- 14.Erdogru T., Teber D., Frede T., Marrero R., Hammady A., Seemann O. Comparison of transperitoneal and extraperitoneal laparoscopic radical prostatectomy using match-pair analysis. Eur Urol. 2004;46:312–320. doi: 10.1016/j.eururo.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z.L., Wu J.T., Wang K., Wang L., Yang D.D., Shi L. Comparison of the extraperitoneal and transperitoneal laparoscopic radical prostatectomy. Chin Med J (Engl) 2006;119:2125–2128. [PubMed] [Google Scholar]
- 16.Horstmann M., Vollmer C., Schwab C., Kurz M., Padevit C., Horton K. Single-centre evaluation of the extraperitoneal and transperitoneal approach in robotic-assisted radical prostatectomy. Scand J Urol Nephrol. 2012;46:117–123. doi: 10.3109/00365599.2011.637957. [DOI] [PubMed] [Google Scholar]
- 17.Hoznek A., Antiphon P., Borkowski T., Gettman M.T., Katz R., Salomon L. Assessment of surgical technique and perioperative morbidity associated with extraperitoneal versus transperitoneal laparoscopic radical prostatectomy. Urology. 2003;61:617–622. doi: 10.1016/s0090-4295(02)02415-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs B.L., Montgomery J.S., Dunn R.L., Weizer A.Z., Miller D.C., Wood D.P. A comparison of extraperitoneal and intraperitoneal approaches for robotic prostatectomy. Surg Innov. 2012;19:268–274. doi: 10.1177/1553350611429028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madi R., Daignault S., Wood D.P. Extraperitoneal v intraperitoneal robotic prostatectomy: analysis of operative outcomes. J Endourol. 2007;21:1553–1557. doi: 10.1089/end.2007.9872. [DOI] [PubMed] [Google Scholar]
- 20.Phinthusophon K., Nualyong C., Srinualnad S., Taweemonkongsap T., Amornvesukij T. Laparoscopic radical prostatectomy: transperitoneal laparoscopic radical prostatectomy versus extraperitoneal endoscopic radical prostatectomy. J Med Assoc Thai. 2007;90:2644–2650. [PubMed] [Google Scholar]
- 21.Porpiglia F., Terrone C., Tarabuzzi R., Billia M., Grande S., Musso F. Transperitoneal versus extraperitoneal laparoscopic radical prostatectomy: experience of a single center. Urology. 2006;68:376–380. doi: 10.1016/j.urology.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Remzi M., Klingler H.C., Tinzl M.V., Fong Y.K., Lodde M., Kiss B. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy versus open retropubic radical prostatectomy. Eur Urol. 2005;48:83–89. doi: 10.1016/j.eururo.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz L., Salomon L., Hoznek A., Vordos D., Yiou R., de la Taille A. Comparison of early oncologic results of laparoscopic radical prostatectomy by extraperitoneal versus transperitoneal approach. Eur Urol. 2004;46:50–56. doi: 10.1016/j.eururo.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira T.M., Jr, Mitre A.I., Duarte R.J., Nascimento H., Barreto F., Falcao E. Transperitoneal versus extraperitoneal laparoscopic radical prostatectomy during the learning curve: does the surgical approach affect the complication rate? Int Braz J Urol. 2010;36:450–457. doi: 10.1590/s1677-55382010000400008. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.S., Ham W.S., Kim W.T., Joo H.J., Lee J.S., Choi Y.D. Comparison of extraperitoneal and transperitoneal robot-assisted radical prostatectomy in prostate cancer: a single surgeon’s experience. Korean J Urol. 2009;50:251–255. [Google Scholar]
- 26.Goldsack J.C., Sonnad S.S. Changing trends in surgical research: an analysis of 30 years of collaborative practices. JAMA Surg. 2014;149:873–874. doi: 10.1001/jamasurg.2014.97. [DOI] [PubMed] [Google Scholar]
- 27.Atug F., Thomas R. Transperitoneal versus extraperitoneal robotic-assisted radical prostatectomy: which one? Minerva Urol Nefrol. 2007;59:143–147. [PubMed] [Google Scholar]
- 28.Stolzenburg J.U., Do M., Pfeiffer H., Konig F., Aedtner B., Dorschner W. The endoscopic extraperitoneal radical prostatectomy (EERPE): technique and initial experience. World J Urol. 2002;20:48–55. doi: 10.1007/s00345-002-0265-4. [DOI] [PubMed] [Google Scholar]
- 29.Stolzenburg J.U., Rabenalt R., Do M., Horn L.C., Liatsikos E.N. Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2006;49:491–500. doi: 10.1016/j.eururo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Stolzenburg J.U., Wasserscheid J., Rabenalt R., Do M., Schwalenberg T., McNeill A. Reduction in incidence of lymphocele following extraperitoneal radical prostatectomy and pelvic lymph node dissection by bilateral peritoneal fenestration. World J Urol. 2008;26:581–586. doi: 10.1007/s00345-008-0327-3. [DOI] [PubMed] [Google Scholar]
- 31.Dahm P., N’Dow J., Holmberg L., Hamdy F. The future of randomised controlled trials in urology. Eur Urol. 2014;66:1–3. doi: 10.1016/j.eururo.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Hirst A., Agha R.A., Rosin D., McCulloch P. How can we improve surgical research and innovation?: the IDEAL framework for action. Int J Surg. 2013;11:1038–1042. doi: 10.1016/j.ijsu.2013.09.016. [DOI] [PubMed] [Google Scholar]