Abstract
Recently, cDNAs encoding prepro-orcokinins were cloned from the crayfish Procambarus clarkii; these cDNAs encode multiple copies of four orcokinin isoforms as well as several other peptides. Using the translated open reading frames of the P. clarkii transcripts as queries, five ESTs encoding American lobster Homarus americanus orthologs were identified via BLAST analysis. From these clones, three cDNAs, each encoding one of two distinct prepro-hormones, were characterized. Predicted processing of the deduced prepro-hormones would generate 13 peptides, 12 of which are conserved between the two precursors: the orcokinins NFDEIDRSGFGFN (3 copies), NFDEIDRSGFGFH (2 copies) and NFDEIDRSGFGFV (2 copies), FDAFTTGFGHN (an orcomyotropin-related peptide), SSEDMDRLGFGFN, GDY(SO3)DVYPE, VYGPRDIANLY and SAE. Additionally, one of two longer peptides (GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV, APARSSPQQDAAAGYTDGAPV) is predicted from each prepro-hormone. MALDI-FTMS analyses confirmed the presence of all predicted orcokinins, the orcomyotropin-related peptide, and three precursor-related peptides, SSEDMDRLGFGFN, GDYDVYPE (unsulfated) and VYGPRDIANLY, in H. americanus neural tissues. SAE and the longer, unshared peptides were not detected. Similar complements of peptides are predicted from P. clarkii transcripts; the majority of these were detected in its neural tissues with mass spectrometry. Truncated orcokinins not predicted from any precursor were also detected in both species. Consistent with previous studies in the crayfish Orconectes limosus, NFDEIDRSGFGFN increased mid-/hindgut motility in P. clarkii. Surprisingly, the same peptide, although native to H. americanus, did not affect gut motility in this species. Together, our results provide the framework for future investigations of the regulation and physiological function of orcokinins/orcokinin precursor-related peptides in astacideans.
Keywords: Homarus americanus, Procambarus clarkii, orcokinin, orcomyotropin, expressed sequence tag (EST), cDNA, sinus gland, commissural ganglion, stomatogastric ganglion, supraoesophageal ganglion, matrix assisted laser desorption/ionization Fourier transform mass spectrometry
1. Introduction
In 1992, Stangier and colleagues reported the isolation and characterization of the peptide NFDEIDRSGFGFN from the nervous system of the crayfish Orconectes limosus, and showed it to be a powerful stimulator of hindgut contractility [25]. Based on its source and physiological function, this peptide was named orcokinin [25]. Subsequent studies have identified a number of other orcokinin isoforms in decapod species [3, 15, 16, 24, 35], as well as in several insects [14, 22]. In addition to myotropic actions on the gut [9, 25], the orcokinins have also been shown to function as neuromodulators, at least in decapod species [16]. Given their tissue distributions and their detection in the hemolymph, it is likely that these peptides exert their actions both as locally-released transmitters/modulators and via hormonal routes [3, 9, 16, 24, 25].
While the orcokinins have been the focus of numerous biochemical/mass spectrometric studies [2, 3, 4, 7, 9, 10, 11, 12, 14, 15, 16, 17, 22, 23, 24, 25, 28, 35], genetic analyses of this peptide family are extremely limited. In fact, only two reports, both focusing on the identification and distribution of the orcokinin precursors from the crayfish Procambarus clarkii, have been published [35, 36]. Interestingly, the assessment of the P. clarkii prepro-orcokinins showed that these transcripts not only encode orcokinins, but also an orcomyotropin-related peptide isoform [9, 35], as well as several other previously unknown peptides [35].
With the identification of the P. clarkii orcokinin-encoding transcripts, we became interested in determining if orthologs might be present in the growing expressed sequence tag (EST) collections that are currently being developed for the American lobster Homarus americanus [e.g. 29, 32], a species of considerable commercial and scientific importance. Here, we report the identification of five H. americanus ESTs encoding putative orcokinin transcripts, the cDNA clones from three of which were fully sequenced. Using peptide prediction programs, the mature structures of the peptides encoded by these transcripts were deduced and mass spectrometry was used to assay tissues for their presence. Comparison of the predicted H. americanus precursors with those of P. clarkii show similar, though not identical, organization and isoform complements. The peptides detected in the two species via mass spectrometry were likewise similar, but not identical. To compare orcokinin function in P. clakii and H. americanus, we examined the effects of one shared isoform, NFDEIDRSGFGFN, on motility in a combined mid-/hindgut preparation in each species. Surprisingly, this peptide had no effect on the H. americanus mid-/hindgut, while it strongly activated contractions in P. clarkii. Collectively, the data presented in our report provide a framework for future investigations of the regulation and physiological function of orcokinins and orcokinin precursor-related peptides in members of the decapod infraorder Astacidea.
2. Materials and methods
2.1. Animals
American lobsters H. americanus were purchased from local (Maine) seafood suppliers and were maintained in aerated natural seawater aquaria at 8-10 °C. Red swamp crayfish P. clarkii were purchased from Carolina Biological Supply Company (Burlington, NC) and were maintained in aerated tanks of aged tap water at 18-20 °C.
2.2. Database searching
The Basic Local Alignment Search Tool (BLAST) program tblastn [search of translated nucleotide database using a protein query; National Center for Biotechnology Information (NCBI), Bethesda, MD; http://www.ncbi.nlm.nih.gov/BLAST/] was employed to search for H. americanus ESTs encoding putative orcokinin precursors using the deduced amino acid sequences of either of two P. clarkii prepro-orcokinins (accession nos. AB029168 and AB029169; [35]) as queries. For these analyses, the database search was limited to non-human, non-mouse ESTs (est_others) and was further restricted to Homarus americanus transcripts (taxid: 6706).
2.3. Characterization of cDNA clones
Complementary DNA clones encoding putative orcokinin precursors were recovered from frozen bacterial stocks [29]. The clones were grown overnight in LB-medium at 37 °C and plasmid DNA was isolated using a Purelink Quick Plasmid Miniprep kit (Invitrogen Corporation, Carlsbad, CA). The cloned insert was then sequenced on an ABI 3100 16-capillary sequencer (Applied Biosystems Incorporated, Foster City, CA) using both vector- and insert-specific forward and reverse primers (Integrated DNA Technologies, Inc., Coralville, IA; Table 1). The sequence trace files resulting from each round of sequencing were analyzed using Chromas 2.31 software (Technelysium Pty Ltd., Tewantin, Queensland, Australia), and the high quality nucleotide sequences were aligned using SeqMan 2.6 (DNASTAR Inc., Madison, WI) and/or ClustalW2 (European Bioinformatics Institute, Hinxton, Cambridge, United Kingdom; http://www.ebi.ac.uk/Tools/clustalw2/index.html) software.
Table 1.
Vector and insert-specific primers used for sequencing of the cDNA from clones derived from DV774522, DV774848, and DV774081. Some insert-specific primers were used for multiple cDNA clones.
| Primer Namea | Direction | Sequence |
|---|---|---|
| Vector Specific Primer | ||
| M13 Reverse Primer | Reverse | 5′-TGAGCGGATAACAATTTCACACAG |
| EST DV774522 (Homarus americanus prepro-orcokinin I) | ||
| OrcI-F1 | Forward | 5′-CCCAGTGCTGAAGTAGAAGTTCCC |
| OrcI-R1 | Reverse | 5′-ACTGGGCCAATACAGTCCTTGTCT |
| OrcI-R2 | Reverse | 5′-AGACAGTAAGTGGTTATATGAAGCGGC |
| OrcI-R3 | Reverse | 5′-TCTTTCTCGGGGATAAAATAGTAA |
| OrcI-R4 | Reverse | 5′-CTCAAGGGTCTGCTTCACTC |
| OrcI-R5 | Reverse | 5′-GCTTGTTGAATCCAAATCCAGAT |
| OrcII-F3 | Forward | 5′-AGTTCCTCTGGTTACTTAGACTCA |
| OrcII-R3 | Reverse | 5′-TTTGTACAGGTTAGCGATGTCC |
| OrcII-R4 | Reverse | 5′-GACGACGCTAAACACCTCCC |
| OrcIII-R2 | Reverse | 5′-CGATCGATCTCGTCAAAGTT |
| EST DV774848 (Homarus americanus prepro-orcokinin II) | ||
| OrcII-F1 | Forward | 5′-ATGGTCCCAGGGACATCGCTA |
| OrcII-F2 | Forward | 5′-TTCCTCCTCACTATTATGGACTGCTC |
| OrcII-F3 | Forward | 5′-AGTTCCTCTGGTTACTTAGACTCA |
| OrcII-R1 | Reverse | 5′-AGTGGTTATATGAAGCGGCTGGCT |
| OrcII-R2 | Reverse | 5′-TACCTCAAGGGTCTGCTTCACT |
| OrcII-R3 | Reverse | 5′-TTTGTACAGGTTAGCGATGTCC |
| OrcII-R4 | Reverse | 5′-GACGACGCTAAACACCTCCC |
| EST DV774081 (Homarus americanus prepro-orcokinin III) | ||
| OrcIII-F1 | Forward | 5′-TTTGCGTCCTCTTCAGTGAGACT |
| OrcIII-R1 | Reverse | 5′-AGTGAGGAGGAAAGAGATGGGCAA |
| OrcIII-R2 | Reverse | 5′-CGATCGATCTCGTCAAAGTT |
| OrcIII-R3 | Reverse | 5′-CGGCGGCAGCGAAGACAG |
| OrcII-F1 | Forward | 5′-ATGGTCCCAGGGACATCGCTA |
| OrcII-R3 | Reverse | 5′-TTTGTACAGGTTAGCGATGTCC |
Named for the product of the cDNA clone (Prepro-orcokinin I, II or III) for which the primer was initially developed, and numbered sequentially from the outside (5′ and 3′ ends) toward the inside.
2.4. Nucleotide translation and structural analysis of the deduced amino acid sequence
Translation of full-length nucleotide sequences was done using the Translate tool of ExPASy (Swiss Institute of Bioinformatics, Basel, Switzerland; http://www.expasy.ch/tools/dna.html). Signal peptide and signal peptide cleavage prediction was done via the online program SignalP 3.0, using both Neural Networks and Hidden Markov Models algorithms (Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark; http://www.cbs.dtu.dk/services/SignalP/ [1]). Pro-hormone cleavage sites were predicted based on the information presented in Veenstra [33]. Prediction of the sulfation state of tyrosine residues was done using the online program Sulfinator (Swiss Institute of Bioinformatics, Geneva, Switzerland; http://www.expasy.org/tools/sulfinator/ [18]).
2.5. Matrix assisted laser desorption/ionization Fourier transform mass spectrometry
2.5.1. Tissue collection
For tissue collection, animals were anesthetized by packing in ice for 20-60 minutes, after which the commissural ganglia (CoGs), stomatogastric ganglion (STG), supraoesophageal ganglion (brain) and sinus glands (SGs) were isolated via micro-dissection in chilled (approximately 10 °C) physiological saline (for H. americanus: 479.12 mM NaCl, 12.74 mM KCl, 13.67 mM CaCl2, 20.00 mM MgSO4, 3.91 mM Na2SO4, 5.00 mM HEPES [pH 7.45]; for P. clarkii: 200 mM NaCl, 5.4. mM KCl, 17.2 mM CaCl2, 5.5. mM MgCl2, 22 mM Tris base, 4.7 mM maleic acid [pH 7.4]). Isolated tissues were then pinned in a Sylgard 184 (KR Anderson, Santa Clara, CA)-lined Petri dish filled with chilled physiological saline, and the sheath covering the cell bodies and the neuropil region of the CoGs, STG and/or brain was removed, again via manual micro-dissection.
