Abstract
The ongoing contractile and metabolic demands of the heart require a tight control over protein quality control, including the maintenance of protein folding, turnover and synthesis. In heart disease, increases in mechanical and oxidative stresses, post-translational modifications (e.g., phosphorylation), for example, decrease protein stability to favour misfolding in myocardial infarction, heart failure or ageing. These misfolded proteins are toxic to cardiomyocytes, directly contributing to the common accumulation found in human heart failure. One of the critical class of proteins involved in protecting the heart against these threats are molecular chaperones, including the heat shock protein70 (HSP70), HSP90 and co-chaperones CHIP (carboxy terminus of Hsp70-interacting protein, encoded by the Stub1 gene) and BAG-3 (BCL2-associated athanogene 3). Here, we review their emerging roles in the maintenance of cardiomyocytes in human and experimental models of heart failure, including their roles in facilitating the removal of misfolded and degraded proteins, inhibiting apoptosis and maintaining the structural integrity of the sarcomere and regulation of nuclear receptors. Furthermore, we discuss emerging evidence of increased expression of extracellular HSP70, HSP90 and BAG-3 in heart failure, with complementary independent roles from intracellular functions with important therapeutic and diagnostic considerations. While our understanding of these major HSPs in heart failure is incomplete, there is a clear potential role for therapeutic modulation of HSPs in heart failure with important contextual considerations to counteract the imbalance of protein damage and endogenous protein quality control systems.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: HSP70, HSP90, carboxy terminus of HSP70-interacting protein, Stub1, BAG-3, heart failure
1. Introduction
According to the World Health Organization, heart disease is a leading cause of death worldwide [1]. An estimated 17.7 million people died of heart disease in 2015, representing 31% of all global deaths [1]. One in nine deaths includes heart failure as a contributing cause, with half of these people developing heart failure dying within 5 years of diagnosis [2]. In the USA, heart failure costs are estimated to be $30.7 billion each year [3]. As heart failure-related mortality rates increase [4], alternative approaches to diagnosing and treating the disease are needed. One just recently recognized therapeutic approach involves targeting protein quality controls systems in heart failure, much like those involved in Alzheimer's disease and other neurodegenerative conditions [5].
The ongoing contractile and metabolic demands of the heart require a tight control over protein quality control, including the folding of proteins, their turnover and protein synthesis process. These processes are best known in the sarcoplasmic reticulum, sarcomeres and mitochondria and are critical to minimizing wear and tear processes. Heart disease, broadly characterized by increases in mechanical and oxidative stresses and changes in pH, induces the accumulation of misfolded proteins, whether caused by myocardial infarction (MI), heart failure or genetic mutations. These misfolded proteins are toxic to cardiomyocytes and can lead directly to heart failure [6] or mimic the proteinopathy that is found in human heart failure [7]. One of the critical class of proteins involved in protecting the heart against these threats are molecular chaperones, which regulate the balance of protein synthesis and degradation, assist with refolding misfolded proteins and can protect against cell death when induced in stressful/pathological conditions (figure 1) [5,8]. The stress-induced cardiomyocyte production of HSPs is appreciated in the context of sarcomere assembly, autophagy, the ubiquitin-proteasome system (UPS) and the normal turnover of proteins in the heart [9].
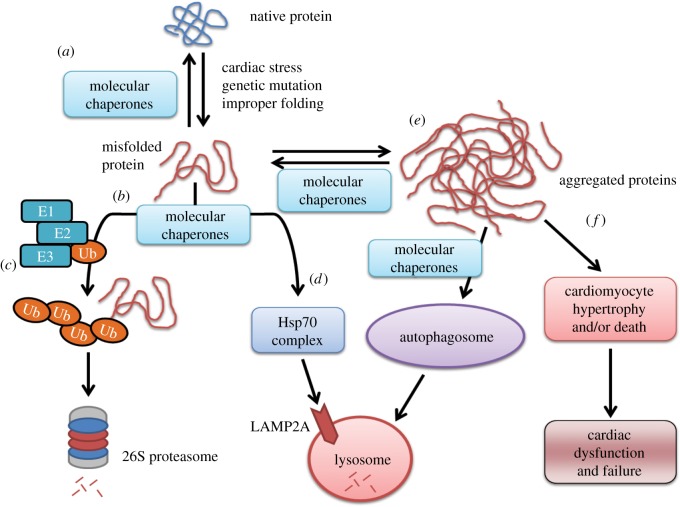
Figure 1.
A schematic of the way molecular chaperones maintain cardiomyocyte protein quality control. (a) Molecular chaperones (i.e. HSP70, HSP90, CHIP and BAG-3) are critical in maintaining a protein's native folding and function in the face of cardiac stress, mutations and improper folding induced by post-translational modifications (e.g., phosphorylation). (b) When misfolded proteins interact with chaperones (which cannot be refolded), they can be shuttled (c) for ubiquitin-dependent proteasome degradation or (d) directly to lysosomal degradation via the HSP70 complex or (e) accumulate as aggregates that can be cleared by the autophagosome (to a certain extent), at which point they (f) start playing a role in proteotoxicity-mediated cardiac dysfunction. Addendum to (c). The ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and the ubiquitin ligase (E3) act in concert to interact specifically with substrates (along with molecular chaperones) to poly-ubiquitinate the substrate, resulting in degradation by the 26S proteasome.
In this review, we focus on the role of the major heat shock proteins (HSPs) in cardiomyocytes (HSP70 and HSP90) and the co-chaperones (CHIP and BAG-3) that are more recently appreciated for their critical roles in maintaining cardiac integrity during stress. We have much to learn about and from these endogenous protein quality control systems as the inherent first line of defence that has been biologically established. Further understanding their roles in heart failure will give insight into ways in which we can therapeutically support HSPs in the treatment of heart failure.
(a). Heat shock protein 70
The 70-kDa heat shock protein (HSP70) family of molecular chaperones is a ubiquitous class of molecular chaperones and conserved protein families throughout evolution. The HSP70 family of molecular chaperones are monomeric proteins found throughout the cytosol, in cell membranes and in the extracellular milieu [10–14]. While originally discovered as a set of genes whose expression increased with thermal stress (heat shock) in Drosophila melanogaster [15], it was later uncovered that HSPs could be induced by ischaemia, nutrient deprivation, irradiation, infections and inflammation, among other cellular stresses [16]. Upregulation of the HSP70 family promotes cell survival in the face of endogenous or exogenous challenges.
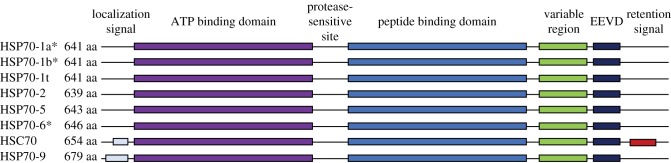
Eight unique gene products make up the human HSP70 family that differ from each other by amino acid sequence, expression levels and localization subcellularly [17,18]. These include three stress-induced isoforms (HSP70-1a, HSP70-1b and HSP70-6) and five not responsive to stress (HSP70-1t, HSP70-2, HSP70-5, HSC70 and HSP70-9) (table 1). The stress-inducible isoforms of HSP70 have high homology, with HSP70-1b having 99% homology and HSP70-6 having 85% homology (figure 2). Alternative names for inducible HSP70 isoforms HSP70-1a and HSP70-1b include HSP70, HSP72 and HSP70-1 (table 1). Notably, the cellular localization of the different isoforms differ with most being in the cytosol and nucleus and others in the ER and mitochondria (figure 2). The inducible HSP70-1a and HSP70-1b can be found in the cytosol, nucleus and lysosomes, while the HSP70-6 is localized to the cytosol and nucleus. The stress-inducible HSC70 isoform (also known as HSP70-8, HSP73) is found in the cytosol and nucleus. Together, these largely highly homologous proteins are found in most compartments of the cell, with critical roles in the health of cells in tissues throughout the body.
Table 1.
HSP70 family of proteins, their alternative names, cellular localization and stress-responsiveness. Adapted from Daugaard et al. [18].
| protein | other names | % homology (versus HSP70-1a) | cellular localization | stress-induced | reference |
|---|---|---|---|---|---|
| HSP70-1a | HSP70, HSP72, HSP70-1 | 100 | cytosol, nucleus, lysosomes | yes | Wu et al. [19] |
| HSP70-1b | HSP70, HSP72, HSP70-1 | 99 | cytosol, nucleus, lysosomes | yes | Wu et al. [19] |
| HSP70-1t | HSP70-hom | 91 | cytosol, nucleus | no | Goate et al. [20] |
| HSP70-2 | HSP70-3, HSPA2 | 84 | cytosol, nucleus | no | Bonnycastle et al. [21] |
| HSP70-5 | Bib, GRP78 | 64 | sarco-/endoplasmic reticulum | no | Munro & Pelham [22] |
| HSP70-6 | HSP70B | 85 | cytosol, nucleus | yes | Leung et al. [23] |
| HSC70 | HSP70-8, HSP73 | 86 | cytosol, nucleus | no | Dworniczak & Mirault [24] |
| HSP70-9 | GRP75, mtHSP75, Mortalin, tumour necrosis factor-associated protein-1 (TRAP1) | 52 | mitochondria | no | Domanico et al. [25] Bhattacharyya et al. [26] |
Figure 2.
The human HSP70 family member protein domain diversity. Of the eight HSP70 family members, three are induced by stress (indicated by *), including HSP70-1a, HSP70-1b and HSP70-6 (aka HSP72). HSC70 and HSP70-9 have localization signals and are found in the cytosol/nucleus and mitochondria, respectively [24–26]. Adapted from Daugaard et al. [18].
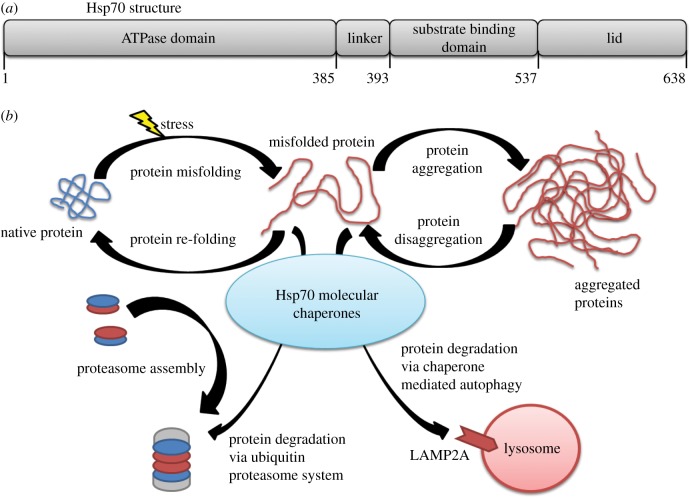
In the heart, HSP70 and its protein homologue, heat shock cognate (HSC) 70, are integral for disease prevention and protecting cardiomyocytes from stress. While HSP70 and HSC70 are protein homologues (as described in figure 2), there are differences between the two. HSC70 is constitutively expressed in the heart, whereas HSP70 expression is induced by a stressful or protective stimuli (table 1). The HSP70 family protein structure consists of a 44-kDa amino terminus nucleotide binding domain that binds and hydrolyses ATP, a central domain with protease-sensitive sites, and a 28-kDa carboxyl terminus substrate binding domain that contains an EEVD motif to bind polypeptides, co-chaperones and other HSPs (figure 3a) [27,28]. Under physiological conditions, HSP70s provide support for protein folding of newly synthesized polypeptides and aberrant proteins and transport of the formed native proteins to protect the intracellular milieu [28]. HSP70s contain unfoldase activity to unfold and then refold misfolded proteins through repeated cycles of binding, ATP-dependent unfolding and finally refolding to the native protein conformation [29,30].
Figure 3.
The HSP70 molecular chaperone structure and molecular pathways. (a) Structure of Hsp70. (b) The many roles the Hsp70 family of molecular chaperones has to maintain cellular proteostasis.
Multiple co-chaperones Hip (HSC70-interacting protein), HSP40 proteins and Hop (HSC70-HSP90 organizing protein) play critical roles in supporting HSP70 function [31,32]. Hip binding to the ATPase domain of HSC70 or HSP70 increases the affinity for substrates by stabilizing the ADP-bound state of HSC70 or HSP70. Members of the HSP40 proteins (also known as DNAJ proteins) are chaperones themselves, binding exposed hydrophobic residues of unfolded proteins and sharing common substrates with HSP70s [33–35]. In most cases, HSP40s are homodimeric proteins with a highly conserved J-region that facilitates interactions with HSP70 family members by binding the N-terminal ATPase domain [36–38]. Hop binds and connects HSP/HSC70 to HSP90 to initiate a partnership, even potentially allowing an exchange of substrates between HSP/HSC70 and HSP90 [31]. HSP70 recognizes short stretches of extended hydrophobic amino acids, such as loosely folded protein complexes, that are exposed by nascent chains, protein translocation intermediates and misfolded proteins [39–42]. When HSP/HSC70 cannot properly refold a misfolded protein, HSP/HSC70 participates in degradation of said protein by forming a complex with other proteins (e.g., CHIP, discussed below; figure 3b). Ubiquitinated proteins are degraded by the proteasome and autophagy when HSP/HSC70 complex with Bcl2-associated athanogene (BAG)-1 and BAG-3 (discussed below), respectively. There is a specific form of autophagy for HSC70, named chaperone-mediated autophagy (CMA). An HSC70 complex recognizes and binds to oxidized or abnormal proteins containing a KFERQ motif and delivers them to the lysosomal membrane where the HSC70 complex binds to a lysosome-associated membrane protein 2A (LAMP2A) receptor for the substrate protein to be internalized for degradation [43]. CMA is similar to macroautophagy but more selective by targeting specific soluble proteins for degradation by the lysosome [44]. A recent study demonstrated that the HSP70 family can not only bring proteins to the proteasome for degradation, but that HSP70 molecular chaperones are integral to the assembly of the proteasome [45]. Yeast lacking HSP70 molecular chaperones exhibited defects in assembly of the proteasome core particle.
