Abstract
Duchenne muscular dystrophy is the most common and severe of the muscular dystrophies, a group of inherited myopathies caused by different genetic mutations leading to aberrant expression or complete absence of cytoskeletal proteins. Dystrophic muscles are prone to injury, and regenerate poorly after damage. Remorseless cycles of muscle fibre breakdown and incomplete repair lead to progressive and severe muscle wasting, weakness and premature death. Many other conditions are similarly characterized by muscle wasting, including sarcopenia, cancer cachexia, sepsis, denervation, burns, and chronic obstructive pulmonary disease. Muscle trauma and loss of mass and physical capacity can significantly compromise quality of life for patients. Exercise and nutritional interventions are unlikely to halt or reverse the conditions, and strategies promoting muscle anabolism have limited clinical acceptance. Heat shock proteins (HSPs) are molecular chaperones that help proteins fold back to their original conformation and restore function. Since many muscle wasting conditions have pathophysiologies where inflammation, atrophy and weakness are indicated, increasing HSP expression in skeletal muscle may have therapeutic potential. This review will provide evidence supporting HSP induction for muscular dystrophy and other muscle wasting conditions.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: skeletal muscle wasting, muscular dystrophy, heat shock proteins
1. Introduction
Muscle wasting is an urgent and unmet health risk associated with many diseases and conditions, including: ageing and frailty; cancer cachexia; sepsis and other forms of catabolic stress; denervation after nerve injury; disuse atrophy with plaster casting (unloading), inactivity and microgravity; burns; HIV–acquired immunodeficiency syndrome (AIDS); chronic kidney or heart failure; chronic obstructive pulmonary disease; inflammatory myopathies and the muscular dystrophies, especially Duchenne muscular dystrophy (DMD). These conditions are linked by the inflammation, atrophy and weakness common to their pathophysiology.
The consequences of muscle wasting can be devastating and include profound weakness, impaired mobility and fatigue, reduced functional independence, and in the worst cases, death from respiratory (diaphragm) or cardiac muscle (heart) failure. In many of these conditions there is the complication of an increased susceptibility to muscle injury and impaired regeneration that compromises healing; e.g. muscles can be injured during sudden falls (especially in the elderly), in the workplace, on the sports field, during recreational pursuits, or as a consequence of surgeries where tourniquet application leads to ischaemia and reperfusion damage. Muscle trauma and loss of mass and physical capacity can impact significantly on the quality of life of patients. Successfully attenuating the loss of muscle mass and improving repair and regeneration can determine the extent of functional recovery [1]. Exercise and nutritional interventions may slow the progression of muscle wasting and weakness in some conditions, but are difficult or impossible for severely ill patients. Several hormonal treatments including growth hormone, insulin-like growth factor-1, testosterone, anabolic steroids, selective androgenic receptor modulators, and β-adrenoceptor agonists have been proposed to enhance muscle growth and function [2,3] but have had limited clinical acceptance owing to serious side effects, highlighting the urgent need to identify non-hormonal treatments for these devastating conditions, especially DMD [4]. Modulation of the stress response by manipulation of the heat shock protein (HSP) family is well known to protect cells, including skeletal muscle fibres, from various sources of cell stress [3,5]. Therapeutic induction of HSPs for muscle wasting conditions is the focus of this review, particularly whether increased expression of HSPs can confer protection in skeletal muscle diseases, including DMD.
DMD is the most common and severe form of muscular dystrophy, affecting 1 in 3500–6000 live born males [6]. It is caused by a variety of mutations and deletions in the dystrophin (DMD) gene on chromosome Xp21, leading to a lack of expression or a non-functional corresponding (dystrophin) protein in muscle. DMD patients exhibit severe and progressive muscle wasting and weakness, and are usually dependent on a wheelchair before their teens. Although early and sustained interventions with corticosteroids and advances in medical care have extended lifespan, affected patients still die prematurely, often from respiratory and/or heart failure [7]. There remains a profound need for interventions that can cure or ameliorate the dystrophic pathology and improve quality of life for patients.
While a cure for DMD will most likely come from gene and cell therapies that can replace the defective or absent dystrophin protein, there are still significant hurdles that plague these approaches [8,9]. It is essential that alternative therapies be developed that can ameliorate the muscle pathology and improve patient lifespan so that they may be able to take advantage of gene or cell therapies when they become available.
A lack of dystrophin in the muscles of DMD patients and well-characterized murine models of DMD renders muscle fibres fragile and prone to injury, and results in significant perturbation in Ca2+ homeostasis. The prolonged elevation of intracellular [Ca2+] triggers chronic inflammation, repeated cycles of degeneration with progressively ineffective regeneration that eventually lead to the loss of muscle fibres, and infiltration of fibrotic and other non-contractile material [10]. Several factors have been implicated in the influx of Ca2+ into dystrophic muscle fibres, including membrane tears, stretch-activated channels and Ca2+ leak channels [11–13]. Improving the function of the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) pump in dystrophic muscles has therefore been suggested as a viable therapeutic target for improving Ca2+ homeostasis and ameliorating the pathology of DMD. Increasing SERCA pump expression and/or activity within dystrophic muscles in transgenic mice or through viral-mediated delivery improves Ca2+-handling and suppresses the pathological cascade of events [14–16]. The role of inflammation in the dystrophic pathology is also well known, particularly that of the pro-inflammatory cytokine tumour necrosis factor-α (TNF-α). TNF-α activates the nuclear factor-kappa B (NFκB) and c-Jun N-terminal kinase (JNK) signalling pathways [17–19]. Identifying ways to maintain Ca2+ homeostasis and regulate inflammation could better protect dystrophic muscle fibres from degeneration and enhance regenerative mechanisms that help maintain muscle mass.
2. Skeletal muscle injury and repair
Skeletal muscle repair is a complex process that involves myofibre degeneration, inflammation and activation of muscle resident stem cells (MuSCs), termed ‘satellite cells’ [20,21], which in healthy uninjured muscle reside in a quiescent state between the sarcolemma of the myofibre and the basal lamina [22].
Severe trauma or direct physical damage disrupts the sarcolemma, resulting in a rapid influx of Ca2+ into the damaged myofibre. If this sudden increase in intracellular Ca2+ is not buffered adequately, calpains and other Ca2+-dependent proteases are activated, which mediate myofibre proteolysis by cleaving various myofibrillar, cytoskeletal and membrane proteins [20]. Muscle degeneration triggers a complex inflammatory response, involving non-resident immune cells (neutrophils and macrophages), which is critical for clearance of necrotic debris and initiation of muscle repair through MuSC activation [21,23]. Upon activation, MuSCs re-enter the cell cycle and undergo several bouts of proliferation and differentiation before fusing with pre-existing multinucleated myofibres or forming new myofibres. Importantly, a subset of MuSCs undergo asymmetric division and self-renewal to reconstitute their pool [20,21]. Each stage of myogenesis is primarily controlled by the basic helix–loop–helix myogenic regulatory factors (MRFs) MyoD, Myf5, myogenin (MyoG) and MRF4.
3. Heat shock proteins in skeletal muscle health
Heat shock proteins are a family of highly conserved proteins that are induced by various stressors including heat, ischaemia, hypoxia, UV irradiation, heavy metals, oxidative stress and infection, to provide protection against cellular damage [24]. HSPs act predominantly as molecular chaperones that maintain cellular proteostasis by regulating protein biosynthesis and folding, transport of polypeptides, assembly of protein complexes and preventing stress-induced protein unfolding and aggregation [25]. In addition to the intracellular functions of HSPs, there is increasing evidence to suggest that HSPs released into the extracellular space following stress or damage are involved in activation of immune cell responses [26–28].