2.5.2. Sample preparation
2.5.2.1. Direct tissue samples
To prepare tissue samples for direct tissue MALDI-FTMS, a small (approximately 2 mm3) fragment of a given tissue was isolated and removed from the saline with fine forceps, rinsed sequentially in two 12 μL droplets of 0.75 M fructose (SigmaAldrich, St. Louis, MO) and placed on a face of a ten-faceted, stainless steel probe tip. The tissue was then sliced 10-20 times with a 0.2 mm needle, gathered together and covered with a 0.5 μL droplet of 1.0 M 2,5-dihydroxybenzoic acid (DHB; SigmaAldrich [sublimed prior to use]), prepared in 1:1 acetonitrile:water containing 2% phosphoric acid. The tissue-matrix mixture was then allowed to co-crystalize at room temperature (approximately 20 °C). For the analysis of some tissue samples, a previously reported salt-doping procedure (see Stemmler et al. [27]) was used to enhance the production of [M+Na]+ and [M+K]+ ions.
2.5.2.2. Tissue extracts
To prepare tissue extracts for mass spectral analyses, paired, desheathed CoGs or single SGs were homogenized with fine dissecting spring scissors in 30 μL of extraction solvent (7% acetic acid, 64% methanol, 29% deionized H2O). The homogenate was sonicated for 2 minutes and centrifuged at 2200 g for 5 minutes in a microcentrifuge (Fischer Scientific, Pittsburgh, PA). The supernatatent was saved, and the pellet resuspended with 5 μL of deionized water. The sonication, centrifugation, and resuspension were repeated two additional times. The supernatants of all cycles were combined. Deionized H2O (20 μL) and CDCl3 (25 μL, SigmaAldrich) were added to the solution. The organic layer was discarded, and the aqueous layer evaporated to dryness, then resuspended in 5 μL of 1:1 acetonitrile:deionized water. For most samples, the resultant extracts were desalted using C18 ZipTip pipette tips (Millipore, Billerica, MA). After their preparation, 0.5 μL of extract was mixed with 0.5 μL of DHB matrix (see Section 2.5.2.1) on one face of the MALDI probe and the extract-matrix mixture was allowed to co-crystallize. In some experiments, as indicated in the text, 1.36 M 3-hydroxypicolinic acid (HPA; SigmaAldrich) prepared in 1.3:1, 1% TFA: acetonitrile with 0.88 M fructose, rather than DHB, was used as the matrix to reduce metastable decay.
For some samples, methyl esterification was performed prior to mass spectral analysis by adding 10 μL of methanolic HCl to the evaporated tissue extracts or to 2 nmol of peptide standard. The methanolic HCl was prepared immediately prior to its use by adding 400 μL acetyl chloride (Alltech, Deerfield, IL) dropwise to 2.5 mL methanol (Alltech) on ice. The methanolic HCl/peptide solution was allowed to react for 2 hours at room temperature, then evaporated to dryness and reconstituted with 5 μL of a 1:1 acetonitrile:deionized water solution. The reconstituted methyl esterified samples were then placed on the MALDI probe tip and mixed with matrix solution as described above.
2.5.3. Peptide Standards
NFDEIDRSGFGFH and NFDEIDRSGFGF peptide standards were custom synthesized by AC Scientific (Duluth, GA). pQFDEY(SO3)GHMRFamide (sulfated Homarus americanus sulfakinin I [8]) was custom synthesized at the Biotechnology Center of the University of Wisconsin (Madison, WI). N-acetyl-DY(SO3)MGWMamide was purchased from the American Peptide Company (Sunnyvale, CA).
2.5.4. Instrumentation
Tissue samples were analyzed using a HiResMALDI Fourier transform mass spectrometer (IonSpec, Lake Forest, CA) equipped with a Cryomagnetics (Oak Ridge, TN) 4.7 Tesla actively-shielded superconducting magnet. Ions were generated using a pulsed nitrogen laser (337 nm) and were transported from the external ion source to the closed cylindrical ICR cell using a quadrupole ion guide. The ion guide radio frequency potential and trapping delay time were optimized to transmit and trap ions of a selected mass range (optimized for m/z 1500 for the results presented here). A pulse of argon was introduced to the vacuum system during trapping to elevate the system pressure transiently for collisional cooling. All spectra were measured using ion accumulation techniques, where ions from seven successive laser shots were accumulated in the cell. A delay of 5-10 s preceded ion detection, which occurred with analyzer pressures of 1-3 × 10−10 Torr. Spectra were obtained in positive ion mode, unless otherwise indicated.
Exact mass measurements were made on internally calibrated spectra using the internal calibration on adjacent samples (InCAS) technique [20], modified to include the accumulation of mass-selected calibrant ions [28]. A mixture of poly(propylene glycol) 725 and 2000 (PPG; SigmaAldrich) was used as the source of calibrant ions.
2.5. Physiological Experiments
2.5.1. Mid- and hindgut preparation
To remove the mid- and hindguts, animals were anesthetized by packing in ice for 20-60 minutes, after which the abdomen was placed into cold (4-10° C) physiological saline and the ventral carapace was dissected away. The underlying muscle was removed to expose the midgut running the length of the abdomen. The ventral carapace overlying the anterior portion of the telson was removed to expose the hindgut, which was left attached to the carapace at the caudal end of the telson. A length of 6/0 suture silk was tied to the midgut, and was used to attach the mid-/hindgut preparation to the force transducer.
2.5.2 Recording techniques and instrumentation
To record the movements of the mid- and hindgut, preparations were pinned to the bottom of a Sylgard 170 (KR Anderson, Santa Clara, CA, USA)-lined dish containing physiological saline. The suture silk from the midgut was attached to a Grass FT-03 force transducer at an angle of approximately 30º, and a baseline stretch of 2 g was applied. Movements of the preparation were recorded using an ETH-250 Bridge/Bio-amplifier (CB Sciences, Inc, Dover, NH), digitized with a MacLab 4/S (AD Instruments, Colorado Springs, CO) and Chart 5 software (AD Instruments).
The preparation was continually superfused, via an Ismatec (Glattbrugg Switzerland) peristaltic pump, with physiological saline at a flow rate of 2-3 ml/min. Saline temperature in the bath was maintained at 10-11° C for H. americanus and 17° C for P. clarkii using a peltier temperature control system (CL-100 Bidirectional controller, SC20 Heater/Cooler, and TCM1 Thermal Cooling Module; Warner Instruments, Hamden, CT). NFDEIDRSGFGFN (Bachem Americas, Inc; Torrance, CA; catalog # H-8839), stored frozen as a 10−3 M solution in deionized water, was diluted to 10−6 M in saline immediately before use, then added to the preparation through the superfusion system. To ensure that our preparations were able to respond to peptide stimulation with rhythmic contractions, we subsequently superfused them with 10−6 M proctolin (RYLPT; Peninsula Laboratories, Belmont, California, USA). Only those preparations (n= 5 H. americanus, n= 7 P. clarkii) that responded to proctolin with strong and regular contractions were analyzed. We thus present two measures of control frequency, one for orcokinin and one for proctolin, since baseline contraction frequency changed somewhat over time in some preparations.
Contraction amplitude, duration and frequency were measured using the built-in functions of Chart 5. Data were graphed and further analyzed using Prism 5 (GraphPad Software, La Jolla, CA). Data were compared statistically using an ANOVA followed by Bonferroni protected t-tests for individual planned comparisons.
3. Results
3.1. In silico searching for Homarus americanus prepro-orcokinin-encoding transcripts
As stated in Section 1, two prepro-orcokinin-encoding transcripts (accession nos. AB029168 and AB029169) were characterized previously from the crayfish P. clarkii [35]. Using the deduced prepro-hormones encoded by these cDNAs as queries, we conducted tblastn searches of the extant H. americanus ESTs for putative homologs. Via these analyses, five ESTs with significant homology (E-values ≤ 4e-63) to the P. clarkii orcokinin precursors were identified, i.e. accession nos. DV771438, DV772231, DV774081, DV774522 and DV774848, all derived from a cDNA library produced from the H. americanus olfactory organ, a paired structure comprised of the lateral flagellum of the antennule and the aesthetascs sensilla, which house the olfactory receptor neurons and their supporting cells [29].
3.2. Nucleotide sequences of putative Homarus americanus prepro-orcokinin cDNAs
Using a combination of vector- and insert-specific forward and reverse sequencing primers (Table 1), three of the five clones from which the above-mentioned ESTs were derived were fully characterized. The cDNA corresponding to DV774522 was 1551 base pairs (bp) in length and consisted of a 56 bp 5′-untranslated region (UTR), a 657 bp open reading frame (ORF), and a 838 bp 3′-UTR containing two AATAAA polyadenylation signal sequences located 12 and 499 bps upstream of a 15 bp poly-A tail (Figs. 1 and 2). The cDNA corresponding to DV774848 was 1527 bp in length and consisted of a 56 bp 5′-UTR and a 624 bp ORF, as well as a 847 bp 3′-UTR containing two AATAAA polyadenylation signal sequences located 19 and 506 bps upstream of a 17 bp poly-A tail (Fig. 1). The cDNA corresponding to DV774081 was 1037 bp in length and consisted of a 56 bp 5′-UTR, a 624 bp ORF, and a 357 bp 3′-UTR, which contained a single AATAAA polyadenylation signal sequence located 13 bp upstream of a 20 bp poly-A tail (Figs. 1 and 3). The full-length cDNAs corresponding to ESTs DV774522, DV774848, and DV774081 were named Homarus americanus prepro-orcokinin I (accession no. EU408473), II (accession no. EU499359) and III (accession no. EU499360), respectively. We were unable to recover the clones corresponding to ESTs DV771438 and DV772231.
Figure 1.

ClustalW alignment of the nucleotide sequences of Homarus americanus prepro-orcokinin I (Orc I; accession no. EU408473), II (Orc II; accession no. EU499359) and III (OrcIII; accession no. EU499360). Nucleotides conserved between all three transcripts are shown in red, while those present in two of the three cDNAs are shown in blue. Nucleotides unique to a single transcript are shown in black. Gaps in the sequences resulting from the best-fit alignments are indicated by dashes. The start codons (atg) for the open reading frames of the transcripts are underlined in green, while the stop codons (tga) for each cDNA is underlined in red. All polyadenylation signal sequences (aataaa) in the 3′-UTRs are underlined in black.
Figure 2.

Amino acid sequence of Homarus americanus prepro-orkokinin I as deduced from the prepro-orcokinin I transcript (accession no. EU408473). The nucleotide sequence of the cDNA is shown in lower case font, while the deduced amino sequence of the prepro-hormone is shown in upper case script. Both the start and stop codons within the coding region of the cDNA (bold font) are underlined, as are the two polyadenylation signal sequences present in the 3′-UTR. The predicted amino acid sequence of the prepro-hormone signal peptide is shown in gray. Predicted dibasic cleavage sites within the prepro-hormone are shown in black. The amino acid sequences of the native H. americanus orcokinins are shown in red. The amino acid sequences of the other peptides present in the pro-hormone are shown in blue. Within the amino acid sequence of the prepro-hormone, the position of the stop codon is denoted with an asterisk.
