Abstract
The molecular chaperone heat shock protein 90 (Hsp90) facilitates metastable protein maturation, stabilization of aggregation-prone proteins, quality control of misfolded proteins and assists in keeping proteins in activation-competent conformations. Proteins that rely on Hsp90 for function are delivered to Hsp90 utilizing a co-chaperone–assisted cycle. Co-chaperones play a role in client transfer to Hsp90, Hsp90 ATPase regulation and stabilization of various Hsp90 conformational states. Many of the proteins chaperoned by Hsp90 (Hsp90 clients) are essential for the progression of various diseases, including cancer, Alzheimer's disease and other neurodegenerative diseases, as well as viral and bacterial infections. Given the importance of these clients in different diseases and their dynamic interplay with the chaperone machinery, it has been suggested that targeting Hsp90 and its respective co-chaperones may be an effective method for combating a large range of illnesses.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: molecular chaperones, drug development, Heat Shock Protein 90
1. Hsp90 inhibitors and their binding sites
(a). Hsp90 N-terminal inhibitors
Hsp90 inhibition gained interest following the discovery of Hsp90 as a target for the natural products geldanamycin (GA) and radicicol (RD) [1–3]. Drugging Hsp90 with GA resulted in reduced cancer cell growth and oncogenic protein depletion [4]. Hsp90 exists as a dimeric protein with each monomer containing an N-terminal ATP-binding domain, a middle co-chaperone and client-binding domain, and a C-terminal dimerization domain. These regions undergo substantial ATP-influenced conformational changes in order to properly chaperone clientele. The absence of bound nucleotide stabilizes the open conformation of Hsp90 characterized by the dimerization of its C-terminal domains. Nucleotide interaction results in the transient dimerization of the N-terminal domains, establishing ATPase competence and strengthening Hsp90–client interactions. Following ATP-hydrolysis, Hsp90 proceeds back to the open conformation. Crystal structure analysis determined the binding site for GA and RD within Hsp90 was located in the N-terminal ATP-binding domain and mimicked the open ADP-bound conformation [3]. Although these compounds efficiently targeted and disrupted Hsp90 function, they were not useful for clinical application due to their toxicity and low stability.
The selectivity of GA and RD toward Hsp90 is due to the unique N-terminal ATP-binding pocket of Hsp90, which contains Bergerat-fold geometry found within the GHKL subgroup of ATPases [5,6]. This selectivity paved the path for new, less toxic and more stable inhibitors that mimic GA and RD interaction within the ATP-binding pocket.
(b). Benzoquinone ansamycin inhibitors
GA is a naturally occurring benzoquinone ansamycin antibiotic isolated from Streptomyces hygroscopicus. Although displaying a planar conformation in solution, upon binding to Hsp90, GA adopts a folded conformation with the planes of the benzoquinone and the ansamycin macrocycle positioned in a parallel configuration [3]. The benzoquinone group binds near the entrance of the pocket and the ansamycin ring, which resembles a five amino acid polypeptide, inserts into the pocket [7]. The most notable site of interaction is within the deepest contacts of the pocket at the highly conserved amino acid Asp93 Hsp90α), located in an otherwise hydrophobic region [7]. Mutation of this position completely disrupts Hsp90 function and inhibits it from binding nucleotide [8,9]. Although GA is effective at inhibiting Hsp90, the bioreduction of the semiquinone radical creates a toxic superoxide radical [10].
