Abstract
Background
There are no current sport concussion assessments that capture the effects of dual-task conditions on gait. Multiple studies have evaluated changes, but none have comprehensively examined literature related to the adolescent and young adult population.
Purpose: The purpose of this systematic review is to synthesize documented changes in gait under dual-task conditions in adolescents and young adults after sustaining a concussion.
Study Design: Systematic Review
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was consulted to guide this systematic review. Six databases were searched: Cinahl, ProQuest, PubMed, Scopus, SPORTdiscus, and Web of Science. Concussion, gait, and dual-task, along with their synonymous terms were the search terms used. Inclusion criteria consisted of adolescent and young adult age groups, acute concussion, dual-tasking, and matched controls. Quality assessment was performed using The Joanna Briggs Institute Critical Appraisal Checklist for Case Control Studies.
Results
Ten full-text articles were selected for inclusion. Concussed individuals demonstrated longer stride times with shorter stride lengths, increased mediolateral displacement with corresponding increases in sagittal and frontal plane peak velocity, and decreased sagittal plane Center of Mass (COM) and Center of Pressure (COP) displacement. The majority of included studies demonstrated moderate to large effect sizes in these gait characteristics.
Conclusion
Concussed individuals demonstrated decreased gait stability while ambulating with a dual-task condition. Though statistically significant differences between concussed individuals and matched controls lasted only 72 hours, concussed individuals demonstrated continued improvements in gait for up to two months post-injury, which has the potential to affect an athlete's ability to perform. Further research is needed to determine if a gait examination with a dual-task condition is a realistic, reliable, and valid measure to be included in return to sport testing.
Level of Evidence
2a
Keywords: Adolescent, concussion, dual-task, gait
INTRODUCTION
The Centers for Disease Control and Prevention report 300,000 traumatic brain injuries per year. However, this number does not reflect the incidence of mild traumatic brain injuries, like concussions, that do not always cause a loss of consciousness. This number may be even higher if accounting for the lack of reporting and limitations in concussion detection.1,2,3 Due to the fact that large numbers of adolescents and young adults participate in sports, particularly football, this population has an increased risk for sustaining a concussion, as well as an increased susceptibility to damage because of brain immaturity.4,5 Additionally, the potential risk for brain re-injury and its resulting long term damage caused by premature return to sport play indicates the need for careful detection of injury before resuming play.
Some standard tools used to detect concussion include symptom checklists and neuropsychological (NP) performance measurements.6 Other common measurements include those related to balance and sway which are assessed by detecting alterations in motoric measures such as center of mass and center of pressure. Despite multiple functional components being examined during a concussion assessment, there have been few objective measures that combine these components. Register-Mihalik et al. suggested the importance of testing cognitive and motor performances together.7 For this reason, testing for gait deficits under dual-task conditions has been developed and is being used to examine the effect of cognitive and motor tasks performed together.7
Dual-tasking is necessary for performing activities within daily life such as obstacle avoidance or maintaining a conversation while walking.8 The necessity for performance in dual-tasking may be even greater with athletes who are competing in higher level activities than walking. A common way the effects of dual-tasking are measured is by evaluating gait combined with a cognitive task. The Stroop test and [modified] Mental Status Exam (MSE) are two examples of commonly used cognitive dual tasks.7 The Stroop test presents a sequence of congruent or incongruent stimuli to be discriminated between9 and the MSE asks a series of questions.10 Both are meant to simulate the type of thinking required for decision making because they utilize the executive functioning areas in the frontal and prefrontal brain regions.9,10,11
An individual who has sustained a concussion may have increased difficulty with dual-task activities because the tasks compete for limited resources in the healing brain.12 Fait et al. report that alterations in locomotor tasks were still present during dual-task activity, even in the absence of other concussion symptoms and normal NP testing.13 Often, NP testing results demonstrate normality after as little as one week.13,14 Dual-tasking, therefore, may be able to detect more residual effects of a concussion.7,13
Currently, it is not standard practice to measure gait deficits under dual-task conditions after injury or for use in return to play decision making following a concussion in adolescents and young adults. The types of gait deficits under dual-task conditions must be identified and described in order to develop a tool that captures the effects which may occur or become apparent during concurrent motor-cognitive activities. There have been some studies about these changes,7-9,12,17-26 but no systematic review of the literature exists which focuses exclusively on this population. The purpose of this systematic review is to synthesize documented changes in gait under dual-task conditions in adolescents and young adults after sustaining a concussion.
METHODS
Registration and protocol
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database under the ID: CRD42016053813.
Search strategy, databases utilized, and study selection
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was consulted to guide this systematic review. The PRISMA checklist is a 27-item list designed with the intent of improving reporting for authors of both systematic reviews and meta-analyses.12 The following databases were searched: Cinahl, ProQuest, PubMed, Scopus, SPORTdiscus, and Web of Science. Gray literature was not intentionally excluded during the systematic search process, however, none was found to include in this review. A hand search was also performed by two reviewers who read the reference list of each included study in search of relevant additional titles. The original search was not restricted with any limiters such as year of publication or language. The search strategy included three main categories (concussion, gait, and dual-task) and synonymous terms were defined and used along with the main category terms to define the search. Similar terms were used across all databases, and specific database terms such as Medical Subject Heading (MESH) terms were used when applicable. A full list of search strategies used can be found in Appendix 1. The final initial search was performed on November 30, 2016.