Figure 3.

Amino acid sequence of Homarus americanus prepro-orcokinin II as deduced from the prepro-orcokinin III transcript (accession no. EU499360). The nucleotide sequence of the cDNA is shown in lower case font, while the deduced amino sequence of the prepro-hormone is shown in upper case script. Both the start and stop codons within the coding region of the cDNA (bold font) are underlined, as is the polyadenylation signal sequence present in the 3′-UTR. The predicted amino acid sequence of the prepro-hormone signal peptide is shown in gray. Predicted dibasic cleavage sites within the prepro-hormone are shown in black. The amino acid sequence of the native H. americanus orcokinins are shown in red. The amino acid sequences of the other peptides present in the pro-hormone are shown in blue. Within the amino acid sequence of the prepro-hormone, the position of the stop codon is denoted with an asterisk.
3.3. Structural analysis of deduced Homarus americanus prepro-orcokinin and prediction of mature orcokinin and precursor-related peptide isoforms
Translation of the ORF of Homarus americanus prepro-orcokinin I predicted a 218 amino acid prepro-peptide, named here Hoam-prepro-orcokinin I (Figs. 1, 2 and 4A). SignalP analysis of this prepro-protein predicted that the first 20 amino acids form a signal peptide, with putative cleavage between Ala20 and Gly21 (Figs. 2 and 4A). Within the pro-hormone sequence, 12 dibasic processing sites are present (11 Lys-Arg and one Arg-Arg; Figs. 2 and 4A), putatively liberating as many as 13 peptides (Figs. 2 and 4A), seven of which are orcokinins: three copies of NFDEIDRSGFGFN, two copies of NFDEIDRSGFGFH and two copies of NFDEIDRSGFGFV. One copy of the orcomyotropin-related peptide FDAFTTGFGHN is also predicted from this precursor, as is one copy of the orcokinin-like peptide SSEDMDRLGFGFN. One copy each of the peptides GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV (Homarus americanus orcokinin precursor-related peptide Ia [Hoam-OPRP Ia]), GDYDVYPE (Hoam-OPRP II), VYGPRDIANLY (Hoam-OPRP III) and SAE (Hoam-OPRP IV) are also predicted from pro-hormone cleavage. Analysis of Hoam-OPRP II using the program Sulfinator suggests that its position 3 Tyr is sulfated (E-value = 42), thereby producing the putative mature structure GDY(SO3)DVYPE. No tyrosine sulfation was predicted for any of the other Tyr-containing peptides.
Figure 4.

Putative processing scenarios for Homarus americanus (Hoam)-prepro-orcokinin I and II. (A) Putative processing scenario for Hoam-prepro-orcokinin I. (B) Putative processing scenario for Hoam-prepro-orcokinin II. In both A and B, the first line represents the prepro-hormone sequence with its respective signal peptidase cleavage locus underlined. The second line of each scenario shows the resultant pro-hormone sequence with putative prohormone convertase cleavage loci underlined. The third line of each scenario shows the putative peptides liberated from the pro-hormone via prohormone convertase activity with their carboxypeptidase E processing sites underlined, while underlined in the fourth line of each scenario are tyrosine residues putatively targeted for sulfation via the action of tyrosylprotein sulfotransferase, with the resultant sulfated peptide shown in the final line of each scenario. In both schematics, the putative mature peptides are shown in bold font. Arrows: 1, signal peptidase; 2, prohormone convertase; 3, carboxypeptidase E; 4, tyrosylprotein sulfotransferase.
Translation of the ORFs of Homarus americanus prepro-orcokinin II and III predicted identical 207 amino acid prepro-peptides, named here Hoam-prepro-orcokinin II (Figs. 1, 3 and 4B). SignalP analyses show that the first 25 amino acids of this prepro-hormone form a signal peptide, with putative cleavage between Ala25 and Ala26 (Figs. 3 and 4B). As with Hoam-prepro-orcokinin I, this prepro-hormone contains 12 dibasic processing sites (identical to those present in Hoam-prepro-orcokinin I) and processing at these sites is predicted to liberate 13 peptides (Figs. 3 and 4B). With the exception of Hoam-OPRP I, the complement of predicted peptides is identical in Hoam-prepro-orcokinin I and II (Figs. 3 and 4B). In the latter protein, Hoam-OPRP I is amino (N)-terminally truncated by 16 amino acids with respect to the former, producing the peptide APARSSPQQDAAAGYTDGAPV (Hoam-OPRP Ib). Like Hoam-OPRP Ia, no sulfation of the tyrosine residue in Hoam-OPRP Ib was predicted by Sulfinator analysis.
As noted in Section 3.1, two additional ESTs putatively encoding prepro-orcokinins were identified via tblastn analyses (i.e. DV771438 and DV772231); however, we were unable to recover these clones in order to characterize either of them fully (see Section 3.2). Regardless, the nucleotide sequence of each EST was translated (Fig. 5). Based on these translations, it appears that DV771438 encodes a full-length prepro-hormone that is identical to Hoam-prepro-orcokinin II, with the exception that it is truncated at its carboxy (C)-terminus (Fig. 5A), missing Hoam-OPRP III and IV, as well as one copy of the orcokinin NFDEIDRSGFGFV. Translation of DV772231 suggests that it encodes an incomplete prepro-hormone (no stop codon present), which is similar to that of Hoam-prepro-orcokinin I. Specifically this EST and Hoam-prepro-orcokinin I are predicted to contain identical signal peptides, as well as the peptide Hoam-OPRP Ia. The remaining peptides predicted from DV772231 are identical to those present in both Hoam-prepro-orcokinin I and II, with the exception of the final predicted orcokinin-like sequence, which is NFHQIDRSGLRLFEE in this EST (Fig. 5B).
Figure 5.

Putative processing scenarios for the putative Homarus americanus (Hoam)-prepro-orcokinins predicted from ESTs DV771438 and DV772231. (A) Putative processing scenario for the prepro-protein predicted from DV771438. (B) Putative processing scenario for the partial prepro-protein predicted from DV772231. Organization and labeling of the processing in each scenario is as per Figure 4.
3.4. Structural analysis of deduced Procambarus clarkii prepro-orcokinins and prediction of mature orcokinin and precursor-related peptide isoforms
As discussed earlier, two orcokinin-encoding cDNAs have been described from the crayfish P. clarkii (accession nos. AB029168 and AB029169 [35], each encoding similar, although not identical, prepro-hormones (Fig. 6). Specifically, the prepro-orcokinin encoded by AB029168 is 251 amino acids in length (Fig. 6A), while that of AB029169 is 266 amino acids long (Fig. 6B). SignalP analysis of each prepro-hormone suggests that the first 20 amino acids form signal peptides. Both of the putative signal peptides are identical in amino acid sequence, with the predicted cleavage locus between Ala20 and Gly21 in each prepro-hormone. The pro-hormone encoded by AB029168 contains 15 dibasic processing sites, 13 Lys-Arg, one Lys-Lys and one Arg-Arg, whose processing is predicted to produce 16 peptides, including 11 orcokinins (seven copies of NFDEIDRSGFGFN, two copies of NFDEIDRSGFGFV and one copy each of NFDEIDRSGFGFA and NFDEIDRTGFGFH), as well as one copy of the orcomyotropin-related peptide FDAFTTGFGHS and one copy each of the precursor-related peptides GTIKTAPARTPSTQDDASFPPDGAPV, VYVPRYIANLY, DYDVFPD and NVE. The pro-hormone encoded by AB029169 is identical to that just described except that it contains one additional Lys-Arg dibasic site, putatively liberating one additional copy of the orcokinin NFDEIDRSGFGFN. Sulfinator analyses of the Tyr-containing peptides predicted from the P. clarkii precursors suggested no sulfations.
Figure 6.

Putative processing scenarios for Procambarus clarkii (Prcl)-prepro-orcokinin I and II. (A) Putative processing scenario for Prcl-prepro-orcokinin I (predicted from translation of the open reading frame [ORF] of AB029168 [35]). (B) Putative processing scenario for Prcl-prepro-orcokinin II (predicted from translation of the ORF of AB029169 [35]). Organization and labeling of the processing in each scenario is as per Figure 4.
3.5. Mass spectral analysis of predicted orcokinin and orcokinin precursor-related peptides in Homarus americanus
To determine if the full complement of peptides predicted from the H. americanus pro-orcokinin precursors is detected in tissue samples, we used MALDI-FTMS to analyze freshly-dissected SG, CoG, brain, and STG tissues, extracts of SG and CoG tissues, and methyl-esterified SG and CoG tissue extracts. As described in detail in Stemmler et al. [28], we have found that orcokinin-family peptides undergo characteristic metastable fragmentations (Asp-Xxx cleavages) under low-pressure MALDI-FTMS conditions. We have used the detection of these characteristic fragments as a fingerprint to confirm orcokinin identity in this study. The MALDI-FT mass spectrum of a synthetic truncated orcokinin, NFDEIDRSGFGF (Orc-[1-12]), provides an example of this metastable fragmentation pattern (Fig. 7A). In addition to the [M+H]+ ion peak at m/z 1403.62, we detect an abundant peak resulting from the loss of NH3 at m/z 1386.60, and two abundant peaks resulting from Asp-Xxx cleavages that yield fragments at m/z 1009.47 and m/z 670.33.
Figure 7.

MALDI-FT mass spectra for underivatized and methyl esterified orcokinin standards. (A) MALDI-FT mass spectrum for Orc-[1-12], NFDEIDRSGFGF, showing peaks produced by loss of NH3 and Asp-Xxx cleavages, which occur by metastable decay under low pressure MALDI-FTMS ionization conditions. (B) MALDI-FT mass spectrum for [His13]-orcokinin, NFDEIDRSGFGFH, showing peaks produced by loss of NH3 and Asp-Xxx cleavages, as well as the loss of the C-terminal basic His residue. These fragmentations all occur by metastable decay under low pressure MALDI-FTMS ionization conditions. (C) MALDI-FT mass spectrum for methyl esterified Orc-[1-12], NFDEIDRSGFGF, where the three acidic amino acid residues and the C-terminal carboxy group have been converted to methyl esters. The conversion of the acidic amino acid residues to methyl esters eliminates Asp-Xxx cleavages. (D) MALDI-FT mass spectrum for methyl esterified [His13]-orcokinin, NFDEIDRSGFGFH, where the three acidic amino acid residues and the C-terminal carboxy group have been converted to methyl esters. The conversion of the acidic amino acid residues to methyl esters eliminates Asp-Xxx cleavages, and conversion of the C-terminal carboxy group to a methyl ester eliminates loss of the C-terminal His residue. All spectra were measured using DHB as the matrix and conditions optimized for the detection of m/z 1500.