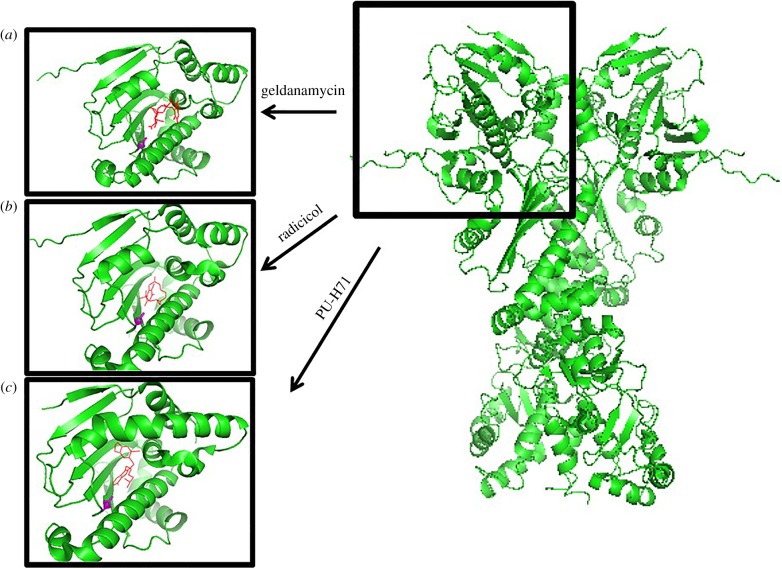
A chemical analogue of GA, 17-allylamino-17-demethoxy geldanamycin (17-AAG) was the first small molecule Hsp90 inhibitor to enter into clinical trials. This compound replaced the 17-methoxy moiety with a 17-alkylamino group to reduce toxicity [11]. Despite displaying anti-cancer efficacy in phase I clinical trials, especially in combination with trastuzumab in HER-2–positive breast cancer patients, 17-AAG development was discontinued due to poor aqueous solubility and patent issues [12]. Other benzoquinone ansamycin analogues that have been used in clinical trials include 17-DMAG, IPI-504 and 17-AG. IPI-504 was the most successful as it advanced to phase II and phase III clinical trials. IPI-504 is a reduced quinone version of GA, displaying enhanced affinity for Hsp90 and reducing hepatotoxicity in patients. Nevertheless, development of IPI-504 was discontinued due to lack of efficacy in clinical trials [12]. Currently, there are no benzoquinone ansamycin compounds remaining under clinical evaluation. The overall binding pose of the benzoquinone ansamycins with Hsp90 can be seen in figure 1a.
Figure 1.
N-terminal inhibitors differentially impact Hsp90 conformation. Right panel: full-length yeast Hsp90 was drawn using PyMOL (2CG9). Left panels: the impact of (a) geldanamycin (1YET), (b) radicicol (4EGK), and (c) PU-H71 (2FWZ) on human Hsp90. N-terminal conformation was created using PyMOL. (Online version in colour.)
(c). Radicicol and analogous inhibitors
RD, another natural Hsp90 inhibitor, is a macrocyclic lactone antibiotic that was isolated from Monosporium bonorden in 1953 [13]. In 1998, RD was found to compete with GA for the Hsp90 ATP-interaction site [14]. Like GA, RD mimics the ADP-bound conformation of Hsp90 and interacts with Asp93 in a similar manner (figure 1b). Unlike GA, however, RD binds in a different orientation than GA and has greater affinity for the ATP-pocket [3]. RD is oriented in the opposite direction of GA with the aromatic ring directed towards the bottom of the pocket and the macrocycle facing towards and making contacts with the top of the pocket. RD also adopts a folded conformation, albeit less dramatic than GA, with the macrocycle and aromatic ring approximately perpendicular instead of parallel [3]. RD does not, however, display anti-tumour activity due to its rapid metabolism in vivo [15]. Using the known structural determinants of RD binding to Hsp90, several synthetic analogues were created.
The electrophilic nature of RD is the cause of its rapid metabolism in vivo. Analogues were created to reduce this electrophilic nature and to increase metabolic stability of the inhibitor. To that end, the 2′-ketone of radicicol was converted to an oxime [16]. This led to the production of KF25706, a metabolically stable compound that displayed potency against several human cancer cell lines and rodent xenograft models [16]. The complexity of this compound, however, made it difficult for large-scale production. The resorcinol moiety of RD behaves in a similar manner to the adenine ring of ATP and is required for Hsp90 inhibition. Employing the resorcinol ring, several inhibitors have included this pharmacophore and are undergoing clinical evaluation. A resorcinol triazole compound created by Synta Pharmaceuticals Corp. called STA-9090 (Ganetespib) has been shown to have a high affinity for Hsp90 and inactivates its functions at concentrations as low as 10 nM. STA-9090 also displays increased tumour penetration with low toxicity profiles [15]. Workman and co-workers screened a library of 56 000 compounds and identified CCT018159, which contains the resorcinol-anchoring unit of radicicol [17]. Further development of CCT018159 resulted in the creation of the resorcinylic isoxazole amide NVP-AUY922/VER52296, licensed by Novartis for clinical evaluation. Additional resorcinol analogues include KW-2478 (Kyowa Hakko Kirin Pharma) and AT13387 (Astex). STA-9090 is no longer under development due to lack of observed benefit in a phase III clinical trial in lung cancer patients. KW-2478 remains in two phase I and II trials (in combination with bortezomib), and AT13387 is being evaluated in phase I clinical trials for metastatic solid tumours and phase II trials for gastrointestinal stromal tumours. NVP-AUY922 is no longer being clinically evaluated due to a failure to show clinically meaningful responses at the maximum tolerated dose [15].