Duplicates between the six databases were eliminated using EndNote duplication system and then manually to ensure all duplicates were removed. The remaining titles were reviewed independently by two authors and then discussed if there was disagreement to determine a final list for abstract review. The same process was performed to screen abstracts to determine a list for full text review and to screen full texts to determine the final included studies. A third reviewer was available to settle any disagreements. A Cohen's unweighted Kappa was calculated for agreement during title, abstract, and full-text selection. A Kappa of less than 0.2 is considered poor agreement; 0.21 to 0.4, fair; 0.41 to 0.6, moderate; 0.61 to 0.8, strong; and more than 0.8, near complete agreement.16
Eligibility criteria
Adolescents and young adults were the primary populations of interest. Adolescents were defined as ages 14 to 18 and young adults as ages 18 through 26. Injury qualifying as a “concussion” was determined by examination and diagnosis by a health care professional. Concussion was defined using either the American Academy of Neurology Practice Parameter or the 3rd International Consensus Statement.17-28 Studies must have included a dual-task defined as the performance of two tasks simultaneously.17-26 The primary task of interest was gait and the secondary task was a cognitive task.
Studies that included a matched control group of non-concussed individuals were included. To ensure the concussion was acute, the concussed groups must have been tested initially within 72 hours post-injury. If a study did not specify the timeline of the concussion in the article, it was excluded. Traumatic brain injuries were also excluded because they suggest a greater severity of brain injury than a concussion. Studies with redundant information were excluded.
Data extraction and synthesis
Data from the final text articles were extracted by two authors and cross checked. For any disagreements about data, a third author was consulted to make the final decision. Sample data extracted included the number of participants, sex, age, mass, and height of participants. Data extracted from the articles included the following outcome measures: center of mass (COM), center of pressure (COP), peak velocities, displacement measures, gait speed, step width, stride length, and max separation distance between COM/COP. Gait variables were analyzed using 3D motion analysis with a range of 25 to 31 retro-reflective markers placed on bony land marks of the subjects. As the subjects walked along a 10-meter walkway, body movement was recorded using a six, eight, or ten camera motion analysis system developed by Motion Analysis Corporation.17-26
Two types of tasks were used as the secondary task to gait. One task was an audio variation of the Stroop test and the other task was a collection of cognitive tasks described within the modified Mental State Exam (MSE). The Single Auditory Stroop (SAS) task required the subject to listen to the words “high” or “low” played in a high or low pitch and then asked to identify the pitch of the word, regardless of whether the pitch was congruent with the meaning.17,21-23 The Multiple Auditory Stroop task was similar to the SAS, except the words were played multiple times per trial rather than once. The Stroop Test has been previously shown to have good test re-test reliability in assessing gait deficits.9 The modified MSE included tasks such as spelling a common five-letter word in reverse, serial subtraction of 7's, and reciting the months of the year in reverse.18-20,23-26 Reliability and validity of the modified MSE has not been previously explored in the existing literature, however it has been used in multiple studies.18-20,23-26
Center of Mass (COM) and Center of Pressure (COP) are two outcome measures that were analyzed within the selected studies. COP can be defined as the point on the ground in which it can be assumed that a resultant ground reaction force is acting upon. To compute COP, ground reaction forces were collected by two force plates. External markers and estimated joint centers were used to calculate the three-dimensional motion for individual body segments and locations of segmental COM. Measurement was taken of the maximum separation between the COM and COP of the supporting foot in the anterior (COM/COP ANTmax) and mediolateral (COM/COP MLmax) directions.24
Quality Assessment
The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Control Studies was used by two authors to assess quality of the included studies. The JBI Critical Appraisal Checklists were collaboratively developed and subsequently reviewed and approved by the JBI International Scientific Committee. The case control checklist in particular consists of ten items regarding study quality plus an additional question at the end referring to the overall opinion of appraisal.29 The opinion of appraisal was subjectively determined based on the number of criteria ranked as “yes” versus “no” for each study. Kohen's unweighted Kappa was calculated between the two authors to determine level of agreement of the quality of the studies between raters.
RESULTS
Study Selection
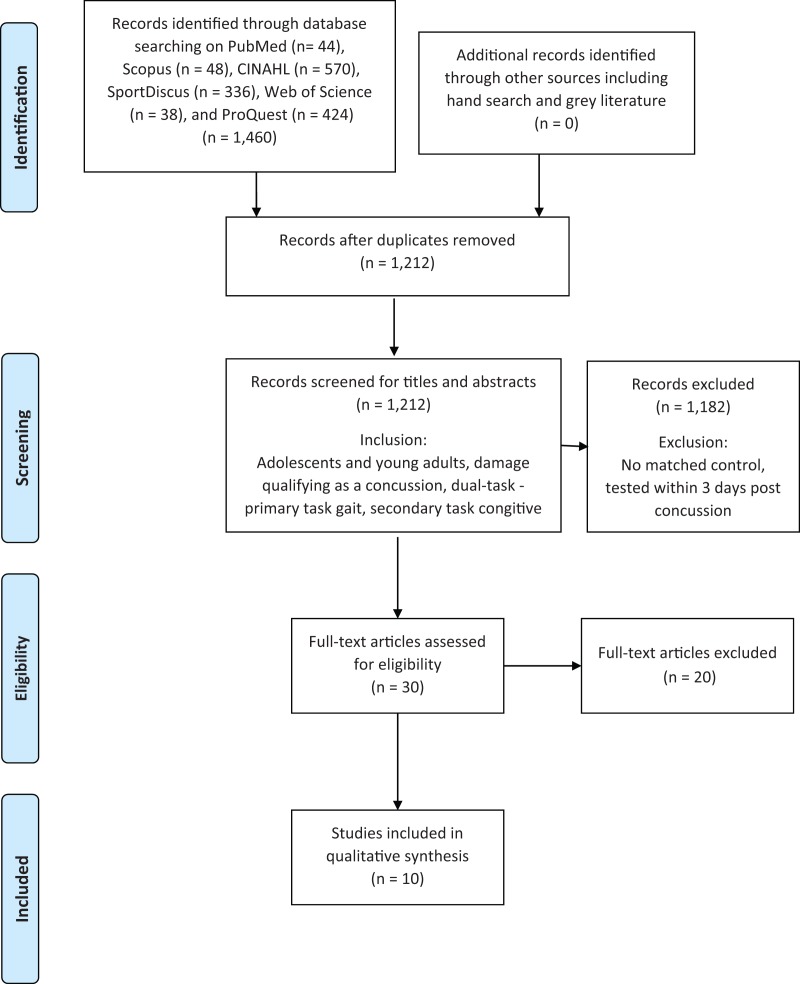
The results of the search criteria produced 1,333 titles which were reviewed for applicability. After title assessment, 74 abstracts were screened; and 30 were deemed appropriate for full-text review. Ten full-text articles were selected after full evaluation and included in the systematic review.
