Abstract
Experimental models of autoimmune diseases have revealed the disease protective role of heat shock proteins (HSPs). Both the administration of exogenous extracellular, mostly recombinant, HSP and the experimental co-induction of endogenous intracellular HSP in models have been shown to lead to production of disease protective regulatory T cells (Tregs). Similar to HSP taken up from extracellular bodily fluids, due to stress-related autophagy upregulated HSP also from intracellular sources is a major provider for the major histocompatibility class II (MHCII) ligandome; therefore, both extracellular and intracellular HSP can be prominent targets of Treg. The development of therapeutic peptide vaccines for the restoration of immune tolerance in inflammatory diseases is an area of intensive research. In this area, HSPs are a target for tolerance-inducing T-cell therapy, because of their wide expression in inflamed tissues. In humans, in whom the actual disease trigger is frequently unknown, HSP peptides offer chances for tolerance-promoting interventions through induction of HSP-specific Treg. Recently, we have shown the ability of a bacterial HSP70-derived peptide, HSP70-B29, to induce HSP-specific Tregs that suppressed arthritis by cross-recognition of their mammalian HSP70 homologues, abundantly present in the MHCII ligandome of stressed mouse and human antigen-presenting cells in inflamed tissues.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: autoimmunity, regulatory T cell, rheumatoid arthritis, inflammation, heat shock protein
1. Heat shock protein and its role in inflammation
Non-resolving inflammation can lead to immunopathology and clinical manifestations of major diseases in humans. As a consequence, much research is oriented towards mechanisms of resolving inflammation. Healthy resolution of inflammatory responses, which also drives tissue damage repair mechanisms and return of homeostasis in the tissue, seems to be an essential factor necessary for ongoing health. Some cellular and molecular mechanisms involved in inflammation resolution have been uncovered in the recent past. These mechanisms include production of specific cytokines, apoptosis of inflammatory leucocytes, macrophage repolarization and production of lipid mediators.
Inflammation resolution through active naturally occurring inflammation-resolving processes may have significant advantages over artificial inhibition of inflammation that can be achieved by passive blockade of pro-inflammatory mediators. Heat shock proteins (HSPs) are produced through inflammatory stress inflicted upon cells that reside at sites of inflammation. This cellular response produced by inflammation provides the immune system with a natural target for control of inflammation, in this case mediated by HSP-specific T-regulatory cells. These T cells can become generated and activated by HSP derived from microbes or released from host cells in the form of exogenous HSP. There are indications that HSP can become actively exported from host cells and that herewith they are present in a soluble form in the peripheral blood, but, in essence, HSPs are not actively secreted molecules. Exogenous HSPs are taken up by antigen-presenting cells, and subsequently processed and presented to responding T cells in the context of major histocompatibility class II (MHCII) molecules. In experimental models, solid evidence was collected that such soluble HSPs, in that case, mostly recombinant HSP, were capable of inducing regulatory T cells (Tregs). Alternatively, under the influence of inflammatory stress, endogenous cellular HSP can become upregulated and its peptides loaded into MHCII molecules, among others through autophagy [1]. Also in this case, experimental findings have shown that this led to the induction of Tregs. Examples illustrating this effect of intracellular HSP can be found in studies where HSP co-inducers were administered to amplify the cellular expression of HSP [2]. Also in such cases, reduction of disease was noted due to the induction of Treg activities [3,4].
Inflammation is characterized by the overproduction of pro-inflammatory cytokines (e.g. TNF-α and interleukin-17 (IL-17)), chemokines (e.g. MCP1) and other inflammatory mediators. Many of these factors are positively regulated by nuclear factor κB (NF-κB). Various studies have shown that due to a close inverse relationship between the induction of endogenous HSP and NF-κB, the promotion of the HSP response by stress-inducing agents results in reducing the NF-κB transcription activation of pro-inflammatory cytokines such as TNF-α. HSP70 was one of the first molecules found to inhibit NF-κB [5]. In addition, transcription factors involved with HSP regulation such as heat shock factor 1 (HSF1) can also exert a direct negative effect on the TNF-α gene promoter [6], which leads to the inhibition of TNF-α production.
2. Regulatory T cells and maintenance of self-tolerance
Despite the fact that many self-antigens simply remain ignored by the immune system, tolerance for self is mainly an actively acquired physiological state, based on various mechanisms. Such mechanisms include the deletion of T cells from the thymus, deletion of B cells from the bone marrow and the lymphoid germinal centres, and functional silencing of T and B cells, known as anergy. More recently, additional critical mechanisms have surfaced, such as immuno-regulatory processes mediated through specialized regulatory cells. Despite the existence of these multiple mechanisms, self-reactive B and T cells are produced and are sometimes activated, giving rise to autoimmune diseases. However, as it seems now, self-reactive T cells may also constitute a major segment of the healthy repertoire that has a determining role in the maintenance of self-tolerance. In this latter sense, these T cells are active regulators that down-modulate other autoreactive cells. As we will discuss in the following sections, in the case of self-HSP-reactive T cells, such active regulatory potential has been shown to exist. These Tregs, also known as natural Tregs (nTregs), propagate an active form of dominant tolerance, which is transferable into naive recipients by adoptive transfer.
The fundamental role of a specialized subset of T cells in the active regulation of self-tolerance was uncovered initially by Sakaguchi et al. in the mouse [7]. The more critical observations here are that the depletion of either CD25+CD4+ T cells or Foxp3+ T cells from mice caused a wide spectrum of autoimmune pathologies [8]. In addition, mutations of Foxp3 in the so-called Scurfy mice were seen to lead to fatal systemic autoimmune and inflammatory diseases, and in humans similar mutations were found to be the basis of IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked syndrome), a disease featuring various autoimmune conditions, such as thyroiditis, inflammatory bowel disease (IBD) and type I diabetes [9]. Since the induction of these pathologies in mice was seen following thymectomy at critical time points at early ages, the thymic origin of a critical Treg population became evident.
It is of critical importance to further define signals or circumstances that lead to the development of the regulatory phenotype in T cells. One probable signal is the micro-environment of accessory signals, such as cytokines. Alternatively, the nature of the ligand that triggers through the T cell receptor (TcR) can be a determining signal. For instance, the relative affinity of the ligand–TcR interactions may well be involved. Detailed studies showed that in the thymus, relatively high affinity interactions with self-antigens, although below those leading to deletion, were leading to selection of tTregs (thymus-derived Treg). Besides this, studies in the mouse have shown that Tregs can be generated in the gut, where dendritic cells (DCs) produce retinoic acid, which, in combination with tissue-produced transforming growth factor-β (TGF-β), can lead to the induction of Foxp3 expression in naive T cells [10,11]. In addition, the intestinal microbiota is supposed to contribute through the stimulation of TGF-β production in intestinal epithelial cells and the production of short chain fatty acids, such as butyrate [12]. The latter Tregs are now known as pTregs (peripherally derived Tregs), which are, therefore, Tregs that differentiated from conventional T cells in the periphery. Tregs obtained by in vitro antigenic stimulation in the presence of IL-2 and TGF-β are usually called induced Treg (iTreg) [13]. In the mouse, all Tregs express CD25, cytotoxic T-lymphocyte protein 4 (CTLA-4) and Foxp3, whereas tTregs also express transcription factor Helios and the cell surface marker neuropilin-1 [14,15].