(d). Additional synthetic Hsp90 inhibitors
Although benzoquinone ansamycin and radicicol analogues have not been approved for patient use, the binding pocket interactions found using those compounds have been useful for creating an additional group of Hsp90 inhibitors. Purine-based compounds were the first fully synthetic derivatives created. These compounds took advantage of the folded structure adopted by GA and RD when bound to Hsp90. These compounds contain an adenine group, a CH2 or S linker, and a right-side aryl group. The adenine binds the pocket in a similar manner and maintains the direct hydrogen bond from N6 to Asp93 on Hsp90. The first inhibitor created was PU3 [18,19]. Although this compound showed efficacy in oncogenic cell lines, it was not as potent as 17-AAG. Further analysis of PU3 and its interaction with Hsp90 resulted in the synthesis of PU-H71. This compound shows higher affinity for oncogenic cells and is only needed in low concentrations to inhibit Hsp90 activity [20,21]. PU-H71 contains an N9 alkane from the adenine group. This amine moiety protrudes from the ATP-binding cavity out into the solvent, as it does not interact directly with the protein and is amenable to further modifications that may improve the pharmacological properties of that compound (figure 1c) [22]. Currently, PU-H71 is being evaluated in a phase I clinical trial for patients with advanced malignancies. Other purine scaffolds have been created, including CNF2024/BIIB021, MPC-3100 (Myriad Pharmaceuticals Inc.) and Debio 0932 (Curis). CNF2024/BIIB021 and Debio 0932 have both been evaluated in phase I and phase II clinical trials.
Benzamide compounds represent another class of synthetic Hsp90 inhibitors. These structures use their benzamide group to mimic adenine with the amide group forming hydrogen bonds with Asp93 and Thr184 [23]. These include SNX-5422, developed by Serenex and acquired by Pfizer, which is a pro-drug of active SNX2112. SNX2112 exists as an indazolone 2-aminobenzamide analogue. Although clinical evaluation of these drugs was discontinued by Pfizer, they have been licensed by Esanex and remain in clinical development. Another benzamide compound, created by Taiho Pharmaceutical, Co. Ltd., is the 4-(1H-pyrazolo[3,4-b]pyridine-1-yl)benzamide TAS-116. This compound is a selective Hsp90 alpha/beta inhibitor that displays anti-tumour activity without inducing eye injury in rats [24]. Clinical trials of this compound will allow for its further evaluation in various cancer indications and perhaps also in other diseases.
(e). Future clinical prospects of ATP-competitive, N-terminally directed Hsp90 inhibitors
While numerous anecdotal responses to Hsp90 inhibitors in heavily pre-treated patients have been reported, the overall clinical activity of these agents has remained modest, and no Hsp90 inhibitor has yet been approved by the US Food and Drug Administration. To date, emphasis in these clinical trials has been placed on impacting client protein levels (with a primary focus on oncogenic kinases), while other critical but more complex activities of Hsp90 that may be associated with the malignant phenotype (e.g. as an organizational hub for signalling proteins) have not been taken into account in understanding clinical response. Perhaps consideration should be given to employing Hsp90 inhibitors as modulators of central signalling hubs including transcriptional control, epigenetic state and maintenance of DNA integrity. Such a re-orientation of Hsp90 inhibitor usage would probably lead to investigating different types of tumours and ultimately selecting different agents for combination. Further, dosing strategies must be reconsidered to avoid global disruption of cellular proteostasis and further activation of HSF1, while providing enough sustained low-level Hsp90 inhibitor exposure to limit development of resistance to other co-administered anti-cancer drugs. Recent developments in isoform-specific inhibitors are also likely to prove beneficial in tailoring Hsp90 inhibitor use to target uniquely deregulated pathways in a tumour-specific manner. For example, only TAS116 (Taiho) is able to distinguish between the two cytosolic Hsp90 proteins and those homologues restricted to the endoplasmic reticulum and mitochondria. Of the other Hsp90 inhibitors in clinical trial, all are unable to distinguish between Hsp90 homologues. Further, a better understanding of the dose-dependent impact of Hsp90 inhibitors on host systemic immunity and the tumour microenvironment may facilitate productive combination of Hsp90 inhibitors with immunotherapy. Finally, N-domain–targeted Hsp90 inhibitors are being repurposed to take advantage of their homing to and concentration in tumours to deliver cytotoxic agents to tumour cells in order to achieve a higher and long-lasting intratumoural concentration with less systemic toxicity compared with delivery of the cytotoxic agent itself. The first so-called ‘Hsp90 drug conjugate’, or HDC, was designed to deliver the active metabolite of irinotecan to tumour cells. Exceptional preclinical in vivo data in paediatric sarcoma models [25] have led to a first-in-human clinical trial of this HDC at the US National Cancer Institute (first patient enrolled in August 2017).