We have recently identified an additional metastable fragmentation pathway that is specific to Arg-containing peptides with a second basic residue (Arg, Lys, or His) at the C- terminal position [26]. Peptides with this combination of amino acid sequence elements undergo the metastable loss of the C-terminal basic residue with retention of the C-terminal hydroxyl group to produce a product known as a [bn−1 + H2O]+ ion [13, 31]. Relevant to this study, we find that [His13]-orcokinin, with a C-terminal His residue, is susceptible to this metastable fragmentation pathway [5]. While the MALDI-FT mass spectrum of synthetic [His13]-orcokinin shows the expected fragments characteristic of other orcokinins, including abundant Asp-Xxx cleavages at m/z 1146.53 and 807.39 (see Fig. 7B), we observe an additional set of peaks resulting from the loss of a His residue. These peaks appear at m/z values (i.e. m/z 1403.62, 1386.60, 1009.4, and 670.33, Fig. 7B) that are identical to those of the truncated orcokinin Orc-[1-12] (Fig. 7A).
Because this metastable fragmentation makes it impossible to distinguish native truncated Orc-[1-12] from that produced by metastable fragmentation, we have used methyl esterification reactions, which convert aspartate, glutamate, and the C-terminal carboxylate groups to methyl esters, to eliminate both metastable Asp-Xxx cleavages and the formation of [bn−1 +H2O]+ ions. For orcokinins, like Orc-[1-12], methyl esterification eliminates metastable Asp-Xxx cleavages (Fig. 7C) because the acidic protons on aspartate and glutamate residues, which catalyze the Asp-Xxx metastable fragmentation, are replaced with a methyl group. In the case of the [His13]-orcokinin standard, Asp-Xxx cleavages are again eliminated by methyl esterification (Fig. 7D). Additionally, methyl esterification of the C-terminal carboxylate group prevents the rearrangement responsible for loss of the C-terminal basic residue [31], and we no longer observe production of fragment ions at the same m/z values as Orc-[1-12] (Fig. 7D). Thus, methyl esterification can be used to clearly distinguish the truncated variant Orc-[1-12] if it is found in the presence of [His13]-orcokinin. We have applied methyl esterification to the analysis of tissue extracts as a second method to assess the peptide complement in SG and CoG tissue samples. For all measurements, we used high-resolution conditions and accurate mass measurements derived from internally calibrated mass spectra.
In our comparative analysis of H. americanus SG, CoG, brain, and STG direct tissue samples, the most abundant orcokinin signals were produced from SG tissues, and the weakest orcokinin signals were observed from the STG. In our analysis of SG and CoG extracts and methyl esterified extracts, where each extract was derived from either a single SG tissue or two CoG tissues (from one individual), we also found that the strongest signals from orcokinin-related peptides were produced from SG samples. Representative MALDI-FT mass spectra for direct-tissue, tissue extracts, and methyl-esterified tissue extracts are shown in Fig. 8 (SG tissues) and Fig. 9 (CoG tissues).
Figure 8.

(A) MALDI-FT mass spectrum of a freshly dissected SG tissue from H. americanus. (B) MALDI-FT mass spectrum of a tissue extract from a single SG tissue from H. americanus. (C) MALDI-FT mass spectrum of a tissue extract from a single SG tissue from H. americanus that was been subjected to methyl esterification to eliminate Asp-Xxx cleavages and loss of the C-terminal His residue. All spectra were measured using DHB as the matrix and conditions optimized for the detection of m/z 1500.
Figure 9.
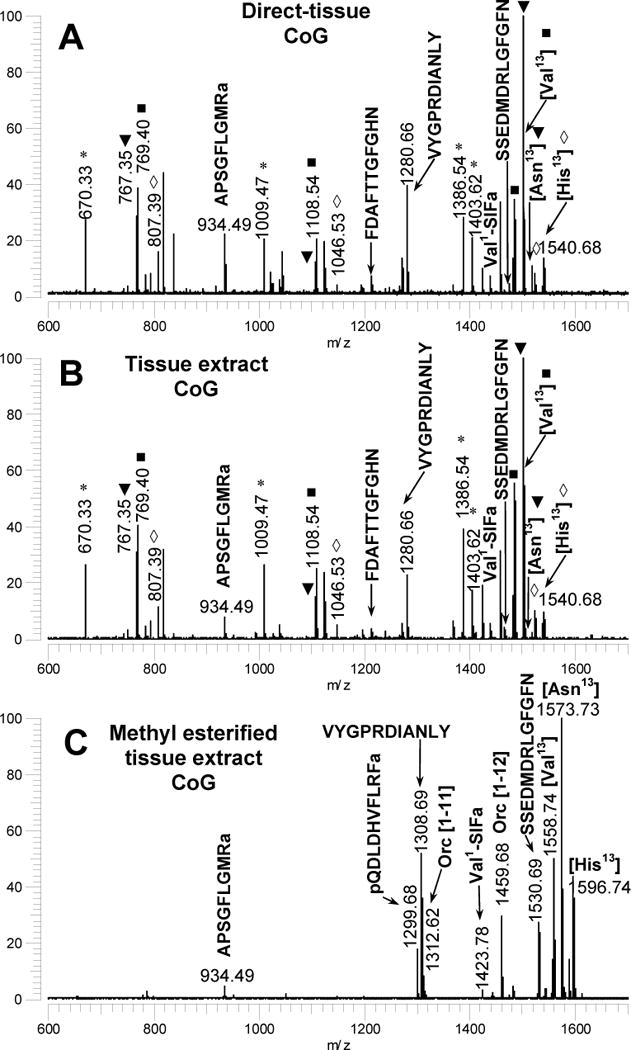
(A) MALDI-FT mass spectrum of a freshly dissected CoG tissue from Homarus americanus. (B) MALDI-FT mass spectrum of a tissue extract from the paired CoG tissues from H. americanus. (C) MALDI-FT mass spectrum of a tissue extract from the paired CoG tissues from H. americanus that was been subjected to methyl esterification to eliminate Asp-Xxx cleavages and loss of the C-terminal His residue. All spectra were measured using DHB as the matrix and conditions optimized for the detection of m/z 1500.
In the MALDI-FT mass spectra of directly analysed SG tissues, we observed characteristic peaks for seven of the ten predicted peptides (Fig. 8A and Table 2). An unsulfated form of GDYDVYPE (Hoam-OPRP II) was detected in SG tissue samples, and the characterization of this peptide is discussed in detail in Section 3.6. With the exception of GDYDVYPE, the same complement of peptides was detected in the methyl-esterified SG extracts (Fig. 8C and Table 3), in CoG and brain tissues (Table 2), and in CoG methyl-esterified tissue extracts (Fig. 9C and Table 3). In STG tissues, incomplete peptide fingerprints were detected and we detected fewer predicted mature peptides than were found in other tissues (Table 2).
Table 2.
Masses of putative peptides produced from (Hoa)-prepro-orcokinin and exact mass measurements for those peptides detected in the direct analysis of tissues freshly dissected from Homarus americanus using MALDI-FTMS.
| Sinus Gland (SG) |
Commisural Ganglion (COG) |
Brain | Stomatogastric ganglion (STG) |
|||
|---|---|---|---|---|---|---|
| Sequence, Peptide | Ion Identity | Calculated m/za |
Measured m/za |
Measured m/za |
Measured m/za |
Measured m/za |
| NFDEIDRSGFGFH [His13]-orcokinin | [M+H]+ | 1540.6815 | 1540.6837 (1.4) |
1540.6845 (1.9) |
1540.6909 (6.1) |
ND |
| [MH-NH3]+ | 1523.6550 | 1523.6577 (1.8) |
1523.6582 (2.1) |
1523.6589 (2.6) |
ND | |
| [y10-H2O]+ | 1146.5327 | 1146.5347 (1.7) |
1146.5312 (−1.3) |
1146.5336 (0.8) |
ND | |
| y7+ | 807.3897 | 807.3894 (−0.4) |
807.3905 (1.0) |
807.3908 (1.4) |
807.3905 (1.0) |
|
| NFDEIDRSGFGFN [Asn13]-orcokinin | [M+H]+ | 1517.6656 | 1517.6661 (0.3) |
1517.6669 (0.9) |
1517.6713 (3.7) |
ND |
| [MH-NH3]+ | 1500.6390 | 1500.6392 (0.2) |
1500.6412 (1.4) |
1500.6429 (2.6) |
1500.6390 (0.02) |
|
| [y10-H2O-NH3]+ | 1106.4902 | 1106.4905 (0.3) |
1106.4894 (−0.7) |
1106.4903 (0.08) |
1106.4929 (2.5) |
|
| y7+ | 784.3737 | 784.3744 (0.9) |
784.3739 (0.2) |
784.3740 (0.5) |
ND | |
| [y7-NH3]+ | 767.3471 | 767.3470 (−0.2) |
767.3466 (−0.7) |
767.3466 (−0.7) |
767.3481 (1.3) |
|
| NFDEIDRSGFGFV [Val13]-orcokinin | [M+H]+ | 1502.6910 | 1502.6898 (−0.8) |
1502.6862 (−3.2) |
1502.6883 (−1.8) |
ND |
| [MH-NH3]+ | 1485.6645 | 1485.6628 (−1.1) |
1485.6643 (−0.2) |
1485.6696 (3.4) |
1485.6653 (0.6) |
|
| [y10-H2O]+ | 1108.5422 | 1108.5415 (−0.6) |
1108.5449 (2.5) |
1108.5471 (4.5) |
1108.5444 (2.0) |
|
| y7+ | 769.3992 | 769.3993 (0.2) |
769.3993 (0.2) |
769.3998 (0.8) |
769.3997 (0.8) |
|
| SSEDMDRLGFGFN Hom-orcokinin | [M+H]+ | 1474.6267 | 1474.6298 (2.1) |
1474.6260 (−0.5) |
1474.6322 (3.7) |
ND |
| [MH-NH3]+ | 1457.6002 | 1457.5985 (−1.2) |
1457.6048 (3.2) |
1457.6090 (6.0) |
1457.6048 (3.2) |
|
| y9+ | 1056.4931 | 1056.4931 (−0.03) | ND | ND | ND | |
| [y9-NH3]+ | 1039.4666 | 1039.4677 (1.08) |
1039.4680 (1.40) |
1039.4702 (3.48) |
1039.4720 (5.21) |
|
| [y7-NH3]+ | 793.3992 | 793.3998 (0.8) |
793.3995 (0.4) |
793.3998 (0.8) |
793.4009 (2.2) |
|
| FDAFTTGFGHN OMT | [M+H]+ | 1213.5273 | 1213.5302 (2.4) |
1213.5267 (−0.5) |
1213.5285 (1.0) |
ND |
| [MH-NH3]+ | 1196.5007 | 1196.5044 (3.0) |
1196.5013 (0.5) |
1196.5003 (−0.3) |
ND | |
| y9+ | 951.4319 | 951.4323 (0.4) |
951.4325 (0.6) |
951.4327 (0.8) |
ND | |
| [y9-NH3]+ | 934.4054 | 934.4050 (−0.4) |
ND | ND | ND | |
| VYGPRDIANLY (Hoa-OPRP III) | [M+H]+ | 1280.6634 | 1280.6639 (0.4) |
1280.6639 (0.4) |
1280.6649 (1.2) |
1280.6651e (1.4) |
| b6+ | 688.3413 | 688.3409 (−0.6) |
688.3413 (0.01) |
688.3418 (0.7) |
ND | |
| GDYDVYPE (Hoa-OPRP II) | [M+H]+ | 957.3836 | 957.3829b (−0.7) |
ND | ND | ND |
| [M+Na]+ | 979.3656 | 979.3669c (1.4) |
ND | ND | ND | |
| [M-H]− | 955.3691 | 955.3716d (3.5) |
ND | ND | ND | |
| [M-H-H2O]− | 937.3585 | 937.3608d (2.4) |
ND | ND | ND | |
| GDY(SO3)DVYPE (Hoa-OPRP II) | [M+H]+ | 1037.3404 | ND | ND | ND | ND |
| [M+Na]+ | 1059.3224 | ND | ND | ND | ND | |
| [M-H]− | 1035.3248 | ND | ND | ND | ND | |
| APARSSPQQDAAA GYTDGAPV (Hoa-OPRP I) | [M+H]+ | 2029.9574 | ND | ND | ND | ND |
| GPIKVRFLSAIFIPI AAPARSSPQQDAA AGYTDGAPV (Hoa-OPRP Ia) | [M+H]+ | 3753.0123 | ND | ND | ND | ND |
| SAE (Hoa-OPRP IV) | [M+H]+ | 306.1296 | ND | ND | ND | ND |
| Truncated Orcokinins | ||||||
| NFDEIDRSGFGF Orc [1-12] | [M+H]+ | 1403.6226 | 1403.6248 (1.6) |
1403.6269 (3.1) |
1403.6305 (5.6) |
ND |
| [MH-NH3]+ | 1386.5961 | 1386.5975 (1.0) |
1386.6014 (3.8) |
1386.6059 (7.0) |
1386.6001 (2.9) |
|
| [y9-H2O]+ | 1009.4738 | 1009.4739 (0.09) |
1009.4754 (1.6) |
1009.4771 (3.3) |
1009.4774 (3.6) |
|
| y6+ | 670.3307 | 670.3306 (−0.2) |
670.3312 (0.6) |
670.3313 (0.8) |
670.3320 (1.8) |
|
| NFDEIDRSGFG Orc [1–11] | [M+H]+ | 1256.5542 | ND | 1256.5575 (2.6) |
ND | ND |
| [MH-NH3]+ | 1239.5277 | 1239.5269 (−0.6) |
1239.5304 (2.2) |
ND | ND | |
| [y8-H2O]+ | 862.4054 | 862.4051 (−0.35) |
862.4057 (0.4) |
ND | ND | |
| y5+ | 523.2623 | 523.2614 (−1.8) |
ND | ND | ND | |
| NFDEIDRSGF Orc [1-10] | [M+H]+ | 1199.5327 | ND | ND | ND | ND |
| [MH-NH3]+ | 1182.5062 | ND | ND | ND | ND | |
| y7+ | 823.3945 | ND | ND | ND | ND | |
| [y7-H2O]+ | 805.3839 | 805.3837 (−0.2) |
ND | ND | ND | |
Exact mass measurements using poly(propylene glycol) 725 and 2000 as calibrants, number in parentheses is the mass measurement error in ppm; ND = not detected;
Peak detected in a tissue extract analyzed using HPA as the matrix with peaks calibrated using known orcokinin m/z values;
Direct tissue samples prepared with salt-doping with NaCl and KCl, as described in Stemmler et al, 2006; peaks calibrated using known orcokinin m/z values.