In humans, nTregs are also defined by the expression of CD4+, CD25+ and Foxp3+. In addition to this, low or negative CD127 is sometimes used for their definition. However, in humans, naive and memory effector T cells also express Foxp3 after TcR triggering. Although this expression is transient, it makes Foxp3 a less suitable marker for Treg in humans than in mice. Furthermore, Helios and neuropilin do not seem to differentiate tTreg from pTreg in humans.
A recent elegant study has revealed the affinity differences for self to select Treg with distinct functional properties [16]. In this mouse study, a distinction was made between GITRhiPD-1hiCD25hi (Triplehi) Treg cells and GITRloPD-1loCD25lo (Triplelo) Treg cells. The first cells were found to be highly self-reactive and capable of controlling lympho-proliferation in peripheral lymph nodes, while the second population was less self-reactive and was found to assist the conversion of conventional CD4+ T cell into iTreg cells.
3. Autoimmune diseases and functioning of regulatory T cells
In various autoimmune conditions, diminished activities of Tregs have been observed, resulting in loss of self-tolerance. In rheumatoid arthritis (RA), CD4+CD25high T cells have a diminished level of inflammatory cytokine inhibition, which could be reversed by anti-TNF interventions [17]. Subsequent findings have suggested that the interaction of CD4+CD25+ Treg cells with activated monocytes in the joint might lead to diminished suppressive activity of CD4+CD25+ Treg cells in vivo, thus contributing to the chronic inflammation in RA [18]. With molecular studies, it was shown that T-cell factor 1 (TCF1), a component of the Wnt signalling pathway, disrupted Foxp3 transcriptional activity. The activation of Wnt signalling was found to reduce Treg-mediated suppression both in vitro and in vivo. As Wnt3a levels were found to be greatly increased in mononuclear cells isolated from synovial fluid versus peripheral blood of RA patients, this study suggested that Wnt produced under inflammatory conditions represses Treg cell function, possibly contributing to the development of disease [19]. Notwithstanding the previous observations, frequencies of peripheral blood Tregs were not found to be consistently diminished in diseases like RA and Tregs of RA patients were shown to have normal suppressive potential. In some cases, however, when obtained from the synovial compartment, the cells were shown to produce IL-17 [20]. In addition, evidence has been presented that inflammatory cytokines present in the inflamed joint may induce resistance to suppression in pathogenic effector T cells [21]. A study in juvenile idiopathic arthritis (JIA) showed that this resistance to suppression was not caused by a memory phenotype of effector T cells or activation status of antigen-presenting cells. Instead, it appeared that activation of protein kinase B (PKB)/c-akt was enhanced in inflammatory effector cells and that inhibition of this kinase restored responsiveness to suppression. The latter study, therefore, indicated that PKB/c-akt hyperactivation causes resistance of effector cells to suppression [22]. Also in JIA, a sustained level of impaired suppression of synovial CD8+ T cells was shown to exist [23].
Given the indications of possible resistance to Treg activities in these inflammatory disorders, restoration of tolerance most likely must depend not only on improvement of Treg presence and function but also on reduction of the pathogenic effector cells. In other words, it may be essential to alter regulator–effector T-cell balances by numerical expansion of the antigen-specific nTreg or iTregs at the expense of effector T-cell populations [24]. This may be achieved using a combination of Treg-inducing interventions with immunosuppressive drugs or biologicals that target inflammatory cytokines or selected co-stimulatory molecules.
4. Antigen recognition is essential for regulatory T-cell function
(a). Self-antigens or autoantigens associated with disease
The thymic selection of tTregs has been the subject of many studies. Selection of T cells in TcR transgenic mice with defined transgenically expressed neo-antigens showed that selection of CD4+CD25+ thymocytes depends on a relatively high TcR affinity for the selecting peptide [25]. On the basis of this, the export of Tregs whose TcRs have the potential to be reactive to self-peptides that might be encountered in the periphery is foreseen. And as already suggested by Miyara et al. [24], the Treg affinity for self-antigens may well be essential for their capacity to suppress autoimmunity.
An early example of effective suppression of disease with self-antigen-reactive Tregs was shown in the non obese diabetic (NOD) model of type I diabetes [26]. A small number of 5 × 105 expanded tTregs obtained from a TcR transgenic mouse with T cells specific for glutamic acid decarboxylase, a diabetes-associated autoantigen, reversed diabetes upon transfer even after diabetes onset. Obviously, the suggestion made on the basis of such findings is that Tregs that express an organ-specific TcR have the capability to home to the target organ. When present at this location, the cells would receive re-activation stimuli by the locally presented self-antigen, and they would mediate local suppression of disease-causing effector cells. Through bystander suppression, such an activity of activated Tregs would include the inhibition of effector T cells also in the absence of well-identified target autoantigens.
The possible nature of the target antigens that are recognized in vivo by the polyclonally expanded tTregs in experimental transfer studies was discussed by Shevach & Thornton [27]. Although it remains difficult to rule out the possibility that polyclonal tTregs do not need activation in vivo to suppress, it is assumed that recognition of self-antigens occurs and is needed. In this case, it is proposed that tTregs are continuously recognizing and activated by ubiquitous self-peptides presented by MHCII molecules.
One study that showed the need for antigen triggering in vivo for Tregs to be functional was based on the acute tamoxifen-inducible ablation of TcRs in Tregs. TracFL mice (which have a loxP-flanked allele encoding the TcR α-chain constant region (Cα or TcRα)) were crossed with Foxp3eGFP-Cre-ERT2 mice (with expression of enhanced green fluorescent protein (eGFP) fused to a Cre recombinase–oestrogen receptor ligand-binding domain protein from the 3′-untranslated region of Foxp3; called ‘Foxp3Cre-ERT2’ here) to achieve tamoxifen-inducible deletion of Trac specifically in Treg cells [28]. The study showed that continuous TcR signalling in Treg cells was essential for their suppressive function, whereas Foxp3, CD25 or GITR expression was not.