HSPs are classified into different families based on their molecular weight: small HSPs (≤34 kDa), HSP40 (35–54 kDa), HSP60 (55–65 kDa), HSP70 (65–80 kDa), HSP90 (81–99 kDa) and high MW HSPs (≥100 kDa) [24]. The important characteristics of the main members of these HSP families are summarized in table 1. Several HSPs are expressed in skeletal muscle and have important roles in muscle development and regeneration.
Table 1.
Characteristics of main members of different HSP families.
| HSP | cellular location | characteristics | proposed functions | references |
|---|---|---|---|---|
| HSP10 | mitochondria | forms complex with HSP60; also has HSP60-independent functions | protein folding, refolding; prevents aggregation of denatured proteins | [29,30] |
|
small HSPs: Hsp25 (murine), HSP27 (human), αB-crystallin |
cytosol; migrates to nucleus with stress | stress inducible; ATP-independent; form oligomers | prevents protein aggregation; microfilament stabilization; anti-apoptotic; cell differentiation | [31–37] |
| HSP40 | cytosol | essential co-chaperone activity with HSP72 | binds unfolded proteins; modulates ATPase activity of HSP72 | [38] |
| HSP60 | mitochondria | constitutively expressed; forms complex with HSP10 | chaperone functions in mitochondrial matrix; pro-apoptotic and anti-apoptotic roles | [30,39] |
| HSP72 | cytosol; migrates to nucleus and nucleolus with stress | highly inducible; protects; cells against further stress | protein folding, transport, complex assembly; prevents stress-induced protein unfolding and aggregation; cytoprotective; anti-apoptotic; anti-inflammatory; maintenance of Ca2+ homeostasis | [14,25,40–42] |
| HSC70 | cytosol; migrates to nucleus and nucleolus with stress | constitutively expressed; only slightly inducible | molecular chaperone | [43] |
| GRP75 (mHSP70) | mitochondria | induced by glucose deprivation and Ca2+ | molecular chaperone | [44] |
| GRP78 | sarcoplasmic reticulum | induced by glucose deprivation and Ca2+ | cytoprotection; molecular chaperone | [44] |
| HSP90 (HSP90α and HSP90β isoforms) | cytosol; sarcoplasmic reticulum; nucleus | component of the steroid receptor complex; regulatory protein | regulation of steroid hormone receptors; role in signal transduction | [45,46] |
| HSP110 | cytosol; migrates to nucleus with stress | constitutively expressed | protein folding | [35] |
(a). Muscle development
Small HSPs, HSP27 and αB-crystallin, are expressed during differentiation of multiple mammalian cell types. Ito et al. [31] detected increased levels of Hsp27 and αB-crystallin during differentiation of murine C2C12 myoblasts into myotubes. Although the authors proposed that the increases in these proteins were dependent on different protein kinase pathways, the mechanism was unclear. Previously, it was reported that the αB-crystallin gene (hspb5) contains an MRF binding site, which is occupied by MyoD or MyoG during C2C12 cell differentiation and can activate hspb5 transcription [47]. It is possible therefore that the protein kinase cascades operate via MyoD or MyoG to increase transcription and protein synthesis of Hsp27 and αB-crystallin during differentiation. Interestingly, overexpression of αB-crystallin in proliferating C2C12 cells reciprocally modulates MyoD levels in a negative feedback manner by mediating increased protein degradation and reduced synthesis, thus delaying differentiation [32]. Further to this regulation of MyoD, Hsp27 and αB-crystallin associate with actin microfilaments in developing myotubes and may have roles in myofibril assembly during early differentiation [33]. In situ hybridization experiments demonstrated that during mouse embryonic development αB-crystallin mRNA is expressed in myotomes and developing skeletal muscles [34], further supporting its role in differentiation. The involvement of Hsp27 in embryonic myogenesis was directly tested by knocking down the hspb1 gene in the zebrafish embryo using antisense oligonucleotides, revealing an important role in craniofacial muscle development [48]. Surprisingly, Hsp27 did not affect the determination and differentiation of myogenic precursors or myofilament organization, but protected myocytes against mechanical and oxidative stress [49].
Similar to αB-crystallin, HSP90 plays a pro-myogenic role in skeletal muscle development. Using geldanamycin to specifically inhibit HSP90 in differentiating C2C12 cells, Wagatsuma and colleagues [50] detected decreased expression of the myogenic markers MyoD, MyoG and sarcomeric myosin heavy chain. This was linked to impaired myotube formation, decreased mitogenic signalling and increased apoptosis [50]. These findings highlight both the pro-myogenic and pro-survival roles of HSP90, but the exact mechanisms require further investigation.
HSP90 has been suggested to play an important role in zebrafish somitogenesis during development, with in situ hybridization of embryos showing co-expression of HSP90α and MyoD mRNA in cells of the paraxial mesoderm; both genes were downregulated in newly formed adult trunk muscles [51]. Moreover, embryos treated with geldanamycin had an abnormally short trunk and tail, further demonstrating the requirement of HSP90 for muscle formation [51,52]. A specific role of HSP90α in the assembly of the thick filament during late stages of myofibril formation has also been suggested [53].
Although the stress adapting functions of HSP72 (the inducible form of HSP70) in skeletal muscle are widely established, there is a paucity of knowledge regarding the roles of HSP72 in muscle development [5,54]. Ogata et al. [55] detected Hsp72 in both soleus and plantaris muscles at embryonic day 22 (e22), specifically in slow type I fibres. The authors also compared expression profiles of Hsp72 and Hsc70 (heat shock cognate 70; constitutively expressed member of Hsp70 family). While Hsp72 levels increased concomitantly with the increasing proportion of soleus type I fibres, Hsc70 levels were unchanged after postnatal day 3 [55]. Taken together, these data demonstrate an overall pro-myogenic role for HSPs, which act to promote differentiation. However, the mechanisms underlying the actions for each HSP appear distinct and the interactions between them is likely complex.
(b). Muscle regeneration
Maintenance of MuSC quiescence is crucial since premature activation of MuSCs can lead to their exhaustion and thus impair regenerative potential [56]. Furthermore, MuSCs must be kept free of oxidative damage and metabolic and biomechanical stresses [56,57]. Following MuSC activation, dramatic changes occur in cell morphology, metabolism and motility which challenge proteostasis [58]. Chaperones such as HSPs play important roles in limiting cellular stresses, maintaining proteostasis and stabilizing signalling complexes, but whether HSPs are specifically involved in MuSC quiescence and activation is not well understood.
Fluorescence-activated cell sorting (FACS) together with advances in microarray technology and whole transcriptome sequencing have facilitated investigations of the molecular signatures of pure populations of stem cells including MuSCs [59–62]. Analysis of extensive whole transcriptome datasets of quiescent and proliferating MuSCs has revealed differential expression of various HSP encoding genes (table 2). In the dataset by Ryall and colleagues [59] in MuSCs cultured ex vivo for 48 h, the expression of hspa1a was reduced 64-fold; dnajb1 and hspb1 decreased nine- and sixfold, while hspa9, hspd1 and hspe1 increased fourfold, threefold and fourfold, respectively. Similar changes in the HSP genes were identified in two other studies using ex vivo MuSCs [60,62], although hspa1a was downregulated 159-fold in MuSCs cultured for 72 h [62]. Two further studies using in vivo activated MuSCs also revealed similar changes but of lesser magnitude [61,62]. Furthermore, in muscles of mdx dystrophic mice hspa1a and hspa1b decreased fivefold and threefold, respectively; hspa9 and hspe1 each decreased twofold, while other HSP genes were unchanged compared with muscles of C57BL/10 mice [63]. These data provide strong preliminary evidence that HSPs play differential roles in quiescent and activated MuSCs and are thus vital for muscle regeneration.