Samples calibrated using known orcokinin m/z values;
Samples calibrated with Cab-TRP Ia, Val1-SIFamide, and pQDLDHVLRFamide.
Table 3.
Masses of methyl esterified forms of putative peptides produced from (Hoa)-prepro-orcokinin and exact mass measurements for those peptides detected in methyl esterified tissue extracts from a single SG or paired CoGs from Homarus americanus using MALDI-FTMS.
| Sinus gland (SG)a |
Commissural ganglia (COG)a |
|||
|---|---|---|---|---|
| Sequence, Peptide | Ion Identity | Calculated m/z |
Measured m/zb |
Measured m/zb |
| NFDEIDRSGFGFH [His13]-orcokinin | [M+H]+ | 1596.7441 | 1596.7437 (−0.2) |
1596.7388 (−3.4) |
| NFDEIDRSGFGFN [Asn13]-orcokinin | [M+H]+ | 1573.7282 | 1573.7339 (3.6) |
1573.7221 (−3.8) |
| NFDEIDRSGFGFV [Val13]-orcokinin | [M+H]+ | 1558.7536 | 1558.7514 (−1.4) |
1558.7446 (−5.8) |
| SSEDMDRLGFGFN Hom-orcokinin | [M+H]+ | 1530.6893 | 1530.6901 (0.5) |
1530.6844 (−3.2) |
| FDAFTTGFGHN OMT | [M+H]+ | 1241.5586 | 1241.5622 (2.9) |
1241.5619 (2.7) |
| VYGPRDIANLY (Hoa-OPRP III) | [M+H]+ | 1308.6947 | 1308.6946 (−0.1) |
1308.6884 (−4.8) |
| GDYDVYPE (Hoa-OPRP II) | [M+H]+ | 1093.4030 | ND | ND |
| [M+Na]+ | 1115.3850 | ND | ND | |
| GDY(SO3)DVYPE (Hoa-OPRP II) | [M+H]+ | 1013.4462 | ND | ND |
| [M+Na]+ | 1035.4282 | ND | ND | |
| APARSSPQQDAAA GYTDGAPV (Hoa-OPRP I) | [M+H]+ | 3781.0436 | ND | ND |
| GPIKVRFLSAIFIPI AAPARSSPQQDAA AGYTDGAPV (Hoa-OPRP Ia) | [M+H]+ | 2072.0043 | ND | ND |
| SAE (Hoa-OPRP IV) | [M+H]+ | 334.16088 | ND | ND |
| Truncated Orcokinins | ||||
| NFDEIDRSGFGF Orc [1-12] | [M+H]+ | 1459.6852 | 1459.6867 (1.0) |
1459.6823 (−2.0) |
| NFDEIDRSGFG Orc [1-11] | [M+H]+ | 1312.6168 | 1312.6193 (1.9) |
1312.6151 (−1.3) |
| NFDEIDRSGF Orc [1-10] | [M+H]+ | 1255.5953 | 1255.5996 (3.4) |
ND |
Single sinus glands or paired commissural ganglia tissues were subjected to solvent extraction followed by methyl esterification. The methyl esterified extracts were analyzed using DHB as the matrix.
Exact mass measurements using poly(propylene glycol) 725 and 2000 as calibrants, number in parentheses is the mass measurement error in ppm, ND= not detected.
Hoam-OPRP Ia, Hoam-OPRP Ib, and Hoam-OPRP IV were not detected in any samples. For Hoam-OPRP Ia and Hoam-OPRP Ib, we also searched for fragment ions resulting from neutral losses or Asp-Xxx cleavages (data not shown) and adjusted the instrument conditions to optimize the collection and detection of these higher m/z peptides. Hoam-OPRP IV (SAE) is a low mass peptide without a basic residue, and it was also not detected in our study.
In addition to those orcokinin-related peptides predicted from the prepro-protein, we also detected three truncated orcokinin family peptides: Orc-[1-12], Orc-[1-11], and Orc-[1-10] (see Tables 2 and 3). As described above, peaks appearing at m/z values for Orc-[1-12] can result from the metastable fragmentation of [His13]-orcokinin. We also showed that this fragmentation pathway is eliminated when the peptide is methyl-esterified. Orc-[1-12] and the other truncated variants (Orc-[1-11], in the SG and CoGs; Orc-[1-10], in the SG) were detected in methyl-esterified extracts from SG and CoG tissues (Fig. 8C, Fig. 9C, and Table 3), supporting the presence of these truncated orcokinin peptides in vivo in H. americanus tissues.
While MALDI is not generally considered a quantitative technique because ion production can vary depending upon sample preparation, sample heterogeneity, and variations in peptide ionization efficiency, we have found that ion yields for peptide isoforms with similar sequences and similar m/z values show little variation (standard deviations < 5 % relative abundance, data not shown) when measurements from different regions from one sample or measurements from different individuals are compared. Using mass spectral peak intensities derived from the analysis of methyl-esterified extracts from three individuals, we have quantified the relative abundances of ion intensities for the three predicted orcokinin peptides and their truncated forms in methyl-esterified SG and CoG tissue extracts (Table 4). We also predicted the relative abundances of the peptides based upon the number of copies in the transcript; these values are also shown for comparative purposes. When we sum the relative abundances of all truncated forms of orcokinin peptides and compare these abundances between tissues, we find no significant differences between the tissues (p > 0.05).
Table 4.
Relative abundances of orcokinin peptides from methyl esterified tissue extracts from a single SG or paired CoGs from Homarus americanus or paired CoGs from Procambarus clarkii analyzed using MALDI-FTMS.
| Homarus americanus Relative peptide abundance | Procambarus clarkii Relative peptide abundance | ||||
|---|---|---|---|---|---|
| Sequence, Peptide | Sinus gland (SG)a |
Commissural ganglia (COG)a |
Predictedb | Commissural ganglia (COG)a |
Predictedc |
| NFDEIDRSGFGFH [His13]-orcokinin | 15.1 (1.4) |
16.2 (1.7) |
28.6 | — | — |
| NFDEIDRSGFGFN [Asn13]-orcokinin | 45.5 (0.9) |
50.3 (3.5) |
42.9 | 67.7 (2.1) |
60/66.7 |
| NFDEIDRSGFGFV [Val13]-orcokinin | 21.1 (1.6) |
20.9 (1.2) |
28.6 | 13.0 (2.7) |
20/16.7 |
| NFDEIDRTGFGFH [Thr8-His13]-orcokinin | — | — | — | 2.4 (0.4) |
10/8.3 |
| NFDEIDRSGFGFV [Ala13]-orcokinin | — | — | — | 6.7 (0.4) |
10/8.3 |
| Truncated Orcokinins | |||||
| NFDEIDRSGFGF Orc [1-12] | 15.6 (2.4) |
10.7 (2.8) |
— | 5.3 (0.9) |
— |
| NFDEIDRSGFG Orc [1-11] | 2.2 (1.7) |
1.9 (1.0) |
— | 3.6 (0.4) |
— |
| NFDEIDRSGF Orc [1-10] | 0.5 (0.2) |
— | — | 0.2 (0.3) |
— |
| NFDEIDRTGFGF [Thr8-Xxx13] | — | — | — | 1.2 (0.1) |
— |
Single sinus glands or paired commissural ganglia tissues were subjected to solvent extraction followed by methyl esterification. The methyl esterified extracts were analyzed using DHB as the matrix.
Relative abundance predicted based upon the number of copies of the orcokinin peptide in the precursor proteins.
3.6 Only the non-sulfated isoform of Hoa-OPRP II is detected
Because the N-terminal Tyr residue of peptide GDYDVYPE (Hoam-OPRP II) was predicted to be sulfated to yield GDY(SO3)DVYPE, we looked for both the sulfated and unsulfated forms using a range of ionization conditions with MALDI-FTMS. The facile metastable loss of SO3 from sulfated Tyr residues has presented challenges for the determination of this post-translational modification by mass spectrometry using either MALDI or electrospray ionization techniques (see the recent review by Monigatti et al. [19]). In general, the analysis of sulfated peptides by MALDI in negative ion mode, producing [M-H]− ions, has yielded signals most resistant to loss of the SO3 group [19, 21, 30, 34].