(b). Microbial antigens
Analysis of antigen specificities of human Tregs also has indicated recognition of microbial recall antigens. Upon in vitro stimulation with these microbial antigens, the cells expanded and kept their regulatory phenotype (CD4+CD25+, CD134+, CD39+) and function [29]. It is possible that such Tregs with specificity for non-self, supposedly pTreg, are actively securing tolerance for dietary, commensal or other environmental antigens. Given the division between the distinct pathways that select for TcR specificities, it is assumed that tTregs are more prone to recognize self-antigen, whereas pTregs are oriented towards non-self-recognition. Such a division was also suggested on the basis of findings showing the relatively non-overlapping antigen recognition repertoires of tTreg and pTreg, despite their closely matched transcriptional signatures [30]. Of note, in the latter study, tTreg alone did not suppress chronic inflammation and autoimmunity. Only with the additional reconstitution with iTreg was tolerance restored. With respect to foreign antigens, most attention has been given until now to antigens from commensal microbes. Although some TcR sequencing studies also have claimed the existence of shared repertoires between intestinal Tregs and thymic Treg [31], other studies have indicated that intestinal Treg was mainly pTreg [32]. The latter studies concur with studies that showed the cure of colitis with the transfer of microbiota-specific intestinal Treg cells [33]. A recent study showed the role of microbiota for pTreg induction. In this case, somatic cell nuclear transfer was used to generate a transnuclear mouse carrying a monoclonal TcR obtained from a pTreg. The study revealed that the Treg TcR did not inevitably predispose T cells to become pTreg and allowed for differentiation of inflammatory CD4+CD8aa+ intraepithelial lymphocytes in the small intestine [34]. In this case, pTreg formation seems more dependent on accessory signals in the environment, such as those dependent on the microbiota, than on TcR affinities or specificities.
(c). Heat shock proteins
Many of the evolutionarily conserved microbial antigens have mammalian homologues present in self-antigens. For example, microbial and mammalian HSPs have strong similarities at the amino acid level. Thus, the tolerance generated for microbiota-derived non-self-epitopes may well assist self-tolerance at the same time, based on cross-recognition of self-antigens by microbe-induced Treg. Moudgil et al. have shown that environmental microbes do induce HSP60-specific T cells which are cross-recognizing self-HSP60 epitopes. Animals kept behind barriers were more prone to arthritis than those kept under conventional circumstances. And the transfer of HSP60 cross-responsive T cells from conventionally reared animals protected the animals that were kept behind barriers from disease [35].
5. Microbial antigens may be essential for tolerance with respect to self-antigens
Indirect evidence for the tolerance-promoting role of our microbial environment may come from the so-called hygiene hypothesis. This hypothesis states that a lack of (early childhood) exposure to infectious agents, symbiotic microorganisms (such as the gut microbiota) and parasites increases susceptibility to allergic diseases by causing an imbalance in the immune system. In particular, the lack of exposure is thought to lead to defects in the establishment of immune tolerance. It is known that the confrontation of the immune system with microbiota soon after birth is decisive for a proper balance between T helper cell 1 (Th1) and T helper cell 2 (Th2) T cells in the immune system. Maintaining the innate Th2 dominance, when not corrected by microbiota-induced Th1 cells, would lead to a tendency to develop allergies. More recently, the hypothesis was also proposed to explain the rising prevalence of autoimmune diseases, such as type 1 diabetes. In this revised variant of the hypothesis, the underlying mechanism was shifted from the Th1/Th2 imbalance to a deficient function of Tregs [36]. And this is, of course, compatible with the current evidence that pTregs are functionally dependent, among others, on the presence of microbiota in the gut. As some of us have argued before, it is possible that HSPs are molecules that are associated with the molecular basis of the hygiene hypothesis [37].
The proposed existence of separate TcR repertoires in Tregs for self- and foreign antigens seems to negate the existence of self–non-self cross-recognition in the immune system. And one may think of various reasons to cast doubt on the reality of such separated repertoires. To begin with, there is ample evidence for broad cross-recognition of distinct antigens in memory T cells, at least with respect to environmental antigens [38]. In T memory repertoires of individuals who had never encountered previously a particular microbial agent, memory T cells were shown to exist with these particular microbial specificities. The interpretation of these findings led to the widely accepted notion that cross-reactivities are a fundamental aspect of the properly functioning immune system. In addition, and also fundamentally critical for the immune system, is the above-mentioned genetic conservation of key molecules in evolution. Many of the most important molecules of hosts and their infectious parasites are closely related and even identical in certain domains. Because of this, it may be out of necessity, in order to avoid critical holes in the repertoire, that self–non-self cross-reactive repertoires exist. Or, alternatively, it may be for reasons of efficiency that these repertoires evolved. It may be efficient to equip cells with receptors that can perceive information from both the microbial environment and the self. As previously argued by Cohen [39], particular self-reactivities could serve as a set of biomarkers that help the immune system to initiate and regulate the inflammatory processes that maintain the body. An efficient immune system is not responding to each possible antigen to the same extent. On theoretical and experimental grounds, Cohen proposed the existence of a set of antigens, central in the repertoire, that receive an above-average attention from the immune system. In a sense, these antigens are the immune system's representation of the body, similar to the neurological homunculus [39,40]. For several reasons, HSPs had a central position in this homunculus theory. And in the case of HSP, cross-recognition between self and non-self may add to a ‘default’ inclination of the immune system to maintain tolerance, as we will discuss in the next section. Apart from this, self–non-self cross-reactivities may be seen as an unavoidable necessity because self–non-self distinction is compounded by the phenomenon of receptor degeneracy [40]. Because ligand–receptor interactions in biology are based on non-covalent bonding allowing flexible interactions at various contact points, secure and precise distinction between antigens is not often possible for the immune system. For this reason, tolerance cannot always depend on self–non-self discrimination and therefore tolerance may need to be assisted by an active form of regulation, mediated by Treg.
6. Immune cross-recognition between microbes and self-HSP acts as a driving force in tolerance
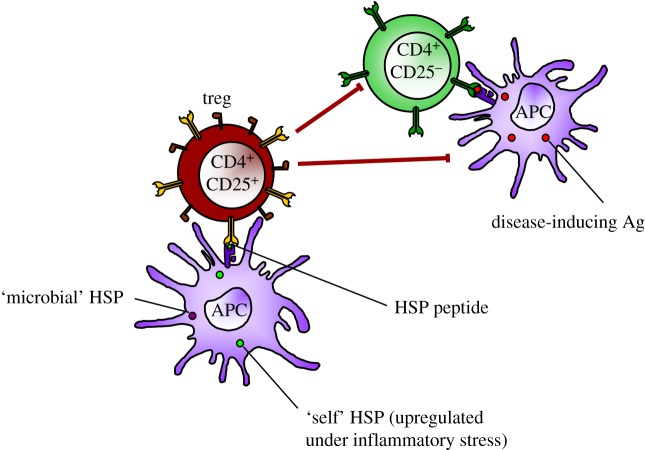
In the case of HSPs, control by Treg seems secured by the evolutionary conservation of molecules. For the mycobacterial HSP60 molecule, one of the most conserved and immunogenic proteins in nature, it was shown in inbred Lewis rats that upon HSP60 immunization, nine distinct T-cell epitopes were seen to act as the target for T cells [41]. Of these nine T-cell epitopes, only the sequence (256–270) that showed maximal homologies with self-HSP60 was found to trigger an arthritis-suppressive population of T cells. The analysis showed that this microbial epitope primed for T-cell responses cross-reactive with the corresponding region of rat hsp60. In addition, the peptide based on the 256–270 sequence conferred upon immunization protection in the disease model of mycobacteria-induced adjuvant arthritis. Interestingly, the peptide also protected against avridine-induced arthritis, indicating that activation of mycobacterial peptide-specific T cells that cross-recognized an epitope in self-hsp60 can protect against autoimmune arthritis induced without mycobacteria. Very similar to the earlier observations, also in HSP70, a conserved sequence was shown to induce cross-reactive and protective T-cell regulation [42]. Therefore, in contrast to the earlier concept that cross-reactive T-cell recognition of foreign and self-antigens might induce aggressive autoimmune disease, these results indicated that cross-reactivity between bacterial and self-hsp60 could be used to maintain a protective self-reactive T-cell population (figure 1).