HSP70 expression is elevated in human myocardial tissues following coronary artery bypass grafting, aortic cross-clamp during surgery or in the setting of ischaemia (as recently reviewed [46]). Ischaemic preconditioning induces HSP70 expression, occurring as soon as 1 h after exposure [47,48]. The closely related HSC70 (not known to be an inducible factor) has been reported to be decreased in the diabetic myocardium, whereas HSP70 is not [49]. Restoration of insulin signalling restores HSC70 levels, demonstrating insulin's major role in HSC70 in cardiomyocytes [49]. In the setting of inducibly elevated HSP70, cardiomyocyte protection has been identified [50]. Additionally, other studies have found a lower incidence of post-operative atrial fibrillation in patients with high levels of HSP70 (also known as HSP70-1a, HSP70-1b), in contrast to those with low HSP70 (or a HSP70 polymorphism with decreased function) who have an increased risk of post-operative atrial fibrillation [42,51,52]. HSP70 expression may also be increased in the phenomena of ischaemic preconditioning and exercise. Ischaemic preconditioning is the induction of resistance to a subsequent ischaemic insult induced by a repeated short episode of ischaemia in the myocardium [53]. The mechanisms of ischaemic preconditioning go beyond the induction of HSPs (e.g., activation of protein kinase C (PKC) isoforms, attenuation of p38 activation) [54], but induction of HSP70 HSPs is involved. When HSP70 and HSC70 are increased using adenoviral vector expressing both, protection from H2O2-induced apoptosis (simulating ischaemic conditions) has been reported by blocking the activation of the apoptosis inducing factor (AIF) [55]. Specifically, HSC70 appears to bind AIF directly to inactivate it [55]. While the cardioprotective effects of HSP70 seem straightforward, it is worth noting that they are context specific, as recently reviewed [50]. In studies using the same HSP70 transgenic animal protected in acute ischaemia reperfusion injury, chronic models of pressure overload-induced cardiac hypertrophy, dilated cardiomyopathy (DCM) and heart failure were not found to be protected when HSP70 was increased [56,57].
Exercise induces HSP70 expression, but the mechanisms by which exercise is cardioprotective are complex and may not be entirely explained by HSP70 expression. Exercise activates an immediate upregulation of HSP70 that remains elevated 24 h following exercise [58–61]. Similarly, increases in HSP70 have been corroborated by another study noting an increase in HSP72 (also known as HSP70-1a, HSP70-1b) expression following endurance exercise, which then elicited cardioprotection against ischaemia–reperfusion (IR) injury compared to sedentary controls that did not have enhanced HSP72 expression [62]. Exercised rats with increased HSP72 expression exhibited smaller infarcts and reduced apoptosis [62]. Induction of HSP70 expression by both ischaemic preconditioning and short-term exercise was shown to be protective against MI [63] and IR injury [58,62], respectively. These cardioprotective effects appear to be secondary to increases in core body temperature, as animals trained in the cold do not demonstrate increases in HSP70, but do have some degree of cardioprotection [64–66]. Given these findings, the mechanisms of exercise-induced HSP70 and associated cardioprotection should be interpreted carefully.
Pathological myocardial stress (e.g., hypertrophy or ischaemia) increases the amount of misfolded proteins that need to be refolded to their native states or removed to avoid protein aggregation [67–69]. To counteract this stress and accumulation of misfolded proteins during myocardial ischaemia, there is a rapid induction of HSP70 expression [47]. Transgenic mice that overexpress HSP70 maintained higher systolic peak pressure and limited cellular injury following ischaemia reperfusion [70–72]. Mice overexpressing HSP70 also exhibited improved myocardial ischaemia recovery of contractile force, high energy phosphate stores and correction of metabolic acidosis [71,73]. During normal conditions, the increased expression of HSP70 did not alter protein synthesis or degradation, nor did it affect cardiac function. Elevated levels of myocardial HSP70 reduced infarct size and improved post-ischaemic recovery [63,74–76]. Blocking the augmented expression of HSP72 resulted in increased cardiomyocyte damage and susceptibility to cell death in isolated cells exposed to hypoxia [77]. Furthermore, there appears to be a direct correlation between the amount of inducible HSP70 and the extent of myocardial protection [78]. This observation was further supported by the finding that in vivo adenovirus (Ad)-mediated gene transfer of HSP70 attenuated IR injury in rabbit hearts [79]. Ad-HSP70 injected rabbit hearts had a nearly 50% reduction in infarct size compared with the rabbit hearts injected with Ad-LacZ or injected with saline alone. Collectively, these findings demonstrate the cardioprotection of increased expression of HSP70 molecular chaperones during myocardial ischaemia.
The heat shock protein HSP75, also known as HSP70-9 (table 1), is a mitochondrially localized member of the HSP90 family [80]. To determine the role of HSP75 in cardiac hypertrophy, cardiac-specific inducible HSP75 transgenic mice were created and challenged to pressure overload-induced hypertrophy [80]. Increasing HSP75 prevented the development of cardiac hypertrophy and fibrosis, assessed by heart weight, echocardiographic and haemodynamic measures, cardiomyocyte cross-sectional areas, collagen markers and the expression of the hypertrophic ‘fetal gene’ program [80]. Increasing HSP75 attenuated the activation of TAK/p38, JNK and Akt signalling pathways, which were suggested to be the mechanisms by which the reduced hypertrophy and fibrosis were regulated [80].
(b). Heat shock protein 90
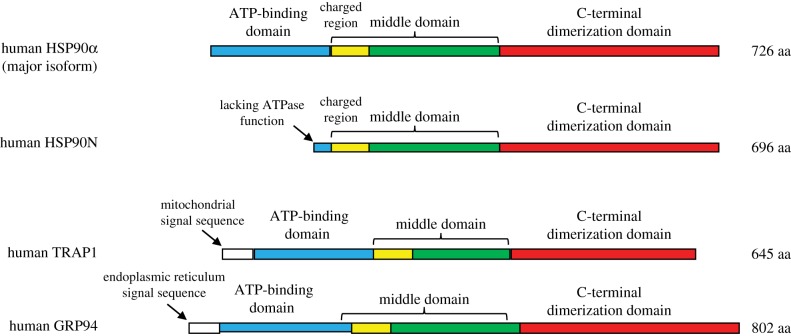
The HSP90 proteins have three functional domains (ATP-binding, protein-binding and dimerizing domain) and assist with stabilizing proteins against heat stress and aid in protein degradation (figure 4). The interaction of HSP90 with non-native structures (misfolded regions) of a substrate protein lends stability to the substrate and decreases aggregation with other misfolded proteins. Moreover, HSP90 appears to be more selective than other chaperones, linking misfolded protein recognition to protein degradation via poly-ubiquitination and destruction by the 26S proteasome. These substrates include the glucocorticoid receptor and immunophilin co-chaperones (e.g., PKBP52) that attach the GR complex to the dynein protein tracking pathway (detailed in figure 5). HSP90 is also involved in the proper functioning of multiple other steroid receptors, including aldosterone, androgen, oestrogen and progesterone and regulates intracellular signal transduction, affecting more than 40 protein kinases [83,84]. Over 200 substrate or client proteins have been identified, as have dozens of co-chaperones [85]. Its prominent role in heart failure, therefore, is not surprising.
Figure 4.
The human HSP90 family member protein domain diversity. The inducible HSP90α is the major isoform found in the cytosol, while HSP90N, TNF receptor-associated protein 1 (TRAP1) and GRP94 are found in the plasma membrane, mitochondria and ER, respectively, as described in the text.
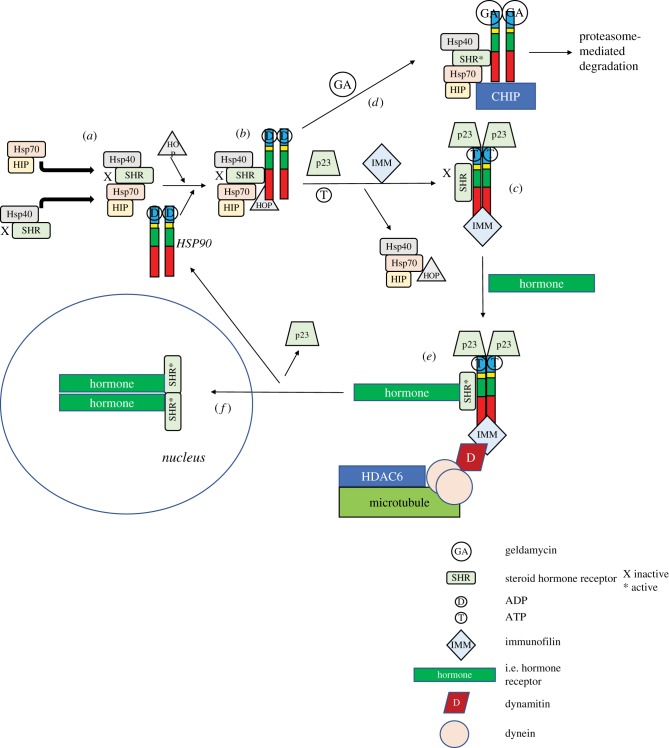
Figure 5.
HSP90-dependent steroid hormone receptor activation as an example of HSP90 activity. (a) The HSP90 complex is established by interactions of HSP70, HSP70-interacting protein (HIP), HSP40 and the steroid hormone receptor (SHR). (b) The SHR is transmitted via interaction with the HSP90 dimer to (c) associate with p23/IMM to change the HSP90 confirmation. Upon binding ATP, the immunophilins bind in place of the HSP70 and co-chaperones. (d) Binding of geldanamycin (a 1,4-benzoquinone ansamycin anti-tumour antibiotic that inhibits HSP90 function by binding to the ADP/ATP-binding pocket [81]) induces the disassociation of p23 and HOP, allowing the carboxyl terminus of HSC70-interacting protein (CHIP) ubiquitin ligase to attached to the complex, to poly-ubiquitinate and target it for proteasome-dependent degradation. (e) The HSP90–immunophilin–p23 complex activates SHR, which then binds steroid hormone, binds dynamitin and dynein (microtubule-associated proteins), which are then (f) trafficked along the cytoskeleton to the nucleus, where they dimerize and interact with the promoters of target genes. GA, geldamycin; SHR, steroid hormone receptor; D (in circle), ADP; T, ATP; IMM, immunofilin; D (in rhombus), dynamitin; peach circle, dynein. Adapted from [82].
Members of the HSP90 family of molecular chaperones are the most abundant chaperones in the cytosol (figure 4). There are two main cytosolic HSP90 isoforms: HSP90α and HSP90β, with HSP90α an inducible isoform, whereas HSP90β is not and is constitutively expressed. In this manner, HSP90 can maintain a high baseline abundance, while increasing expression inducible by stress [86,87]. Other members of the HSP90 family include HSP90N, TNF receptor-associated protein 1 (TRAP1) and glucose-regulated protein with molecular mass of 94 kDa (Grp94; figure 4) [88,89]. HSP90N interacts with plasma membrane-localized rapidly accelerated fibrosarcoma gene (Raf) [90], while TRAP1 is localized in mitochondrial matrix and Grp94 is most abundant in the endoplasmic reticulum, with 50% and 35% homology to cytoplasmic HSP90 forms, respectively [88,89,91] (figure 4).
Cardiac ischaemia induces HSP90 (aka HSP90α) levels approximately 16-fold [92], driven by concurrent increases in the HSF1 transcription factor, driven by the accumulation of reactive oxygen species (ROS) in ischaemia/reperfusion [93] or ATP concentration [94]. HSP90 is regulated by co-chaperones Hop, p23 and the cyclophillins (as outlined in figure 5) [89,95]. Briefly, Hop (discussed above) can bind HSP/HSC70 and Hsp90. The p23 protein can then bind the amino terminus of HSP90. The cyclophillins are a family of co-chaperones that contain peptidyl-prolylisomerase activity to aid in the final folding of HSP90 substrates [96]. Substrates targeted by HSP90 tend to be bound in a more compact structure having near-native conformations and resemble late-stage folding intermediates [89]. Misfolded proteins that cannot be refolded to their native state by HSP90 can remain bound to HSP90, thereby keeping the misfolded protein in a holding state to prevent it from aggregating with other misfolded proteins and maintenance of a folding-competent state. HSP90 can then pass the misfolded protein onto a HSP70 complex for unfolding and attempted refolding [29,86,97].
