Abstract
Mood disorders affect nearly a quarter of the world's population. Therefore, understanding the molecular mechanisms underlying these conditions is of great importance. FK-506 binding protein 5 (FKBP5) encodes the FKBP51 protein, a heat shock protein 90 kDa (Hsp90) co-chaperone, and is a risk factor for several affective disorders. FKBP51, in coordination with Hsp90, regulates glucocorticoid receptor (GR) activity via a short negative feedback loop. This signalling pathway rapidly restores homeostasis in the hypothalamic–pituitary–adrenal (HPA) axis following stress. Expression of FKBP5 increases with age through reduced DNA methylation. High levels of FKBP51 are linked to GR resistance and reduced stress coping behaviour. Moreover, common allelic variants in the FKBP5 gene are associated with increased risk of developing affective disorders like anxiety, depression and post-traumatic stress disorder (PTSD). This review highlights the current understanding of the Hsp90 co-chaperone, FKBP5, in disease from both human and animal studies. In addition, FKBP5 genetic implications in the clinic involving life stress exposure, gender differences and treatment outcomes are discussed.
This article is part of the theme issue ‘Heat shock proteins as modulators and therapeutic targets of chronic disease: an integrated perspective’.
Keywords: FKBP51, depression, PTSD, anxiety, glucocorticoids, stress
1. Introduction
Over the past two decades, scientific literature has greatly increased our understanding of how stressful situations affect our psychological and physical health. Considering that adverse life events and exposure to trauma are very common, there is great interest in understanding the impact that disruption of stress signalling homeostasis has on the development of central nervous system (CNS) diseases. The 90 kDa heat shock protein (Hsp90) is an integral component in the molecular chaperone machine that regulates hormone signalling and stress response. Hsp90 regulates glucocorticoid receptor (GR) activity through several signalling feedback loops able to restore hypothalamic–pituitary–adrenal (HPA) homeostasis after stressful situations [1]. The regulation of these feedback loops is primarily accomplished through interactions with two Hsp90 co-chaperones, FK-506 binding protein 51 (FKBP51) and 52 (FKBP52).
In addition to the glucocorticoid signalling, these molecular chaperones are known to participate in a variety of processes in the CNS. In the cytosol, these chaperones are responsible for regulating protein aggregation, protein trafficking and cellular metabolism. While Hsp90, FKBP51 and FKBP52 are primarily cytoplasmic residents, they have also been found to be released from the cell in exosomes [2,3]. Extracellular Hsp90 has been shown to regulate neuronal migration and extracellular protein aggregation [4,5], as well as a variety of other functions [6], but the function of extracellular FKBP51 and FKBP52 has not been well-characterized. On the cytoplasmic side of the plasma membrane, FKBP51 and FKBP52 have been shown to regulate synaptic plasticity and ion homeostasis by interacting with transmembrane channels such as the store-operated calcium (SOC) channels. In endothelial cells, overexpression of FKBP51 led to a decrease of calcium entry through SOC, while FKBP52 enhanced SOC entry [7]. Changes in calcium levels are known to disrupt essential cortical processes including neuronal excitability, neurotransmitter release and cell metabolism. In addition to FKBP51's participation in key cellular functions, genetic variants are common in patients with mental health disorders, suggesting FKBP51 may be a therapeutic target for the treatment of these disorders. This review will focus on the role of Hsp90/FKBP51 heterocomplexes in GR signalling and how dysregulation of this pathway has been associated with stress-related illnesses such as anxiety, depression and post-traumatic stress disorder (PTSD). Advances in using FKBP51 as a biomarker and potential therapeutics targeting this pathway will also be discussed.
2. Hsp90/FKBP51 heterocomplexes
(a). Hypothalamic–pituitary–adrenal axis regulation and glucocorticoid receptor signalling
Our central stress response system is the HPA axis. Upon stress, the paraventricular nucleus of the hypothalamus secretes corticotrophin-releasing hormone (CRH), which stimulates the release of the adrenocorticotropic hormone (ACTH) from the pituitary gland into the blood. ACTH activates the synthesis and secretion of glucocorticoids (like cortisol) from the adrenal glands, leading to higher cortisol levels in blood and tissues. The stress hormone, cortisol, targets two main receptors: mineralocorticoid receptor (MR) and GR. In particular, GR is highly expressed in key regions of the HPA axis, including the hippocampus, amygdala and hypothalamus [8]. As a negative feedback mechanism, cortisol binds to hypothalamic and pituitary GR. This interaction leads to the inhibition of secreted hormones ACTH and CRH, and restores basal cortisol levels [9]. The HPA stress response is essential for survival and enables triggering of the fight-or-flight response. However, an imbalance between activation and negative feedback in this system is often observed in psychiatric patients.