(f). Consequences of different N-terminal inhibitors on Hsp90 specificity and conformation
Through protein crystallography it is clear that these Hsp90 inhibitors bind the N-terminal domain ATP-pocket and share many of the amino acid contacts. However, based on co-crystal structures, there are subtle differences in the consequences of their interaction with Hsp90 on its local N-domain conformation. Figure 1 displays some of the different N-terminal inhibitor interactions with Hsp90. Each type of inhibitor causes slight variations to the structure of the N-terminal domain, which may have consequences for drug recognition of Hsp90 in one or more subtly different conformational states. For example, using several rounds of GA-agarose pulldowns followed by additional rounds of PU-H71–agarose pulldown and vice versa, it was determined that GA and PU-H71 bound to distinct but overlapping Hsp90 populations and that these populations are likely to be at least partially determined by the cellular environment, including unique post-translational modifications. Compared with GA, PU-H71 also interacts more strongly with Hsp90 bound to the kinase delivering co-chaperone p50Cdc37 as well as to client kinases [26]. Therefore, a better understanding of each of the inhibitors' impacts on client specificity and Hsp90 conformation may allow for a more informed, perhaps disease-focused, use of these agents.
(g). Hsp90 C-terminal inhibitors
Using nucleotide affinity cleavage, a second ATP-binding pocket was discovered within the C-terminus of Hsp90 [27]. The natural product novobiocin was the first C-terminal inhibitor discovered. The site of novobiocin interaction is proximal to the C-terminal dimerization domain and novobiocin binds in a bent ADP-like state. This pocket does not interact with GA or RD, and novobiocin does not require the same amino acids for binding as used in GA and RD interaction at the Hsp90 N-terminus [28]. The interaction of novobiocin to the C-terminus resulted in client degradation; however, its interaction with Hsp90 is weak. Novobiocin structure contains three features: a benzamide side-chain, a coumarin core and a noviose sugar. Using the structure of novobiocin, the compound A4 and its analogues were synthesized. These A4 analogues were coumarin-modified ring systems that mimic adenine and guanine with additional strategically placed hydrogen bond acceptors and donors to fit the pocket with higher specificity [29]. The most potent novobiocin analogue created was KU-174, which was created to mimic the GTP-bound conformation [30]. This compound has shown potency in several cancer cell lines as it degrades clients without reported heat shock response (HSR) [31]. Another C-terminal inhibitor is the green tea extract, Epigallocatechin-3-Gallate (EGCG). This compound has reported anti-cancer activity and was found to interact within the same region of Hsp90 as novobiocin [32–34]. Other C-terminal inhibitors include the platinum-containing chemotherapeutic agent cisplatin and the microtubule stabilizer taxol [35]. To date, none of the C-terminal inhibitors have been evaluated in the clinic and the exact location of their interaction with Hsp90 remains unknown. The ability of these compounds to inhibit Hsp90 function without the consequence of HSR induction addresses one of the drawbacks of N-domain–targeted agents and makes C-terminal inhibitors interesting candidates for future development.