Insulin-like growth factor (IGF)-1 and downstream Akt signalling is best known as the pathway activated in physiological muscle (and cardiac) hypertrophy growth in response to exercise. However, in pathological cardiac hypertrophy induced by pressure overload (e.g., hypertension), multiple signalling pathways are activated, including Akt [98,99]. In the context of cardiac disease, we focus here on Akt signalling because of its importance in pathological cardiac hypertrophy and heart failure [100]. The critical role of HSP90 in supporting Akt signalling was first identified in human osteosarcoma cells and human embryonic kidney 293 cells [101]. In these studies, platelet-derived growth factor (PDGF) stimulation leads to increased Akt association with the actin skeleton through direct contact with the Akt pleckstrin homology domain (PH domain) [102]. Other studies have reported that Akt then associates with HSP90, which supports active Akt by preventing dephosphorylation by protein phosphatase 2A (PP2A) by competing for binding of the Akt 229–309 amino acid residues [103]. Akt interacts with HSP90 to associate and activate endothelial nitric oxide synthase (eNOS), whereby HSP90 acts as a scaffold to help Akt-mediated phosphorylation of eNOS in caveolae [104–106]. Experimental evidence for these pathways in cardiomyocytes include observational studies of elevated HSP90 and Akt levels in hypoxia-challenged cardiomyocytes [107]. Subsequent studies demonstrated that the anti-apoptotic effect of HSP90 on hypoxia-mediated cardiomyocyte damage was due to PI3 K/Akt signalling pathways [108].
Another major HSP90 client is HER2 (also known as erbB2), a member of the tyrosine kinase epidermal growth factor receptor (EGFR or erbB1) family. HER2 is an orphan receptor found in both neuronal and non-neuronal tissues in embryos and adult animals, including the heart [109]. Mice lacking erbB2 are embryonic lethal, but crossing erb2−/− mice with cardiac transgenic erbB2 rescues the embryos to allow development to birth (but still having a severe neurological defect) [109]. Mice conditionally lacking cardiac-specific erbB2 develop heart failure, characterized by left ventricular dilation, wall thinning and decreased systolic function [109]. When humans are treated with Herceptin (anti-erbB2 monoclonal therapy against breast cancers overexpressing erbB2), a subset have developed cardiac dysfunction, potentially by similar mechanisms [109]. HSP90's support of HER2 function is most likely relevant in the context of the development of pathological cardiac hypertrophy. Central to this pathophysiology is the activation of the angiotensin II receptor by angiotensin. Pharmacological inhibition of angiotensin II-stimulated cardiomyocyte hypertrophy by increasing EGFR first suggested a link between HER2 and pathological hypertrophy [110]. Specific inhibition of HER2 acted as a dominant-negative inhibitor co-transfected with full-length EGFRs. While the role of HSP90 in mediating angiotensin II-induced cardiac hypertrophy has not been tested directly, there is emerging evidence linking HSP90 to HER2 expression [111–113].
HSP90 may be cardioprotective in doxorubicin-induced heart failure experimentally, through its support of Akt signalling. Using mice treated with 3 mg kg−1 doxorubicin twice weekly for four weeks (cumulative does 24 mg kg−1), approximately 30% of mice survived 35 days [114]. Noticing the doxorubicin activation of poly ADP ribose polymerase (PARP), the PARP inhibitor L-2286 was tested and found to protect against the cytotoxic effects of doxorubicin and improved survival (more than 60% survived at 35 days) [114]. These cardioprotective effects of the PARP inhibitor L-2286 included the observation that Akt (also known as protein kinase B) signalling was enhanced (Akt-1 Ser473 phosphorylation), as was GSK-3β Ser9 phosphorylation (a downstream target of Akt-1Ser473) and significant increases in HSP90 [114]. Furthermore, decreases in HSP90 substrates p-p38 and p-JNK were identified, consistent with previously reported activity of elevated HSP90 (also found) [114]. The cardioprotective effects of the PARP inhibitor on function by echocardiographic measurements illustrate the role of HSP90 and other potential mechanisms in protection against doxorubicin-induced heart failure [114].
HSP90 also appears to be cardioprotective in high-glucose-induced cell injury. The systemic high glucose (HG) found in diabetes has a role in the pathogenesis of diabetic cardiomyopathy. Recent studies have found that exogenous hydrogen sulfide (H2S) protects against HG-induced cardiac injury and modifies HSP90 and Akt [115]. When the H9c2 cardiomyocyte cell line was challenged to 35 mM glucose (HG) for 1–24 h, a marked reduction in both HSP90 and phosphorylated (p-)Akt over time was identified [115]. Treatment of H9c2 cells with a donor of H2S prior to HG challenge significantly inhibited the HG-induced decrease in HSP90 and p-Akt levels, increasing cell viability, decreasing apoptosis, attenuating ROS and decreasing the mitochondrial membrane potential [115]. Inhibiting HSP90 with geldanamycin aggravated the inhibition of the p-Akt expression by HG, which treatment with the Akt inhibitor LY294002 blocked the cardioprotective effects of H2S [115]. Together, these findings reveal that the cardioprotection of H2S is mediated by both HSP90 and p-Akt activities, giving context in heart failure to HSP90 and its support of p-Akt signalling [115].
The role of cardiomyocyte HSP90 in the remodeling associated with cardiac hypertrophy and heart failure has recently been reported, involving a rich crosstalk between cardiomyocytes and fibroblasts [116]. The pathological remodelling of the heart involves both increases in left ventricular mass and deposition of extracellular matrix, which contributes to the development of heart failure. Release of transforming growth factor beta (TGFβ) from cardiomyocyte is a driving mechanism of this remodelling, activating adjacent fibroblasts to deposit extracellular matrix. In fibroblasts, the TGFβ receptor I (TGFβRI) complexes with HSP90, which in silico studies predict are made up of HSP90 dimers and TGFβRI extracellular domain interactions [116]. Inhibiting the extracellular HSP90 decreased collagen production and activation of the canonical TGFβ signalling cascade [116]. Collagen protein synthesis was significantly reduced in mice lacking Hsp90aa1 (HSP90−/−), implicating a link between the activity of HSP90 at the plasma membrane and the cooperative relationship of HSP90 and TGFβRI [116]. Other investigators have found that myocyte-derived HSP90 modulates collagen by the biphasic activation of Stat-3 in fibroblasts [117]. Cardiomyocyte-targeted knockdown of HSP90 in rats challenged with pressure overload (via renal artery ligation) exhibited a downregulation of collagen synthesis [117]. When cardiac fibroblasts were conditioned with HSP90-inhibited myocyte supernatant, a role in the regulation of collagen expression in fibroblasts was established [117]. The mechanism by which myocyte-derived HSP90 orchestrates myocyte IL-6 synthesis has been detailed at multiple levels in cardiac hypertrophy [117]. Cardiomyocyte HSP90 regulates IL-6 release by supporting p65-mediated IL-6 synthesis and its release by regulating exosomal vesicles release [117]. Myocyte-derived HSP90 then orchestrates the biphasic activation of STAT-3 in cardiac fibroblasts that results in excess collagen synthesis, resulting in defects in cardiac function [117]. Together, these mechanisms act in unison to activate Stat-3 in cardiac fibroblasts, which then synthesizes excess collagen and leads to the eventual cardiac dysfunction that occurs [117]. These recent findings give context to the role of HSP90 isoforms during pathological cardiac remodelling in pressure overload models and the detection of circulating HSP90 [116].
Cardiac HSP90 proteins have critical roles in supporting protein maturation and have roles in the development of mutation-related cardiac arrhythmias. The human ether-a-go-go-related (hERG) potassium channels in cardiomyocytes are essential for the normal coordinated electrical activity in the heart. Mutations in the hERG gene cause long QT syndrome, which predisposes individuals to life-threatening arrhythmias. More than 100 mutations have been linked to long QT syndrome in the hERG channels, mainly due to single-nucleotide changes causing amino acid substitutions [118]. HSP90 proteins prevent the aggregations of misfolded protein in vitro, with HSP90 inhibition (via geldenamycin) preventing the maturation of wild-type hERG channels, which promoted the ubiquitination and degradation of the protein. A proposed mechanism for the depleted hERG channels is linked to their retention by the quality control mechanism in cells [118] because the G601S and R752 W hERG mutations have been observed to have increased binding with HSP90 compared to wild-type HERG [119]. A more detailed review of hERG channel trafficking and HSP90 mechanisms can be found in Dennis et al. [120].
(c). Carboxyl terminus of HSC70-interacting protein
Both the chaperone and the UPSs function to maintain cardiomyocyte protein quality control, however, a link between these two systems remained illusive [121,122]. CHIP, a protein that is ubiquitously expressed but most prominently in striated muscle (cardiac and skeletal muscle) and the brain, was initially identified in a screen for tetratricopeptide repeat (TPR) motifs [123]. CHIP possesses three domains: a TPR domain at its amino terminus responsible for protein–protein interactions, a central charged domain and the U-box domain at its carboxyl terminus, which binds E2-conjugating enzymes and harbours ubiquitin ligase capabilities (figure 6a). Through its TPR domain CHIP interacts with the EEVD motif at the carboxyl terminus of HSPs HSP70, HSC70 and HSP90 [89,124]. The U-box structure is similar to RING domains, except that the U-box lacks a zinc-binding motif, instead using salt-bridges to stabilize the structure [125]. The co-chaperone CHIP plays a key role in both protein folding by regulating its co-chaperones and in protein degradation as a ubiquitin ligase. This unique combination of molecular chaperone and ubiquitin ligase capabilities allows CHIP to make protein triage decisions: refold the misfolded protein with its co-chaperones or if the protein cannot be repaired, ubiquitinate the misfolded protein targeting for protein degradation by the proteasome [122]. Thereby CHIP is a primary effector of cardiomyocyte protein quality control, but how does CHIP discriminate between what is to be refolded and those to be degraded? When a co-chaperone cannot refold a misfolded protein, CHIP will bind to its co-chaperone and ubiquitinate the misfolded protein for degradation [67].
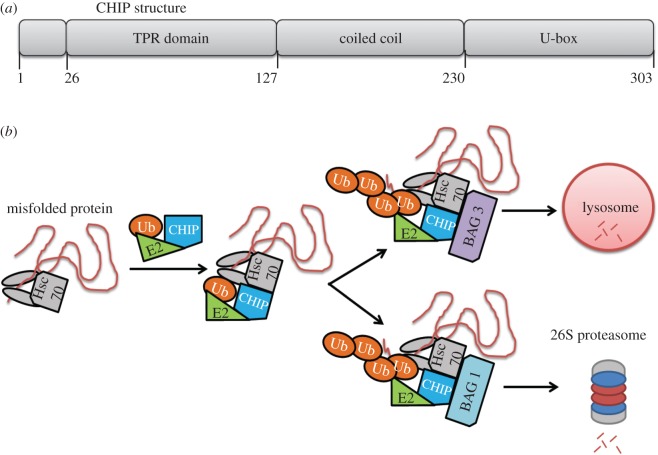
Figure 6.
Structure and function of the Carboxyl terminus of HSC70-interacting protein (CHIP) encoded by the Stub1 gene. (a) The CHIP complex will recognize an Hsp70 molecular chaperone (e.g., Hsc70) that is bound to a substrate, ubiquitinate the protein substrate, and then (b) shuttle the protein to the lysosome or proteasome for degradation if CHIP is complexed with Bag-3 or Bag1, respectively. Ub, ubiquitin.
CHIP forms a multi-protein complex to facilitate protein degradation. BAG-1 connects the HSC70–CHIP complex with the proteasome through binding Hsc70 and the proteasome simultaneously [126]. It has also been speculated that Bag-1 recruits and binds its cofactor, HSC70, thus CHIP to the proteasome [127,128]. CHIP was immunoprecipated with the α7 subunit (also known as the HC8 subunit) of the 26S proteasome in COS-7 cells [129], however, the presence of BAG-1 was not assessed. CHIP is also linked to protein degradation by autophagy during exercise [130]. It has been speculated that CHIP shuttles proteins towards autophagy when in complex with BAG-3 [128,131]. In neurons, CHIP was revealed to facilitate the degradation of α-synuclein mediated by either the proteasome or autophagy route [132]. Collectively, these findings link the detection of misfolded proteins to their ubiquitination to their shuttling for protein degradation to the proteasome or lysosome, all of which are dependent on the proteins that CHIP is in complex with (figure 6b).
CHIP overexpression or deletion does not alter basal cardiac function. However, once the heart is challenged, CHIP modulation has a profound cardiac effect [133]. CHIP−/− mice develop exaggerated cardiac hypertrophy, evidenced by increased cardiomyocyte size, heart weights and wall thicknesses in response to exercise or pressure overload [130,134]. Following voluntary wheel running exercise, CHIP−/− mouse hearts displayed a further increase in heart weight to body weight, cardiomyocyte cross-sectional area and wall thickness compared with wild-type mice [130]. Subjecting CHIP−/− mice to pressure overload resulted in a 35% mortality within the first week while the wild-type mice had no mortality in the same period [134]. CHIP−/− mice also exhibited exaggerated cardiac hypertrophy and fibrosis along with a dramatic decline in cardiac function compared with control mice following pressure overload. Interestingly, CHIP−/− cardiomyocytes also had a decline in mitochondrial density, which was attributed to decreased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) and oxidative phosphorylation (OxPhos) in CHIP−/− myocardium [134]. These data suggest CHIP has a role in maintaining mitochondria during the development of left ventricular hypertrophy.