The agreement between authors throughout the multiple stages of selecting articles varied at each stage of the process: κ = -0.22 (poor agreement) for the abstract screen; κ = 0.51(medium agreement) for the full text review screen; and κ = 1.0 (complete agreement) for which full text articles were selected for inclusion. Detailed characteristics of the study selection process are presented in Figure 1.
Figure 1.
PRISMA Figure.
Seven of the ten included studies involved young university aged participants reported as having a Grade 2 concussive brain injury as defined by the American Academy of Neurology Practice Parameter.17-20,24-26 In three of the studies that studied young university22,23 and adolescent participants21 the definition of concussion was consistent with that of the 3rd International Consensus Statement on Concussion in Sport.21-23 The control groups of all studies were matched by age, sex, mass, and height to non-concussed individuals as indicated in Table 1.
Table 1.
Included Study demographics, measurement timing, and concussion definition
| Concussed Participants | Non-Concussed Participants | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | N (Gender) | Mean Age (SD) | Mass (SD) | N (Gender) | Mean Age (SD) years | Mass (SD) kg | Time(s) Measured Post Injury (D) | Concussion Definition |
| Catena (2011) | 10 (5M, 5F) | 21.0 (3.1) | 71.1 (10.5) | 10 (5M,5F) | 20.7 (4.1) | 72.6 (10.5) | 2, 6, 14, 28 | AAN |
| Catena (2006) | 14 (8M, 6F) | 22.29 (4.46) | 75.24 (15.36) | 14(8M,6F) | 22.29 (3.05) | 75.07 (16.93) | 2 | AAN |
| Catena (2009) | 30 (16M, 14F) | 21.5 (3.3) | 83.2 (24.7) | 30 (16M,14F) | 21.7 (3.1) | 82.6 (23.9) | 2 ,6, 14, 28 | AAN |
| Howell (2014) | 23 (20M, 3F) | 15.4 (1.3) | 73.0 (16.1) | 23 (20M, 3F) | 15.7 (1.3) | 70.4 (12.7) | 3, 7, 14, 1mo, 2mo | ICSC3 |
| Howell (2015) | YA: 19 (9M, 10F) AD: 19 (17M, 2F) | YA: 23 (2.4) AD: 15.1 (1.1) | YA: 71.8 (15.4) AD: 74.2 (16.9) | YA: 19 (9M, 10F) AD: 19 (17M, 2F) | YA: 20.4 (2.1) AD: 15.6 (1.1) | YA: 71.1 (11.1) AD: 68.8 (11.1) | 3, 7, 14, 1mo, 2mo | ICSC3 |
| Howell (2013) | 20 (18M, 2F) | 15.3 (1.3) | 74.8 (16.6) | 20 (18M,2F) | 15.6 (1.0) | 70.7 (13.6) | 3, 7, 14, 1mo, 2mo | ICSC3 |
| Parker (2006) | 15 (9M, 6F) | 20.6 (1.55) | 91.0 (28.67) | 15 (9M, 6F) | 20.6 (1.8) | 89.03 (30.03) | 2, 5, 14, 28 | AAN |
| Parker (2007) | 29 (15M, 14W) | 21.6 (3.26) | 81.82 (24.16) | 29 (15M, 14W) | 21.38 (3.4) | 83.31 (23.66) | 2, 5, 14, 28 | AAN |
| Parker (2005) | 10 (4M, 6F) | 20.2 (1.7) | 84.20 (20.10) | 10 (4M,6F) | 19.9 (1.9) | 83.40 (25.30) | 2 | AAN |
| Chen (2015) | 15 (9M, 6F) | 21.3 (3.3) | 88.5 (18.2) | 15 (9M, 6F) | 21.2 (3.4) | 84.8 (22.1) | 2 | AAN |
NOTE: Mass is reported in kg, and age is reported in years.