Although we did not have a standard of GDY(SO3)DVYPE to assess how well we would be able to detect Tyr sulfation in this sequence, we did analyze two sulfated peptide standards, a sulfakinin, pQFDEY(SO3)GHMRFamide, and a cholecystokinin derivative, N-acetyl-DY(SO3)MGWMamide, by MALDI-FTMS using both positive and negative ion modes. For both peptide standards, we were unable to detect signals for the molecular ion using the matrix DHB in the positive ion mode; however, [M-H]− ions were detected in negative ion mode at 50% and 10% relative abundance, respectively. The most abundant negative ion fragments for each standard showed retention of the sulfate groups. Accompanying the [M-H]− ion was an [M-H-H2O]− fragment for pQFDEY(SO3)GHMRFamide, which was the base peak in the spectrum. For N-acetyl-DY(SO3)MGWMamide, a metastable y5− fragment, with retention of the sulfate group, was the base peak. We also found that a different MALDI matrix, HPA, could be used to reduce fragmentation significantly in both positive and negative ion modes. While sequence specific differences in ionization characteristics may change the ionization behavior of the peptide of interest for this study, GDY(SO3) DVYPE, the data supports our ability to produce [M-H]− ions for peptides sulfated at a tyrosine residue, and we used both negative and positive ion modes, and both matrices (DHB and HPA), for our experiments.
When we analyzed the SG and CoGs from H. americanus in negative and positive ion modes using DHB as the matrix, and analyzed a SG extract using HPA as the matrix, we found no evidence for the O-sulfated isoform, GDY(SO3)DVYPE; however, we did detect the unsulfated form, GDYDVYPE, using a variety of ionization conditions. As shown in Fig. 10A, we detected [M+H]+, [M+Na]+, and [M+K]+ ions for GDYDVYPE using HPA in the positive ion mode, [M-H]−, [M-H-H2O]−, and [M-H-2H2O]− ions using DHB in the negative ion mode (Fig. 10B), and [M+Na]+ and [M+K]+ ions using DHB doped with NaCl and KCl in the positive ion mode (Fig. 10C). We used the addition of NaCl and KCl to improve our ability to detect GDYDVYPE, which does not have a basic amino acid in the peptide sequence and consequently is less susceptible to positive ionization, using sample preparation conditions that have been described in an earlier publication [27]. Thus, our mass spectral measurements show that GDYDVYPE (Hoa-II) is present in SG tissues, while we found no evidence for the sulfated isoform.
Figure 10.
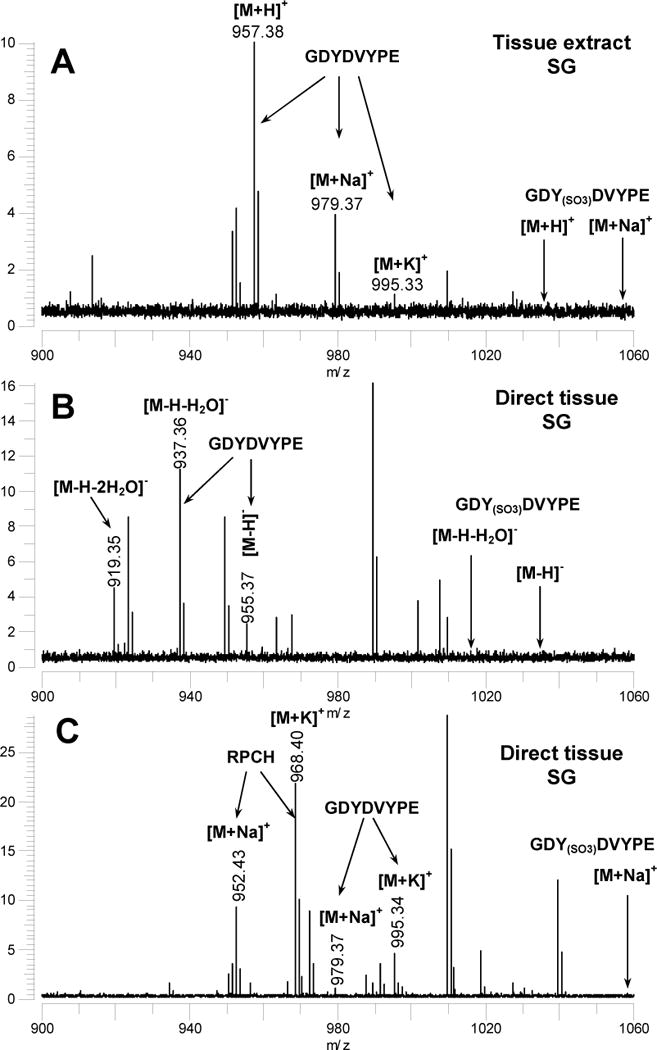
(A) MALDI-FT mass spectrum of a tissue extract from a single SG tissue from Homarus americanus analysed using 3-hydroxypicolinic acid (HPA) as a matrix to reduce metastable loss of SO3 from sulfated peptides. The m/z values where peaks corresponding to GDY(SO3)DVPE (not detected) would appear are indicated. (B) MALDI-FT mass spectrum of a SG tissue sample from H. americanus measured in the negative ion mode using DHB as the MALDI matrix. The m/z values where peaks corresponding to GDY(SO3)DVPE (not detected) would appear are indicated. (C) MALDI-FT mass spectrum of a a SG tissue sample from H. americanus measured using DHB as the MALDI matrix and following the addition of aqueous NaCl and KCl to promote the formation of [M+Na]+ and [M+K]+ ions. All spectra were measured using conditions optimized for the detection of m/z 1500.
3.7. Mass spectral analysis of predicted orcokinin, orcokinin precursor-related peptides and truncated orcokinins in Procambarus clarkii
For comparative purposes, we used MALDI-FTMS to analyze freshly-dissected CoG tissues from the crayfish, P. clarkii, to determine if the full complement of peptides predicted from the P. clarkii pro-orcokinin precursors were detected. All of the predicted orcokinin peptides ([Thr8-His13], [Asn13], [Val13], and [Ala13]) were observed at the predicted m/z values (Fig. 11A and Table 5). Furthermore, m/z values corresponding to the orcomyotropin-related peptide FDAFTTGFGHS and the peptide VYVPRYIANLY were detected as well. We did not detect m/z values for GDYDVYPE, NVE or GTIKTAPARTPSTQDDASFPPDGAPV.
Figure 11.

(A) MALDI-FT mass spectrum of a freshly dissected CoG tissue from Procambarus clarkii using DHB as the matrix and spectra measured using conditions optimized for the detection of m/z 1500. (B) MALDI-FT mass spectrum of a tissue extract from the paired CoG tissues from P. clarkii. (C) MALDI-FT mass spectrum of a tissue extract from the paired CoG tissues from P. clarkii that was been subjected to methyl esterification to eliminate Asp-Xxx cleavages and loss of the C-terminal His residue. All spectra were measured using DHB as the matrix and conditions optimized for the detection of m/z 1500.
Table 5.
Masses of putative peptides produced from (Pro)-prepro-orcokinin A and B with exact mass measurements for those peptides detected in the direct analysis of CoG tissues freshly dissected from Procambarus clarkii using MALDI-FTMS.
| COG | |||
|---|---|---|---|
| Sequence, Peptide | Ion Identity | Calculated m/za |
Measured m/za |
| NFDEIDRTGFGFH [Thr8-His13]-orcokinin | [M+H]+ | 1554.6972 | 1554.7060 (5.7) |
| [MH-NH3]+ | 1537.6706 | 1537.6748 (2.7) |
|
| [y10-H2O]+ | 1160.5483 | 1160.5509 (2.2) |
|
| y7+ | 821.4053 | 821.4049 (−0.5) |
|
| NFDEIDRSGFGFN [Asn13]-orcokinin | [M+H]+ | 1517.6656 | 1517.6669 (0.9) |
| [MH-NH3]+ | 1500.6390 | 1500.6401 (0.7) |
|
| [y10-H2O-NH3]+ | 1106.4902 | 1106.4887 (−1.3) |
|
| y7+ | 784.3737 | 784.3728 (−1.1) |
|
| [y7-NH3]+ | 767.3471 | 767.3453 (−2.4) |
|
| NFDEIDRSGFGFV [Val13]-orcokinin | [M+H]+ | 1502.6910 | 1502.6846 (−4.3) |
| [MH-NH3]+ | 1485.6645 | 1485.6621 (−1.6) |
|
| [y10-H2O]+ | 1108.5422 | 1108.5465 (3.8) |
|
| y7+ | 769.3992 | 769.3985 (−0.9) |
|
| NFDEIDRSGFGFA [Ala13]-orcokinin | [M+H]+ | 1474.6597 | 1474.6619 (1.5) |
| [MH-NH3]+ | 1457.6332 | 1457.6418 (5.9) |
|
| [y10-H2O]+ | 1080.5109 | 1080.5127 (1.7) |
|
| y7+ | 741.3678 | 741.3672 (−0.8) |
|
| FDAFTTGFGHS OMT | [M+H]+ | 1186.5164 | 1186.5148 (−1.3) |
| y9+ | 924.4210 | 924.4212 (0.2) |
|
| [y9-H2O]+ | 906.4104 | 906.4138 (3.7) |
|
| VYVPRYIANLY | [M+H]+ | 1370.7472 | 1370.7506 (2.5) |
| GDYDVYPE | [M+H]+ | 870.3516 | ND |
| [M+Na]+ | 892.3335 | ND | |
| [M-H]− | 868.3370 | ND | |
| NVE | [M+H]+ | 361.1718 | ND |
| GTIKTAPARTPSTQ DDASFPPDGAPV | [M+H]+ | 2597.2842 | ND |
| Truncated Orcokinins | |||
| NFDEIDRTGFGF [Thr8-Xxx13] | [M+H]+ | 1417.6383 | 1417.6378 (−0.4) |
| [MH-NH3]+ | 1400.6117 | 1400.6158 (2.9) |
|
| [y9-H2O]+ | 1023.4894 | 1023.4887 (−0.7) |
|
| y6+ | 684.3464 | 684.3461 (−0.4) |
|
| NFDEIDRSGFGF Orc [1–12] | [M+H]+ | 1403.6226 | 1403.6285 (4.2) |
| [MH-NH3]+ | 1386.5961 | 1386.6049 (6.4) |
|
| [y8-H2O]+ | 1009.4738 | 1009.4779 (4.0) |
|
| y5+ | 670.3307 | 670.3310 (0.4) |
|
Exact mass measurements using poly(propylene glycol) 725 and 2000 as calibrants, number in parentheses is the mass measurement error in ppm.
In addition to the peptides predicted from the orcokinin precursor, m/z values for two truncated orcokinin peptides, [Thr8-Xxx13] and Orc-[1-12], were detected in directly analyzed CoG tissues from P. clarkii and in tissue extracts (Fig. 11A and B and Table 5). The peaks appearing at m/z values corresponding to [Thr8-Xxx13] could result from the same metastable fragmentation reaction described above for [His13]-orcokinin, with loss of the C-terminal His residue from [Thr8-His13] producing the observed ions. To determine if truncated forms of orcokinin peptides were found in CoG tissues from P. clarkii, we methyl-esterified extracts of paired CoG tissues to eliminate metastable loss of the C-terminal His residue, and analyzed the samples by MALDI-FTMS. For samples from three individual animals, truncated orcokinin peptides (Orc-[1-12], Orc-[1-11], Orc-[1-10], and Orc-[Thr8-Xxx13]) were detected upon MALDI-FTMS analysis of the methyl-esterified extracts (Fig. 11C). The Orc-[1-12] isoform consistently appeared with higher relative abundance compared with the Orc-[Thr8-Xxx13] isoform in all analyzed samples (Table 4). We did not detect any other truncated [Thr8-His13]-Orc isoforms in the methyl-esterified extracts.