MI/IR injury damages intracellular proteins, impairs protein degradation processes, leads to cardiomyocyte apoptosis and reduces cardiac function [135]. In response to MI or IR injury, CHIP−/− mice developed 50% larger infarcts, had a higher incidence of arrhythmias, decreased survival and a failure to upregulate Hsp70 in comparison to wild-type control mice [133]. There was also a dramatic increase in cardiomyocyte apoptosis in CHIP−/− in response to IR injury [133]. Subsequent investigations have revealed this is in part due to an accumulation of the tumour suppressor/pro-apoptotic, p53, in CHIP−/− hearts during IR [136]. Conversely, mice with cardiac-restricted CHIP overexpression were protected from MI as evidenced by a decreased infarct size, cardiomyocyte apoptosis, inflammation, fibrosis and heart weight to body weight compared with wild-type controls [136,137]. CHIP overexpression mice also exhibited improved cardiac function and a prolonged lifespan after suffering an MI [136,137]. Additionally, mice with CHIP overexpression showed increased post-MI angiogenesis in the heart [137]. Taken together, these data suggest that mechanisms increasing myocardial CHIP levels act as a novel therapeutic strategy for cardiac hypertrophy and myocardial ischaemia to improve cardiac function.
An emerging area of interest is the role CHIP has in regulating intracellular protein signalling. CHIP knockdown in HL-1 cells increased protein kinase B (Akt) phosphorylation at S473 at baseline and in response to IGF stimulation [130]. Akt was also phosphorylated at S473 more in CHIP knockout mice following cardiac pressure overload compared with their WT littermates [134]. Activation of the IGF-1/Akt signalling pathway induces the development of cardiac hypertrophy [138,139]. Interestingly, during stress, CHIP was shown to ubiquitinate Akt for degradation by the proteasome, thereby acting as a negative regulator of Akt signalling [140]. In response to cardiac pressure overload, there is an increase in AMP-activated protein kinase (AMPK) signalling in WT mice but not in CHIP−/− mice. AMPK and its protein substrate ACC are less phosphorylated in CHIP−/− mice during pressure overload, indicating decreased AMPK activity, despite an increased amount of LKB1, an upstream-positive regulator of AMPK [134]. The authors propose CHIP enhances LKB1 phosphorylation, thus activation, of AMPK during cardiac stress [134]. CHIP knockout/knockdown results in increased Akt activation and decreased AMPK activation. The mechanistic target of rapamycin (mTOR), an activated and essential protein kinase during cardiac hypertrophy [141], is by regulation by Akt (positively) and AMPK (negatively). Indeed, an enhancement of mTOR activity, as measured by phosphorylation of its protein substrates, was detected in CHIP−/− mouse hearts during cardiac hypertrophy [134].
In the heart, CHIP emerged as a protein containing a TPR domain (binding molecular chaperones) and ubiquitin ligase activity allowing it to connect the molecular chaperone system and the UPS. With these properties, CHIP was identified as a co-chaperone with regulatory functions in protein quality control (recognizing damaged/misfolded protein and ubiquitinating them to target proteasome-mediated degradation), a rheostat of cardiac disease mediating the pathogenesis of cardiac hypertrophy and myocardial ischaemia reperfusion injury, and as a regulator of AMPK sensor of cardiac stress (recognizing ATP depletion/increased AMP) and downstream AMPK-mediated intracellular signalling. While we have accumulated a vast array of knowledge about CHIP's functions, targets and role in cardiac disease, we have no information on how to pharmacologically affect CHIP functions. Discovering the mechanisms that regulate the activity or expression of CHIP within cardiomyocytes may translate the powerful cardioprotective function of CHIP to the clinic.
2. Bcl-2 associated athanogene 3
Bcl-2 associated athanogene 3 (BAG-3) is a member of a highly conserved, six-member family of anti-apoptotic BAG proteins, all of which are characterized by an approximately 40 kDa BAG domain located near the C-terminus. It is found predominantly in cardiac and skeletal muscle but also in subsidiary amounts in the brain, nervous system and cancer cells. As its name suggests, one of its first known binding partners is Bcl-2, with which it interacts to suppress apoptosis [142]. BAG proteins are involved in a variety of cellular mechanisms such as stress response, protein folding, apoptosis, autophagy, CMA/chaperone-assisted selective autophagy (CASA) and the UPS. BAG-3 has recently been the focus of an influx of research, as it is thought to be involved in a variety of disease models such as Alzheimer's disease [143], cancer [144–146], skeletal muscle/myofibrillar myopathies (MFM) [147–150] and heart failure [151–156]. This section will focus on what is known about the relationship between BAG-3 and HSP70, the implication of BAG-3 in heart disease and its role in protein quality control.
One of the first discoveries concerning BAG-3 was its role as a co-chaperone to HSP70/HSC70. BAG-1 was discovered by the interaction of its BAG domain and the ATPase domain of HSP/HSC 70 [157]. Of the BAG proteins, the relationship between BAG-1's four isoforms and HSP70 has been a large focus of study, with the inhibition or promotion of substrate release isoform dependent [158,159]. Along with BAG-2, BAG-4 and BAG-5, BAG-3 was discovered via a yeast-two hybrid screen using HSP70 as bait [160]. BAG-3 is now well known to be a co-chaperone to many different HSPs. The ability of BAG-3 to be such a successful co-chaperone is influenced by its various domains. In addition to the BAG domain, BAG-3 comprises a WW domain, a proline-rich region (PxxP) and two isoleucine–proline–valine (IPV) motifs (figure 7). The WW and PxxP domains are believed to facilitate complexes among BAG-3, HSP/HSC 70 and other proteins, while the IPV motifs mediate an interaction between BAG-3 and the small heat shock proteins (sHSP/HSPB8), which are involved in autophagy [161,162]. As HSPs are induced when the cell experiences stress, when exposed to heat and heavy metal exposure, HeLa cells exhibit an increase in expression of BAG-3 and HSP70, indicating that both chaperones play a role in protecting against cellular stress and that this stress response could be activated by stresses beyond temperature and heavy metals [163].
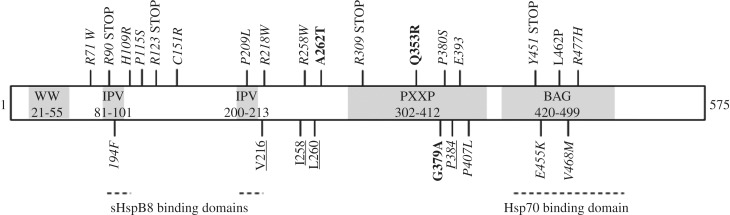
Figure 7.
Schematic of BAG-3 domains and mutations that have a potential DCM or MFM implication. Additional information on each mutation is detailed in table 2. Bold represents frameshift mutations, underlined represent deletions and italicized represent missense mutations. IPV, isoleucine–proline–valine.
BAG-3 and its interaction with HSP70 are critical for cardiac and skeletal muscle development and in vascular disease pathogenesis. Constitutive deletion of BAG-3 is not embryonic lethal, but results in multiple defects, including delayed growth compared with wild-type litter mates and premature death by day 25 [147]. BAG-3−/− mice also develop cardiomyopathy and non-inflammatory MFM [147]. While expression of BAG-3's two well-known binding partners Bcl-s and HSP70 do not change with decreased amounts of BAG-3, it can be inferred that without BAG-3, these proteins are unable to contribute to cell maintenance as they would in a control model [147]. From a clinical perspective, it was observed that patients with end stage heart failure have a decrease in BAG-3 in their heart tissue [154], while other studies have detected increased amounts of BAG-3 in the serum of heart failure patients, suggesting that cardiomyocytes release BAG-3 upon the influx of stress [164]. Additionally, BAG-3 has been implicated in the maintenance of the vasculature. BAG-3 is capable of evoking a dose-dependent vasorelaxation in resistance vessels, typically involved in blood pressure regulation [165]. The mechanism appears to be BAG-3's effect on the activation of the PI3 K/Akt signalling pathway in endothelial cells, leading to nitric oxide release [165]. Subsequent studies have found that BAG-3 is involved in advanced glycation end product (AGE)-induced proliferation and migration of vascular smooth muscle cells, implicated in the vascular dysfunction in diabetes [166]. AGEs were found to increase ROS production, promoting the proliferation and migration of vascular smooth muscle cells by increasing the expression of BAG-3 [166]. Together, these studies demonstrate a critical role for BAG-3 in both cardiac and skeletal muscle development and implicate BAG-3 in potentially having critical roles in vascular biology and disease.
An important study investigated the relationship between BAG-3 and HSC70 in a BAG-3 knockdown model [167]. Using rat neonatal ventricular cardiomyocytes (NVCM), BAG-3 was knocked down via shRNA and the cells were stretched to simulate mechanical stress. Increased stretch resulted in increased myofibrillar disarray and z-disc destabilization. Further, it was hypothesized that BAG-3 plays a role in maintaining structural stability by facilitating the bond between capZβ1, an actin capping protein, and Hsc70. It was observed that BAG-3 and Hsc70 formed a complex with capZβ1, promoting myofibril stability and facilitating the localization of HSC70 from the cytoplasm to the z-disc. In the absence of BAG-3 to localize capZβ1, it is degraded by the proteasome. In addition to being vital for myofilament structure, BAG-3 is also important for cellular contraction. In adult mouse, left ventricular myocytes BAG-3 localizes to the sarcolemma and t-tubules and acts to modulate myocyte contraction through its interactions with β1-andrenergic receptor and L-type Ca2+ channels [168]. Whether this represents a shift in BAG-3 signalling between neonatal and adult myocytes is not yet clear. Mutations in BAG-3 (P209 L) have resulted in a long QT phenotype, making it possible that BAG-3 acts to support ion channel expression/function (in addition to L-type Ca2+ channels) [169]. Further work is necessary to determine how BAG-3 and HSC70 signalling change as the cardiomyocyte matures, and what other proteins may be involved.
Upwards of 20 potentially pathogenic BAG-3 mutations have been discovered (table 2). About one-third of these are found in the BAG domain or cause a deletion or premature stop codon responsible for preventing transcription of most of the N-terminus (figure 7). While other mutations seem to have no known deleterious effects on the patient, many cause heart disease such as DCM, which presents as a heart with enlarged, thin-walled chambers and an inability to pump blood effectively [152,154,170,173–175]. Other mutations result in MFMs that may or may not be present with cardiomyopathies [148,151,152,171,172,176–179]. In many cases, a patient is initially diagnosed with MFM, but later develops and succumbs to issues stemming from heart failure. MFMs affect skeletal muscle, and result in progressive muscle weakness and atrophy. Mutations that cause this disease are not limited to BAG-3, and have been found in vital intermediate filament proteins such as desmin [180–188], the client protein of the chaperone αB-crystallin (encoded by HspB5) [189,190]. In many of these mutations that lead to cardiac and MFMs, sarcomere disorganization is characteristic. Given that contraction of a myocyte depends on the ability of filaments to slide past one another, a disruption in the localization of these myofilaments greatly and drastically impacts the cell, and as a result, the whole heart/skeletal muscle's ability to function properly. Contributing to impeded contraction, these myopathies are characterized by aggregations of proteins such as desmin, αB-crystallin, dystrophin and many others that are indicative of myofilament disintegration [171].
Table 2.
A list of known BAG-3 mutations, the type of mutation and the disease they were associated with.
| mutation | type of mutation | associated disease | reference |
|---|---|---|---|
| R71 W | missense | DCM | Norton et al. [151] |
| R90 STOP | missense | DCM | Norton et al. [151] |
| I94F | missense | DCM | Villard et al. [170] |
| H109R | missense | DCM | Norton et al. [151] |
| P115S | missense | DCM | Villard et al. [170] |
| R123 STOP | missense | DCM | Norton et al. [151] |
| C151R | missense | DCM | Villard et al. [170] |
| P209 L | missense | MFM | Selcen et al. [171] |
| V216 | deletion | DCM | Norton et al. [151] |
| R218 W | missense | DCM | Arimura et al. [152] |
| I258 | deletion | DCM | Villard et al. [170] |
| R258 W | missense | MFM | Lee et al. [172] |
| L260 | deletion | MFM | Sato et al. [149] |
| A262T | missense | DCM | Norton et al. [151] |
| R309 STOP | missense | DCM | Villard et al. [170] |
| Q353R | frameshift | DCM | Franaszczyk et al. [173] |
| G379A | frameshift | DCM | Franaszczyk et al. [173] |
| P380S | missense | DCM | Villard et al. [170] |
| P384 | deletion | DCM | Villard et al. [170] |
| E393 | deletion | DCM | Villard et al. [170] |
| P407 L | missense | DCM | Villard et al. [170] |
| Y451 STOP | missense | DCM | Franaszczyk et al. [173] |
| E455 K | missense | DCM | Villard et al. [170] |
| L462P | missense | DCM | Arimura et al. [152] |
| V468M | missense | DCM | Villard et al. [170] |
| R477H | missense | DCM | Norton et al. [151] |
One particular mutation, Pro209Leu, causes severe MFM and hypertrophic/restrictive cardiomyopathy, often presenting in early childhood [171]. This mutation has been investigated in multiple models, all of which have slightly differing phenotypes. It is hypothesized that the difference in phenotype seen among human, cell and animal models is due to different amounts of induced Pro209Leu mutant expression [179]. When investigating muscle biopsies of patients with this mutation, BAG-3 was discovered to remain localized at the z-disc regions despite its disintegration and large protein deposits. There was a severe decrease in both BAG-3 and HSP70 compared with control patients [169], indicating that BAG-3 and Hsp70 are important for z-disc maintenance. By contrast, when this mutation was expressed in neonatal rat cardiomyocytes and cardiomyocyte cell line H9c2s, there were no reductions in function compared with control cells. However, when expressed in the skeletal muscle cell line C2C12, this mutation affected the multi-nucleation of the cells into myotubes [152]. This study concluded that this mutation does not affect cardiomyocyte function, but affects cellular development. When put in the context of an inducible myocyte-specific transgenic mouse model [179,191], the Pro209 L mutation induces left ventricular dysfunction indicated by depressed fractional shortening. As seen in human patients, there were protein accumulations identified as pre-amyloid oligomers, a condition often observed in HSP mutations, which is unsurprising given that this mutation occurs in the IPV domain of BAG-3, which is known to be vital for interaction with HSPB proteins [191,192]. In addition, wild-type BAG-3 becomes haploinsufficient, and there is an increase in p38 signalling, all of which can be hypothesized to contribute to heart failure [179]. This study also saw an alteration in mitochondrial dynamics with the expression of mutant BAG-3. Wild-type BAG-3 and HSP70 have been linked to mitochondrial quality control in cardiomyocytes, with a knockdown in BAG-3 resulting in reduced mitophagy, lending itself to the hypothesis that BAG-3 is necessary to maintain mitochondrial homeostasis under cellular stresses induced with disease [193].