SD = Standard deviation, D = days post injury, YA = Young Adult, AD = Adolescent, AAN = American Academy of Neurology, ICSC3 = 3rd International Consensus Statement on Concussion
Quality Assessment
There are currently no studies establishing reliability or validity for the JBI Critical Appraisal Checklists, however, the Cohen's unweighted Kappa agreement between two independent reviewers was κ = 0.713 (substantial agreement). The ten included studies all received at least 8 out of 10 affirmative scores as indicated in Table 2. JBI category seven was the most missed as four studies failed to state strategies used to deal with confounding factors. While the JBI was a useful guide to measure quality, it did not detect the lack of data reporting throughout the majority of included studies. Most studies did not report the group mean for each measured variable which led to an inability to calculate effect sizes. The calculated effect sizes that are available are indicated in Table 3.
Table 2.
Joanna-Briggs Institute Checklist Results for included studies
| JBI criterion number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | |
| Catena 2006 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 9/10 | |
| Catena 2009 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 9/10 | |
| Catena 2011 | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 8/10 | |
| Chen 2015 | Yes | Unclear | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 8/10 | |
| Howell 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
| Howell 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
| Howell 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
| Parker 2006 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
| Parker 2007 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
| Parker 2005 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/10 | |
Notes: JBI = Joanna-Briggs Institute Critical Appraisal Checklist for Case Control Studies, Criterion numbers 1-10 represent the corresponding criterion listed in the JBI Appraisal Checklist.
Table 3.
Statistical outcomes between gait parameters and cognitive dual-task, including effect sizes when available
| Study | Cognitive dual-task used | Gait parameter (Change vs Control) | Interaction | p-value | Cohens D |
|---|---|---|---|---|---|
| Catena 2011 | Auditory Stroop Task (high/low pitch) | 1. COM-AV (DEC) 2. Sagittal COM/COP Separation (DEC) 3. Day 14 COM M/L ROM (INC) 4. Peak M/L COM V |
1. G*G 2. G*G, G*D 3. G*G 4. NS |
1. p = 0.015* 2. p = 0.041*/0.001* 3. p = 0.00625* 4. NR |
NC |
| Howell 2014 | 3. MSE (5wrd reverse, -7s, month reverse) | 1. M/L COM Disp. (INC) 2. M/L COM Peak V 3. Peak COM Ant V 4. AVG Walking Speed (DEC) |
1. G*G 2. NS 3. G*G 4. G*D |
1. p = 0.004* 2. P = > 0.125 3. p = 0.008* 4. p = 0.015* |
1-4. NC |
| Howell 2015 | Auditory Stoop 4stim (high vs. low pitch) | 1. Adolescent M/L COM disp. (INC) 2. Peak M/L COM V (INC) 3. Peak COM ANT V (DEC) 4. Young Adult M/L COM Displacement 5. Young Adult Peak M/L COM V |
1. G*G 2. G*G 3. G*G 4. NS 5. NS |
1. p = 0.001* 2. p = 0.001* 3. p = 0.01* 4. p = 0.149 5. NR |
1-5. NC |
| Howell 2013 | Auditory Stoop 4stim (high vs. low pitch) | 1. Peak Ant COM V (DEC) 2. Peak M/L COM V (INC) 3. M/L COM DISP. (INC) 4. Average Walking speed (m/s) 5. Step Length (m) (INC) |
1. G*G 2. G*G 3. G*G 4. NS 5. G*D |
1. p = 0.001* 2. p = 0.027* 3. p = 0.014* 4. NR 5. p = 0.012* |
1-3. NC 4. -0.106 (trivial) 5. -0.905 (large) |
| Catena 2006 | 1. MSE (5wrd reverse, Sub X 7, months reverse) | 1. Gait V (DEC) 2. Stride Time (INC) 3. Stride Length (m) 4. Step Width (m) 5. COM A/P DISP 6. COM A/P V (DEC) 7. COM/COP SMAX (DEC) 8. COM M/L DISP (INC) 9. COM Peak M/L V 10. COM M/L Max |
1. G*G 2. G*G 3. NS 4. NS 5. NS 6. G*G 7. NS 8. G*G 9. G*G 10. NS |
1. p = 0.007* 2. p = 0.020* 3. NR 4. NR 5. NR 6. p = 0.007* 7. NR 8. p = 0.041* 9. p = 0.046* 10. NR |
1. -1.19 (large) 2. 1.01 (large) 3. - 0.52 (Mod) 4. 0.739 (Mod) 5. - 0.517 (Mod) 6. - 1.197 (large) 7. -0.712 (Mod) 8. 0.899 (Mod) 9. 0.871 (Mod) 10. 0.200 (Small) |
| Catena 2009 | 1. MSE (5wrd reverse, Sub X 7, months reverse) | 1. Day 2 COM AP Disp. (DEC) 2. COM A/P V (DEC) 3. COM/COP M/L SMAX 4. COM M/L V |
1. G*G 2. G*G 3. NS 4. NS |
1. p = 0.0143* 2. p = 0.0135* 3. NR 4. NR |