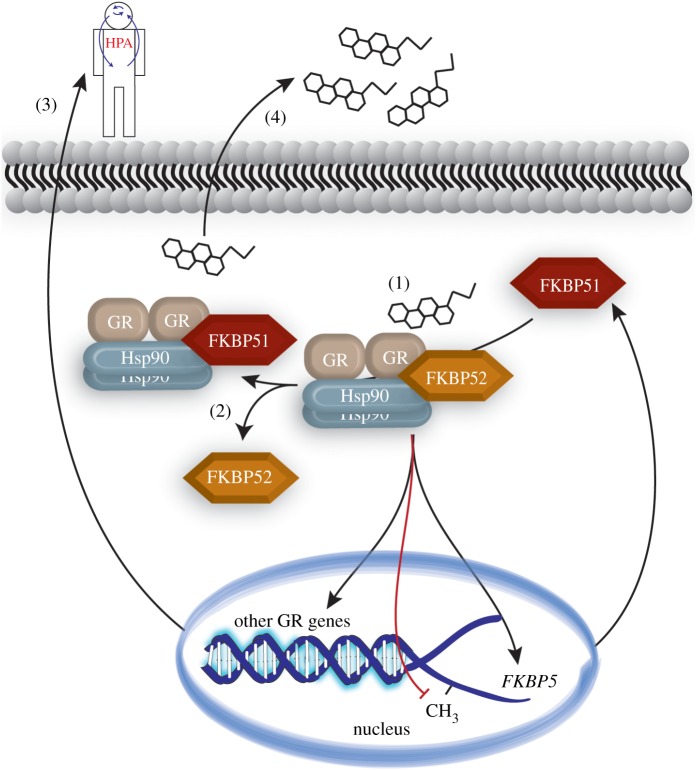
Following stress, cortisol diffuses into the cytosol where it binds to GR. Intracellularly, GR binds to a multimeric chaperone complex. These heterocomplexes are comprised of Hsp90, FKBP51 or FKBP52, Hsp70 and p23. Together, these complexes play a key role in modulating steroid receptor-associated activity, which regulates processes such as sexual reproduction, metabolism and stress adaptation [1,10,11]. Hsp90 serves as the centre point of this interaction through direct binding to GR and the FKBPs. In fact, inhibiting the ATPase activity of Hsp90 leads to reduced GR activity [12]. Both FKBP51 and FKBP52 bind directly to Hsp90 through their tetratricopeptide (TPR) domain [13]. Although FKBP51 and FKBP52 share high structural similarity and compete for binding [1,14], they are divergent in their regulatory role on GR activity. A complex of Hsp90 and FKBP51 slows GR translocation into the nucleus, reducing its activity [15]; inversely, FKBP52 enhances GR nuclear translocation and signalling [16]. Upon glucocorticoid binding to GR, FKBP51 dissociates from the Hsp90-heterocomplex and is replaced by FKBP52, leading to dynein-dependent GR nuclear translocation. Following nuclear translocation, GR homodimers bind to glucocorticoid response elements (GRE) to induce or inhibit the expression of various genes. One of the genes induced by this GR activity is the gene that encodes FKBP51, FKBP5 [17]. High intracellular levels of FKBP51 lower the binding affinity of GR for glucocorticoids leading to GR resistance, as seen in squirrel monkeys and New World primates [14,18]. Therefore, the Hsp90/FKBP51 complex serves as a short, negative feedback regulator of GR signalling by diminishing GR ligand binding affinity (figure 1). This regulation is important to the understanding of several affective diseases, since these disorders have been associated with both increased and decreased GR sensitivity along with changes in basal and stimulated cortisol levels.
Figure 1.