Table 2.
Differential expression of HSP genes following MuSC activation (fold changes relative to quiescent MuSCs). Blank areas, not determined in the study dataset.
| gene | protein | Ryall et al. [59] |
Fukada et al. [60] |
Pallafacchina et al. [62] |
Liu et al. [61] |
Pallafacchina et al. [62] |
Tseng et al. [63] |
|---|---|---|---|---|---|---|---|
| RNAseq, in vitro MuSC activation 48 h | microarray, in vitro MuSC activation 48 h | microarray, in vitro MuSC activation 72 h | microarray, in vivo MuSC activation 60 h post BaCl2 injury | microarray, MuSCs from 1 week old mice | microarray, gastroc. muscles from 16 week old C57Bl10 or mdx mice | ||
| dnajb1 | DnaJ (Hsp40) homologue, subfamily B, member 1 (Hsp40) | ↓ 9-fold | ↓ 15-fold | ↓ 10-fold | ↓ 8-fold | ↓ 2-fold | ↔ |
| hspa1a | heat shock protein 1A (Hsp72) | ↓ 64-fold | ↓ 76-fold | ↓ 159-fold | ↓ 7-fold | ↓ 2-fold | ↓2 5-fold |
| hspa1b | heat shock protein 1B (Hsp70.1) | ↓ 23-fold | ↓ 24-fold | ↓ 4-fold | ↓ 2-fold | ↓ 3-fold | |
| hspa4 | heat shock protein 4 (Hsp110) | ↑ 4-fold | ↑ 3-fold | ↑ 2-fold | ↑ 2-fold | ↔ | |
| hspa5 | heat shock protein 5 (Grp78) | ↓ 2-fold | ↓ 2-fold | ↓ 2-fold | ↔ | ||
| hspa9 | heat shock protein 9 (Grp75) | ↑ 4-fold | ↑ 2-fold | ↑ 2-fold | ↓ 2-fold | ||
| hspb1 | heat shock protein 1 (Hsp25) | ↓ 6-fold | ↓ 4-fold | ↓ 11-fold | ↓ 2-fold | ||
| hspd1 | chaperonin (Hsp60) | ↑ 3-fold | ↑ 5-fold | ||||
| hspe1 | chaperonin 10 (Hsp10) | ↑ 4-fold | ↑ 3-fold | ↓ 2-fold | |||
| hsph1 | heat shock protein H1 (Hsp105) | ↓ 2-fold | ↓ 3-fold | ↓ 2-fold | ↓ 2-fold | ||
| hsp90ab1 | heat shock protein 90 alpha (cytosolic; Hsp90) | ↑ 2-fold | ↔ |
Senf and colleagues [64] provided the first evidence that Hsp72 is necessary for muscle regeneration and recovery following acute muscle injury. They showed that following induction of muscle injury with cardiotoxin, mice with deletion of the Hsp72 encoding hspa1a and hspa1b genes (Hsp72−/– mice) had a severely delayed inflammatory response, which was associated with deficient regeneration, persistent necrosis, inflammatory lesions, fibrosis and Ca2+ deposits, for up to 42 days post-injury [64]. By conducting rescue experiments in which an Hsp72 plasmid was reintroduced specifically in the muscles of Hsp72−/− mice 4 days after injury, they confirmed a role for muscle-derived Hsp72 in muscle fibre regrowth during regeneration [64]. Furthermore, when Hsp72 was reintroduced into the muscle prior to injury, it prevented the sustained inflammatory lesions and persistent necrotic muscle fibres in Hsp72−/− mice [64], indicating a critical role for Hsp72 in the first 4 days after injury. Hsp72 is released into the extracellular microenvironment after tissue injury [26,27] and can interact with immune cell receptors to regulate neutrophil [28] and macrophage [27] activation and chemotaxis. Hence, it is plausible that Hsp72 released from skeletal muscle might regulate the early inflammatory response. In support of this notion, simulated release of Hsp72 at the onset of injury through direct injection of recombinant Hsp72 completely restored immune cell infiltration into the muscles of Hsp72−/− mice [64]. Overall, these findings indicate that intracellularly and extracellularly localized HSP72 have divergent functions in facilitating efficient muscle repair. Combined therapeutic strategies that differentially target intracellular and extracellular HSP72 could optimally promote regeneration and restoration of muscle function and are thus relevant for treating conditions associated with increased susceptibility to muscle injury such as muscular dystrophy and sarcopenia.
4. Therapeutic potential of heat shock proteins for muscular dystrophy
The most widely studied HSP in skeletal muscle is HSP72. HSP72 inhibits inflammatory mediators including p-JNK, TNF-α, and the NFκB pathway, and binds and preserves SERCA function under conditions of cellular stress [40,41,65,66]. While some studies have shown HSP72 to be elevated in muscles of DMD patients [67], there is little consensus since expression data for young patients are variable and sourcing age-matched controls is problematic. Nevertheless, the endogenous heat shock response in DMD is insufficient to be protective [14].
To investigate whether elevating HSP72 could protect the dystrophic pathology, our laboratory bred dystrophin-null mdx dystrophic mice with transgenic (TG) mice over-expressing Hsp72 to produce mdxTG(+) mice and mdx littermate controls. Serum levels of the muscle damage marker creatine kinase (CK) were reduced in mdxTG(+) mice compared with littermate control mice lacking the transgene (designated ‘mdxTG(−)’), and these mice had improved whole body strength and endurance as well as improved markers of diaphragm muscle pathophysiology, including reduced Evans blue dye (EBD) infiltration (another indicator of damaged muscle fibres), reduced collagen infiltration, and improved normalized force production. The improvements in diaphragm structure and function are particularly important because respiratory failure is the cause of death in up to 90% of DMD patients, and diaphragm function is an accurate predictor of respiratory insufficiency.
There is a vast body of evidence to suggest that intracellular [Ca2+] is elevated in dystrophin-deficient muscle fibres [11,68,69]. Impaired SERCA function potentially contributes to the loss of Ca2+ homeostasis. Findings from our laboratory have demonstrated that in dystrophic mdx muscle homogenates, not only is the maximal SERCA activity compromised but also the sensitivity of the pumps to cytosolic [Ca2+] is reduced (higher mean [Ca2+]50) [14]. These observations are consistent with increased susceptibility of SERCA pumps to inactivating modifications during stress conditions owing to the presence of a high number of cysteine residues [70]. Indeed, inactivating post-translational modifications of SERCA have been identified during ageing, hyperthermia, strenuous exercise and neuromuscular diseases [66,71,72], all conditions characterized by excessive reactive oxygen species (ROS) or reactive nitrogen species (RNS) production. Interestingly, Hsp72 has been found to bind SERCA and protect it from functional inactivation [66]. In agreement with this, we observed that maximal SERCA activity of muscle homogenates (and in single muscle fibres) was increased in mdxTG(+) compared with mdxTG(−) mice [14]. Preservation of SERCA function through Hsp72 induction has important therapeutic implications, since attenuating the Ca2+ overload could delay disease progression and ameliorate the dystrophic pathophysiology.