1. NC 2. -0.483 (Small) 3. 0.017 (Trivial) 4. -0.029 (Trivial) |
| Parker 2006 | MSE (5wrd reverse, Sub X 7, months reverse) | 1. Day 2 Gait Velocity (DEC) 2. Day 2 Stride Length (DEC) 3. Stride Time (s) 4. Step Width (m) 5. COM ANT DISP (m) 6. COM ANT Inst. V 7. COM/COP SMAX (DEC) 8. COM M/L Inst. V 9. COM M/L Disp. (INC) 10. COM/COP M/L SMAX |
1. G*G 2. G*G 3. NS 4. NS 5. NS 6. NS 7. G*G 8. NS 9. G*G 10. NS |
1. p = 0.012* 2. p = 0.016* 3. NR 4. NR 5. NR 6. NR 7. p = 0.005* 8. NR 9. p = 0.013* 10. NR |
1. NC 2. -0.990 (large) 3. 0.40 (Small) 4. 0.084 (trivial) 5. -0.845 (large) 6. -0.907 (large) 7. NC 8. 0.030 (trivial) 9. NC 10. - 0.038 (trivial) |
| Parker 2007 | MSE (5wrd reverse, Sub X 7, months reverse) | 1. COM M/L DISP (INC) 2. COM/ COP ANT SEP (DEC) |
1. G*D 2. G*D |
1. p = 0.001* 2. p = 0.040* |
1. NC 2. NC |
| Parker 2005 | MSE (5wrd reverse, Sub X 7, months reverse) | 1. Gait Velocity (m/s) 2. Step Width (m) 3. Stride Length(DEC) 4. Stride time (S) 5. COM AP ROM (m) 6. COM AP V (DEC) 7. COM AP SMAX 8. COM ML ROM (INC) 9. COM ML V (m/s) 10. COM ML SMAX (m) |
1. NS 2. NS 3. G*G 4. NS 5. NS 6. NS 7. NS 8. G*G 9. NS 10. NS |
1. NR 2. NR 3. p=0.042* 4. NR 5. NR 6. p = 0.041* 7. NR 8. p = 0.021* 9. NR 10. NR |
1. -1.09 (large) 2. -0.44 (small) 3. -1.29 (large) 4. 0.328 (small) 5. -1.43 (large) 6. -1.09 (large) 7. -0.69 (Mod) 8. 0.59 (Mod) 9. -0.29 (Small) 10. -0.53 (Mod) |
| Chen 2015 | MSE (5wrd reverse, Sub X 7, months reverse) | 1. Gait V (m/s) (DEC) 2. Stride Length (m) 3. Step Width (m) |
1. G*G 2. NS 3. NS |
1. p = 0.04* 2. p = 0.22 3. p = 0.70 |
1. -1.02 (Large) 2. -0.57 (Mod) 3. -0.197 (Trivial) |
Cognitive task: MSE = Mental state exam 3 task Q/A.
Gait Parameters: COM = Center of Mass, COP = Center of Pressure, ANT = Anterior, V = Velocity (m/s), A/P = Anterior/Posterior, M/L = Medial/Lateral, DISP = Displacement, SMAX = Separation Maximum (m), SEP = Separation (m), INC = Increased as compared to control, DEC = Decreased as compared to control
Significance: * = p < 0.05, NR = Value Not Reported
Interaction: G*G= Between Group Interaction (Concussion vs control), G*D = Between testing day interaction within the concussion group, NS = No Significant Interaction
Cohen's: Not calculated due to a lack of study reporting (NC)
GAIT VARIABLES
Gait Velocity/Walking Speed/Average Walking Speed
Four of the six studies that measured gait velocity/walking speed found a significant decrease in velocity/speed when compared to the control group.17,20,24,26 Three of those studies reported on gait velocity showed a large effect size.18,20,26 In the two studies reporting on walking speed, effect size of only one of the studies could be calculated and was trivial.22
Stride Time
Three studies measured stride time; one study showed an increased stride time in concussed versus control with a large effect size,18 whereas the two studies that did not find a significant difference had small effect sizes.24,26
Step Length/Stride Length
One study found that step length significantly decreased in subjects with concussion as compared with control and had a large effect size.22 Four studies looked at stride length in the concussed group, and two showed a significant decrease versus control and had large effect sizes,24,26 while two showed no significant difference between groups but still revealed moderate effect sizes.18,20
Step Width
Four studies measured step width during gait with dual-task conditions and none reported a significant difference compared to control.18,20,24,26 The results of these studies demonstrate conflicting trends and varied effect sizes.18,26
COM ANTERIOR TO POSTERIOR (A/P) PLANE VARIABLES
A/P COM Velocity, Peak Velocity
Four studies measured COM velocity in the sagittal plane and all four showed that subjects with concussion had a decreased A/P COM velocity compared to the control subjects.17,18,19,26 Only three studies reported enough information to calculate effect size; two were large,18,26 while one was considered small.19 Three of the included studies measured peak velocity in the sagittal plane and all showed a significant decrease versus the control.21,22,23 Not enough information from the three studies was reported on peak velocity to calculate effect size.