2. Extracellular Hsp90 inhibition
Extracellular Hsp90α, known as eHsp90, was initially discovered as an important factor in ERK1/2 activation as well as lymphoid cell growth [36,37]. Further analysis determined a role for eHsp90 in protecting cells against various environmental stresses. Secretion of eHsp90 from regions of cellular stress result in its interaction with the surface receptor LRP-1/CD91, increasing cell migration required for beneficial events such as wound healing [38–40]. These properties are also useful for tumour cell protection, invasion, growth and metastasis [41,42]. HIF-1α has been identified as a key upstream regulator of eHsp90 secretion. Overexpression of HIF-1α has been reported to occur in approximately 40% of human tumours and is associated with increased tumour survival and growth [43]. It is not surprising, therefore, that eHsp90 has been identified in several different cancer cell lines. A separate study focused on identifying proteins required for cancer invasion using two separate proteomic screens identified eHsp90 in both assays. This study determined that eHsp90 is required for matrix metalloproteinase MMP2 activation, a process that is required for cell invasion. Furthermore, use of extracellularly restricted GA beads resulted in a 45% reduction of HT-1080 fibrosarcoma invasion and an 80% decrease of active MMP2 protein [41]. Interestingly, eHsp90 was also found to facilitate stem cell heterogeneity in prostate cancer cells, resulting in a drug-resistant phenotype [44]. These features make eHsp90 a promising target for cancer therapy.
To better understand the impact of targeting eHsp90 in tumour cell growth, an Hsp90-specific, Cy5-tethered, cell impermeable inhibitor was created, HS-131 [45]. This inhibitor binds strongly and specifically to the N-terminal ATP-binding pocket of Hsp90. HS-131 was used to monitor the internalization of eHsp90. Internalization of eHsp90 was found to be a unique phenomenon associated with highly transformed tumour cells, and the extent of the internalization correlated with the aggressiveness of the tumour [45]. Surprisingly, the inhibitor itself had no impact on eHsp90 internalization, demonstrating the nucleotide-bound state is not important for this function. Further use of eHsp90-targeting compounds, such as HS-131, may, therefore, best be utilized for tumour-specific drug delivery. Obstruction of eHsp90 through the use of neutralizing antibodies results in complete inhibition of hypoxia-induced motility, alluding to a possible target to inhibit tumour growth [38]. As eHsp90 is not mechanistically affected by standard N-terminal inhibitors, the region within Hsp90 required for secretion was determined. Interestingly, the region of eHsp90 required for cell migration and wound healing maps to a 115-amino acid region (aa 236–350), termed F5, which is independent of its ATPase and C-terminal dimerization regions [40]. Future work, therefore, seeks to design an inhibitor that specifically targets this region of the protein. Use of novobiocin-based C-terminal Hsp90 inhibitors reduces cell migration, although direct targeting of eHsp90 by C-terminal inhibitors has yet to be established [46].
As eHsp90 expression is elevated in regions of inflammation, its abundance and function was monitored in new-onset type 1 diabetes. In this study, human beta cells and cadaveric islets released elevated levels of eHsp90 due to exposure to pro-inflammatory cytokines [47]. Upon pro-inflammatory cytokine exposure, eHsp90 levels increased within exosomes, a process regulated by JNK activation. Inhibition of the JNK pathway using SP600125 resulted in attenuated eHsp90 release in response to cytokine stress. Thus, eHsp90 can be utilized as an early indicator for beta cell stress in type 1 diabetes [47].
3. Hsp90 co-chaperone inhibitors
Hsp90 function relies on its many nucleotide-influenced conformations. Throughout its ATPase cycle, helper co-chaperone proteins bind and release Hsp90 in order to assist in processes such as conformational dynamics, nucleotide and client interactions and ATPase activity. Although the functional significance of all the co-chaperones has yet to be fully characterized, studies focused on individual co-chaperones demonstrate they each have distinct functions when it comes to Hsp90 regulation. Co-chaperone proteins are also uniquely regulated in different illnesses, making their inhibition of interest for pharmaceutical development. p50Cdc37 is a co-chaperone that binds the open conformation of Hsp90 and is known for its role in recruiting kinase clients to the Hsp90 chaperone machinery; si-RNA knockdown of p50Cdc37 results in client kinase degradation [48]. Furthermore, ATP-competitive kinase inhibitors such as vemurafenib and lapatinib have been found to disrupt kinase-p50Cdc37 interaction, also resulting in the degradation of oncogenic kinases such as B-Raf and ErbB2 [49]. Another client-recruiting co-chaperone, Hop, binds the open conformation of Hsp90 and bridges Hsp70 to Hsp90 for client loading. Disruption of Hop-Hsp90 interaction leads to the proteasomal degradation of a diverse set of clients as well as cell cycle arrest, cell adhesion inhibition and apoptosis [50].