Overall regulation of FKBP5 expression by GR following stress. (1) Stress produces CORT, which binds to GR, activating the GR/Hsp90/FKBP52 heterocomplex. GR forms a homodimer and translocates to the nucleus where it binds the GRE in the promoter region. This leads to reduced FKBP5 methylation and increased FKBP51 production. (2) Increased FKBP51 outcompetes FKBP52 and decreases affinity of GR/Hsp90 for CORT and prevents GR translocation. (3) Impaired feedback inhibition of HPA axis. (4) Decreased CORT use by GR/Hsp90 leads to higher circulating CORT levels (hypercortisolemia). CORT = cortisol or corticosterone; FKBP51 = FK506 binding protein 51; FKBP52 = FK506 binding protein 52; GR = glucocorticoid receptor; Hsp90 = 90 kDa heat shock protein; HPA = hypothalamic–pituitary–adrenal axis; CH3 = methyl group. (Online version in colour.)
(b). FKBP51: stress, epigenetic and genetic regulation
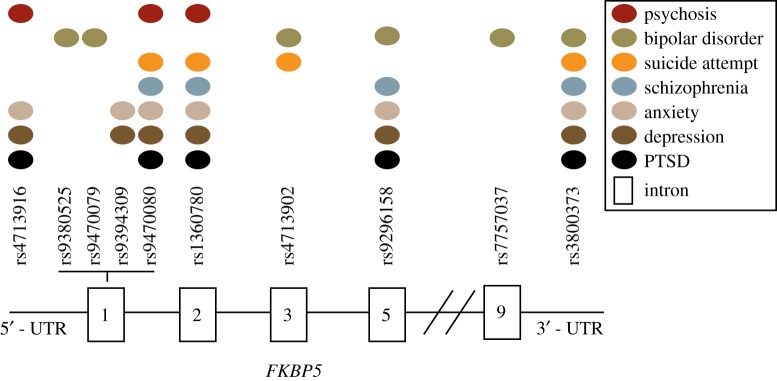
Here we discuss how dysregulation of the Hsp90/FKBP51 heterocomplex may increase the susceptibility to developing psychiatric disorders. Variations in the function of this chaperone complex can be caused by changes in FKBP5 levels due to genetic, epigenetic and environmental factors. In fact, several studies have demonstrated that the interaction of stress with FKBP5 single nucleotide polymorphisms (SNP), such as rs1360780, increases the risk for developing PTSD (see [19] for review). Additionally, this allelic variant (rs1360780) has been shown to increase FKBP5 expression through decreased DNA methylation [20,21], which can also lead to GR resistance [22]. Similarly, other FKBP5 allelic variations have been linked to several mental disorders such as anxiety, depression and schizophrenia [23–28] (figure 2). This suggests that, in addition to environmental factors such as stress, our genetic predisposition may alter our neurophysiology including biochemical processes, brain connectivity and gene expression (figure 3).
Figure 2.
Schematic overview of the FKBP5 single nucleotide polymorphisms (SNPs) and their association with mental health disorders. As represented, there is a genetic overlap between mental health disorders. Four FKBP5 SNPs (rs9470080, rs1360780, rs9296158, rs3800373) are significantly associated with at least five diseases. Most of these SNPs have been reported in bipolar disorder, anxiety, depression and PTSD. Introns are represented by rectangles. (Online version in colour.)
Figure 3.
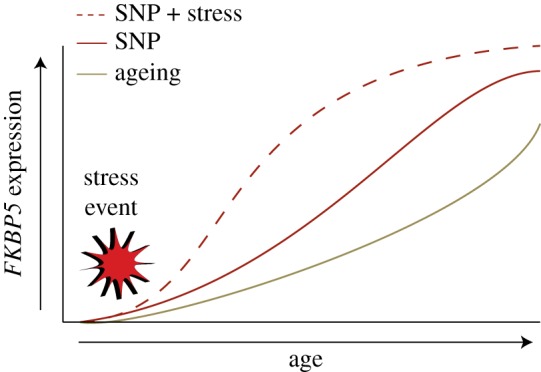
Graphic representation of how SNPS and stress regulate FKBP5 expression through ageing. Along with ageing, common variants of the FKBP5 gene are known to increase expression of FKBP5. These SNPs and their interaction with environmental factors (ex. stress event) are associated with augmented FKBP5 expression leading to a higher risk of developing psychiatric disorders. (Online version in colour.)