Figure 1.
Evolutionarily conserved epitopes present in microbial HSP can activate and expand HSP-specific Tregs. At sites of inflammation, HSP-specific Treg do cross-recognize the upregulated and presented homologous HSP peptides on antigen-presenting cells (APCs). Upon recognition, the Tregs are activated and capable of downregulating (suppressing) the activities of CD4+CD25− effector (inflammatory) T cells and/or APCs.
As already suggested by Shevach & Thornton [27], the target molecules of antigen-specific Tregs at sites of inflammation need to be ubiquitous in order for Tregs to have anti-inflammatory effects. For HSP, it is well known that intracellular levels of HSP are increased under cellular stress [43]. For this reason, HSPs are also called stress proteins. Cytokines and other mediators produced during inflammation are inflicting stress on cells. It is, therefore, no surprise that synovial cells obtained from inflamed joints have shown upregulated HSP60 and HSP70 molecules [44,45]. Similarly, pro-inflammatory cytokines (TNF-α, IL-1α and IL-6) were found to induce activation of HSF1 DNA binding leading to raised hsp70 expression in cultivated synovial fluid-derived cells. Furthermore, shear stress also induced a complete heat shock response in cultivated synovial cells. Therefore, the data suggested that induction of hsp70 expression in rheumatoid synovial tissue is based on transcriptional activation of HSF1 due to the presence of pro-inflammatory cytokines (and possibly also shear stress) [46]. Altogether, HSPs are ubiquitous antigens, with raised levels of expression in inflamed tissues.
HSP60 was already known as the common antigen of gram-negative bacteria, before its molecular characterization. In other words, upon immunization with bacteria, high levels of antibodies were seen to be directed to an antigen of which we now know that it was bacterial HSP60. This, in itself, may well illustrate the dominating immunogenicity of bacterial HSP. Knowing now the ease with which cross-reactivity, certainly at the level of T cells, with self-homologues appeared, we may accept that a rich repertoire of self-HSP cross-reactive T cells is present. The induction of self-HSP-specific immunomodulatory T cells has been shown in a variety of studies, in arthritis [47], other autoimmune conditions [48,49], transplant survival [50] and also protein deposition disorders [51]. The presence of a wide abundance of studies that provide examples of HSP-induced disease down-modulatory T-cell responses is very suggestive for the existence of a rich HSP-specific Treg repertoire. It is clear that this Treg repertoire can be manipulated by HSP-derivative peptides, as we will discuss in the next section. In addition to this, however, some studies have suggested that Treg may become recruited in a non-specific manner via the interactions of HSP with Toll-like receptors (TLRs). For example, HSP60 was shown to enhance the function of Treg through signalling via TLR2 [52].
7. HSP offers chances for a novel tolerance therapy in autoimmune diseases
Current targeted biotherapeutics (inhibitors of TNF, IL-6, CTLA-4 and anti-CD20 B-cell therapy) have improved the treatment of various autoimmune diseases. However, these medications are limited by inefficacy or sub-optimal responses in a significant proportion of patients, while others may be at risk of adverse events such as infections due to impaired immune function. In addition, in the case of the biologicals in the form of, for instance, humanized monoclonal antibodies, the development of resistance to treatment over time occurs in many cases. As a next step, immune tolerance approaches would revolutionize the treatment of diseases like RA by inducing drug-free remissions. And for the healthy but at-risk patient, this possibly would offer a ‘vaccination’ treatment for the prevention of disease.
Therapeutic vaccination, with the goal of re-establishing self-tolerance, constitutes a new approach to immune regulation. In the case of therapeutic vaccination based on HSP, the approach is likely to be both effective and safe, because HSPs are used by the immune system itself as physiological regulators and orchestrators of the inflammatory reaction; this conclusion is based on a series of experimental systems that have disclosed the various ways by which the mammalian immune system, both rodent and human, is poised to respond to mammalian HSP molecules. The anti-inflammatory effect appears to be limited to the site of autoimmune inflammation and herewith the T cells elsewhere in the body remain fully capable of mediating Th1 effector responses. Through this form of selective regulation, the HSP-targeting approach may account for an unprecedented level of safety and possibly effectiveness.
8. HSP70-derived peptide B29 is a peptide with immuno-regulatory and disease suppressive potential
Recently, we have uncovered an epitope in the HSP70 molecule that may function as an endogenous regulator of inflammation as demonstrated in the model of proteoglycan-induced arthritis (PGIA) in Balb/c mice [42]. The identification of this epitope resulted from immunization of Balb/c mice with a recombinant mycobacterial HSP70 and the monitoring of induced T-cell responses with sets of overlapping synthetic peptides spanning the HSP70 molecule. Subsequently, we selected from the peptides that showed positive responses those that were relatively conserved, having sequence similarities with homologous sequences present in mammalian HSP70 family members. Immunizations with a pooled collection of such conserved peptides revealed that dominant T-cell responses were seen in the context of a peptide, which we had named B29. Interestingly, of all conserved peptides tested, B29 stood out not only in its quality of inducing T-cell responses but also in its degree of evolutionary conservation. As summarized in table 1, there are in the sequence of 15 amino acids, two amino acid changes between the mycobacterial B29 and its mammalian homologues mB29a and mB29b. mB29a is present in HspA9 (earlier known as GRP75), which is a mitochondrial chaperone, and mB29b is present in one of the most abundantly stress-inducible cellular proteins: HSPA1A (also known as HSP72). HspA8 (or Hsc70) is a rather ubiquitous protein [53].
Table 1.
Origin and sequence of highly conserved B29 peptides. Human and mouse peptides of the same protein were completely identical. Altered residues compared with Mycobacterium tuberculosis are in bold and underlined. ID, GeneID in the National Center for Biotechnology Information (NCBI) Entrez Gene database (www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene).
| peptide | protein | origin | ID | sequence |
|---|---|---|---|---|
| B29 | DnaK (Hsp70) | Mycobacterium tuberculosis | 885946 | VLRIVNEPTAAALAY |
| mB29aa | HspA9 | Mus musculus | 15526 | VLRVINEPTAAALAY |
| (GRP75) | Homo sapiens | 3313 | ||
| mB29ba | HspA1A | Mus musculus | 193740 | VLRIINEPTAAAIAY |
| (Hsp72) | Homo sapiens | 3303 | ||
| HspA8 | Mus musculus | 15481 | VLRIINEPTAAAIAY | |
| (Hsc70) | Homo sapiens | 3312 |
amB29a and mB29b are mammalian homologues of mycobacterial Hsp70 peptide B29.
Given the close sequence homologies between B29 and its mammalian homologues, we tested responses of B29-induced T cells to these homologs. Spleen cells obtained from B29-immunized mice showed a proliferative response in the presence of both mammalian homologues and, in addition, these cells were seen to produce cytokines such as INF-γ and IL-10 upon this cross-stimulation [42].