Following ATP interaction, Hsp90 proceeds into the closed conformation, characterized by its N-terminal dimerization. While in this conformation, Hsp90 interacts with immunophilin proteins at its C-terminus. These immunophilins are most well studied for their impact on steroid hormone regulation. FKBP51 is an immunophilin made up of two FKBP-like domains, which contain its peptidyl-prolyl isomerase activity (PPIase), as well as a tetratricopeptide repeat (TPR) domain for its interaction with the Hsp90 C-terminal MEEVD motif. Although FKBP51 is inhibited by non-selective compounds such as cyclosporin A, rapamycin and FK506, a recent study revealed a new class of ligands that selectively interact with and inhibit FKBP51 [51,52]. The use of these ligands in mice displayed improved endocrine feedback and stress-coping behaviour in mice, suggesting a new basis for antidepressant development [52]. p23 interacts with the N-terminal domain of Hsp90 while it is in complex with the immunophilin proteins and stabilizes the closed conformation. As Hsp90 interacts strongly with clients while in the closed conformation, inhibition of p23 results in client instability and degradation. Celastrol inhibits p23 by altering its three-dimensional structure, resulting in selective destabilization of hormone receptors [53]. Recently, the natural product Gedunin was found to bind directly to p23 and inactivate its function as well as disrupt its interaction with Hsp90. This inhibition resulted in destabilized steroid hormone receptors, with no impact on client kinases, as well as cancer cell death via apoptosis [54]. Further analysis of co-chaperone interaction and regulation of Hsp90 may allow for an alternative method in inhibiting Hsp90 function.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl Acad. Sci. USA 91, 8324–8328. ( 10.1073/pnas.91.18.8324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mimnaugh EG, Chavany C, Neckers L. 1996. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 271, 22 796–22 801. ( 10.1074/jbc.271.37.22796) [DOI] [PubMed] [Google Scholar]
- 3.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. 1999. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266. ( 10.1021/jm980403y) [DOI] [PubMed] [Google Scholar]
- 4.Workman P, Burrows F, Neckers L, Rosen N. 2007. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann. NY Acad. Sci. 1113, 202–216. ( 10.1196/annals.1391.012) [DOI] [PubMed] [Google Scholar]
- 5.Grenert JP, et al. 1997. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 272, 23 843–23 850. ( 10.1074/jbc.272.38.23843) [DOI] [PubMed] [Google Scholar]
- 6.Dutta R, Inouye M. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25, 24–28. ( 10.1016/S0968-0004(99)01503-0) [DOI] [PubMed] [Google Scholar]
- 7.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. 1997. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89, 239–250. ( 10.1016/S0092-8674(00)80203-2) [DOI] [PubMed] [Google Scholar]
- 8.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. 1998. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 143, 901–910. ( 10.1083/jcb.143.4.901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panaretou B, Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW Pearl LH. 1998. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17, 4829–4836. ( 10.1093/emboj/17.16.4829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang W, Caldwell GW, Li J, Leo GC, Jones WJ, Masucci JA. 2007. Biotransformation of geldanamycin and 17-allylamino-17-demethoxygeldanamycin by human liver microsomes: reductive versus oxidative metabolism and implications. Drug Metab. Dispos. 35, 21–29. ( 10.1124/dmd.106.009639) [DOI] [PubMed] [Google Scholar]
- 11.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. 1998. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 58, 2385–2396. [PubMed] [Google Scholar]
- 12.Sidera K, Patsavoudi E. 2014. HSP90 inhibitors: current development and potential in cancer therapy. Recent Patents Anticancer Drug Discov. 9, 1–20. ( 10.2174/15748928113089990031) [DOI] [PubMed] [Google Scholar]
- 13.Delmotte P, Delmotte-Plaque J. 1953. A new antifungal substance of fungal origin. Nature 171, 344 ( 10.1038/171344a0) [DOI] [PubMed] [Google Scholar]