We also compared the relative abundance of truncated orcokinin isoforms from the methyl-esterified CoG tissue extracts from P. clarkii with those detected in CoG tissue extracts from H. americanus. When we summed the relative abundance values for all orcokinin isoforms, we found no significant difference (p > 0.05) between the two species (Table 4).
3.8 Physiological effects of orcokinins in Homarus and Procambarus
To determine whether the effects of orcokinins were similar to those that have been described in the crayfish O. limosus [25], we examined the effects of [Asn13]-orcokinin (NFDEIDRSGFGFN), which we identified in both H. americanus and P. clarkii, on the movements of the mid- and hind-guts of both species. Although only 57% (4 of 7) of the P. clarkii preparations we tested were spontaneously active, superfusion with 10−6 M [Asn13]-orcokinin reliably activated contractions in the remaining preparations, and increased contraction frequency in all preparations (from an average of 1.0 to 4.6 contractions/min; Fig. 12A). As an added control, we bath applied the peptide proctolin to the same preparations, and found that it too increased motility to similar levels (from an average of 0.8 to 6.5 contractions/min; Fig. 12A). Frequencies in both peptides were significantly higher than their respective control frequencies (ANOVA/Bonferroni, p<0.05 for [Asn13]-orcokinin; p<0.001 for proctolin), but frequencies recorded in proctolin and in [Asn13]-orcokinin did not differ significantly from one another (ANOVA/Bonferroni, p>0.05).
Figure 12.

[Asn13]-orcokinin enhanced mid-/hindgut motility in Procambarus clarkii (A), but not in Homarus americanus (B). The rate of spontaneous contractions of the combined mid-/hindgut preparation was measured in control saline (Cont) and during superfusion with 10−6 M [Asn13]-orcokinin ([N13]Orc). To ensure that the preparations were healthy and responsive to neuropeptides, all preparations were also superfused with 10−6 M proctolin (Proct), which evoked or enhanced motility in both species. Both [Asn13]-orcokinin and proctolin increased activity in P. clarkii (n=7), while only proctolin did so in H. americanus (n=5). * indicates significantly different from control, p<0.05; ** indicates significantly from control, p<0.001 (ANOVA, followed by post-hoc protected Bonferroni t-tests).
In H. americanus, somewhat fewer preparations (30%) showed spontaneous contractions, although there was no significant difference in the average control frequencies recorded in the two species (P. clarkii 0.8 and 1.0 contractions/min; H americanus, 0.5 and 1.1 contractions/min; ANOVA/Bonferroni, p>0.05). Surprisingly, [Asn13]-orcokinin did not activate contractions in any of the 5 preparations in which we tested it (control frequency 1.1 contractions/min, [Asn13]-orcokinin frequency 0.9 contractions/min; ANOVA/Bonferroni, p>0.05; Fig. 12B). These preparations did, however, respond to proctolin with increased contraction frequency (control 0.5 contractions/min, proctolin 8.6 contractions/min; ANOVA/Bonferroni, p<0.001; Fig. 12B).
4. Discussion
4.1. Identification and characterization of Homarus americanus orcokinin-encoding cDNAs
Using the deduced amino acid sequences of known crayfish prepro-orcokinins as queries, we identified five American lobster ESTs encoding putative orcokinin prepro-hormones. Three of the five clones from which the ESTs were generated were fully characterized, each encoding a unique transcript. The ORF of one of the cDNAs (Homarus americanus-prepro-orcokinin I) was predicted to produce a 218 amino acid prepro-hormone (Hoam-prepro-orcokinin I), while the other two cDNAs (Homarus americanus-prepro-orcokinin II and III) were predicted to encode an identical 207 amino acid prepro-protein (Hoam-prepro-orcokinin II). Comparison of the two prepro-hormones showed them to contain distinct signal sequences, but to encode an essentially common set of neuropeptides. Specifically, 12 of 13 of the peptides putatively liberated from each protein were fully conserved, both in terms of their structures and their position within the two precursors. The remaining peptide in each of the two prepro-orcokinins was also highly conserved: the 21 amino acid C-termini are identical, but the peptide predicted to be released from Hoam-prepro-orcokinin I possesses a 16 amino acid N-terminal extension. At present it is unknown if the three transcripts are derived from a common or distinct gene and/or whether the transcripts might represent individual-specific variants. Regardless, the nearly identical set of peptides contained within the predicted prepro-homones suggests a strong conservation in function for the individual peptides present.
4.2. The encoded peptides and structure of the Homarus americanus prepro-orcokinin is similar, though not identical, to those of Procambarus clarkii
Comparison of the prepro-orcokinins deduced from H. americanus with those deduced from the crayfish P. clarkii [35] shows extensive conservation in the overall peptide complement of the prepro-hormones from these two species. Specifically, the prepro-proteins of both species include multiple orcokinin isoforms, as well as a single copy of an orcomyotropin-related peptide. Moreover, the majority of the precursor-related peptides predicted for the two species are also similar, i.e. the prepro-orcokinins of both H. americanus and P. clarkii possess single copies of a similar 21+ amino acid peptide, an acid-rich peptide, an undecapeptide and a tripeptide.
Despite the similar overall peptide complements that are present in the H. americanus and P. clarkii prepro-orcokinins, variation is present in the specific isoforms found in the two species. For example, while both species possess the orcokinin isoforms NFDEIDRSGFGFN and NFDEIDRSGFGFV, NFDEIDRSGFGFH was present only in H. americanus and NFDEIDRSGFGFA and NFDEIDRTGFGFH were found only in P. clarkii. Similarly, the isoforms of the encoded orcomyotropin-related peptide (FDAFTTGFGHN in H. americanus versus FDAFTTGFGHS in P. clarkii) and the other precursor-related peptides (GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV or APARSSPQQDAAAGYTDGAPV versus GTIKTAPARTPSTQDDASFPPDGAPV; GDYDVYPE versus DYDVFPD; VYGPRDIANLY versus VYVPRYIANLY; SAE versus NVE) varied between the two species. Moreover, one copy of the orcokinin-like peptide, SSEDMDRLGFGFN, was present only in the H. americanus precursors. Regardless, the presence of such a highly conserved overall complement of peptides suggests strong conservation in function, although variation in the roles served due to the species-specific isoforms is also possible.
One striking difference that was observed in comparisons of the H. americanus and P. clarkii prepro-orcokinins is the organization of the peptides within the proteins. Specifically, the P. clarkii precursors include more copies of the native orcokinin isoforms and the order of the acidic and undecapeptides in the proteins is reversed in the two species. Molecular analysis on members of the astacidean infraorder [6], including both H. americanus and P. clarkii, suggests that the former is more basal in the phylogeny of this taxon. Thus, the additional copies and isoforms of orcokinin, as well as the reordering of the two precursor-related peptides present in P. clarkii, may reflect gene duplications and rearrangement that have occurred in the evolution of the more derived crayfish.
4.3. Some, but not all, peptides predicted from Homarus americanus and Procambarus clarkii pro-orcokinins are detectable via direct tissue MALDI-FTMS
In P. clarkii, six of the nine, and in H. americanus seven of the nine predicted peptides from the prepro-orcokinin transcript processing were detected. All of the orcokinins predicted by the orcokinin prepro-protein transcript were detected in the respective species. Additionally, in both species orcomyotropin-related peptide isoforms, FDAFTTGFGHS (P. clarkii) and FDAFTTGFGHN (H. americanus), and VYVPRYIANLY (P. clarkii) and VYGPRDIANLY (H. americanus) were detected. Of the DYDVFPD and GDYDVYPE peptides in P. clarkii and H. americanus, respectively, the H. americanus isoform was detected only in the SGs of that species. The sulfation predicted for Tyr3 of GDYDVYPE was not observed, even using MALDI-FTMS conditions where sulfate retention was demonstrated for two sulfated peptide standards, suggesting that the sulfated peptide is not present in vivo. No mass spectral evidence was found for the short peptide products SAE in H. americanus and NVE in P. clarkii, and the larger peptides GTIKTAPARTPSTQDDASFPPDGAPV in P. clarkii and GPIKVRFLSAIFIPIAAPARSSPQQDAAAGYTDGAPV or APARSSPQQDAAAGYTDGAPV in H. americanus. These peptide isoforms may not have any biological activity, but may serve as structural domains in the precursor peptide that are rapidly degraded in vivo during processing.
In the analysis of SG, CoG, and brain tissues from H. americanus, we consistently detected the same complement of orcokinin prepro-derived peptides, with the exception of GDYDVYPE, which was detected only in the SG. Fewer peptides were detected in the STG, which may be a consequence of the lower apparent concentration of orcokinin-related peptides in these samples or the differential expression of peptides, as has been documented within the brain and pericardial organ of Cancer borealis [7].
4.4. Evidence for biochemically-derived truncated orcokinins for H. americanus and P. clarkii
While all of the orcokinins predicted by the transcript were observed in the mass spectra, additional truncated orcokinins were observed in this study and in others that have characterized H. americanus tissues using MALDI-TOF [16, 23], MALDI-FTMS [28] or ESI-LC-MS [4,10,11]. In this study, we used methyl esterification reactions to eliminate the metastable loss of the basic C-terminal His residue, which we found to be a source of m/z values corresponding to Orc-[1-12] from [His13]-orcokinin. The fact that Orc-[1-12], and other less abundant truncated orcokinins were observed in methylated extracts from SG and CoG tissues (H. americanus) and from CoG tissues (P. clarkii), suggests that these peptides are generated by further processing or degradation of full-length orcokinins. As the neuromodulatory or hormonal roles of orcokinin family peptides have yet to be firmly established, it will be interesting to determine if these truncated forms play a role in the generation of active orcokinin isoforms.
4.5. Physiological effects of orcokinin peptides in H. americanus and P. clarkii
The orcokinin with the largest number of copies in the predicted prepro-hormones of both species is [Asn13]-orcokinin, with 3 copies in H. americanus and 7 copies in P. clarkii. Because of this, because it is one of only two orcokinin isoforms shared by the two species, and because this is the isoform initially identified and studied in O. limosus [25], we chose to test the biological activity of this isoform. All three species are closely related, being members of the infraorder Astacidea of the decapod crustaceans; we therefore expected to record increases in hindgut motility in P. clarkii and H. americanus that were similar to those previously reported in O. limosus. Not surprisingly, we did record similar increases in P. clarkii. In contrast, in none of the five preparations of H. americanus mid-/hindguts was there any increase in movements. Given the close phylogenetic relationships of these species, this finding is surprising. At the present time, neither the evolutionary nor the mechanistic reasons for this difference are clear. We considered the possibility that the H. americanus preparations, most of which did not show spontaneous activity, were simply less responsive or less healthy than our P. clarkii preparations. To control for this possibility, we compared the effects of the peptide proctolin on mid-/hindgut preparations in the two species, and found that both species responded equally to proctolin. We also considered the possibility that the H. americanus gut responds selectively to other orcokinins, but found no response in H. americanus to either [His13]-orcokinin or Orc-[1-12] (data not shown). Thus, our data suggest that the hindgut of H. americanus, in contrast to those of related crayfish species (O. limosus and P. clarkii), is not a target of orcokinins.