FKBP5 DNA methylation is a dynamic process. It is well-documented that stress exposure can induce epigenetic changes, such as alterations in DNA methylation. DNA methylation consists in transferring a methyl group onto a CpG site (or CG-rich region), which can affect gene transcription and expression [29]. Studies in tissue from human and mouse brains demonstrate that FKBP5 DNA methylation is inversely correlated with FKBP51 expression [20,30–32]. Altered FKBP51 expression is significant since FKBP51 competes with other TPR-containing proteins for the same binding site on Hsp90. Since FKBP51 is one of the strongest-binding Hsp90 co-chaperones [33], imbalances in FKBP51 expression can lead to dramatic alterations in Hsp90 activity, client selection and the fate of client proteins that are dependent on Hsp90 interactions and regulation [32]. Taken together, epigenetic changes, in tandem with or in addition to functional genetic variants, can provide mechanisms to regulate FKBP5 gene expression. This suggests that individual differences in basal or stress-induced FKBP5 expression could affect one's resiliency to stress-related psychiatric disorders [34–37]. Understanding the interplay between genetic predisposition and environmental factors in the development of stress-related disorders may facilitate individualized treatment for these patients.
3. Risk for psychiatric disorders
(a). Depression
Depression is a polysymptomatic disorder, with presentations varying by individual but often including nervousness, irritability, sleep problems and decreased energy [38,39]. Each of these symptoms has been linked to a physiological response to stress through cortisol regulation [40,41]. FKBP5 SNPs interact with childhood abuse to increase risk for major depression or depressive symptoms [42–44]. This increased risk may be mediated by changes in limbic circuit structure and function observed with FKBP5 genotypes. In support of this, altered brain activity was measured by functional magnetic resonance imaging (fMRI) during an attentional focus task in patients with major depression who carry the T risk allele of the FKBP5 SNP rs1360780 [42]. Additionally, risk SNP carriers with major depression presented with reduced FKBP5 DNA methylation as well as reduced grey matter volume in the prefrontal cortex [45].
There is also evidence that suggests that these genetic, neurophysiological and neuroanatomical changes can impact the efficacy of current antidepressant therapeutics. For example, the rs1360780 risk allele was correlated with increased FKBP51 protein in lymphocytes, more depressive episodes and faster responses to antidepressants [21,46]. Other researchers found the rs4713916 risk allele (A compared to G) positively correlates with better antidepressant treatment [46]. In another study, levels of FKBP5 were found to be lower in patients who responded favourably to antidepressant treatment [47]. Since FKBP51 operates in several adaptive feedback loops, it is difficult to draw conclusions about the meaning of altered FKBP5 mRNA levels in these studies. Studies in mammalian cells, which were correlated to antidepressant response in human ex vivo cell culture models, suggest that this response is regulated through the levels of FKBP51 altering autophagy activation [48,49]. Taken together, these studies demonstrate that FKBP5 expression can regulate response to antidepressants; however, additional clinical studies are needed to further understand the role of FKBP51 in the clinical manifestation of anxiety and depressive disorders.
(b). Post-traumatic stress disorder
PTSD is characterized by flashbacks of traumatic events, increased arousal or startle state, evasion of places and people, and a decline in cognitive and emotional functions [50]. Patients with PTSD have been shown to exhibit low basal levels of cortisol and enhanced cortisol suppression following dexamethasone (DEX) [34,51,52]. Interestingly, patients with PTSD displayed increased GR protein levels in peripheral lymphocytes without changes in other GR signalling regulators like Hsp90 [53,54]. However, another study reported unchanged cortisol and HPA function in patients with PTSD [55]. This inconsistency may be attributed to factors such as type of trauma, number of individuals in the study, comorbidity with other illness or genetic predisposition.