- 14.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. 1998. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 3, 100–108. ( 10.1379/1466-1268(1998)003%3C0100:ARBTTN%3E2.3.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandelwal A, Crowley VM, Blagg BS. 2016. Natural product inspired N-Terminal Hsp90 inhibitors: from bench to bedside? Med. Res. Rev. 36, 92–118. ( 10.1002/med.21351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soga S, et al. 1999. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res. 59, 2931–2938. [PubMed] [Google Scholar]
- 17.Rowlands MG, Newbatt YM, Prodromou C, Pearl LH, Workman P, Aherne W. 2004. High-throughput screening assay for inhibitors of heat-shock protein 90 ATPase activity. Anal. Biochem. 327, 176–183. ( 10.1016/j.ab.2003.10.038) [DOI] [PubMed] [Google Scholar]
- 18.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. 2001. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol. 8, 289–299. ( 10.1016/S1074-5521(01)00015-1) [DOI] [PubMed] [Google Scholar]
- 19.Chiosis G, Lucas B, Shtil A, Huezo H, Rosen N. 2002. Development of a purine-scaffold novel class of Hsp90 binders that inhibit the proliferation of cancer cells and induce the degradation of Her2 tyrosine kinase. Bioorg. Med. Chem. 10, 3555–3564. ( 10.1016/S0968-0896(02)00253-5) [DOI] [PubMed] [Google Scholar]
- 20.Caldas-Lopes E, et al. 2009. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl Acad. Sci. USA 106, 8368–8373. ( 10.1073/pnas.0903392106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trendowski M. 2015. PU-H71: an improvement on nature's solutions to oncogenic Hsp90 addiction. Pharmacol. Res. 99, 202–216. ( 10.1016/j.phrs.2015.06.007) [DOI] [PubMed] [Google Scholar]
- 22.Immormino RM, Kang Y, Chiosis G, Gewirth DT. 2006. Structural and quantum chemical studies of 8-aryl-sulfanyl adenine class Hsp90 inhibitors. J. Med. Chem. 49, 4953–4960. ( 10.1021/jm060297x) [DOI] [PubMed] [Google Scholar]
- 23.Patel HJ, Modi S, Chiosis G, Taldone T. 2011. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin Drug Discov. 6, 559–587. ( 10.1517/17460441.2011.563296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkubo S, et al. 2015. TAS-116, a highly selective inhibitor of heat shock protein 90α and β, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol. Cancer Ther. 14, 14–22. ( 10.1158/1535-7163.MCT-14-0219) [DOI] [PubMed] [Google Scholar]
- 25.Heske CM, Mendoza A, Edessa LD, Baumgart JT, Lee S, Trepel J, Proia DA, Neckers L, Helman LJ. 2016. STA-8666, a novel HSP90 inhibitor/SN-38 drug conjugate, causes complete tumor regression in preclinical mouse models of pediatric sarcoma. Oncotarget 7, 65 540–65 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beebe K, et al. 2013. Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors. Oncotarget 4, 1065–1074. ( 10.18632/oncotarget.1099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soti C, Vermes A, Haystead TA, Csermely P. 2003. Comparative analysis of the ATP-binding sites of Hsp90 by nucleotide affinity cleavage: a distinct nucleotide specificity of the C-terminal ATP-binding site. Eur. J. Biochem. 270, 2421–2428. ( 10.1046/j.1432-1033.2003.03610.x) [DOI] [PubMed] [Google Scholar]
- 28.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. 2000. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 275, 37 181–37 186. ( 10.1074/jbc.M003701200) [DOI] [PubMed] [Google Scholar]
- 29.Yu XM, Shen G, Neckers L, Blake H, Holzbeierlein J, Cronk B, Blagg BSJ. 2005. Hsp90 inhibitors identified from a library of novobiocin analogues. J. Am. Chem. Soc. 127, 12 778–12 779. ( 10.1021/ja0535864) [DOI] [PubMed] [Google Scholar]
- 30.Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BSJ. 2008. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J. Org Chem. 73, 8901–8920. ( 10.1021/jo801312r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskew JD, et al. 2011. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer 11, 468 ( 10.1186/1471-2407-11-468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Z, Henry EC, Gasiewicz TA. 2009. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry 48, 336–345. ( 10.1021/bi801637q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khandelwal A, Hall JA, Blagg BS. 2013. Synthesis and structure-activity relationships of EGCG analogues, a recently identified Hsp90 inhibitor. J. Org Chem. 78, 7859–7884. ( 10.1021/jo401027r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses MA, Henry EC, Ricke WA, Gasiewicz TA. 2015. The heat shock protein 90 inhibitor, (−)-epigallocatechin gallate, has anticancer activity in a novel human prostate cancer progression model. Cancer Prev. Res. (Phila). 8, 249–257. ( 10.1158/1940-6207.CAPR-14-0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly A, Blagg BS. 2008. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr. Med. Chem. 15, 2702–2717. ( 10.2174/092986708786242895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. 2000. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J. Biol. Chem. 275, 189–196. ( 10.1074/jbc.275.1.189) [DOI] [PubMed] [Google Scholar]