BAG-3 has been hypothesized to be involved in protein quality control in macroautophagy, CMA and the UPS. Many studies have attributed the onset of disease to a failure of one or more of these systems [194–196]. While not specific to the heart, the role of BAG-3 in autophagy and the UPS is starting to be elucidated. It has been hypothesized that under normal physiological conditions, BAG-1-HSP70 are mainly responsible for degrading misfolded proteins via the UPS. During ageing or pathological stress, BAG-3 begins interacting with HSP70 and starts clearing these proteins via this CMA. This change in degradation pathways has been referred to as a ‘BAG-1/BAG-3 switch’ [196,197].
Several studies have investigated the relationship among BAG-3, Hsp70 and HspB8 in macroautophagy and the UPS to degrade misfolded substrates [131,197,198]. This BAG-3 complex interacts with the microtubule motor dynein and contributes to shuttling misfolded and aggregated proteins to the aggresome, a perinuclear compartment where degradation substrates are sequestered [198]. When BAG-3 is blocked from binding with dynein, aggregation-prone proteins are ineffectively degraded and are connected with neurodegenerative diseases such as ALS [199].
As some cardiomyopathies and MFMs involved the accumulation of misfolded proteins, an alteration to any protein quality control system may contribute to this accumulation. A mutation in the small HSP αβ-crystallin (CryAB) gene has been attributed to MFM [190,200] and overexpression of this mutation leads to toxic oligomers detected in the heart [7,201]. It was observed that BAG-3 forms a complex with CryAB to inhibit protein aggregation and suppress cell death caused by a CryAB mutant [202]. HSP70 is known to bind to CHIP to inhibit aggregation and it is thought that there may be a connection between the BAG-3 and HSP–CHIP complexes to cooperatively inhibit aggregation of mutant CryAB. This relationship is more fully investigated in 1(c).
3. Therapeutic implications: extracellular HSP70, HSP90 and BAG-3 in cardiovascular disease
There is limited information currently on the role of extracellular HSPs in the pathogenesis of heart failure, with the entirety of this review focusing on intracellular HSPs. Therapeutics to increase HSPs would likely be most applicable in acute ischaemic heart diseases. However, more chronic diseases (e.g., hypertension) may not be helpful. For example, while increasing HSP70 in the heart protects against acute ischaemia reperfusion injury, elevated HSP70 in transgenic animals accelerated heart failure in settings of pressure overload or DCM [56,57]. That said, there are pharmacological options that increase intracellular HSP70 (e.g., carbenoxolone, 2-cyclopenten-1-one) that have been primarily tested in cancers and infections, as recently reviewed [50].
Extracellular HSP70 has been identified as an independent prognostic marker of mortality in patients with heart failure [203] and found to be an independent prognostic marker of cardiac arrest patients [204]. In the development of hypertension-induced cardiac hypertrophy and fibrosis, extracellular HSP70 has been identified. Interestingly, HSP70 is a ligand for damage-associated molecular pattern receptors (DAMPS), which can induce inflammation in the myocardium [205]. In hypertensive animals, HSP70 was found elevated [205]. Transcriptional inhibition of (intracellular) HSP70 promoted cardiomyocyte hypertrophy and dysfunction while protecting animals from cardiac fibrosis development [205]. Conversely, inhibiting extracellular HSP70 with anti-HSP70 antibodies attenuated hypertension-induced cardiac hypertrophy and fibrosis without affecting haemodynamics [205]. The cardioprotective response was thought to be due to the inhibition of the extracellular interaction with DAMPs, reducing infiltrating macrophages, decreasing pro-inflammatory factors such as MCP-1 (monocyte chemoattractant protein-1) and the profibrotic TGFβ1, attenuating p38 and ERK MAPK signalling pathways [205].
Extracellular HSP90 has recently been described in the context of the heart. During the development of pathological cardiac hypertrophy, increases in left ventricular mass occur in parallel with extracellular matrix deposition, leading to fibrosis and heart failure. The major mediator of this process is cardiomyocyte-derived transforming growth factor beta (TGFβ), which is secreted by cardiomyocytes to act upon fibroblasts, which then secrete collagen. The extracellular HSP90 seems to play a role in the stabilization of TGFβ signalling by stabilizing the TGFβ cascade [116]. In these studies, extracellular HSP90 was identified complexed with fibroblast TGFβ receptor, with in silico studies predicting that HSP90 dimers and the TGFβ receptor domain interact [116]. Both Hsp90aa1 and Hsp90ab1 isoforms were identified in complex with the TGFβ receptor. Inhibiting extracellular HSP90 decreased collagen production and stimulation of the canonical TGFβ signalling pathway [116]. As fibrosis is a major contributor to the aetiology of many chronic heart diseases including ischaemic heart disease and heart failure, targeting extracellular HSP90 may be a therapeutic consideration in the future, with functions distinctly different from those found intracellularly.
Like HSP70 and HSP90, elevated circulating extracellular BAG-3 has recently been identified in patients with advanced heart failure, suggesting a useful biomarker in monitoring heart failure progression [155,164,206]. Subsequent studies investigated the possible role of elevated BAG-3 in regulating haemodynamics [165]. In these studies, BAG-3 was found to evoke a dose-dependent vasorelaxation, exerting its effects on resistance vessels typically involved in blood pressure regulation [165]. BAG-3's effects were through activation of PI3 K/Akt signalling, resulting in endothelial cell release of nitric oxide [165]. While complementary to the studies of intracellular BAG-3, the increases in extracellular BAG-3 seen in heart failure may be one previously unrecognized compensatory mechanism in regulating blood pressure. By contributing to increased endothelial cell NO release, extracellular BAG-3 may have therapeutic value regulating blood pressure outside of its growing important repertoire of intracellular functions in cardiovascular disease.
4. Conclusion
Thematically, genetic regulation of the expression of intracellular HSPs and their co-chaperones has revealed their critical roles in supporting protein stability (with anti-aggregate activities) and signalling pathways in the heart. Therapeutically enhancing intracellular HSP activities by drugs on the market (e.g., geranylgeranylacetone, an anti-ulcer medicine supporting HSP70) and protecting against MI experimentally [207] may be one way in which our current knowledge can be applied soon. In addition, emerging evidence for extracellular HSP70, HSP90 and BAG-3's role in mechanistically regulating the pathophysiology of disease is revealing important new pathways for heart failure diagnosis/prognosis and possibly therapy. However, a better understanding of how both intracellular and extracellular HSPs mediate disease will be necessary to best optimize therapies for specific diseases and stages, given the plethora of contributors to protein misfolding and proteinopathies (including those caused by mutations) in heart failure.
Data accessibility
This article has no additional data.
Competing interests
The authors do not have competing interests to report.
Funding
M.J.R. is supported by funding from the American Heart Association (no. 16POST29090003). J.A.K. is funded by the American Heart Association (14SDG20380148) and the National Institutes of Health (R01HL136737). M.S.W. receives funding from the National Institutes of Health (RO1HL104129) and the Leducq Foundation (11CVD04).
References
- 1.WHO. 2017. Cardiovascular diseases (CVDs) Fact Sheet. See http://www.who.int/mediacentre/factsheets/fs317/en/. (accessed 8 July 2017).
- 2.Writing GM, et al. 2016. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation 133, e38–360. ( 10.1161/CIR.0000000000000350) [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, et al. 2011. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944. ( 10.1161/CIR.0b013e31820a55f5) [DOI] [PubMed] [Google Scholar]
- 4.Ni H, Xu J. 2015. Recent trends in heart failure-related mortality: United States, 2000–2014. NCHS Data Brief. 231, 1–8. [PubMed] [Google Scholar]
- 5.Willis MS, Patterson C. 2013. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N. Engl. J. Med. 368, 455–464. ( 10.1056/NEJMra1106180) [DOI] [PubMed] [Google Scholar]
- 6.Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. 2008. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation 117, 2743–2751. ( 10.1161/CIRCULATIONAHA.107.750232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. 2004. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc. Natl Acad. Sci. USA 101, 10 132–10 136. ( 10.1073/pnas.0401900101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarone G, Brancaccio M. 2014. Keep your heart in shape: molecular chaperone networks for treating heart disease. Cardiovasc. Res. 102, 346–361. ( 10.1093/cvr/cvu049) [DOI] [PubMed] [Google Scholar]
- 9.Willis MS, Schisler JC, Portbury AL, Patterson C. 2009. Build it up–tear it down: protein quality control in the cardiac sarcomere. Cardiovasc. Res. 81, 439–448. ( 10.1093/cvr/cvn289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pockley AG, Henderson B, Multhoff G. 2014. Extracellular cell stress proteins as biomarkers of human disease. Biochem. Soc. Trans. 42, 1744–1751. ( 10.1042/BST20140205) [DOI] [PubMed] [Google Scholar]
- 11.Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, Zilch T, Multhoff G. 2005. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 12, 38–51. ( 10.1038/sj.cdd.4401510) [DOI] [PubMed] [Google Scholar]
- 12.Hantschel M, Pfister K, Jordan A, Scholz R, Andreesen R, Schmitz G, Schmetzer H, Hiddemann W, Multhoff G. 2000. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 5, 438–442. ( 10.1379/1466-1268(2000)005%3C0438:HPMEOP%3E2.0.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. 1995. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 61, 272–279. ( 10.1002/ijc.2910610222) [DOI] [PubMed] [Google Scholar]
- 14.Multhoff G, Hightower LE. 1996. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones 1, 167–176. ( 10.1379/1466-1268(1996)001%3C0167:CSEOHS%3E2.3.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tissieres A, Mitchell HK, Tracy UM. 1974. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J. Mol. Biol. 84, 389–398. ( 10.1016/0022-2836(74)90447-1) [DOI] [PubMed] [Google Scholar]
- 16.Lindquist S, Craig EA. 1988. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677. ( 10.1146/annurev.ge.22.120188.003215) [DOI] [PubMed] [Google Scholar]
- 17.Tavaria M, Gabriele T, Kola I, Anderson RL. 1996. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones 1, 23–28. ( 10.1379/1466-1268(1996)001%3C0023:AHSGTT%3E2.3.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daugaard M, Rohde M, Jaattela M. 2007. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581, 3702–3710. ( 10.1016/j.febslet.2007.05.039) [DOI] [PubMed] [Google Scholar]
- 19.Wu B, Hunt C, Morimoto R. 1985. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol. Cell Biol. 5, 330–341. ( 10.1128/MCB.5.2.330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goate AM, Cooper DN, Hall C, Leung TK, Solomon E, Lim L. 1987. Localization of a human heat-shock HSP 70 gene sequence to chromosome 6 and detection of two other loci by somatic-cell hybrid and restriction fragment length polymorphism analysis. Hum. Genet. 75, 123–128. ( 10.1007/BF00591072) [DOI] [PubMed] [Google Scholar]
- 21.Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, Weber JL, Patterson D, Schellenberg GD. 1994. Cloning, sequencing, mapping of the human chromosome 14 heat shock protein gene (HSPA2). Genomics 23, 85–93. ( 10.1006/geno.1994.1462) [DOI] [PubMed] [Google Scholar]
- 22.Munro S, Pelham HR. 1986. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46, 291–300. ( 10.1016/0092-8674(86)90746-4) [DOI] [PubMed] [Google Scholar]