In a similar protocol, CD25+Foxp3+ T cells were selected from the spleens of B29-immunized mice and analysed for surface markers after in vitro restimulations. The classical phenotypic markers of Tregs were seen to appear, also after cross-stimulation with the mammalian homologues Nrp-1 and IL-10.
When tested in a classical in vitro suppression assay, the B29-induced CD25+ T cells were seen to suppress CD25− T cells obtained from the same responder mice, or naive mice or unrelated (PG-specific) TcR transgenic T cells.
When transferred into naive mice, the CD25+ T cells from B29-immunized animals were capable of suppressing PGIA. Relatively low numbers of 3 × 105 transferred cells were effectively preventing induction of disease, whereas only 1 × 106 T cells already sufficed to suppress ongoing joint inflammation. Importantly, we have shown that T cells recognizing HSP70 epitopes home to inflamed tissues. Transferred B29-induced Tregs were traceable with congenic markers in not only spleen and lymph nodes but also the joints of recipient mice 50 days after a process of arthritis. Moreover and most interestingly, peptide B29 was found capable of inducing a LAG3+ Foxp3+ CD25+ Treg population that at numbers as low as 4000 cells fully suppressed disease upon adoptive transfer [42].
Subsequent antibody-mediated depletion of these Tregs in vivo, using a depleting antibody specific for the CD90.1 congenic marker present in the transferred T cells, abrogated the effect, proving the lasting anti-inflammatory activity of these transferred antigen-specific Tregs.
9. HSP70 sequences are among the most frequent MHCII natural ligand sources
To investigate whether our B29 homologues would be present in the MHCII molecules of the relevant antigen-presenting cells of Balb/c mice, we analysed the peptide repertoire eluted from peptide–MHC complexes collected from bone marrow-derived dendritic cells. As shown in table 2, our homologous peptide mB29a was identified and herewith shown to be an HSP70-derived sequence naturally present in the MHCII groove of mouse antigen-presenting cells. With such a finding, we can assume that B29-induced T cells would be able to find their cross-cognate targets in the MHCII grooves of cells present at the site of inflammation. Earlier peptide elution studies have already indicated that MHCII molecules may have a loading preference for molecules such as HSP70, as shown in supplementary data tables of Paludan et al. [54]. Hsc70, Hsp70 and GAPDH were the three most frequently obtained ligand donor molecules. As if in the default status of self-tolerance, MHCII is presenting these molecular fragments to the resting T-cell populations. The preferential loading of HSP into MHCII molecules can be concluded even more convincingly when we take into account that HSP70 is functionally involved with the cell biological phenomenon of chaperone-mediated autophagy (CMA). Autophagy is the process that cells adopt when they are under stress and that leads to the MHCII presentation of peptides from intracellular source proteins. As nicely described in Dengjel et al. [1] for a human B-cell line under the stress of nutrient deprivation, the HLA-DR4 molecules on the surface of this cell line were found to be loaded with HSP70 sequences that remarkably enough included exactly our mB29b sequence!
Table 2.
MHCII-presented Hsp70 peptides eluted from in vitro cultured murine bone marrow-derived DCs. Peptides were analysed by data-dependent nanoscale liquid chromatography–mass spectrometry (LC–MS). Several homologues of the mB29a peptide varying in length are depicted, and their relative abundance compared with all eluted mB29a variants is shown [42].
| sequence | relative abundance (%) |
|---|---|
| VLRVIN | 4 |
| VLRVINE | 4 |
| VLRVINEP | 13 |
| VLRVINEPT | 1 |
| VLRVINEPTA | 2 |
| VLRVINEPTAA | 9 |
| VLRVINEPTAAA | 6 |
| VLRVINEPTAAAL | 55 |
| LRVINEPTAAAL | 5 |
| Total | 100 |
For a protein to be a CMA substrate, it must have, in its sequence, a CMA-targeting motif that is recognized by HSP70 (hsc70) and that targets the substrate to the lysosome surface [55]. This substrate protein–HSP70 complex binds to lysosome-associated membrane protein type 2A (LAMP-2A), which is the receptor for this pathway. Substrate proteins undergo unfolding after binding to LAMP-2A in a process likely mediated by the membrane-associated hsc70 and some co-chaperones. Substrate translocation requires the presence of hsc70 inside the lysosomal lumen, which may act by either pulling substrates into the lysosomes or preventing their return to the cytosol.
Herewith, it is possible that cellular stress has the additional effect of giving some of the upregulated HSP70 family members a preferential access to the MHCII loading compartments of the cell. At the same time, when perceived by T cells as a sign of cell stress, these HSP70 sequences may control inflammation by a preferential induction of Tregs.
10. Translational steps to use HSP70 peptide B29 for human anti-inflammatory therapies
For the translation of our mouse experimental findings for use in humans, we have characterized the B29 binding and specific CD4+ T-cell responses in the context of human MHC (human leucocyte antigen, HLA) molecules. We found a highly promiscuous HLA class II-binding pattern of our mouse defined peptide [56]. For this, HLA–DR molecules were immuno-purified from Epstein–Barr virus (EBV)-transformed cell lines. The binding of B29 peptides to HLA–DR molecules was then assessed by competitive ELISA. Interestingly, the binding assays revealed a relatively high affinity of B29 homologous peptides for the RA-associated HLA-DR4 and HLA-DQ8 molecules. Based on this broad and promiscuous binding pattern, the peptide can be used for the induction of tolerance-promoting Treg in more than 80% of RA patients. In order to prove the functionality of the peptide in the context of human MHC molecules, we showed B29-induced suppression of PGIA in HLA-DQ8 transgenic mice. HLA-DQ8 transgenic mice were treated intranasally with peptide B29 or with PBS or pOVA as control. Subsequently, arthritis was induced, and disease development was monitored using a visual scoring system. Prophylactic intranasal treatment with B29 significantly reduced arthritis development compared with treatment with PBS or pOVA. When splenocytes from the pretreated mice were restimulated ex vivo with proteoglycan alone or in combination with B29 or pOVA, it was seen that pretreatment with peptide B29 reduced all PG responses and that this response was further reduced when B29 was present during restimulation, but only when the mice had been pretreated with B29. These results indicate that binding of peptide B29 to HLA-DQ8 is functional and induces immune regulatory cells in vivo, which also become active during ex vivo restimulation in the presence of peptide B29 [56].
In vitro priming and subsequent restimulation of peripheral blood mononuclear cells (PBMCs) obtained from healthy blood bank donors with B29 peptide-loaded DCs also revealed the presence and expansion of B29-specific T cells, which were cross-reactive with the mammalian homologues. Because we did not know whether B29-specific T cells would be present as naive or T effector (Teff) cells, we used a sensitive assay that allows in vitro priming and restimulation of naive human T cells. As expected, CD4+ T cells that had been cultured for 14 days in the presence of unloaded autologous monocyte-derived DCs did not show any peptide-specific response above the background level upon restimulation. However, priming of CD4+ T cells with B29-loaded monocyte-derived DCs resulted in an average 3.1-fold increase in IFN-γ+CD40 L+ cells in responding donors after restimulation with B29 compared with restimulation with medium only [56].