- 37.Kuroita T, Tachibana H, Ohashi H, Shirahata S, Murakami H. 1992. Growth stimulating activity of heat shock protein 90 alpha to lymphoid cell lines in serum-free medium. Cytotechnology 8, 109–117. ( 10.1007/BF02525493) [DOI] [PubMed] [Google Scholar]
- 38.Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. 2007. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J. 26, 1221–1233. ( 10.1038/sj.emboj.7601579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng CF, et al. 2008. Transforming growth factor alpha (TGFα)-stimulated secretion of HSP90α: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFβ-rich environment during wound healing. Mol. Cell. Biol. 28, 3344–3358. ( 10.1128/MCB.01287-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng CF, et al. 2011. A fragment of secreted Hsp90α carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J. Clin. Invest. 121, 4348–4361. ( 10.1172/JCI46475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eustace BK, et al. 2004. Functional proteomic screens reveal an essential extracellular role for hsp90 α in cancer cell invasiveness. Nat. Cell Biol. 6, 507–514. ( 10.1038/ncb1131) [DOI] [PubMed] [Google Scholar]
- 42.Tsutsumi S, Neckers L. 2007. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 98, 1536–1539. ( 10.1111/j.1349-7006.2007.00561.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenza GL. 2007. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 12, 853–859. ( 10.1016/j.drudis.2007.08.006) [DOI] [PubMed] [Google Scholar]
- 44.Nolan KD, Kaur J, Isaacs JS. 2017. Secreted heat shock protein 90 promotes prostate cancer stem cell heterogeneity. Oncotarget 8, 19 323–19 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe LB, et al. 2017. A fluorescent Hsp90 probe demonstrates the unique association between extracellular hsp90 and malignancy in vivo. ACS Chem. Biol. 12, 1047–1055. ( 10.1021/acschembio.7b00006) [DOI] [PubMed] [Google Scholar]
- 46.Ghosh S, Shinogle HE, Garg G, Vielhauer GA, Holzbeierlein JM, Dobrowsky RT, Blagg BSJ. 2015. Hsp90 C-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90α/Aha1 complex in PC3-MM2 cells. ACS Chem. Biol. 10, 577–590. ( 10.1021/cb5008713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ocaña GJ, Pérez L, Guindon L, Deffit SN, Evans-Molina C, Thurmond DC, Blum JS. et al. 2017. Inflammatory stress of pancreatic beta cells drives release of extracellular heat-shock protein 90α. Immunology 151, 198–210. ( 10.1111/imm.12723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith JR, Clarke PA, de Billy E, Workman P. 2009. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene 28, 157–169. ( 10.1038/onc.2008.380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polier S, Samant RS, Clarke PA, Workman P, Prodromou C, Pearl LH. 2013. ATP-competitive inhibitors block protein kinase recruitment to the Hsp90-Cdc37 system. Nat. Chem. Biol. 9, 307–312. ( 10.1038/nchembio.1212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Liu Y, Zhao Z, Xie C, Xu Y, Hu Y, Quan H, Lou L. 2016. Y-632 inhibits Hsp90 function through disrupting the interaction of Hsp90-Hop and exerts antitumor activity in vitro and in vivo. Cancer Sci. 107, 782–790. ( 10.1111/cas.12934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. 1995. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol. Cell. Biol. 15, 4395–4402. ( 10.1128/MCB.15.8.4395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaali S, et al. 2015. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 11, 33–37. ( 10.1038/nchembio.1699) [DOI] [PubMed] [Google Scholar]
- 53.Chadli A, Felts SJ, Wang Q, Sullivan WP, Botuyan MV, Fauq A, Ramirez-Alvarado M, Mer G. 2010. Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the Co-chaperone p23. J. Biol. Chem. 285, 4224–4231. ( 10.1074/jbc.M109.081018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BS, Chadli A. 2013. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J. Biol. Chem. 288, 7313–7325. ( 10.1074/jbc.M112.427328) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.