- 23.Leung TK, Rajendran MY, Monfries C, Hall C, Lim L. 1990. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B') and isolation of its cDNA and genomic DNA. Biochem. J. 267, 125–132. ( 10.1042/bj2670125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworniczak B, Mirault ME. 1987. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res. 15, 5181–5197. ( 10.1093/nar/15.13.5181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domanico SZ, DeNagel DC, Dahlseid JN, Green JM, Pierce SK. 1993. Cloning of the gene encoding peptide-binding protein 74 shows that it is a new member of the heat shock protein 70 family. Mol. Cell Biol. 13, 3598–3610. ( 10.1128/MCB.13.6.3598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. 1995. Cloning and subcellular localization of human mitochondrial hsp70. J. Biol. Chem. 270, 1705–1710. ( 10.1074/jbc.270.4.1705) [DOI] [PubMed] [Google Scholar]
- 27.Flaherty KM, DeLuca-Flaherty C, McKay DB. 1990. Three-dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature 346, 623–628. ( 10.1038/346623a0) [DOI] [PubMed] [Google Scholar]
- 28.Hartl FU. 1996. Molecular chaperones in cellular protein folding. Nature 381, 571–579. ( 10.1038/381571a0) [DOI] [PubMed] [Google Scholar]
- 29.Finka A, Sharma SK, Goloubinoff P. 2015. Multi-layered molecular mechanisms of polypeptide holding, unfolding and disaggregation by HSP70/HSP110 chaperones. Front. Mol. Biosci. 2, 29 ( 10.3389/fmolb.2015.00029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma SK, De los Rios P, Christen P, Lustig A, Goloubinoff P. 2010. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat. Chem. Biol. 6, 914–920. ( 10.1038/nchembio.455) [DOI] [PubMed] [Google Scholar]
- 31.Frydman J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647. ( 10.1146/annurev.biochem.70.1.603) [DOI] [PubMed] [Google Scholar]
- 32.Hohfeld J, Cyr DM, Patterson C. 2001. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2, 885–890. ( 10.1093/embo-reports/kve206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez MP, Chadli A, Toft DO. 2002. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 277, 11 873–11 881. ( 10.1074/jbc.M111445200) [DOI] [PubMed] [Google Scholar]
- 34.Goffin L, Georgopoulos C. 1998. Genetic and biochemical characterization of mutations affecting the carboxy-terminal domain of the Escherichia coli molecular chaperone DnaJ. Mol. Microbiol. 30, 329–340. ( 10.1046/j.1365-2958.1998.01067.x) [DOI] [PubMed] [Google Scholar]
- 35.Szabo A, Korszun R, Hartl FU, Flanagan J. 1996. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 15, 408–417. [PMC free article] [PubMed] [Google Scholar]
- 36.Clare DK, Saibil HR. 2013. ATP-driven molecular chaperone machines. Biopolymers 99, 846–859. ( 10.1002/bip.22361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene MK, Maskos K, Landry SJ. 1998. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc. Natl Acad. Sci. USA 95, 6108–6113. ( 10.1073/pnas.95.11.6108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michels AA, Kanon B, Konings AW, Ohtsuka K, Bensaude O, Kampinga HH. 1997. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J. Biol. Chem. 272, 33 283–33 289. ( 10.1074/jbc.272.52.33283) [DOI] [PubMed] [Google Scholar]
- 39.Ungermann C, Neupert W, Cyr DM. 1994. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science 266, 1250–1253. ( 10.1126/science.7973708) [DOI] [PubMed] [Google Scholar]
- 40.Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. 1994. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370, 111–117. ( 10.1038/370111a0) [DOI] [PubMed] [Google Scholar]
- 41.Bukau B, Horwich AL. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92, 351–366. ( 10.1016/S0092-8674(00)80928-9) [DOI] [PubMed] [Google Scholar]
- 42.Afzal AR, Mandal K, Nyamweya S, Foteinos G, Poloniecki J, Camm AJ, Jahangiri M, Xu Q. 2008. Association of Met439Thr substitution in heat shock protein 70 gene with postoperative atrial fibrillation and serum HSP70 protein levels. Cardiology 110, 45–52. ( 10.1159/000109406) [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Robbins J. 2014. Proteasomal and lysosomal protein degradation and heart disease. J. Mol. Cell Cardiol. 71, 16–24. ( 10.1016/j.yjmcc.2013.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi L, Zhang XD, Wu JC, Lin F, Wang J, DiFiglia M, Qin ZH. 2012. The role of chaperone-mediated autophagy in huntingtin degradation. PLoS ONE 7, e46834 ( 10.1371/journal.pone.0046834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammack LJ, Firestone K, Chang W, Kusmierczyk AR. 2017. Molecular chaperones of the Hsp70 family assist in the assembly of 20S proteasomes. Biochem. Biophys. Res. Commun. 486, 438–443. ( 10.1016/j.bbrc.2017.03.059) [DOI] [PubMed] [Google Scholar]
- 46.de Jong PR, Schadenberg AW, Jansen NJ, Prakken BJ. 2009. Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones 14, 117–131. ( 10.1007/s12192-008-0066-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knowlton AA, Brecher P, Apstein CS. 1991. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J. Clin. Invest. 87, 139–147. ( 10.1172/JCI114963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian YZ, Bernardo NL, Nayeem MA, Chelliah J, Kukreja RC. 1999. Induction of 72-kDa heat shock protein does not produce second window of ischemic preconditioning in rat heart. Am. J. Physiol. 276(1 Pt 2), H224–H234. [DOI] [PubMed] [Google Scholar]
- 49.Chen HS, et al. 2006. Downregulation of the constitutively expressed Hsc70 in diabetic myocardium is mediated by insulin deficiency. J. Endocrinol. 190, 433–440. ( 10.1677/joe.1.06692) [DOI] [PubMed] [Google Scholar]
- 50.Bernardo BC, Weeks KL, Patterson NL, McMullen JR. 2016. HSP70: therapeutic potential in acute and chronic cardiac disease settings. Future Med. Chem. 8, 2177–2183. ( 10.4155/fmc-2016-0192) [DOI] [PubMed] [Google Scholar]
- 51.Mandal K, Torsney E, Poloniecki J, Camm AJ, Xu Q, Jahangiri M. 2005. Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann. Thorac. Surg. 79, 865–871; discussion 71 ( 10.1016/j.athoracsur.2004.08.018) [DOI] [PubMed] [Google Scholar]
- 52.St Rammos K, Koullias GJ, Hassan MO, Argyrakis NP, Voucharas CG, Scarupa SJ, Cowte TG. 2002. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc. Surg. 10, 228–232. ( 10.1016/S0967-2109(01)00138-7) [DOI] [PubMed] [Google Scholar]
- 53.Murry CE, Jennings RB, Reimer KA. 1986. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74, 1124–1136. ( 10.1161/01.CIR.74.5.1124) [DOI] [PubMed] [Google Scholar]
- 54.Lochner A, Marais E, Genade S, Huisamen B, du Toit EF, Moolman JA. 2009. Protection of the ischaemic heart: investigations into the phenomenon of ischaemic preconditioning. Cardiovasc. J. Afr. 20, 43–51. [PMC free article] [PubMed] [Google Scholar]
- 55.Goussetis D, Mestril R. 2006. Experimental biology. FASEB J. 20(4), A119. [Google Scholar]
- 56.Weeks KL, et al. 2012. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3 K gene therapy rescues cardiac dysfunction. Circ. Heart Fail. 5, 523–534. ( 10.1161/CIRCHEARTFAILURE.112.966622) [DOI] [PubMed] [Google Scholar]
- 57.Sapra G, et al. 2014. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 5, 5705 ( 10.1038/ncomms6705) [DOI] [PubMed] [Google Scholar]
- 58.Melling CW, Thorp DB, Milne KJ, Noble EG. 2009. Myocardial Hsp70 phosphorylation and PKC-mediated cardioprotection following exercise. Cell Stress Chaperones 14, 141–150. ( 10.1007/s12192-008-0065-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, Shanely RA, Jessup J. 1998. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am. J. Physiol. 275, R1468–R1477. [DOI] [PubMed] [Google Scholar]
- 60.Xu T, Zhang B, Yang F, Cai C, Wang G, Han Q, Zou L. 2015. HSF1 and NF-κB p65 participate in the process of exercise preconditioning attenuating pressure overload-induced pathological cardiac hypertrophy. Biochem. Biophys. Res. Commun. 460, 622–627. ( 10.1016/j.bbrc.2015.03.079) [DOI] [PubMed] [Google Scholar]
- 61.Lee BJ, Emery-Sinclair EL, Mackenzie RW, Hussain A, Taylor L, James RS, Thake CD. 2014. The impact of submaximal exercise during heat and/or hypoxia on the cardiovascular and monocyte HSP72 responses to subsequent (post 24 h) exercise in hypoxia. Extrem. Physiol. Med. 3, 15 ( 10.1186/2046-7648-3-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. 2007. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J. Appl. Physiol. 103, 1056–1062. ( 10.1152/japplphysiol.00263.2007) [DOI] [PubMed] [Google Scholar]
- 63.Marber MS, Latchman DS, Walker JM, Yellon DM. 1993. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88, 1264–1272. ( 10.1161/01.CIR.88.3.1264) [DOI] [PubMed] [Google Scholar]
- 64.Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. 2001. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am. J. Physiol. Heart Circ. Physiol. 281, H1346–H1352. [DOI] [PubMed] [Google Scholar]
- 65.Harris MB, Starnes JW. 2001. Effects of body temperature during exercise training on myocardial adaptations. Am. J. Physiol. Heart Circ. Physiol. 280, H2271–H2280. [DOI] [PubMed] [Google Scholar]
- 66.Taylor RP, Harris MB, Starnes JW. 1999. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am. J. Physiol. 276, H1098–H1102. [DOI] [PubMed] [Google Scholar]
- 67.Willis MS, Patterson C. 2010. Hold me tight: role of the heat shock protein family of chaperones in cardiac disease. Circulation 122, 1740–1751. ( 10.1161/CIRCULATIONAHA.110.942250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Su H, Ranek MJ. 2008. Protein quality control and degradation in cardiomyocytes. J. Mol. Cell Cardiol. 45, 11–27. ( 10.1016/j.yjmcc.2008.03.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parry TL, Melehani JH, Ranek MJ, Willis MS. 2015. Functional amyloid signaling via the inflammasome, necrosome, signalosome: new therapeutic targets in heart failure. Front. Cardiovasc. Med. 2, 25 ( 10.3389/fcvm.2015.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. 1995. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J. Clin. Invest. 95, 1854–1860. ( 10.1172/JCI117865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. 1995. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Invest. 95, 1446–1456. ( 10.1172/JCI117815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH. 1998. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J. Clin. Invest. 101, 855–862. ( 10.1172/JCI265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radford NB, et al. 1996. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc. Natl Acad. Sci. USA 93, 2339–2342. ( 10.1073/pnas.93.6.2339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yellon DM, Pasini E, Cargnoni A, Marber MS, Latchman DS, Ferrari R. 1992. The protective role of heat stress in the ischaemic and reperfused rabbit myocardium. J. Mol. Cell Cardiol. 24, 895–907. ( 10.1016/0022-2828(92)91102-B) [DOI] [PubMed] [Google Scholar]
- 75.Donnelly TJ, Sievers RE, Vissern FL, Welch WJ, Wolfe CL. 1992. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation 85, 769–778. ( 10.1161/01.CIR.85.2.769) [DOI] [PubMed] [Google Scholar]
- 76.Currie RW, Tanguay RM, Kingma JG Jr. 1993. Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation 87, 963–971. ( 10.1161/01.CIR.87.3.963) [DOI] [PubMed] [Google Scholar]
- 77.Nakano M, Mann DL, Knowlton AA. 1997. Blocking the endogenous increase in HSP 72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation 95, 1523–1531. ( 10.1161/01.CIR.95.6.1523) [DOI] [PubMed] [Google Scholar]
- 78.Hutter MM, Sievers RE, Barbosa V, Wolfe CL. 1994. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation 89, 355–360. ( 10.1161/01.CIR.89.1.355) [DOI] [PubMed] [Google Scholar]
- 79.Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. 2001. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation 103, 877–881. ( 10.1161/01.CIR.103.6.877) [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Jiang DS, Yan L, Cheng KJ, Bian ZY, Lin GS. 2011. HSP75 protects against cardiac hypertrophy and fibrosis. J. Cell Biochem. 112, 1787–1794. ( 10.1002/jcb.23091) [DOI] [PubMed] [Google Scholar]
- 81.Bedin M, Gaben AM, Saucier C, Mester J. 2004. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest. Int. J. Cancer 109, 643–652. ( 10.1002/ijc.20010) [DOI] [PubMed] [Google Scholar]
- 82.Pałyga J, Kozłowski Ł. 2007. Structure and function of molecular chaperone HSP90. Sowriemiennyj Naucznyj Wiestnik Ser Biologija Chimija. 15, 46–65. [Google Scholar]
- 83.Zhao R, et al. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727. ( 10.1016/j.cell.2004.12.024) [DOI] [PubMed] [Google Scholar]
- 84.Pratt WB, Toft DO. 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228, 111–133. ( 10.1177/153537020322800201) [DOI] [PubMed] [Google Scholar]
- 85.Picard D. 2017. HSP90 Interactors. See https://www.picard.ch/downloads/Hsp90interactors.pdf (Accessed July 8, 2017).