To determine whether pharmacological induction of Hsp72 could produce similar effects, mdx and more severely dystrophic dko (dystrophin : utrophin null; dys : utrn−/−) mice were treated with BGP-15, a co-inducer of HSP72, previously shown to protect against obesity-induced insulin resistance [40,73]. BGP-15 treatment increased Hsp72 protein expression in the diaphragm, reduced CK levels and reduced EBD infiltration in muscles of dystrophic mice. BGP-15-treated dystrophic mice had improved overall strength and endurance, reduced fibrosis and increased maximal SERCA activity in diaphragm muscle homogenates, similar to that in transgenic mice with elevated muscle levels of Hsp72. In dys : utrn−/− mice treated from 3–4 weeks until 10 weeks of age, BGP-15 treatment markedly reduced kyphosis (spinal curvature), reduced serum CK levels and collagen infiltration in the diaphragm, and improved force producing capacity of diaphragm muscle strips and intact limb (tibialis anterior; TA) muscles. Lifelong treatment of dys : utrn−/− mice with BGP-15 extended survival, with a 27% increase in median lifespan; this is especially relevant to DMD [14]. Taken together, these results indicated that HSP72 induction could be an important and novel therapeutic approach to improve the dystrophic pathology and attenuate disease progression to allow many patients to benefit from perfected gene- or cell-based treatments.
Although these results were exciting, it should be noted that Hsp72 induction was beneficial in attenuating the progression of the dystrophic pathophysiology when mice were treated from a very young age. In a follow-up study, our laboratory investigated some of the more clinically relevant questions regarding Hsp72 upregulation for muscular dystrophy, especially whether initiating BGP-15 treatment in older dystrophic mice (at a later stage in the pathology) would confer similar improvements in muscle structure and function [74]. We also investigated whether this therapeutic approach would benefit the heart since cardiomyopathy and dysfunction is evident in many DMD patients and in mdx and especially dys : utrn−/− dystrophic mice. Treatments that can ameliorate cardiomyopathy will therefore improve quality of life for DMD patients and improve lifespan.
Later stage treatment of mdx or dys : utrn−/− mice with BGP-15 did not improve maximal force of TA muscles (in situ) or diaphragm muscle strips (in vitro). Fibrosis was reduced in TA muscles of BGP-15-treated dys : utrn−/− mice but unchanged in TA muscles of treated mdx mice and diaphragm of treated mdx and dys : utrn−/− mice. In young dys : utrn−/− mice BGP-15 treatment ameliorated aspects of the cardiac pathology, with reduced collagen deposition and improved membrane integrity and systolic function. Although BGP-15 could improve aspects of the dystrophic pathology, it did so with differing efficacies in heart and skeletal muscles and at different stages of the disease progression, highlighting the notion of a therapeutic ‘window of opportunity’ for HSP72 induction for muscular dystrophy. Treating early in the pathology would likely produce maximal benefits in skeletal muscles and although these effects were less pronounced when treatment commenced later, the heart remained responsive with favourable effects on cardiac muscle structure and function with later induction. Taken together, these findings support a role for HSP72 induction among a suite of pharmacological therapies attempting to ameliorate the pathology in DMD and related muscle disorders (figure 1).
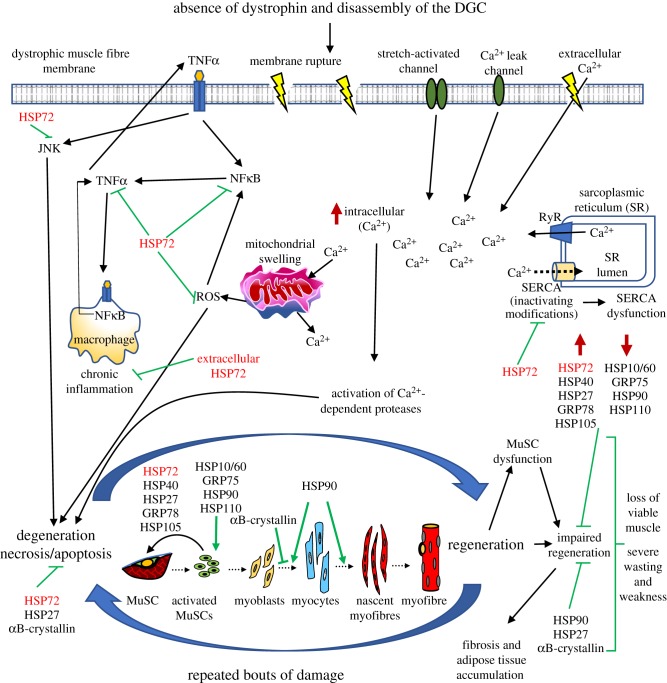
Figure 1.
Known expression levels and functions of heat shock proteins in muscular dystrophy. The absence of the dystrophin glycoprotein complex (DGC) renders dystrophic muscle fibres fragile and prone to damage, resulting in membrane tears and an influx of extracellular Ca2+. Compromised SERCA function contributes to an inability to buffer the increased intracellular [Ca2+], triggering inflammatory pathways that lead to muscle fibre degeneration. Although muscle stem cells (MuSC) can regenerate damaged fibres during the early stages of the pathology, remorseless cycles of injury/repair during the disease progression eventually overwhelm the regenerative capacity because of MuSC dysfunction, disrupted sarcomere assembly, impaired regeneration, and accumulation of connective tissue and fat. The heat shock proteins are carefully regulated both temporally and spatially during these processes. Our work and that of others suggest that modulation of HSP expression, particularly HSP72, has therapeutic potential for treating muscular dystrophy by ameliorating the dystrophic pathophysiology.
Since its discovery, the importance of dystrophin in force transmission has been studied extensively. However, what (if any) role dystrophin may play in MuSCs is less clear [75]. In one of the first studies to document a role for dystrophin in MuSCs (and regeneration of dystrophic muscle), Dumont et al. [76] demonstrated that dystrophin played a vital role in regulating asymmetric cell division and the commitment of MuSCs to the myogenic lineage. In this study, the authors used extensive immunofluorescence experiments to show that cultured ex vivo MuSCs exhibited high levels of dystrophin protein, with the protein becoming polarized to one side of the cell immediately prior to cell division. These results strongly support a role for dystrophin in the process of polarization as in its absence MuSCs were unable to undergo successful cell polarization and asymmetric division, which typically allows both expansion of committed muscle progenitors and self-renewal of the MuSC pool. Finally, the authors linked the decrease in asymmetric MuSC division to a depletion of committed muscle progenitor number (and a concomitant expansion of MuSC numbers), leading to the observed impairment in skeletal muscle regeneration.
The results from Dumont et al. [76] have important implications for the development of future therapies for DMD because they suggest that any successful therapy must target both skeletal muscle and the endogenous population of MuSCs. While our laboratory has previously demonstrated that induction of Hsp72 in skeletal muscle can improve the dystrophic phenotype in mice, no study to date has thoroughly investigated the effect of targeting HSP72 in MuSCs. As several whole transcriptome studies have shown a significant reduction in Hsp72 gene expression in activated MuSCs (up to 159-fold in some cases, table 2), an intriguing hypothesis surrounds the role of HSP72 in MuSC activation and self-renewal. Whether targeting HSP72 can also overcome the defects in dystrophic MuSCs has yet to be investigated.
In addition to HSP72, the expression of other HSPs has been documented to change in DMD. Proteomic profiling and subsequent immunostaining of muscles from mdx mice showed expression of Hsp20, Grp75 and Hsp90 was decreased, Hsp25, Hsp60 and Hsp72 unchanged, and cvHsp and Hsp110 dramatically increased, in muscle fibres of the diaphragm [77]. Similar studies in limb muscles showed reduced Hsp25 expression in TA muscles from old mdx mice [78], and reduced Hsp25, Hsp40 and Hsp60 expression in hearts of mdx mice compared with control [79,80]. In muscles of DMD patients, HSP65, HSP72 and HSP72/73 were specifically increased in hypercontracted muscle fibres, whereas HSP90 was specifically upregulated in regenerating muscle fibres [67].