A/P Displacement, COM
Three studies measured sagittal plane COM displacement; one reported a decrease in the concussed group when compared to the control group17 (effect size could not be calculated) while two did not find a significant difference between groups and showed moderate and large effect sizes.18,24 Parker et al in 2005 did not find a significant difference in sagittal plane COM range of motion between concussion and control groups; however, the calculated effect size was large.26
COM MEDIOLATERAL (M/L) VARIABLES
M/L Velocity, Peak Velocity, Velocity at Separation Max
Nine total studies measured a form of mediolateral COM velocity; however, they measured the variable in three different ways. Six studies reported on peak M/L velocity, and they were evenly split; three reported a significant increase in M/L velocity in the concussion group compared to control18,21,22 with a large calculated effect size,18 while the other three reported no significant difference between groups17,23,24 and no effect sizes could be calculated. The study completed by Howell et al in 2015 reported a significant increase versus control in the adolescent age group but not in the young adult group.21 One study reported no significant relationship between groups for M/L velocity at separation maximum26 while the two remaining studies reported the M/L velocity relationship between groups to have no significance and trivial19 to small effect sizes.26
M/L Displacement, COM
Seven studies measured M/L COM displacement. Six showed a significant increase in displacement between concussion and control groups,18,21-25 but of the six, only one effect size could be calculated and was reported as moderate.18 The study completed by Howell et al in 2015 showed the concussion group exhibited a significant increase versus control in the adolescent group, but not in the young adult group.21 Two studies also reported that M/L COM range of motion was significantly increased compared with controls,17,26 with the one calculated effect size being moderate.26
COM/COP SEPARATION VARIABLES
A/P COM/COP Separation
Five studies measured sagittal plane COM/COP separation. Three reported a significant decrease in separation in the concussion group (effect sizes could not be calculated)17,24,25 while two studies found no significant difference between groups, but showed moderate effect size.18,26
M/L COM/COP Separation
Two studies compared the mediolateral separation between COM and COP, but neither showed a significant difference.19,24 The calculated effect sizes were trivial.19,24
CONCUSSION SYMPTOMS OVER TIME
The majority of significant gait deviations in the concussed group were observed during the first two days post-injury, however, all included studies showed a trend that concussed patients continued to show improvement in gait abnormalities over the course of two months. Of the seven studies that took measurements up to the 28 days post injury, four studies noted significant differences between concussed and control past the day two measurement mark. Howell et al noted concussed individuals had increased M/L COM displacement (2 months post-injury23), increased step length (1 week post-injury; large effect size22), decreased peak sagittal plane COM velocity (2 weeks post-injury23) and walking speed (1 week post-injury23). Catena et al noted COM/COP separation decreases up to a week post-injury.17 Parker et al reported significant differences between single and dual-task COM/COP separation and COM M/L displacement up to 28 days post-injury.26
DISCUSSION
The purpose of this systematic review is to synthesize documented changes in gait under dual-task conditions in adolescents and young adults after sustaining a concussion. When tested under two distinct dual-task conditions (Stroop, MSE), subjects who experienced a concussion exhibited a variety of gait deficits within four main categories: gait characteristics, sagittal COM, medial/lateral (M/L) COM, and COM/COP changes. These variables combined suggest that individuals who have sustained a concussion exhibit poorer outcomes while dual-tasking compared to matched controls, specifically in the adolescent and young adult population.
One possible explanation for the deviations in gait is the disrupted attention allocation in concussed adolescents and young adults.7,8,12 Kahneman's (1973) theory about divided attention suggests that there is a limited capacity available for processing information, with different tasks taking up different amounts of space. This capacity can be influenced by external variables. Due to the nature of a concussion, an individual who has sustained a concussion must devote more attention to what are typically automatic processes in the uninjured population. Cognitive deficits are typically associated with sustaining a concussion, which may make proper allocation of attentional resources more difficult, and explain why dual-task performance is typically decreased in concussed individuals.7,12
A second potential explanation for the deviations in gait is postural instability caused by concussion deficits. Previously, postural instability has been demonstrated in individuals with a concussion as deviations in M/L and A/P COP time series in static balance, postural sway, and COM deficits.30,31,32 This is consistent with the current findings. Additionally, this instability is thought to decrease gait velocity to serve as a protective mechanism.31 A contributing factor to decreased gait velocity is the increased time in double stance phase to compromise for instability, adopting a more conservative gait strategy post-concussion.7,8,33 The combination of all of the deviations seen, suggest that sport performance could be compromised. Because performing in a sporting event requires a higher level of stability and cognitive function compared to basic daily tasks, deficits in performance may be amplified. The potential amplified deficits could negatively impact an athlete's ability to compete at their previous level or at a level consistent with non-concussed peers.34,35
Adolescents and young adults have a heightened risk for increased susceptibility to damage because of brain immaturity.4,5 Greatest deficits were seen 48-72 hours post-injury, however these deficits may not have fully resolved until two months post-injury, suggesting this brain immaturity may play a role in the healing time. The prolonged recovery, though currently not clinically significant in the adolescent and young adult population, is consistent with healing times in the brain, specifically in the regions where dual-tasking is processed.12 This discrepancy in healing times may indicate that healing is still occurring even though it is not detected in current testing measures. This increase in healing time could potentially affect an athlete if they return to play too early putting them at a higher risk for re-injury.
LIMITATIONS
One potential limitation of this systematic review was poor initial agreement between authors when selecting articles for inclusion based on title. This may have led to exclusion of articles that may have been appropriate for this systematic review. The two subsequent levels of agreement showed much higher agreement, once both authors had re-discussed and clarified in further detail the study's inclusion and exclusion criteria.
The nature of concussion limits the studies in the review to only case control studies; as using a randomized control trial research design would not be feasible or ethical. Therefore, causation cannot be inferred, only correlated with the findings.
The studies selected focused on the adolescent and young adult age group of both athletes and non-athletes. Because of this, the information presented within this study cannot be generalized to other populations outside of the population parameters. The methods used in attaining the information on gait parameters requires an extensive use of equipment that may not be feasible to keep within the general clinic due to costs.
CONCLUSION AND IMPLICATIONS FOR FURTHER RESEARCH
The majority of current standard concussion tests only test motor or neurophysiological performance, but often not together.6 Performing baseline gait examination during dual-task conditions is suggested to provide another outcome measurement for rehabilitation deficits not captured with separate motor or neurophysiological tests. Further research is needed to discover if examining gait deficits during a dual-task condition is a realistic, reliable, and valid measure that can be included in return to sport test batteries and clinical settings.