- 86.Wegele H, Muller L, Buchner J. 2004. Hsp70 and Hsp90—a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 151, 1–44. ( 10.1007/s10254-003-0021-1) [DOI] [PubMed] [Google Scholar]
- 87.Pearl LH, Prodromou C. 2006. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75, 271–294. ( 10.1146/annurev.biochem.75.103004.142738) [DOI] [PubMed] [Google Scholar]
- 88.Sreedhar AS, Kalmar E, Csermely P, Shen YF. 2004. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 562, 11–15. ( 10.1016/S0014-5793(04)00229-7) [DOI] [PubMed] [Google Scholar]
- 89.Young JC, Moarefi I, Hartl FU. 2001. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154, 267–273. ( 10.1083/jcb.200104079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grammatikakis N, Vultur A, Ramana CV, Siganou A, Schweinfest CW, Watson DK, Raptis L. 2002. The role of Hsp90N, a new member of the Hsp90 family, in signal transduction and neoplastic transformation. J. Biol. Chem. 277, 8312–8320. ( 10.1074/jbc.M109200200) [DOI] [PubMed] [Google Scholar]
- 91.Picard D. 2002. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol. Life Sci. 59, 1640–1648. ( 10.1007/PL00012491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishizawa J, Nakai A, Higashi T, Tanabe M, Nomoto S, Matsuda K, Ban T, Nagata K. 1996. Reperfusion causes significant activation of heat shock transcription factor 1 in ischemic rat heart. Circulation 94, 2185–2192. ( 10.1161/01.CIR.94.9.2185) [DOI] [PubMed] [Google Scholar]
- 93.Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. 1999. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation 99, 934–941. ( 10.1161/01.CIR.99.7.934) [DOI] [PubMed] [Google Scholar]
- 94.Chang J, Knowlton AA, Xu F, Wasser JS. 2001. Activation of the heat shock response: relationship to energy metabolites. A (31)P NMR study in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 280, H426–H433. [DOI] [PubMed] [Google Scholar]
- 95.Caplan AJ. 1999. Hsp90's secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 9, 262–268. ( 10.1016/S0962-8924(99)01580-9) [DOI] [PubMed] [Google Scholar]
- 96.Cyr DM, Hohfeld J, Patterson C. 2002. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 27, 368–375. ( 10.1016/S0968-0004(02)02125-4) [DOI] [PubMed] [Google Scholar]
- 97.Mattoo RU, Goloubinoff P. 2014. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol. Life Sci. 71, 3311–3325. ( 10.1007/s00018-014-1627-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frey N, Olson EN. 2003. Cardiac hypertrophy: the good, the bad, the ugly. Annu. Rev. Physiol. 65, 45–79. ( 10.1146/annurev.physiol.65.092101.142243) [DOI] [PubMed] [Google Scholar]
- 99.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. 2010. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128, 191–227. ( 10.1016/j.pharmthera.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 100.Shimizu I, Minamino T. 2016. Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 97, 245–262. ( 10.1016/j.yjmcc.2016.06.001) [DOI] [PubMed] [Google Scholar]
- 101.Cenni V, Sirri A, Riccio M, Lattanzi G, Santi S, de Pol A, Maraldi NM, Marmiroli S. 2003. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol. Life Sci. 60, 2710–2720. ( 10.1007/s00018-003-3349-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abeyrathna P, Su Y. 2015. The critical role of Akt in cardiovascular function. Vascul. Pharmacol. 74, 38–48. ( 10.1016/j.vph.2015.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato S, Fujita N, Tsuruo T. 2000. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl Acad. Sci. USA 97, 10 832–10 837. ( 10.1073/pnas.170276797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. 2001. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J. Biol. Chem. 276, 32 663–32 669. ( 10.1074/jbc.M101371200) [DOI] [PubMed] [Google Scholar]
- 105.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. 2001. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ. Res. 89, 866–873. ( 10.1161/hh2201.100319) [DOI] [PubMed] [Google Scholar]
- 106.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. 2002. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 90, 866–873. ( 10.1161/01.RES.0000016837.26733.BE) [DOI] [PubMed] [Google Scholar]
- 107.Zhao XH, Peng YZ, Wang YY, Huang YS. 2007. Influence of heat shock protein 90 on protein serine threonine kinases expression in hypoxic cardiomyocytes. Zhonghua Shao Shang Za Zhi. 23, 265–268. [PubMed] [Google Scholar]
- 108.Downward J. 1999. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1, E33–E35. ( 10.1038/10026) [DOI] [PubMed] [Google Scholar]
- 109.Negro A, Brar BK, Lee KF. 2004. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 59, 1–12. ( 10.1210/rp.59.1.1) [DOI] [PubMed] [Google Scholar]
- 110.Chan HW, Jenkins A, Pipolo L, Hannan RD, Thomas WG, Smith NJ. 2006. Effect of dominant-negative epidermal growth factor receptors on cardiomyocyte hypertrophy. J. Recept. Signal Transduct. Res. 26, 659–677. ( 10.1080/10799890600923187) [DOI] [PubMed] [Google Scholar]
- 111.Slotta-Huspenina J, Becker KF, Feith M, Walch A, Langer R. 2014. Heat Shock Protein 90 (HSP90) and Her2 in Adenocarcinomas of the Esophagus. Cancers 6, 1382–1393. ( 10.3390/cancers6031382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drecoll E, Nitsche U, Bauer K, Berezowska S, Slotta-Huspenina J, Rosenberg R, Langer R. 2014. Expression analysis of heat shock protein 90 (HSP90) and Her2 in colon carcinoma. Int. J. Colorectal Dis. 29, 663–671. ( 10.1007/s00384-014-1857-3) [DOI] [PubMed] [Google Scholar]
- 113.Berezowska S, Novotny A, Bauer K, Feuchtinger A, Slotta-Huspenina J, Becker K, Langer R, Walch A. 2013. Association between HSP90 and Her2 in gastric and gastroesophageal carcinomas. PLoS ONE 8, e69098 ( 10.1371/journal.pone.0069098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartha E, et al. 2011. Regulation of kinase cascade activation and heat shock protein expression by poly(ADP-ribose) polymerase inhibition in doxorubicin-induced heart failure. J. Cardiovasc. Pharmacol. 58, 380–391. ( 10.1097/FJC.0b013e318225c21e) [DOI] [PubMed] [Google Scholar]
- 115.Ke X, et al. 2017. Heat shock protein 90/Akt pathway participates in the cardioprotective effect of exogenous hydrogen sulfide against high glucose-induced injury to H9c2 cells. Int. J. Mol. Med. 39, 1001–1010. ( 10.3892/ijmm.2017.2891) [DOI] [PubMed] [Google Scholar]
- 116.Garcia R, Merino D, Gomez JM, Nistal JF, Hurle MA, Cortajarena AL, Villar AV. 2016. Extracellular heat shock protein 90 binding to TGFβ receptor I participates in TGFβ-mediated collagen production in myocardial fibroblasts. Cell. Signal. 28, 1563–1579. ( 10.1016/j.cellsig.2016.07.003) [DOI] [PubMed] [Google Scholar]
- 117.Datta R, Bansal T, Rana S, Datta K, Datta Chaudhuri R, Chawla-Sarkar M, Sarkar S. 2017. Myocyte-derived Hsp90 modulates collagen upregulation via biphasic activation of STAT-3 in fibroblasts during cardiac hypertrophy. Mol. Cell Biol. 37, e00611 ( 10.1128/MCB.00611-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Delisle BP, Anson BD, Rajamani S, January CT. 2004. Biology of cardiac arrhythmias: ion channel protein trafficking. Circ. Res. 94, 1418–1428. ( 10.1161/01.RES.0000128561.28701.ea) [DOI] [PubMed] [Google Scholar]
- 119.Ficker E, Dennis AT, Wang L, Brown AM. 2003. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 92, e87–e100. ( 10.1161/01.RES.0000079028.31393.15) [DOI] [PubMed] [Google Scholar]
- 120.Dennis A, Wang L, Wan X, Ficker E. 2007. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem. Soc. Trans. 35, 1060–1063. ( 10.1042/BST0351060) [DOI] [PubMed] [Google Scholar]
- 121.Murata S, Chiba T, Tanaka K. 2003. CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int. J. Biochem. Cell Biol. 35, 572–578. ( 10.1016/S1357-2725(02)00394-1) [DOI] [PubMed] [Google Scholar]
- 122.McClellan AJ, Frydman J. 2001. Molecular chaperones and the art of recognizing a lost cause. Nat. Cell Biol. 3, E51–E53. ( 10.1038/35055162) [DOI] [PubMed] [Google Scholar]
- 123.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell Biol. 19, 4535–4545. ( 10.1128/MCB.19.6.4535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kettern N, Dreiseidler M, Tawo R, Hohfeld J. 2010. Chaperone-assisted degradation: multiple paths to destruction. Biol. Chem. 391, 481–489. ( 10.1515/bc.2010.058) [DOI] [PubMed] [Google Scholar]
- 125.Aravind L, Koonin EV. 2000. The U box is a modified RING finger—a common domain in ubiquitination. Curr. Biol. 10, R132–R134. ( 10.1016/S0960-9822(00)00398-5) [DOI] [PubMed] [Google Scholar]
- 126.Demand J, Alberti S, Patterson C, Hohfeld J. 2001. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr. Biol. 11, 1569–1577. ( 10.1016/S0960-9822(01)00487-0) [DOI] [PubMed] [Google Scholar]
- 127.Luders J, Demand J, Hohfeld J. 2000. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J. Biol. Chem. 275, 4613–4617. ( 10.1074/jbc.275.7.4613) [DOI] [PubMed] [Google Scholar]
- 128.Kettern N, Rogon C, Limmer A, Schild H, Hohfeld J. 2011. The Hsc/Hsp70 co-chaperone network controls antigen aggregation and presentation during maturation of professional antigen presenting cells. PLoS ONE 6, e16398 ( 10.1371/journal.pone.0016398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3, 93–96. ( 10.1038/35070170) [DOI] [PubMed] [Google Scholar]
- 130.Willis MS, Min JN, Wang S, McDonough H, Lockyer P, Wadosky KM, Patterson C. 2013. Carboxyl terminus of Hsp70-interacting protein (CHIP) is required to modulate cardiac hypertrophy and attenuate autophagy during exercise. Cell Biochem. Funct. 31, 724–735. ( 10.1002/cbf.2962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arndt V, et al. 2010. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr. Biol. 20, 143–148. ( 10.1016/j.cub.2009.11.022) [DOI] [PubMed] [Google Scholar]
- 132.Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ. 2005. The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates α-synuclein degradation decisions between proteasomal and lysosomal pathways. J. Biol. Chem. 280, 23 727–23 734. ( 10.1074/jbc.M503326200) [DOI] [PubMed] [Google Scholar]
- 133.Zhang C, Xu Z, He XR, Michael LH, Patterson C. 2005. CHIP, a cochaperone/ubiquitin ligase that regulates protein quality control, is required for maximal cardioprotection after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 288, H2836–H2842. ( 10.1152/ajpheart.01122.2004) [DOI] [PubMed] [Google Scholar]
- 134.Schisler JC, Rubel CE, Zhang C, Lockyer P, Cyr DM, Patterson C. 2013. CHIP protects against cardiac pressure overload through regulation of AMPK. J. Clin. Invest. 123, 3588–3599. ( 10.1172/JCI69080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Powell SR, Herrmann J, Lerman A, Patterson C, Wang X. 2012. The ubiquitin-proteasome system and cardiovascular disease. Prog. Mol. Biol. Transl. Sci. 109, 295–346. ( 10.1016/B978-0-12-397863-9.00009-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Naito AT, et al. 2010. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ. Res. 106, 1692–1702. ( 10.1161/CIRCRESAHA.109.214346) [DOI] [PubMed] [Google Scholar]
- 137.Xu CW, Zhang TP, Wang HX, Yang H, Li HH. 2013. CHIP enhances angiogenesis and restores cardiac function after infarction in transgenic mice. Cell Physiol. Biochem. 31, 199–208. ( 10.1159/000343361) [DOI] [PubMed] [Google Scholar]
- 138.McMullen JR, Izumo S. 2006. Role of the insulin-like growth factor 1 (IGF1)/phosphoinositide-3-kinase (PI3 K) pathway mediating physiological cardiac hypertrophy. Novartis Found Symp. 274, 90–111; discussion -7, 52–5, 272–6 ( 10.1002/0470029331.ch7) [DOI] [PubMed] [Google Scholar]
- 139.McMullen JR, et al. 2004. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J. Biol. Chem. 279, 4782–4793. ( 10.1074/jbc.M310405200) [DOI] [PubMed] [Google Scholar]
- 140.Dickey CA, et al. 2008. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc. Natl Acad. Sci. USA 105, 3622–3627. ( 10.1073/pnas.0709180105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sciarretta S, Volpe M, Sadoshima J. 2014. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 114, 549–564. ( 10.1161/CIRCRESAHA.114.302022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee J-H, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. 1999. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 18, 6183–6190. ( 10.1038/sj.onc.1203043) [DOI] [PubMed] [Google Scholar]
- 143.Seidel K, et al. 2012. The HSPB8-BAG3 chaperone complex is upregulated in astrocytes in the human brain affected by protein aggregation diseases. Neuropathol. Appl. Neurobiol. 38, 39–53. ( 10.1111/j.1365-2990.2011.01198.x) [DOI] [PubMed] [Google Scholar]
- 144.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Büchler MW. 2001. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 503, 151–157. ( 10.1016/S0014-5793(01)02728-4) [DOI] [PubMed] [Google Scholar]
- 145.Boiani M, Daniel C, Liu X, Hogarty MD, Marnett LJ. 2013. The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J. Biol. Chem. 288, 6980–6990. ( 10.1074/jbc.M112.414177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim JA, Kim Y, Kwon B-M, Han DC. 2013. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated Athanogene Domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 288, 28 713–28 726. ( 10.1074/jbc.M113.488346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. 2006. BAG3 deficiency results in fulminant myopathy and early lethality. Am. J. Pathol. 169, 761–773. ( 10.2353/ajpath.2006.060250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Odgerel Z, et al. 2010. Inheritance patterns and phenotypic features of myofibrillar myopathy associated with a BAG3 mutation. Neuromuscul. Disord. 20, 438–442. ( 10.1016/j.nmd.2010.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sato T, Hayashi YK, Keduka E, Noguchi S, Osawa M, Nonaka I, Nishino I. 2011. P5. 54 Novel BAG3 mutations in myofibrillar myopathy patients. Neuromuscul. Disord. 21, 740 ( 10.1016/j.nmd.2011.06.1083) [DOI] [Google Scholar]