Altered HSP expression has been similarly observed in other muscle diseases. A localization study showed selective HSP90 upregulation in inflammatory cells in polymyositis and inclusion body myositis (IBM), indicating selective inhibition of HSP90 as a potential therapeutic strategy for these diseases [81]. In the laminin-deficient dy/dy mouse model of congenital muscular dystrophy, the abundances of αB-crystallin, Hsp25 and p20 were all increased in fast-twitch TA muscles and decreased in slow-twitch soleus muscles [82]. HSP47 was increased in both the fibrotic tissue and the muscle membrane in DMD and in active inflammatory myopathy [83]. In addition to HSP72, increased expression of αB-crystallin [84], HSP65 [85] and HSP90 [67] were shown in sporadic inclusion body myositis (sIBM). Together these studies demonstrate that changes in expression of specific HSPs occur in many muscle diseases but their expression is dependent on the severity of the pathology.
In general, the main involvement of HSPs in skeletal muscle disease involves increased or decreased expression in affected muscle, but evidence for their direct role in disease aetiology comes from mutations in the DnaJ heat shock protein family (HSP40) member B6 (DNAJB6), directly responsible for a severe early-onset limb girdle muscular dystrophy [86]. These mutations are hypothesized to disrupt the sarcoplasmic function of DNAJB6, suggesting that DNAJB6 plays a key role in Z-disc organization and stress granule kinetics [87]. Mutations within the αB-crystallin protein are similarly linked to a small number of cases of myofibrillar myopathies [88,89]. The tight regulation of these HSPs is therefore required for the maintenance of skeletal muscle health.
5. Therapeutic potential of heat shock proteins for other muscle wasting conditions
Age-related muscle wasting (sarcopenia) is characterized by a progressive loss of muscle mass, a gradual decline in muscle force generation, and an increased susceptibility to contraction-induced injury with impaired recovery [90]. These deficits in functional performance could be explained, in part, by the diminished capacity of old individuals and rodents to induce HSPs in skeletal muscles after contractile activity [91,92]. Therapeutic strategies that preserve the stress response during ageing could conceivably improve quality of life for the elderly.
McArdle et al. [93] showed that transgenic overexpression of Hsp72 ameliorated functional deficits in old mice following a protocol of severe lengthening contractions. Extensor digitorum longus (EDL) muscles of adult and old transgenic mice had a reduced force deficit at day 3 post-injury relative to their wild-type (WT) counterparts, indicating protection from secondary damage after injury. EDL muscles of transgenic mice recovered by day 14 post-injury, whereas muscles from old WT mice had a persistent force deficit even at 28 days post-injury. In agreement with these findings, pharmacological induction of Hsp72 with the Hsp90 inhibitor 17-(allylamino)-17-demethoxygeldanamycin (17AAG) restored maximal force in muscles of old WT mice compared with DMSO-treated controls [94]. Neither Hsp72 overexpression nor 17AAG treatment prevented the age-related decline in muscle cross-sectional area but Hsp72 overexpression maintained specific force in muscles of old mice [93,94]. Although the mechanisms responsible for functional improvements in old Hsp72 transgenic mice are not completely understood, prevention of age-related accumulation of oxidative species has been proposed as a potential explanation [95]. In support of this, Hsp72 overexpression reduced markers of oxidative damage, normalized levels of antioxidant enzymes and preserved NFκB-mediated transcriptional activity in muscles of old mice [95]. These findings highlight the therapeutic potential of HSP72 induction to preserve muscle function and enhance functional recovery after injury, especially during ageing.
The roles of other HSPs in age-related muscle dysfunction have also been considered. Transgenic overexpression of Hsp10 in muscles of old mice prevented the decline in maximal force, preserved muscle cross-sectional area and protected from contraction-induced damage [96]. These improvements were hypothesized to be related to Hsp10 preserving mitochondrial function. Defective mitochondria (with mutations in key components of the electron transport chain) accumulate in muscle fibres during ageing, resulting in aberrant ROS production and damage to mitochondrial proteins and membranes [97]. Lifelong overexpression of Hsp10 resulted in a threefold increase in Hsp10 levels in the mitochondria, associated with a reduction in accumulated oxidized proteins presumably due to targeting of abnormal proteins for degradation [96]. Thus, increased levels of HSP10 can preserve mitochondrial function and prevent aberrant ROS production, implicating it as a potential therapeutic target for attenuating age-related muscle wasting and weakness.
Skeletal muscle atrophy also occurs in response to disuse associated with limb unloading, immobilization, denervation and space flight [98]. Loss of muscle mass is primarily the consequence of increased protein degradation through the ubiquitin–proteasome pathway, which is regulated, in part, by NFκB and FOXO signalling and the downstream muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF1 [99,100]. Several studies have reported that gene and protein expression of HSPs is downregulated with muscle disuse and inactivity [65,101–103], suggesting a potential link between HSP levels and the regulation of muscle mass.
Plasmid-mediated overexpression of Hsp72 in the soleus muscles of rats via electroporation prevented muscle fibre atrophy induced by 7 days of bilateral hindlimb immobilization [65,101,103]. This was associated with reduced NFκB and FOXO3a transcriptional activities and downregulation of the ubiquitin ligases atrogin-1 and MuRF1 [65]. A later study showed that Hsp72 maintained muscle mass during immobilization by blocking FOXO3a-dependent transcription of atrogin-1 [103]. Furthermore, Dodd et al. [101] showed in both young and old rats that Hsp72 overexpression suppressed NFκB activity by preventing the decline in IκBα expression after immobilization. Miyabara and colleagues [104] later examined the effect of transgenic overexpression of Hsp72 in mice using a model of unilateral limb immobilization and observed that Hsp72 overexpression enhanced structural and functional recovery by maintaining the MuSC pool [104]. Since immobilization reduces MuSC number [105], any strategy that can maintain the number and function of MuSCs is relevant for retaining skeletal muscle mass.
Plasmid-mediated overexpression of Hsp27 in the soleus muscles of rats had similar effects to Hsp72. It prevented muscle fibre atrophy, reduced expression of atrogin-1 and MuRF1 and suppressed NFκB transcriptional activity, but had no effect on FOXO activity after bilateral hindlimb immobilization [102]. Together, these findings suggest that Hsp72 or Hsp27 overexpression is sufficient to preserve muscle mass and strength in disuse atrophy. Future studies should investigate pharmacological approaches that upregulate these HSPs in vivo and determine whether dual upregulation of HSP72 and HSP27 have additive benefits for preserving muscle mass and strength.
6. Conclusion
Muscle wasting and weakness are consequences of many diseases, most notably the devastating muscular dystrophies, but also with many cancers, frailty in ageing, disuse and other conditions. Effective treatments that can attenuate the severity of muscle trauma and wasting, hasten muscle repair, and restore muscle function, would help reduce the economic burden of healthcare/rehabilitation and the costs for government-sponsored return-to-work schemes, but especially alleviate the personal suffering and financial hardship of affected patients. Hormonal (and related) treatments for muscle wasting disorders have had limited efficacy and acceptance clinically because of side effects, highlighting the need for non-hormonal treatment options for these devastating conditions. HSPs are essential for proteostasis by helping to maintain normal protein structure and function. The capacity for treatments to induce specific HSPs to levels sufficient to confer protection to affected muscles in DMD and other wasting disorders would represent a major clinical advance. Such treatments would have the potential to delay progression of the dystrophic pathology, enabling patients to live longer and with a better quality of life until such time that a clinically viable cure is identified.
Acknowledgement
BGP-15 was obtained from N-Gene Research Laboratories Inc. (USA).
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed ideas and wrote sections for the review which were coordinated and edited by S.S.T. and G.S.L.
Competing interests
G.S.L. is a consultant for N-Gene Research Laboratories Inc. (USA).