Appendix 1.
Search Strategy
| Database | Search Strategy |
|---|---|
| CINAHL | (TX concuss* OR TX “mild traumatic brain injury” OR TX mtbi OR MW brain concussion) AND (TX gait OR TX ambulat* OR TX walk* OR MW gait OR MW gait disorders, neurologic OR MW gait analysis OR MW walking) AND (TX “dual task” OR TX “dual-task” OR TX multitask* OR TX “multi-task*” OR TX “divided attention” OR TX distrac* OR TX “split attention” OR TX “simultaneous task” OR TX “secondary task” OR TX “task perform*” OR MW ( task performance and analysis ) OR MW ( motor activity ) OR MW ( physical activity )) |
| ProQuest | (Concuss* OR (“Mild traumatic brain injury”) OR mTBI OR mesh(Brain Concussion) OR su(Concussion) OR su(Traumatic Brain Injury)) AND (gait OR mesh(gait) or mesh(Gait disorder, neurologic) OR ambulat* OR su(walking) OR walk* OR mesh(walking)) AND (“dual task” OR dual-task OR multitask* OR multi-task OR “simultaneous task” OR su(multitasking) OR “divided attention” OR “split attention” OR distract* OR mesh(Motor activity) OR mesh(task performance)) |
| PubMed | ((((Concuss*[Text Word]) OR “Mild Traumatic Brain Injury”[Text Word]) OR mTBI[Text Word]) OR brain concussion[MeSH Terms]) AND ((((((gait[MeSH Terms]) OR gait disorder, neurologic[MeSH Terms]) OR gait[Text Word]) OR ambulat*[Text Word]) OR walk*[Text Word]) OR walking[MeSH Terms]) AND ((((((((((“Dual Task”[Text Word]) OR “Dual-Task”[Text Word]) OR multitask[Text Word]) OR “multi-task”[Text Word]) OR “Simultaneous Task”[Text Word]) OR “divided attention”[Text Word]) OR “split attention”[Text Word]) OR distrac*[Text Word]) OR motor activity[MeSH Terms]) OR performance, task[MeSH Terms]). |
| Scopus | ( TITLE-ABS-KEY ( concuss* ) OR TITLE-ABS-KEY ( “mild traumatic brain injury” ) OR TITLE-ABS-KEY ( mtbi ) ) AND ( TITLE-ABS-KEY ( gait ) OR TITLE-ABS-KEY ( ambulat*) OR TITLE-ABS-KEY ( walk* ) ) AND (( TITLE-ABS-KEY ( “dual task” OR “dual-task” ) OR TITLE-ABS-KEY ( multitask* OR “multi-task” ) OR TITLE-ABS-KEY ( “divided attention” ) OR TITLE-ABS-KEY ( distrac* ) OR TITLE-ABS-KEY ( “split attention” ) OR TITLE-ABS-KEY ( “simultaneous task” ) OR TITLE-ABS-KEY ( “secondary task” ) OR TITLE-ABS-KEY ( “task perform*” ) ) |
| SportDiscus | (TX concuss* OR TX “mild traumatic brain injury” OR TX mtbi OR SU brain concussion) AND (TX gait OR TX ambulat* OR TX walk* OR SU gait disorders OR SU walking) AND (TX ( “dual task” OR “dual-task” ) OR TX ( multitask* OR “multi-task*” ) OR TX “divided attention” OR TX distrac* OR TX “split attention” OR TX “simultaneous task” OR TX “secondary task” OR TX “task perform*” OR SU performance evaluation OR SU motor ability testing) |
| Web of Science | (TOPIC: (Concuss*) OR TOPIC: (“Mild Traumatic Brain Injury”) OR TOPIC: (mTBI) OR TOPIC: (Brain Concussion)) AND (TOPIC: (Gait) OR TOPIC: (Ambutlat*) OR TOPIC: (walk*)) AND (TOPIC: (“Dual Task”) OR TOPIC: (“Dual-task”) OR TOPIC: (Multitask*) OR TOPIC: (“Multi-task”) OR TOPIC: (“simultaneous task”) OR TOPIC: (“divided attention”) OR TOPIC: (“split attention”) OR TOPIC: (distract*)) |
REFERENCES
- 1.Langlois JA Rutland-Brown W Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006; 21(5):375–378. [DOI] [PubMed] [Google Scholar]
- 2.Theodom A Starkey NJ Dowell T, et al. Sports-related brain injury in the general population: An epidemiological study. J Sci Med Sport. 2014;17(6):591-596. [DOI] [PubMed] [Google Scholar]
- 3.Rose SC Weber KD Collen JB, et al. The Diagnosis and Management of Concussion in Children and Adolescents. Pediatr Neurol. 2015;53:108-118. [DOI] [PubMed] [Google Scholar]
- 4.Marar M McIlvain NM Fields SK, et al. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4). [DOI] [PubMed] [Google Scholar]
- 5.Patel DR Shivdasani V Baker RJ. Management of Sport-Related Concussion in Young Athletes. Sports Med. 2005;35(8):671-684. [DOI] [PubMed] [Google Scholar]
- 6.McCrory P Meeuwisse W Aubry M, et al. Consensus statement on concussion in sport - the 4th international conference on concussion in sport held in Zurich, November 2012. Clin J Sport Med. 2013;23(2)89-117. [DOI] [PubMed] [Google Scholar]
- 7.Register-Mihalik JK Littleton AC, and Guskiewicz KM. Are Divided Attention Tasks Useful in the Assessment and Management of Sport-Related Concussion? Neuropsychol Rev. 2013;23:300–313. [DOI] [PubMed] [Google Scholar]
- 8.Weerdesteyn V Schillings AM Van Galen GP, et al. Distraction affects the performance of obstacle avoidance during walking. J Motor Behav. 2003;35(1): 53-63. [DOI] [PubMed] [Google Scholar]