As discussed above, previously we showed in mice that B29 immunization resulted in the induction of Foxp3+CD4+CD25+ Treg cells that suppressed PG-induced arthritis [42]. To analyse whether B29 can also induce Treg cells in humans susceptible to RA, we expanded CD4+CD25− T cells from a healthy HLA-DR4+ donor with B29 peptide or hemagglutinin (HA) peptide as control. After 14 days, expanded cells were stained with tetramers specific for B29, mB29b or human CLIP. After 14 days of culture, CD4+ T cells showed enhanced expression of CD25 (approx. 16%) and Foxp3 (approx. 12%). However, culturing with peptide B29 specifically expanded B29 tetramer-positive and mB29b tetramer-positive cells, in both the total CD4+ T-cell population and the FoxP3+CD4+CD25+ T-cell population. The HA control peptide did not induce B29 tetramer-positive or mB29b tetramer-positive T cells. These data suggest that peptide B29 activated and expanded B29-specific Treg cells, which were cross-reactive with the human homologues [56].
On the basis of these findings, we have concluded that the data indicate a conserved specificity and functionality of B29-induced Treg responses in the context of the human MHC. Therefore, a translational path for the clinical development of peptide B29 may be a real possibility in the near future.
11. Development of HSP-based immune tolerance therapies
Various HSP peptides have been used for the exploration of immune tolerance therapies. First, clinical trials were performed in type I diabetes with an HSP60-derived peptide called p277. Following an initial phase I trial, a successful phase II clinical trial was performed in newly diagnosed type 1 diabetes. It seemed that peptide p277 was able to preserve endogenous insulin production, perhaps through induction of a shift from T-helper-1 to T-helper-2 cytokines produced by the autoimmune T cells [57]. Phase III clinical trials have been initiated subsequently and are not finished at this moment.
In RA, first trials were done with dnaJ peptides. The dnaJ family of proteins, also known as HSP40, contain a 70 amino acid consensus sequence known as the J domain. The J domain of DnaJ interacts with Hsp70. DnaJ HSPs play a role in regulating the ATPase activity of Hsp70. dnaJP1 is a 15-mer synthetic peptide that shares homology with the ‘shared epitope’ sequence conferring susceptibility to RA that is present in certain HLA class II alleles. A group of patients with early RA were treated for six months orally with dnaJP1. Immunological analysis showed a change from pro-inflammatory to regulatory T-cell function. In fact, dnaJP1-induced T-cell production of IL-4 and IL-10 increased, whereas dnaJP1-induced T-cell proliferation and production of IL-2, IFN-γ and tumour necrosis factor-α decreased. The total number of dnaJP1-specific cells did not change over time, whereas expression of Foxp3 by CD4+CD25(bright) cells increased, suggesting that the treatment affected regulatory T-cell function. Thus, rather than clonal deletion, the observed change in immune reactivity to dnaJP1 seemed the outcome of treatment-induced emergence of T cells with a different functional phenotype [58].
A subsequent phase I/II trial of oral dnaJP1 showed again a reduction in the percentage of T cells producing TNF-α and a corresponding trend towards an increased percentage of T cells producing IL-10. Co-expression of a cluster of molecules (programmed cell death protein 1 (PD-1) and its ligands) associated with T-cell regulation was also found to be a prerequisite for successful tolerization in clinical responders. Analysis of the primary efficacy endpoint showed a modest positive clinical effect [59].
The dnaJP1 intervention was aimed at restoring self-regulation by inducing mucosal tolerance to a dominant pro-inflammatory epitope. As with all other HSP-based interventions, the independence of HSP from any primary trigger of autoimmune inflammation is an important conceptual difference from experimental animal models that have used the inciting antigen as the tolerogen and from prior attempts in humans to achieve tolerance for purported antigenic disease triggers [60].
Based on preclinical data obtained from mice and rats, a first-phase clinical trial with an altered peptide ligand (APL) of a human HSP60 (E18-3) sequence was performed in RA. In the preclinical models, the peptide called APL1 was able to increase the percentage of CD4+CD25+FoxP3+ Treg cells in vivo after inoculation into BALB/c mice and in the rat adjuvant arthritis model, where it was able to control histological damage and clinical signs of arthritis, an effect that was associated with increased proportions of FoxP3+ Tregs in the spleen. Furthermore, the APL1 induced Tregs ex vivo in PBMCs obtained from RA patients [61].
In a recently performed open-labelled phase I/II safety trial, the peptide was subcutaneously administered in 18 RA patients, with moderate disease activity and temporally without conventional treatment. No serious adverse events were noted. Furthermore, a reduction in pro-inflammatory cytokine levels was seen. Interestingly, by MRI scoring of hand joint erosions and oedema, a clinical improvement was noted in a significant number of patients (M del Carmen Domínguez 2016, personal communication).
Another potentially attractive and innovative approach for the administration of tolerance-promoting peptides is the loading and re-infusing of tolerized dendritic cells. Herewith, HSP tolerance therapies may join the bandwagon of novel cell-based therapies. The former director of the American Immune Tolerance Network (ITN), J. Bluestone, mentioned the advent of cellular medicines for the next decades [62]: ‘Two decades ago, the pharmaceutical industry was revolutionized by the discovery that biological processes can be harnessed to make medical products (biologics). Today, biomedicine sits on the cusp of a new revolution: the use of human cells as versatile therapeutic engines.’
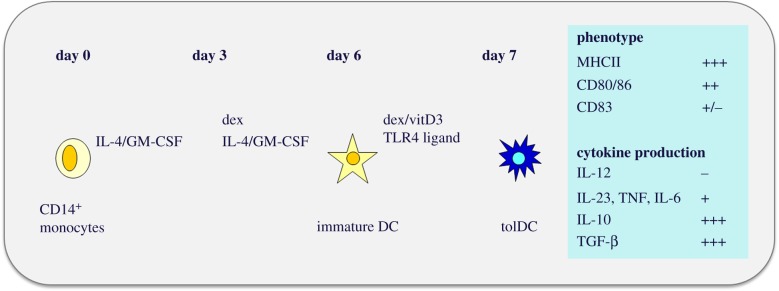
Studies in experimental arthritis models have demonstrated the efficacy of tolerized dendritic cells (tolDC) in preventing and ameliorating disease activity by the induction of Tregs [63]. Two recent proof-of-concept trials in RA patients confirmed the feasibility and safety of antigen-pulsed tolDC. A first study was done in Brisbane (Australia) with autologous DCs modified with a NF-κB inhibitor and loaded with four citrullinated peptide antigens, in a single-centre, open-labelled, first-in-human phase I/II trial. The tolDC were injected intradermally at two progressive dose levels to 18 patients with citrullinated peptide-specific autoimmunity. At one month after treatment, a reduction in effector T cells and an increased ratio of regulatory to effector T cells were observed [64]. No adverse effects were seen. A second study was done in Newcastle (UK) using synovial fluid-loaded autologous DC, differentiated from CD14+ monocytes and that were tolerized with Dexamethasone and vitamin D3, followed by a maturation step using TLR4 ligand MPLA (figure 2). The tolDC were administered by intra-articular injections. Also in this second trial, safety was shown. However, no systemic clinical or immunomodulatory effects were seen [65]. Building on the experience obtained in the Newcastle trial, we are planning a trial to be carried out in the Utrecht University Hospital in collaboration with Hilkens and Isaacs from the Newcastle group. Using the HSP70-derived B29 peptide (or one of the mammalian B29 homologues) we will be able, after this antigen-specific tolerance-promoting intervention, to monitor the immunomodulatory effect at the level of B29-specific T cells. In addition, whereas the patients of the Newcastle trial were relatively advanced RA cases, which enabled the collection of autologous synovial fluid for tolDC loading, the use of B29 will allow for the selection of patients with relatively inactive or less advanced disease states. For tolerance induction to be successful, such an inactive state of disease may well turn out be essential for reaching the desired effect.