Funding
This study was supported by research grants from the National Health and Medical Research Council of Australia (APP1009114; APP1065456; APP1103571), Australian Research Council (DP150100206), the Muscular Dystrophy Association, USA (MDA255153), CASS Foundation (SM/12/4269) and Dutch Parent Project (DPPNL, muscular dystrophy). S.S.T. is supported by a Australian Government Research Training Program Scholarship, a June Opie Fellowship and a Lionel Murphy Endowment Scholarship.
References
- 1.Gehrig SM, Lynch GS. 2011. Emerging drugs for treating skeletal muscle injury and promoting muscle repair. Exp. Opin. Emerg. Drugs 16, 163–182. ( 10.1517/14728214.2010.524743) [DOI] [PubMed] [Google Scholar]
- 2.Lynch GS. 2011. Sarcopenia – age-related muscle wasting and weakness: mechanisms and treatments, 1st edn Berlin, Germany: Springer. [Google Scholar]
- 3.Swiderski K, Lynch GS. 2015. Therapeutic potential of orphan drugs for the rare skeletal muscle diseases. Expert Opin. Orphan Drugs 3, 1397–1425. ( 10.1517/21678707.2015.1085858) [DOI] [Google Scholar]
- 4.Sanchis-Gomar F, Gomez-Cabrera MC, Vina J. 2011. The loss of muscle mass and sarcopenia: non hormonal intervention. Exp. Gerontol. 46, 967–969. ( 10.1016/j.exger.2011.08.012) [DOI] [PubMed] [Google Scholar]
- 5.Senf SM. 2013. Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front. Physiol. 4, 330 ( 10.3389/fphys.2013.00330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushby K, et al. 2010. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 9, 77–93. ( 10.1016/S1474-4422(09)70271-6) [DOI] [PubMed] [Google Scholar]
- 7.Wong BL, Rybalsky I, Shellenbarger KC, Tian C, McMahon MA, Rutter MM, Sawnani H, Jefferies JL. 2017. Long-term outcome of interdisciplinary management of patients with Duchenne muscular dystrophy receiving daily glucocorticoid treatment. J. Pediatr. 182, 296–303. ( 10.1016/j.jpeds.2016.11.078) [DOI] [PubMed] [Google Scholar]
- 8.Bengtsson NE, Seto JT, Hall JK, Chamberlain JS, Odom GL. 2016. Progress and prospects of gene therapy clinical trials for the muscular dystrophies. Hum. Mol. Genet. 25, R9–R17. ( 10.1093/hmg/ddv420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. 2005. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. FASEB J. 19, 880–891. ( 10.1096/fj.04-1956rev) [DOI] [PubMed] [Google Scholar]
- 10.Serrano AL, Muñoz-Cánoves P. 2017. Fibrosis development in early-onset muscular dystrophies: mechanisms and translational implications. Semin. Cell Dev. Biol. 64, 181–190. ( 10.1016/j.semcdb.2016.09.013) [DOI] [PubMed] [Google Scholar]
- 11.Blake DJ, Weir A, Newey SE, Davies KE. 2002. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82, 291–329. ( 10.1152/physrev.00028.2001) [DOI] [PubMed] [Google Scholar]
- 12.Burr AR, Molkentin JD. 2015. Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ. 22, 1402–1412. ( 10.1038/cdd.2015.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cully TR, Launikonis BS. 2016. Leaky ryanodine receptors delay the activation of store overload-induced Ca2+ release, a mechanism underlying malignant hyperthermia-like events in dystrophic muscle. Am. J. Physiol. Cell Physiol. 310, C673–C680. ( 10.1152/ajpcell.00366.2015) [DOI] [PubMed] [Google Scholar]
- 14.Gehrig SM, et al. 2012. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 484, 394–398. ( 10.1038/nature10980) [DOI] [PubMed] [Google Scholar]
- 15.Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD. 2011. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Invest. 121, 1044–1052. ( 10.1172/JCI43844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morine KJ, Sleeper MM, Barton ER, Sweeney HL. 2010. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum. Gene Ther. 21, 1735–1739. ( 10.1089/hum.2010.077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharyya S, et al. 2007. Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 117, 889–901. ( 10.1172/JCI30556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodziejczyk SM, et al. 2001. Activation of JNK1 contributes to dystrophic muscle pathogenesis. Curr. Biol. 11, 1278–1282. ( 10.1016/S0960-9822(01)00397-9) [DOI] [PubMed] [Google Scholar]
- 19.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. 2003. Activation of nuclear factor-κB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 60, 993–997. ( 10.1212/01.wnl.0000049913.27181.51) [DOI] [PubMed] [Google Scholar]
- 20.Chargé SBP, Rudnicki MA. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238. ( 10.1152/physrev.00019.2003) [DOI] [PubMed] [Google Scholar]
- 21.Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. 2015. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 5, 1027–1059. ( 10.1002/cphy.c140068) [DOI] [PubMed] [Google Scholar]
- 22.Mauro A. 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495. ( 10.1083/jcb.9.2.493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tidball JG. 2005. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R345–R353. ( 10.1152/ajpregu.00454.2004) [DOI] [PubMed] [Google Scholar]
- 24.Macario AJ, Conway de Macario E. 2005. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 353, 1489–1501. ( 10.1056/NEJMra050111) [DOI] [PubMed] [Google Scholar]
- 25.Mayer MP, Bukau B. 2005. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 62, 670–684. ( 10.1007/s00018-004-4464-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. 2002. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 105, 685–690. ( 10.1161/hc0602.103617) [DOI] [PubMed] [Google Scholar]
- 27.Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY. 2006. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 14, 129–137. ( 10.1111/j.1743-6109.2006.00102.x) [DOI] [PubMed] [Google Scholar]
- 28.Giraldo E, Martin-Cordero L, Garcia JJ, Gehrmann M, Multhoff G, Ortega E. 2010. Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur. J. Appl. Physiol. 108, 217–225. ( 10.1007/s00421-009-1201-8) [DOI] [PubMed] [Google Scholar]
- 29.Czarnecka AM, Campanella C, Zummo G, Cappello F. 2006. Heat shock protein 10 and signal transduction: a ‘capsula eburnea’ of carcinogenesis? Cell Stress Chaperones 11, 287–294. ( 10.1379/CSC-200.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deocaris CC, Kaul SC, Wadhwa R. 2006. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11, 116–128. ( 10.1379/CSC-144R.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. 2001. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp. Cell Res. 266, 213–221. ( 10.1006/excr.2001.5220) [DOI] [PubMed] [Google Scholar]
- 32.Singh BN, Rao KS, Rao CHM. 2010. Ubiquitin-proteasome–mediated degradation and synthesis of MyoD is modulated by αB-crystallin, a small heat shock protein, during muscle differentiation. Biochim. Biophys. Acta 1803, 288–299. ( 10.1016/j.bbamcr.2009.11.009) [DOI] [PubMed] [Google Scholar]
- 33.Dubinska-Magiera M, Jablonska J, Saczko J, Kulbacka J, Jagla T, Daczewska M. 2014. Contribution of small heat shock proteins to muscle development and function. FEBS Lett. 588, 517–530. ( 10.1016/j.febslet.2014.01.005) [DOI] [PubMed] [Google Scholar]
- 34.Benjamin IJ, Shelton J, Garry DJ, Richardson JA. 1997. Temporospatial expression of the small HSP/αB-crystallin in cardiac and skeletal muscle during mouse development. Dev. Dyn. 208, 75–84. ( 10.1002/(SICI)1097-0177(199701)208:1%3C75::AID-AJA7%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 35.Knowlton AA. 1995. The role of heat shock proteins in the heart. J. Mol. Cell. Cardiol. 27, 121–131. ( 10.1016/S0022-2828(08)80012-0) [DOI] [PubMed] [Google Scholar]