- 9.Teel E Register-Mihalik J. Balance and cognitive performance during a dual-task: Preliminary implications for use in concussion assessment. J Sci Med Sport. 2013;16(3):190-194. [DOI] [PubMed] [Google Scholar]
- 10.Norris DR Clark MS Shipley S. The Mental Status Examination. Am Fam Physician. 2016;94(8):635-641. [PubMed] [Google Scholar]
- 11.McDonald Flashman LA Saykin AJ. Executive dysfunction following traumatic brain injury: Neural substrates and treatment strategies. NeuroRehabilitation. 2002;17:333–344. [PubMed] [Google Scholar]
- 12.Martini DN Goulet GC Gates DH, et al. Long-term effects of adolescent concussion history on gait, across age. Gait & Posture. 2016;49:264–270. [DOI] [PubMed] [Google Scholar]
- 13.Fait P Swaine B Cantin JF Leblond J, et al. Altered Integrated Locomotor and Cognitive Function in Elite Athletes 30 Days Postconcussion: A Preliminary Study. J Head Trauma Rehabil. 2013; 28(4):293–301. [DOI] [PubMed] [Google Scholar]
- 14.Belanger HG Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. J Int Neuropsych Soc. 2005;11:345–357. [DOI] [PubMed] [Google Scholar]
- 15.Moher D Liberati A, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009; 151(4):264-269. [DOI] [PubMed] [Google Scholar]
- 16.Landis J Koch G. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
- 17.Catena RD Van Donkelaar P Chou L. The effects of attention capacity on dynamic balance control following concussion. J Neuroeng Rehabil. 2011;8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catena RD Van Donkelaar P Chou L. Cognitive task effects on gait stability following concussion. Exp Brain Res. 2006;23-31. [DOI] [PubMed] [Google Scholar]
- 19.Catena RD Van Donkelaar P Chou L. Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J Neuroeng Rehabil 2009;7:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H-L Lu T-W Chou L-S. Effect of Concussion on Inter-joint Coordination During Divided-Attention Gait. J. Med. Biol. Eng. 2015;35:28–33. [Google Scholar]
- 21.Howell DR Osternig LR Chou L-S. Adolescents Demonstrate Greater Gait Balance Control Deficits After Concussion Than Young Adults. Am J Sports Med. 2015;43(3):625-632. [DOI] [PubMed] [Google Scholar]
- 22.Howell DR Osternig LR Chou LS. Dual-task effect on gait balance control in adolescents with concussion. Arch Phys Med Rehabil. 2013;94(8):1513-1520. [DOI] [PubMed] [Google Scholar]
- 23.Howell DR Osternig LR Koester MC, et al. The effect of cognitive task complexity on gait stability in adolescents following concussion. Exp Brain Res. 2014;1773-1782. [DOI] [PubMed] [Google Scholar]
- 24.Parker TM Osternig LR Van Donkelaar P, et al. Gait Stability following Concussion. Med Sci Sports Exerc. 2006;(21). [DOI] [PubMed] [Google Scholar]
- 25.Parker TM Osternig LR Van Donkelaar P, et al. Recovery of cognitive and dynamic motor function following concussion. Br J Sports Med. 2007;868-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker TM Osternig LR Lee H, et al. The effect of divided attention on gait stability following concussion. Clin Biomech. 2005; 20:389-395. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Neurology. Practice parameter: the management of concussion in sports [summary statement]. Neurology. 1997;48:581–5. [DOI] [PubMed] [Google Scholar]
- 28.McCrory P Meeuwisse W Johnson K, et al. Consensus statement on concussion in sport: The 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J Ath Train. 2009;44:434-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2016 edition. Australia: The Joanna Briggs Institute; 2016 [Google Scholar]
- 30.Cavanaugh J Guskiewicz K Giuliani C, et al. Recovery of Postural Control After Cerebral Concussion: New Insights Using Approximate Entropy. J Athl Train. 2006;41(3):305-313. [PMC free article] [PubMed] [Google Scholar]
- 31.Guskiewicz K Gansneder B. Effect of Mild Head Injury on Postural Stability in Athletes. J Athl Train. 1996;31(4):300-306. [PMC free article] [PubMed] [Google Scholar]
- 32.Powers K Kalmar J Cinelli M. Recovery of static stability following a concussion. Gait & Posture. 2014;39(1):611-614. [DOI] [PubMed] [Google Scholar]
- 33.Martini D Sabin M Depesa S et al. The Chronic Effects of Concussion on Gait. Br J Sports Med. 2011;45(4):361-362. [DOI] [PubMed] [Google Scholar]
- 34.Guskiewicz KM McCrea M Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA – J Am Med Assoc. 290:2549–2555, 2003. [DOI] [PubMed] [Google Scholar]
- 35.McCrea M Guskiewicz K Randolph C, et al. Effects of a Symptom Free Waiting Period on Clinical Outcome And Risk Of Reinjury After Sport Related Concussion. Neurosurg. 2009;65(5):876-883. . [DOI] [PubMed] [Google Scholar]