Figure 2.
Protocol for the generation of tolDC and phenotypic characterization (as used for the AUTODECRA study performed by Hilkens and Isaacs in the University of Newcastle, UK) [65].
Apart from this tolDC-mediated tolerance approach, preferably as a next step following a proven Treg induction with the B29/tolDC approach, we will explore the possibilities of using peptide B29 as a direct in vivo immunomodulatory compound or therapeutic vaccine. For this, it will be essential to deliver such a peptide in the context of the right delivery systems for reaching the natural tolDCs, as present in the tissues, and in the presence of adjuvants that favour the expansion of Tregs. Such a therapy would benefit patients not only with established disease but also before the onset of disease to halt the disease process at the early immune initiation phase of the disease, before any serious damage has occurred.
12. Conclusion
Mechanistic understanding of chronic inflammatory diseases has improved. Until now, this has led to significantly better ‘therapeutic’ possibilities. However, in most cases, these possibilities are based on the non-specific inhibition of immunity, which leads to side effects such as reduced resistance to infection or cancer. Now with the understanding of active tolerance mechanisms, such as those mediated by Tregs, innovative possibilities are in sight for the induction of therapeutic tolerance. HSPs can be targets for the actions of Treg given their upregulated expression at sites of inflammation. Experimental models have shown the anti-inflammatory activities of both intracellular and extracellular HSPs. In combination with novel immuno-manipulative strategies such as the incorporation of the relevant antigen into tolerized DCs, HSP may offer chances of re-inducing physiological tolerance in patients with autoimmune diseases. It seems that only this can lead to a permanent and drug-free remission in these debilitating conditions.
Acknowledgements
Dr Ruurd van der Zee is acknowledged for critical reading of the manuscript.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests. WvE has shares in Trajectum Pharma, SME.
Funding
Part of work described was financed by the Dutch Arthritis Foundation.
References
- 1.Dengjel J, et al. 2005. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 102, 7922–7927. ( 10.1073/pnas.0501190102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigh L, et al. 1997. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat. Med. 3, 1150–1154. ( 10.1038/nm1097-1150) [DOI] [PubMed] [Google Scholar]
- 3.Wieten L, van der Zee R, Spiering R, Wagenaar-Hilbers J, van Kooten P, Broere F, van Eden W. 2010. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 62, 1026–1035. ( 10.1002/art.27344) [DOI] [PubMed] [Google Scholar]
- 4.Wieten L, Broere F, van der Zee R, Koerkamp EK, Wagenaar J, van Eden W. 2007. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett. 581, 3716–3722. ( 10.1016/j.febslet.2007.04.082) [DOI] [PubMed] [Google Scholar]
- 5.Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. 1996. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFκB activation. J. Biol. Chem. 271, 17 724–17 732. ( 10.1074/jbc.271.30.17724) [DOI] [PubMed] [Google Scholar]
- 6.Ferat-Osorio E, et al. 2014. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. 11, 19 ( 10.1186/1476-9255-11-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133, 775–787. ( 10.1016/j.cell.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 8.Lahl K, et al. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204, 57–63. ( 10.1084/jem.20061852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildin RS, Freitas A. 2005. IPEX and FOXP3: clinical and research perspectives. J. Autoimmun. 25, 56–62. ( 10.1016/j.jaut.2005.04.008) [DOI] [PubMed] [Google Scholar]
- 10.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774. ( 10.1084/jem.20070719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun CM, et al. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785. ( 10.1084/jem.20070602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atarashi K, et al. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. ( 10.1038/nature12331) [DOI] [PubMed] [Google Scholar]
- 13.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. 2004. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173, 7259–7268. ( 10.4049/jimmunol.173.12.7259) [DOI] [PubMed] [Google Scholar]
- 14.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. 2010. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441. ( 10.4049/jimmunol.0904028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav M, et al. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722. ( 10.1084/jem.20120822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyss L, et al. 2016. Affinity for self antigen selects Treg cells with distinct functional properties. Nat. Immunol. 17, 1093–1101. ( 10.1038/ni.3522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285. ( 10.1084/jem.20040165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. 2007. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 56, 732–742. ( 10.1002/art.22414) [DOI] [PubMed] [Google Scholar]
- 19.van Loosdregt J, et al. 2013. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 39, 298–310. ( 10.1016/j.immuni.2013.07.019) [DOI] [PubMed] [Google Scholar]
- 20.Afzali B, et al. 2013. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur. J. Immunol. 43, 2043–2054. ( 10.1002/eji.201243296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrath J, et al. 2011. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur. J. Immunol. 41, 2279–2290. ( 10.1002/eji.201041004) [DOI] [PubMed] [Google Scholar]
- 22.Wehrens EJ, et al. 2011. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood 118, 3538–3548. ( 10.1182/blood-2010-12-328187) [DOI] [PubMed] [Google Scholar]
- 23.Petrelli A, van Wijk F. 2016. CD8+ T cells in human autoimmune arthritis: the unusual suspects. Nat. Rev. Rheumatol. 12, 421–428. ( 10.1038/nrrheum.2016.74) [DOI] [PubMed] [Google Scholar]
- 24.Miyara M, Ito Y, Sakaguchi S. 2014. TREG-cell therapies for autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 10, 543–551. ( 10.1038/nrrheum.2014.105) [DOI] [PubMed] [Google Scholar]
- 25.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306. ( 10.1038/86302) [DOI] [PubMed] [Google Scholar]
- 26.Tang Q, et al. 2004. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465. ( 10.1084/jem.20040139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevach EM, Thornton AM. 2014. tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102. ( 10.1111/imr.12160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AG, Arvey A, Jin W, Rudensky AY. 2014. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078. ( 10.1038/ni.3004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seddiki N, et al. 2014. Human antigen-specific CD4+ CD25+ CD134+ CD39+ T cells are enriched for regulatory T cells and comprise a substantial proportion of recall responses. Eur. J. Immunol. 44, 1644–1661. ( 10.1002/eji.201344102) [DOI] [PubMed] [Google Scholar]