- 36.Koh TJ, Escobedo J. 2004. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am. J. Physiol. Cell Physiol. 286, C713–C722. ( 10.1152/ajpcell.00341.2003) [DOI] [PubMed] [Google Scholar]
- 37.Bruey JM, et al. 2000. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell. Biol. 2, 645–652. ( 10.1038/35023595) [DOI] [PubMed] [Google Scholar]
- 38.Fan CY, Lee S, Cyr DM. 2003. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones 8, 309–316. ( 10.1379/1466-1268(2003)008%3C0309:MFROHF%3E2.0.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ornatsky OI, Connor MK, Hood DA. 1995. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem. J. 311, 119–123. ( 10.1042/bj3110119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung J, et al. 2008. HSP72 protects against obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA 105, 1739–1744. ( 10.1073/pnas.0705799105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. 2001. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20, 446–456. ( 10.1093/emboj/20.3.446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang B, Xiao W, Shi Y, Liu M, Xiao X. 2005. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones 10, 252–262. ( 10.1379/CSC-124R.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch WJ, Feramisco JR. 1984. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J. Biol. Chem. 259, 4501–4513. [PubMed] [Google Scholar]
- 44.Locke M, Noble EG. 1995. Stress proteins: the exercise response. Can. J. Appl. Physiol. 20, 155–167. ( 10.1139/h95-011) [DOI] [PubMed] [Google Scholar]
- 45.Botos J, Xian W, Smith DF, Smith CL. 2004. Progesterone receptor deficient in chromatin binding has an altered cellular state. J. Biol. Chem. 279, 15 231–15 239. ( 10.1074/jbc.M309718200) [DOI] [PubMed] [Google Scholar]
- 46.Tago K, Tsukahara F, Naruse M, Yoshioka T, Takano K. 2004. Regulation of nuclear retention of glucocorticoid receptor by nuclear Hsp90. Mol. Cell Endocrinol. 213, 131–138. ( 10.1016/j.mce.2003.10.057) [DOI] [PubMed] [Google Scholar]
- 47.Gopal-Srivastava R, Piatigorsky J. 1993. The murine alpha B-crystallin/small heat shock protein enhancer: identification of alpha BE-1, alpha BE-2, alpha BE-3, and MRF control elements. Mol. Cell Biol. 13, 7144–7152. ( 10.1128/MCB.13.11.7144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middleton RC, Shelden EA. 2013. Small heat shock protein HSPB1 regulates growth of embryonic zebrafish craniofacial muscles. Exp. Cell Res. 319, 860–874. ( 10.1016/j.yexcr.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 49.Tucker NR, Shelden EA. 2009. Hsp27 associates with the titin filament system in heat-shocked zebrafish cardiomyocytes. Exp. Cell Res. 315, 3176–3186. ( 10.1016/j.yexcr.2009.06.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagatsuma A, Shiozuka M, Kotake N, Takayuki K, Yusuke H, Mabuchi K, Matsuda R, Yamada S. 2011. Pharmacological inhibition of HSP90 activity negatively modulates myogenic differentiation and cell survival in C2C12 cells. Mol. Cell. Biochem. 358, 265–280. ( 10.1007/s11010-011-0977-0) [DOI] [PubMed] [Google Scholar]
- 51.Krone PH, Evans TG, Blechinger SR. 2003. Heat shock gene expression and function during zebrafish embryogenesis. Semin. Cell Dev. Biol. 14, 267–274. ( 10.1016/j.semcdb.2003.09.018) [DOI] [PubMed] [Google Scholar]
- 52.Lele Z, Hartson SD, Martin CC, Whitesell L, Matts RL, Krone PH. 1999. Disruption of zebrafish somite development by pharmacologic inhibition of Hsp90. Dev. Biol. 210, 56–70. ( 10.1006/dbio.1999.9262) [DOI] [PubMed] [Google Scholar]
- 53.Hawkins TA, et al. 2008. The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development 135, 1147–1156. ( 10.1242/dev.018150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Gampert L, Nething K, Steinacker JM. 2006. Response and function of skeletal muscle heat shock protein 70. Front. Biosci. 11, 2802–2827. ( 10.2741/2011) [DOI] [PubMed] [Google Scholar]
- 55.Ogata T, Oishi Y, Roy RR, Ohmori H. 2003. Endogenous expression and developmental changes of HSP72 in rat skeletal muscles. J. Appl. Physiol. 95, 1279–1286. ( 10.1152/japplphysiol.00353.2003) [DOI] [PubMed] [Google Scholar]
- 56.Cheung TH, Rando TA. 2013. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340. ( 10.1038/nrm3591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tower J. 2012. Stress and stem cells. Wiley Interdiscip. Rev. Dev. Biol. 1, 789–802. ( 10.1002/wdev.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang AH, Rando TA. 2014. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 33, 2782–2797. ( 10.15252/embj.201488278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryall JG, et al. 2015. The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16, 171–183. ( 10.1016/j.stem.2014.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. 2007. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25, 2448–2459. ( 10.1634/stemcells.2007-0019) [DOI] [PubMed] [Google Scholar]
- 61.Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA. 2013. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 4, 189–204. ( 10.1016/j.celrep.2013.05.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M. 2010. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 4, 77–91. ( 10.1016/j.scr.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 63.Tseng BS, Zhao P, Pattison JS, Gordon SE, Granchelli JA, Madsen RW, Folk LC, Hoffman EP, Booth FW. 2002. Regenerated mdx mouse skeletal muscle shows differential mRNA expression. J. Appl. Physiol. 93, 537–545. ( 10.1152/japplphysiol.00202.2002) [DOI] [PubMed] [Google Scholar]
- 64.Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR. 2013. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS ONE 8, e62687 ( 10.1371/journal.pone.0062687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senf SM, Dodd SL, McClung JM, Judge AR. 2008. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 22, 3836–3845. ( 10.1096/fj.08-110163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tupling AR, et al. 2004. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J. Biol. Chem. 279, 52 382–52 389. ( 10.1074/jbc.m409336200) [DOI] [PubMed] [Google Scholar]
- 67.Bornman L, Polla BS, Lotz BP, Gericke GS. 1995. Expression of heat-shock/stress proteins in Duchenne muscular dystrophy. Muscle Nerve 18, 23–31. ( 10.1002/mus.880180105) [DOI] [PubMed] [Google Scholar]
- 68.Han R, Grounds MD, Bakker AJ. 2006. Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18. Cell Calcium 40, 299–307. ( 10.1016/j.ceca.2006.04.016) [DOI] [PubMed] [Google Scholar]
- 69.Williams DA, Head SI, Bakker AJ, Stephenson DG. 1990. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J. Physiol. 428, 243–256. ( 10.1113/jphysiol.1990.sp018210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tupling AR, Bombardier E, Vigna C, Quadrilatero J, Fu M. 2008. Interaction between Hsp70 and the SR Ca2+ pump: a potential mechanism for cytoprotection in heart and skeletal muscle. Appl. Physiol. Nutr. Metab. 33, 1023–1032. ( 10.1139/H08-067) [DOI] [PubMed] [Google Scholar]
- 71.Fu MH, Tupling AR. 2009. Protective effects of Hsp70 on the structure and function of SERCA2a expressed in HEK-293 cells during heat stress. Am. J. Physiol. Heart Circ. Physiol. 296, H1175–H1183. ( 10.1152/ajpheart.01276.2008) [DOI] [PubMed] [Google Scholar]
- 72.Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. 2006. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem. J. 394, 605–615. ( 10.1042/BJ20051214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Literáti-Nagy B, et al. 2009. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 41, 374–380. ( 10.1055/s-0028-1128142) [DOI] [PubMed] [Google Scholar]