Acknowledgments
Financial support for this work was provided by National Science Foundation grants IBN-0111040 (P.S.D.) and MRI-0116416 (E.A.S.), NIH Grant Number P20 RR-016463 from the INBRE Program of the National Center for Research Resources (to MDIBL; Patricia Hand Ph.D., PI), the Arnold and Mabel Beckman Foundation (C.R.C), the Merck Foundation (C.R.C), a Mount Desert Island Biological Laboratory New Investigator Award through the Salisbury Cove Research Fund provided by the Thomas H. Maren Foundation (A.E.C), and through institutional funds provided by MDIBL (A.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Billimoria CP, Li L, Marder E. Profiling of neuropeptides released at the stomatogastric ganglion of the crab, Cancer borealis with mass spectrometry. J Neurochem. 2005;95:191–199. doi: 10.1111/j.1471-4159.2005.03355.x. [DOI] [PubMed] [Google Scholar]
- 3.Bungart D, Hilbich C, Dircksen H, Keller R. Occurrence of analogues of the myotropic neuropeptide orcokinin in the shore crab, Carcinus maenas: evidence for a novel neuropeptide family. Peptides. 1995;16:67–72. doi: 10.1016/0196-9781(94)00145-v. [DOI] [PubMed] [Google Scholar]
- 4.Cape SS, Rehm KJ, Mingming M, Marder E, Li L. Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic Homarus americanus. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05154.x. In press. [DOI] [PubMed] [Google Scholar]
- 5.Cashman CR, Dickinson PS, Christie AE, Stemmler EA. C-Terminal histidine loss from orcokinin family neuropeptides: Implications for the identification of truncated neuropeptide variants by MALDI-FTMS” 233rd National Meeting of the American Chemical Society. Chicago, IL: 2007. Mar 25–29, 2007. [Google Scholar]
- 6.Crandall KA, Harris DJ, Fetzner JW. The monophyletic origin of freshwater crayfish estimated from nuclear and mitochondrial DNA sequences. Proc R Soc Lond B. 2000;267:1679–1686. doi: 10.1098/rspb.2000.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeKeyser SS, Kutz-Naber KK, Schmidt JJ, Barrett-Wilt GA, Li L. Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J Proteome Res. 2007;6:1782–1791. doi: 10.1021/pr060603v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson PS, Stevens JS, Rus S, Brennan HR, Goiney CC, Smith CM, Li L, Towle DW, Christie AE. Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J Exp Biol. 2007;210:2278–2289. doi: 10.1242/jeb.004770. [DOI] [PubMed] [Google Scholar]
- 9.Dircksen H, Burdzik S, Sauter A, Keller R. Two orcokinins and the novel octapeptide orcomyotropin in the hindgut of the crayfish Orconectes limosus: identified myostimulatory neuropeptides originating together in neurones of the terminal abdominal ganglion. J Exp Biol. 2000;203:2807–2818. doi: 10.1242/jeb.203.18.2807. [DOI] [PubMed] [Google Scholar]
- 10.Fu Q, Goy MF, Li L. Identification of neuropeptides from the decapod crustacean sinus glands using nanoscale liquid chromatography tandem mass spectrometry. Biochem Biophys Res Commun. 2005;337:765–778. doi: 10.1016/j.bbrc.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, Kutz KK, Schmidt JJ, Hsu YW, Messinger DI, Cain SD, de la Iglesia HO, Christie AE, Li L. Hormone complement of the Cancer productus sinus gland and pericardial organ: an anatomical and mass spectrometric investigation. J Comp Neurol. 2005;493:607–626. doi: 10.1002/cne.20773. [DOI] [PubMed] [Google Scholar]
- 12.Fu Q, Li L. Investigation of several unique tandem mass spectrometric fragmentation patterns of NFDEIDR, an orcokinin analog, and its N-terminal dimethylated form. Rapid Commun Mass Spectrom. 2006;20:553–562. doi: 10.1002/rcm.2337. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez J, Besada V, Garay H, Reyes O, Padron G, Tambara Y, Takao T, Shimonishi Y. Effect of the position of a basic amino acid on C-terminal rearrangement of protonated peptides upon collision-induced dissociation. J Mass Spectrom. 1996;31:150–158. doi: 10.1002/(SICI)1096-9888(199602)31:2<150::AID-JMS287>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Hofer S, Dircksen H, Tollback P, Homberg U. Novel insect orcokinins: characterization and neuronal distribution in the brains of selected dicondylian insects. J Comp Neurol. 2005;490:57–71. doi: 10.1002/cne.20650. [DOI] [PubMed] [Google Scholar]
- 15.Huybrechts J, Nusbaum MP, Bosch LV, Baggerman G, De Loof A, Schoofs L. Neuropeptidomic analysis of the brain and thoracic ganglion from the Jonah crab, Cancer borealis. Biochem Biophys Res Commun. 2003;308:535–544. doi: 10.1016/s0006-291x(03)01426-8. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E. Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol. 2002;444:227–244. doi: 10.1002/cne.10139. [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Kutz-Naber KK, Li L. Methyl esterification assisted MALDI FTMS characterization of the orcokinin neuropeptide family. Anal Chem. 2007;79:673–681. doi: 10.1021/ac061536r. [DOI] [PubMed] [Google Scholar]
- 18.Monigatti F, Gasteiger E, Bairoch A, Jung E. The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics. 2002;18:769–770. doi: 10.1093/bioinformatics/18.5.769. [DOI] [PubMed] [Google Scholar]
- 19.Monigatti F, Hekking B, Steen H. Protein sulfation analysis - a primer. Biochem Biophys Acta. 2006;1764:1904–1913. doi: 10.1016/j.bbapap.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor PB, Costello CE. Internal calibration on adjacent samples (InCAS) with Fourier transform mass spectrometry. Anal Chem. 2000;72:5881–5885. doi: 10.1021/ac000770t. [DOI] [PubMed] [Google Scholar]
- 21.Önnerfjord P, Heathfield TF, Heinegård D. Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J Bio Chem. 2004;279:26–33. doi: 10.1074/jbc.M308689200. [DOI] [PubMed] [Google Scholar]
- 22.Pascual N, Castresana J, Valero ML, Andreu D, Belles X. Orcokinins in insects and other invertebrates. Insect Biochem Mol Biol. 2004;34:1141–1146. doi: 10.1016/j.ibmb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Skiebe P, Dreger M, Borner J, Meseke M, Weckwerth W. Immunocytochemical and molecular data guide peptide identification by mass spectrometry: orcokinin and orcomyotropin-related peptides in the stomatogastric nervous system of several crustacean species. Cell Mol Biol (Noisy-le-grand) 2003;49:851–871. [PubMed] [Google Scholar]
- 24.Skiebe P, Dreger M, Meseke M, Evers JF, Hucho F. Identification of orcokinins in single neurons in the stomatogastric nervous system of the crayfish, Cherax destructor. J Comp Neurol. 2002;444:245–259. doi: 10.1002/cne.10145. [DOI] [PubMed] [Google Scholar]
- 25.Stangier J, Hilbich C, Burdzik S, Keller R. Orcokinin: a novel myotropic peptide from the nervous system of the crayfish, Orconectes limosus. Peptides. 1992;13:859–864. doi: 10.1016/0196-9781(92)90041-z. [DOI] [PubMed] [Google Scholar]
- 26.Stemmler EA, Cashman CR, Dickinson PS. C-Terminal fragmentation of singly-protonated, arginine-containing peptides: Metastable loss of C-terminal histidine, lysine, and arginine characterized by MALDI-FTMS; Proceedings of the 55th ASMS Conference on Mass Spectrometry and Allied Topics; Indianapolis, Indiana. June 3–7, 2007.2007. [Google Scholar]
- 27.Stemmler EA, Gardner NP, Guiney ME, Bruns EA, Dickinson PS. The detection of red pigment-concentrating hormone (RPCH) in crustacean eyestalk tissues using matrix-assisted laser desorption/ionization-Fourier transform mass spectrometry: [M+Na]+ ion formation in dried droplet tissue preparations. J Mass Spectrom. 2006;41:295–311. doi: 10.1002/jms.989. [DOI] [PubMed] [Google Scholar]
- 28.Stemmler EA, Provencher HL, Guiney ME, Gardner NP, Dickinson PS. Matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for the identification of orcokinin neuropeptides in crustaceans using metastable decay and sustained off-resonance irradiation. Anal Chem. 2005;77:3594–3606. doi: 10.1021/ac0502347. [DOI] [PubMed] [Google Scholar]
- 29.Stepanyan R, Day K, Urban J, Hardin DL, Shetty RS, Derby CD, Ache BW, McClintock TS. Gene expression and specificity in the mature zone of the lobster olfactory organ. Physiol Genomics. 2006;25:224–233. doi: 10.1152/physiolgenomics.00276.2005. [DOI] [PubMed] [Google Scholar]
- 30.Talbo G, Roepstorff P. Determination of sulfated peptides via prompt fragmentation by UV matrix-assisted laser desorption/ionization mass spectrometry. Rapid Comm Mass Spec. 1993;7:201–204. [Google Scholar]
- 31.Thorne GC, Ballard KD, Gaskell SJ. Metastable decomposition of peptide [M+H]+ ions via rearrangement involving loss of the c-terminal amino-acid residue. J Am Soc Mass Spectrom. 1989;1:249–257. [Google Scholar]
- 32.Towle DW, Smith CM. Gene discovery in Carcinus maenas and Homarus americanus via expressed sequence tags. Integr Comp Biol. 2006;46:912–918. doi: 10.1093/icb/icl002. [DOI] [PubMed] [Google Scholar]
- 33.Veenstra JA. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch Insect Biochem Physiol. 2000;43:49–63. doi: 10.1002/(SICI)1520-6327(200002)43:2<49::AID-ARCH1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Wolfender JL, Chu F, Ball H, Wolfender F, Fainzilber M, Baldwin MA, Burlingame AL. Identification of tyrosine sulfation in Conus pennaceus conotoxins α-PnIA and α-PnIB: Further investigations of labile sulfo- and phosphopeptides by electrospray, matrix-assisted laser desorption/ionization (MALDI) and atmospheric pressure MALDI mass spectrometry. J Mass Spec. 1999;43:447–454. doi: 10.1002/(SICI)1096-9888(199904)34:4<447::AID-JMS801>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda-Kamatani Y, Yasuda A. Identification of orcokinin gene-related peptides in the brain of the crayfish Procambarus clarkii by the combination of MALDI-TOF and on-line capillary HPLC/Q-Tof mass spectrometries and molecular cloning. Gen Comp Endocrinol. 2000;118:161–172. doi: 10.1006/gcen.1999.7453. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda-Kamatani Y, Yasuda A. Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii. J Comp Neurol. 2006;496:135–147. doi: 10.1002/cne.20903. [DOI] [PubMed] [Google Scholar]