- 30.Haribhai D, et al. 2011. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35, 109–122. ( 10.1016/j.immuni.2011.03.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cebula A, et al. 2013. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497, 258–262. ( 10.1038/nature12079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lathrop SK, et al. 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254. ( 10.1038/nature10434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12 204–12 209. ( 10.1073/pnas.0909122107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilate AM, Bousbaine D, Mesin L, Agudelo M, Leube J, Kratzert A, Dougan SK, Victora GD, Ploegh H. 2016. Tissue-specific emergence of regulatory and intraepithelial T cells from a clonal T cell precursor. Sci. Immunol. 1, eaaf7471 ( 10.1126/sciimmunol.aaf7471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moudgil KD, Kim E, Yun OJ, Chi HH, Brahn E, Sercarz EE. 2001. Environmental modulation of autoimmune arthritis involves the spontaneous microbial induction of T cell responses to regulatory determinants within heat shock protein 65. J. Immunol. 166, 4237–4243. ( 10.4049/jimmunol.166.6.4237) [DOI] [PubMed] [Google Scholar]
- 36.Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GAW. 2006. Mechanisms of disease: the hygiene hypothesis revisited. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 275–284. ( 10.1038/ncpgasthep0471) [DOI] [PubMed] [Google Scholar]
- 37.van Eden W, Koets A, van Kooten P, Prakken B, van der Zee R. 2003. Immunopotentiating heat shock proteins: negotiators between innate danger and control of autoimmunity. Vaccine 219, 897–901. ( 10.1016/S0264-410X(02)00538-8) [DOI] [PubMed] [Google Scholar]
- 38.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. 2013. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity 38, 373–383. ( 10.1016/j.immuni.2012.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen IR. 2007. Biomarkers, self-antigens and the immunological homunculus. J. Autoimmun. 29, 246–249. ( 10.1016/j.jaut.2007.07.016) [DOI] [PubMed] [Google Scholar]
- 40.van Eden W, Cohen IR. 2000. Autoimmunity and microbial infection. In Effects of microbes on the immune system (eds Cunningham MW, Fujinami RS), pp. 569–591. Philadelphia, PA: Lippincott-Raven Press. [Google Scholar]
- 41.Anderton SM, van der Zee R, Prakken B, Noordzij A, van Eden W. 1995. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J. Exp. Med. 181, 943–952. ( 10.1084/jem.181.3.943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Herwijnen MJ, et al. 2012. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc. Natl Acad. Sci. USA 109, 14 134–14 139. ( 10.1073/pnas.1206803109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartl FU, Hayer-Hartl M. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858. ( 10.1126/science.1068408) [DOI] [PubMed] [Google Scholar]
- 44.Boog CJ, et al. 1992. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J. Exp. Med. 175, 1805–1810. ( 10.1084/jem.175.6.1805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedlackova L, Nguyen TT, Zlacka D, Sosna A, Hromadnikova I. 2009. Cell surface and relative mRNA expression of heat shock protein 70 in human synovial cells. Autoimmunity 42, 17–24. ( 10.1080/08916930802227466) [DOI] [PubMed] [Google Scholar]
- 46.Schett G, Redlich K, Xu Q, Bizan P, Groger M, Tohidast-Akrad M, Kiener H, Smolen J, Steiner G. 1998. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J. Clin. Invest. 102, 302–311. ( 10.1172/JCI2465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moudgil KD, Durai M. 2008. Regulation of autoimmune arthritis by self-heat-shock proteins. Trends Immunol. 29, 412–418. ( 10.1016/j.it.2008.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pockley AG, Multhoff G. 2008. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found. Symp. 291, 86–95; discussion 96–100, 137–40 ( 10.1002/9780470754030.ch7) [DOI] [PubMed] [Google Scholar]
- 49.Pockley AG, Muthana M, Calderwood SK. 2008. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 33, 71–79. ( 10.1016/j.tibs.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 50.Borges TJ, et al. 2010. Prolonged survival of allografts induced by mycobacterial Hsp70 is dependent on CD4+CD25+ regulatory T cells. PLoS ONE 5, e14264 ( 10.1371/journal.pone.0014264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labrador-Garrido A, et al. 2014. Chaperoned amyloid proteins for immune manipulation: α-Synuclein/Hsp70 shifts immunity toward a modulatory phenotype. Immun. Inflamm. Dis. 2, 226–238. ( 10.1002/iid3.39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. 2006. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 116, 2022–2032. ( 10.1172/JCI28423) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Vos MJ, Hageman J, Carra S, Kampinga HH. 2008. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 47, 7001–7011. ( 10.1021/bi800639z) [DOI] [PubMed] [Google Scholar]
- 54.Paludan C, et al. 2005. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307, 593–596. ( 10.1126/science.1104904) [DOI] [PubMed] [Google Scholar]
- 55.Xilouri M, Stefanis L. 2016. Chaperone mediated autophagy in aging: starve to prosper. Ageing Res. Rev. 32, 13–21. ( 10.1016/j.arr.2016.07.001) [DOI] [PubMed] [Google Scholar]
- 56.de Wolf C, et al. 2016. An arthritis-suppressive and Treg Cell-Inducing CD4+ T cell epitope is functional in the context of HLA-restricted T cell responses. Arthritis Rheumatol. 68, 639–647. ( 10.1002/art.39444) [DOI] [PubMed] [Google Scholar]
- 57.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. 2001. β-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 358, 1749–1753. ( 10.1016/S0140-6736(01)06801-5) [DOI] [PubMed] [Google Scholar]
- 58.Prakken BJ, et al. 2004. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 101, 4228–4233. ( 10.1073/pnas.0400061101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koffeman EC, et al. 2009. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 60, 3207–3216. ( 10.1002/art.24916) [DOI] [PubMed] [Google Scholar]
- 60.Weiner HL, da Cunha AP, Quintana F, Wu H. 2011. Oral tolerance. Immunol. Rev. 241, 241–259. ( 10.1111/j.1600-065X.2011.01017.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbera A, et al. 2016. APL1, an altered peptide ligand derived from human heat-shock protein 60, increases the frequency of Tregs and its suppressive capacity against antigen responding effector CD4+T cells from rheumatoid arthritis patients. Cell Stress Chaperones 21, 735–744. ( 10.1007/s12192-016-0698-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bluestone JA, Trotta E, Xu D. 2015. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert. Opin. Ther. Targets 19, 1091–1103. ( 10.1517/14728222.2015.1037282) [DOI] [PubMed] [Google Scholar]
- 63.Stoop JN, Robinson JH, Hilkens CM. 2011. Developing tolerogenic dendritic cell therapy for rheumatoid arthritis: what can we learn from mouse models? Ann. Rheum. Dis. 70, 1526–1533. ( 10.1136/ard.2011.151654) [DOI] [PubMed] [Google Scholar]
- 64.Benham H, et al. 2015. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 7, 290ra87 ( 10.1126/scitranslmed.aaa9301) [DOI] [PubMed] [Google Scholar]
- 65.Bell GM, et al. 2017. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 76, 227–234. ( 10.1136/annrheumdis-2015-208456) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.