- 74.Kennedy TL, et al. 2016. BGP-15 improves aspects of the dystrophic pathology in mdx and dko mice with differing efficacies in heart and skeletal muscle. Am. J. Pathol. 186, 3246–3260. ( 10.1016/j.ajpath.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 75.Keefe AC, Kardon G. 2015. A new role for dystrophin in muscle stem cells. Nat. Med. 21, 1391–1393. ( 10.1038/nm.4006) [DOI] [PubMed] [Google Scholar]
- 76.Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA. 2015. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21, 1455–1463. ( 10.1038/nm.3990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doran P, Martin G, Dowling P, Jockusch H, Ohlendieck K. 2006. Proteome analysis of the dystrophin-deficient MDX diaphragm reveals a drastic increase in the heat shock protein cvHSP. Proteomics 6, 4610–4621. ( 10.1002/pmic.200600082) [DOI] [PubMed] [Google Scholar]
- 78.Carberry S, Zweyer M, Swandulla D, Ohlendieck K. 2012. Profiling of age-related changes in the tibialis anterior muscle proteome of the mdx mouse model of dystrophinopathy. J. Biomed. Biotechnol. 2012, 691641 ( 10.1155/2012/691641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holland A, Dowling P, Zweyer M, Swandulla D, Henry M, Clynes M, Ohlendieck K. 2013. Proteomic profiling of cardiomyopathic tissue from the aged mdx model of Duchenne muscular dystrophy reveals a drastic decrease in laminin, nidogen and annexin. Proteomics 13, 2312–2323. ( 10.1002/pmic.201200578) [DOI] [PubMed] [Google Scholar]
- 80.Lewis C, Jockusch H, Ohlendieck K. 2010. Proteomic profiling of the dystrophin-deficient MDX heart reveals drastically altered levels of key metabolic and contractile proteins. J. Biomed. Biotechnol. 2010, 648501 ( 10.1155/2010/648501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Paepe B, Creus KK, Weis J, Bleecker JL. 2012. Heat shock protein families 70 and 90 in Duchenne muscular dystrophy and inflammatory myopathy: balancing muscle protection and destruction. Neuromuscul. Disord. 22, 26–33. ( 10.1016/j.nmd.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 82.Sakuma K, Watanabe K, Totsuka T, Kato K. 1998. Pathological changes in levels of three small stress proteins, αB crystallin, HSP 27 and p20, in the hindlimb muscles of dy mouse. Biochim. Biophys. Acta 1406, 162–168. ( 10.1016/s0925-4439(97)00094-x) [DOI] [PubMed] [Google Scholar]
- 83.Higuchi I, Hashiguchi A, Matsuura E, Higashi K, Shiraishi T, Hirata N, Arimura K, Osame M. 2007. Different pattern of HSP47 expression in skeletal muscle of patients with neuromuscular diseases. Neuromuscul. Disord. 17, 221–226. ( 10.1016/j.nmd.2006.11.008) [DOI] [PubMed] [Google Scholar]
- 84.Banwell BL, Engel AG. 2000. αB-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology 54, 1033–1041. ( 10.1212/wnl.54.5.1033) [DOI] [PubMed] [Google Scholar]
- 85.Hohlfeld R, Engel AG. 1992. Expression of 65-kd heat shock proteins in the inflammatory myopathies. Ann. Neurol. 32, 821–823. ( 10.1002/ana.410320619) [DOI] [PubMed] [Google Scholar]
- 86.Sarparanta J, et al. 2012. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat. Genet. 44, 450–455. ( 10.1038/ng.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bengoechea R, Pittman SK, Tuck EP, True HL, Weihl CC. 2015. Myofibrillar disruption and RNA-binding protein aggregation in a mouse model of limb-girdle muscular dystrophy 1D. Hum. Mol. Genet. 24, 6588–6602. ( 10.1093/hmg/ddv363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selcen D, Engel AG. 2003. Myofibrillar myopathy caused by novel dominant negative αB-crystallin mutations. Ann. Neurol. 54, 804–810. ( 10.1002/ana.10767) [DOI] [PubMed] [Google Scholar]
- 89.Vicart P, et al. 1998. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95. ( 10.1038/1765) [DOI] [PubMed] [Google Scholar]
- 90.Ryall JG, Schertzer JD, Lynch GS. 2007. Attenuation of age-related muscle wasting and weakness in rats after formoterol treatment: therapeutic implications for sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 62, 813–823. ( 10.1093/gerona/62.8.813) [DOI] [PubMed] [Google Scholar]
- 91.Vasilaki A, Iwanejko LM, McArdle F, Broome CS, Jackson MJ, McArdle A. 2003. Skeletal muscles of aged male mice fail to adapt following contractile activity. Biochem. Soc. Trans. 31, 455–456. ( 10.1042/bst0310455) [DOI] [PubMed] [Google Scholar]
- 92.Vasilaki A, Jackson MJ, McArdle A. 2002. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 25, 902–905. ( 10.1002/mus.10094) [DOI] [PubMed] [Google Scholar]
- 93.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. 2004. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 18, 355–357. ( 10.1096/fj.03-0395fje) [DOI] [PubMed] [Google Scholar]
- 94.Kayani AC, Close GL, Broome CS, Jackson MJ, McArdle A. 2008. Enhanced recovery from contraction-induced damage in skeletal muscles of old mice following treatment with the heat shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. Rejuvenation Res. 11, 1021–1030. ( 10.1089/rej.2008.0795) [DOI] [PubMed] [Google Scholar]
- 95.Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. 2006. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 20, 1549–1551. ( 10.1096/fj.05-4935fje) [DOI] [PubMed] [Google Scholar]
- 96.Kayani AC, Close GL, Dillmann WH, Mestril R, Jackson MJ, McArdle A. 2010. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle cross-sectional area. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R268–R276. ( 10.1152/ajpregu.00334.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herbst A, Pak JW, McKenzie D, Bua E, Bassiouni M, Aiken JM. 2007. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: evidence for a causal role in muscle fiber loss. J. Gerontol. A Biol. Sci. Med. Sci. 62, 235–245. ( 10.1093/gerona/62.3.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandri M. 2008. Signaling in muscle atrophy and hypertrophy. Physiology 23, 160–170. ( 10.1152/physiol.00041.2007) [DOI] [PubMed] [Google Scholar]
- 99.Cai D, et al. 2004. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119, 285–298. ( 10.1016/j.cell.2004.09.027) [DOI] [PubMed] [Google Scholar]
- 100.Sandri M, et al. 2004. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412. ( 10.1016/S0092-8674(04)00400-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dodd S, Hain B, Judge A. 2009. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10, 605–611. ( 10.1007/s10522-008-9203-1) [DOI] [PubMed] [Google Scholar]
- 102.Dodd SL, Hain B, Senf SM, Judge AR. 2009. Hsp27 inhibits IKKβ-induced NF-κB activity and skeletal muscle atrophy. FASEB J. 23, 3415–3423. ( 10.1096/fj.08-124602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Senf SM, Dodd SL, Judge AR. 2010. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol. 298, C38–C45. ( 10.1152/ajpcell.00315.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyabara EH, Nascimento TL, Rodrigues DC, Moriscot AS, Davila WF, AitMou Y, deTombe PP, Mestril R. 2012. Overexpression of inducible 70-kDa heat shock protein in mouse improves structural and functional recovery of skeletal muscles from atrophy. Pflugers Arch. 463, 733–741. ( 10.1007/s00424-012-1087-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brooks NE, Myburgh KH. 2014. Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 5, 99 ( 10.3389/fphys.2014.00099) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.