- 150.Ruparelia AA, Oorschot V, Vaz R, Ramm G, Bryson-Richardson RJ. 2014. Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathol. 128, 821–833. ( 10.1007/s00401-014-1344-5) [DOI] [PubMed] [Google Scholar]
- 151.Norton N, et al. 2011. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am. J. Hum. Genet. 88, 273–282. ( 10.1016/j.ajhg.2011.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arimura T, Ishikawa T, Nunoda S, Kawai S, Kimura A. 2011. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum. Mutat. 32, 1481–1491. ( 10.1002/humu.21603) [DOI] [PubMed] [Google Scholar]
- 153.Cheung JY, et al. 2015. Cardiac dysfunction in HIV-1 transgenic mouse: role of stress and BAG3. Clin. Transl. Sci. 8, 305–310. ( 10.1111/cts.12331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feldman AM, et al. 2014. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J. Cell Physiol. 229, 1697–1702. ( 10.1002/jcp.24615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.De Marco M, et al. 2014. BAG3 protein in advanced-stage heart failure. JACC: Heart Fail. 2, 673–675. ( 10.1016/j.jchf.2014.05.012) [DOI] [PubMed] [Google Scholar]
- 156.Knezevic T, Myers VD, Gordon J, Tilley DG, Sharp TE, Wang J, Khalili K, Cheung JY, Feldman AM. 2015. BAG3: a new player in the heart failure paradigm. Heart Fail. Rev. 20, 423–434. ( 10.1007/s10741-015-9487-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takayama S, Bimston DN, Matsuzawa S-I, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. 1997. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16, 4887 ( 10.1093/emboj/16.16.4887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brive L, Takayama S, Briknarova K, Homma S, Ishida SK, Reed JC, Ely KR. 2001. The carboxyl-terminal lobe of Hsc70 ATPase domain is sufficient for binding to BAG1. Biochem. Biophys. Res. Commun. 289, 1099–1105. ( 10.1006/bbrc.2001.6087) [DOI] [PubMed] [Google Scholar]
- 159.Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI. 1998. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 17, 6871 ( 10.1093/emboj/17.23.6871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takayama S, Xie Z, Reed JC. 1999. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J. Biol. Chem. 274, 781–786. ( 10.1074/jbc.274.2.781) [DOI] [PubMed] [Google Scholar]
- 161.Fuchs M, Poirier Dominic J, Seguin Samuel J, Lambert H, Carra S, Charette Steve J, Landry J. 2009. Identification of the key structural motifs involved in HspB8/HspB6/Bag3 interaction. Biochem. J. 425, 245 ( 10.1042/BJ20090907) [DOI] [PubMed] [Google Scholar]
- 162.Rauch JN, Tse E, Freilich R, Mok S-A, Makley LN, Southworth DR, Gestwicki JE. 2017. BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J. Mol. Biol. 429, 128–141. ( 10.1016/j.jmb.2016.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pagliuca MG, Lerose R, Cigliano S, Leone A. 2003. Regulation by heavy metals and temperature of the human BAG3 gene, a modulator of Hsp70 activity. FEBS Lett. 541, 11–15. ( 10.1016/S0014-5793(03)00274-6) [DOI] [PubMed] [Google Scholar]
- 164.De Marco M, et al. 2013. Detection of soluble BAG3 and anti-BAG3 antibodies in patients with chronic heart failure. Cell Death Dis. 4, e495 ( 10.1038/cddis.2013.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carrizzo A, et al. 2016. The prosurvival protein BAG3: a new participant in vascular homeostasis. Cell Death Dis. 7, e2431 ( 10.1038/cddis.2016.321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li C, Chang Y, Li Y, Chen S, Chen Y, Ye N, Dai D, Sun Y. 2017. Advanced glycation end products promote the proliferation and migration of primary rat vascular smooth muscle cells via the upregulation of BAG3. Int. J. Mol. Med. 39, 1242–1254. ( 10.3892/ijmm.2017.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hishiya A, Kitazawa T, Takayama S. 2010. BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ. Res. 107, 1220 ( 10.1161/CIRCRESAHA.110.225649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feldman AM, et al. 2016. BAG3 regulates contractility and Ca2+ homeostasis in adult mouse ventricular myocytes. J. Mol. Cell Cardiol. 92, 10–20. ( 10.1016/j.yjmcc.2016.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kostera-Pruszczyk A, et al. 2015. BAG3-related myopathy, polyneuropathy and cardiomyopathy with long QT syndrome. J. Muscle Res. Cell Motil. 36, 423–432. ( 10.1007/s10974-015-9431-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Villard E, et al. 2011. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur. Heart J. 32, 1065–1076. ( 10.1093/eurheartj/ehr105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. 2009. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol. 65, 83–89. ( 10.1002/ana.21553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee HC, Cherk SW, Chan SK, Wong S, Tong TW, Ho WS, Chan AY, Lee KC, Mak CM. 2011. BAG3-related myofibrillar myopathy in a Chinese family. Clin. Genet. 81, 394–398. ( 10.1111/j.1399-0004.2011.01659.x) [DOI] [PubMed] [Google Scholar]
- 173.Franaszczyk M, et al. 2014. The BAG3 gene variants in Polish patients with dilated cardiomyopathy: four novel mutations and a genotype–phenotype correlation. J. Transl. Med. 12, 192 ( 10.1186/1479-5876-12-192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Toro R, Perrez-Serra A, Campuzano O, Moncayo-Arlandi J, Allegue C, Iglesias A, Mangas A, Brugada R. 2016. Familial dilated cardiomyopathy caused by a novel frameshift in the BAG3 gene. PLoS ONE 11, e0158730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chami N, et al. 2014. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in french canadians. Can. J. Cardiol. 30, 1655–1661. ( 10.1016/j.cjca.2014.09.030) [DOI] [PubMed] [Google Scholar]
- 176.Jaffer F, et al. 2012. BAG3 mutations: another cause of giant axonal neuropathy. J. Peripher. Nerv. Syst. 17, 210–216. ( 10.1111/j.1529-8027.2012.00409.x) [DOI] [PubMed] [Google Scholar]
- 177.Konersman CG, et al. 2015. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul. Disord. 25, 418–422. ( 10.1016/j.nmd.2015.01.009) [DOI] [PubMed] [Google Scholar]
- 178.Semmler A-L, et al. 2014. Unusual multisystemic involvement and a novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet J. Rare Dis. 9, 121 ( 10.1186/s13023-014-0121-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Quintana MT, et al. 2016. Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209 L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3 haploinsufficiency, activates p38 signaling. Am. J. Pathol. 186, 1989–2007. ( 10.1016/j.ajpath.2016.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goldfarb LG, et al. 1998. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat. Genet. 19, 402–403. ( 10.1038/1300) [DOI] [PubMed] [Google Scholar]
- 181.Munoz-Marmol AM, et al. 1998. A dysfunctional desmin mutation in a patient with severe generalized myopathy. Proc. Natl Acad. Sci. USA 95, 11 312–11 317. ( 10.1073/pnas.95.19.11312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sjoberg G, Saavedra-Matiz CA, Rosen DR, Wijsman EM, Borg K, Horowitz SH, Sejersen T. 1999. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, exerts a dominant negative effect on filament formation. Hum. Mol. Genet. 8, 2191–2198. ( 10.1093/hmg/8.12.2191) [DOI] [PubMed] [Google Scholar]
- 183.Dalakas MC, Park K-Y, Semino-Mora C, Lee HS, Sivakumar K, Goldfarb LG. 2000. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. New England J. Med. 342, 770–780. ( 10.1056/NEJM200003163421104) [DOI] [PubMed] [Google Scholar]
- 184.Park K-Y, Dalakas MC, Goebel HH, Ferrans VJ, Semino-Mora C, Litvak S, Takeda K, Goldfarb LG. 2000. Desmin splice variants causing cardiac and skeletal myopathy. J. Med. Genet. 37, 851–857. ( 10.1136/jmg.37.11.851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sugawara M, et al. 2000. A novel de novo mutation in the desmin gene causes desmin myopathy with toxic aggregates. Neurology 55, 986–990. ( 10.1212/WNL.55.7.986) [DOI] [PubMed] [Google Scholar]
- 186.Goudeau B, Dagvadorj A, Rodrigues-Lima F, Nedellec P, Casteras-Simon M, Perret E, Langlois S, Goldfarb L, Vicart P. 2001. Structural and functional analysis of a new desmin variant causing desmin-related myopathy. Hum. Mutat. 18, 388–396. ( 10.1002/humu.1210) [DOI] [PubMed] [Google Scholar]
- 187.Schroeder R, et al. 2003. On noxious desmin: functional effects of a novel heterozygous desmin insertion mutation on the extrasarcomeric desmin cytoskeleton and mitochondria. Hum. Mol. Genet. 12, 657–669. ( 10.1093/hmg/ddg060) [DOI] [PubMed] [Google Scholar]
- 188.Dagvadorj A, et al. 2003. Respiratory insufficiency in desminopathy patients caused by introduction of proline residues in desmin c-terminal α-helical segment. Muscle Nerve. 27, 669–675. ( 10.1002/mus.10370) [DOI] [PubMed] [Google Scholar]
- 189.Vicart P, et al. 1998. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95. ( 10.1038/1765) [DOI] [PubMed] [Google Scholar]
- 190.Selcen D, Engel AG. 2003. Myofibrillar myopathy caused by novel dominant negative αB-crystallin mutations. Ann. Neurol. 54, 804–810. ( 10.1002/ana.10767) [DOI] [PubMed] [Google Scholar]
- 191.Quintana M, Yates C, Takayama S, Willis M. 2015. Bag3+ P209 L transgene provides a cardiac-specific murine model of protein misfolding and aggregation. FASEB J. 29, 46.6. [Google Scholar]
- 192.Eaton SC, Takayama S, Sidorova TN, Murray KT, Willis MS. 2016. Pharmacological inhibition of p38/MAPK improves cardiac function in cardiac-specific Bag3-P209 L transgenic mice. FASEB J. 30(1 Supplement), 306. [Google Scholar]
- 193.Tahrir FG, Knezevic T, Gupta MK, Gordon J, Cheung JY, Feldman AM, Khalili K. 2017. Evidence for the role of BAG3 in mitochondrial quality control in cardiomyocytes. J. Cell Physiol. 232, 797–805. ( 10.1002/jcp.25476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132, 27–42. ( 10.1016/j.cell.2007.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. 2008. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075. ( 10.1038/nature06639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Behl C. 2011. BAG3 and friends: co-chaperones in selective autophagy during aging and disease. Autophagy 7, 795–798. ( 10.4161/auto.7.7.15844) [DOI] [PubMed] [Google Scholar]
- 197.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. 2009. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 28, 889–901. ( 10.1038/emboj.2009.29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. 2011. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 12, 149–156. ( 10.1038/embor.2010.203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SDM, Rubinsztein DC. 2005. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat. Genet. 37, 771–776. ( 10.1038/ng1591) [DOI] [PubMed] [Google Scholar]
- 200.Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. 1999. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl Acad. Sci. USA 96, 6137–6142. ( 10.1073/pnas.96.11.6137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K-I, Murphy E, Robbins J. 2005. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in α-B-crystallin desmin related cardiomyopathy. Circulation 112, 3451–3461. ( 10.1161/CIRCULATIONAHA.105.572552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hishiya A, Salman MN, Carra S, Kampinga HH, Takayama S. 2011. BAG3 directly interacts with mutated αB-crystallin to suppress its aggregation and toxicity. PLoS ONE 6, e16828 ( 10.1371/journal.pone.0016828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jenei ZM, Gombos T, Forhecz Z, Pozsonyi Z, Karadi I, Janoskuti L, Prohaszka Z. 2013. Elevated extracellular HSP70 (HSPA1A) level as an independent prognostic marker of mortality in patients with heart failure. Cell Stress Chaperones 18, 809–813. ( 10.1007/s12192-013-0425-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jenei ZM, Szeplaki G, Merkely B, Karadi I, Zima E, Prohaszka Z. 2013. Persistently elevated extracellular HSP70 (HSPA1A) level as an independent prognostic marker in post-cardiac-arrest patients. Cell Stress Chaperones 18, 447–454. ( 10.1007/s12192-012-0399-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cai WF, et al. 2010. Intracellular or extracellular heat shock protein 70 differentially regulates cardiac remodelling in pressure overload mice. Cardiovasc. Res. 88, 140–149. ( 10.1093/cvr/cvq182) [DOI] [PubMed] [Google Scholar]
- 206.Gandhi PU, Gaggin HK, Belcher AM, Harisiades JE, Basile A, Falco A, Rosati A, Piscione F, Januzzi JL Jr. 2015. Turco MC. Analysis of BAG3 plasma concentrations in patients with acutely decompensated heart failure. Clin. Chim. Acta. 445, 73–78. ( 10.1016/j.cca.2015.02.048) [DOI] [PubMed] [Google Scholar]
- 207.Zhou C, Bai J, Jiang C, Ye L, Pan Y, Zhang H. 2017. Geranylgeranylacetone attenuates myocardium ischemic/reperfusion injury through HSP70 and Akt/GSK-3β/eNOS pathway. Am. J. Transl. Res. 9, 386–395. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.