It is important to mention that even though most people will experience a traumatic situation in their lives, only some will develop PTSD [55]. Current exposure therapy and pharmacological interventions can alleviate some PTSD symptoms [56], but, similar to depression, these therapies have not been effective for all PTSD patients [34]. Some differences in ‘resiliency’ have been attributed to the interaction between FKBP5 variants or expression differences and environmental factors [57,58]. An increased number of studies highlight the influence of FKBP5 SNPs and their interaction with stressful environments as a risk factor for the development of PTSD. Additionally, Mehta and colleagues observed an increase in GR sensitivity only in PTSD carriers of FKBP5 SNP, rs9296158 [59]. In general, four SNPs in FKBP5 have been associated with child abuse severity and increased risk to develop PTSD in adulthood [26,60]. Moreover, Watkins et al. reported that child abuse coupled with distinct FKBP5 SNPs significantly correlated with a higher PTSD symptom severity in a sample of European-American U.S. military veterans [61]. Another study duplicated this association in African-Americans but not in European-Americans [62]. Taken together, this suggests that the effect of FKBP5 on PTSD risk may be influenced by other factors such as context-specific trauma.
(c). Anxiety
Similar to depression, anxiety disorders can present with a variety of symptoms including nervousness, irritability, sleep problems and decreased energy [38]. Despite these commonalities, the role of FKBP5 in the development and heritability of anxiety disorders has gone largely unexplored in the patient population. Recently, however, FKBP5 polymorphisms were associated with increased risk of anxiety in patients with cancer [35]. Since other studies have failed to link FKBP5 variants with anxiety [63–65], this suggests that FKBP5 may specifically affect stress-induced anxiety.
To elucidate the potential roles for FKBP5 in anxiety disorders, we must examine the literature pertaining to the extensive studies conducted in animal models. Recent studies in rodent models have provided supporting evidence and further insight into the role of FKBP5 in psychiatric disease (table 1). Many studies have demonstrated that anxiety is controlled by the amygdala [77–79]. Although a global knockout of FKBP5 in mice did not alter anxiety-like behaviour under basal [36] and stress conditions [72,80], selectively reducing FKBP51 in the amygdala with viral vectors reduces stress-induced anxiety-like behaviours in mice [75]. Similarly, pharmacological disruption of FKBP51 signalling locally in the amygdala also produces an anxiolytic effect [69]. In support of this, viral-mediated overexpression of FKBP51 in the basolateral or central amygdala enhanced anxiety-like behaviour [69]. In contrast, anxiety was not altered by overexpressing FKBP51 in the dorsal hippocampus of mice [69] or by knocking down FKBP5 in the prelimbic cortex of rats [76]. The region-specific influence of FKBP51 in the amygdala is critical for anxiety regulation. In fact, stress-related mechanisms have also been shown to modulate FKBP51 in the amygdala. One study found that chronic stress increases amygdalar FKBP51 in mice through a mechanism involving neuropsin-mediated cleavage of the tyrosine receptor kinase, EphB2 [75]. A more recent study found that microRNA-15a inhibits amygdalar FKBP51 expression, which reduced anxiety levels in mice [81]. They also found that selectively reducing amygdalar microRNA-15a increased FKBP51 and anxiety-like behaviour in a chronic stress paradigm. Besides anxiety, FKBP51 can also modulate stress resiliency in rodents. FKBP5−/− mice have been shown to be more resilient to the effects of various stress paradigms compared to wild-type littermates [72,73,80]. Moreover, FKBP5 knockout mice have lower HPA activity and reduced changes in sleep, demonstrating a pro-resilience sleep phenotype that is a common symptom in anxiety disorders [82]. While relating these results to humans can be difficult, the data demonstrate that FKBP51 has a clear role in regulating stress response and risk of phenotypes in animal models, reminiscent of the role of FKBP51 in human affective disorders.
Table 1.
Summary of rodent studies manipulating FKBP51 expression. Affected brain structures and behavioural observations are described according to the stress model or molecular manipulation performed. FKBP51 = FK506 binding protein 51; CORT = corticosterone; DEX = dexamethasone; CA1 = Cornu Ammonis 1; DG = dentate gyrus; PVN = paraventricular nucleus; CeA = central amygdala; CSDS = chronic social defeat stress; CMS = chronic mild stress; HPA = hypothalamic–pituitary–adrenal axis; KO, knock-out; SAFit2 = selective antagonist of FKBP51 by induced fit; shRNA, short hairpin RNA; IL = infralimbic cortex; Increase ( ) or decrease (
) or decrease ( ) FKBP51 levels or activity. (Online version in colour.)
) FKBP51 levels or activity. (Online version in colour.)
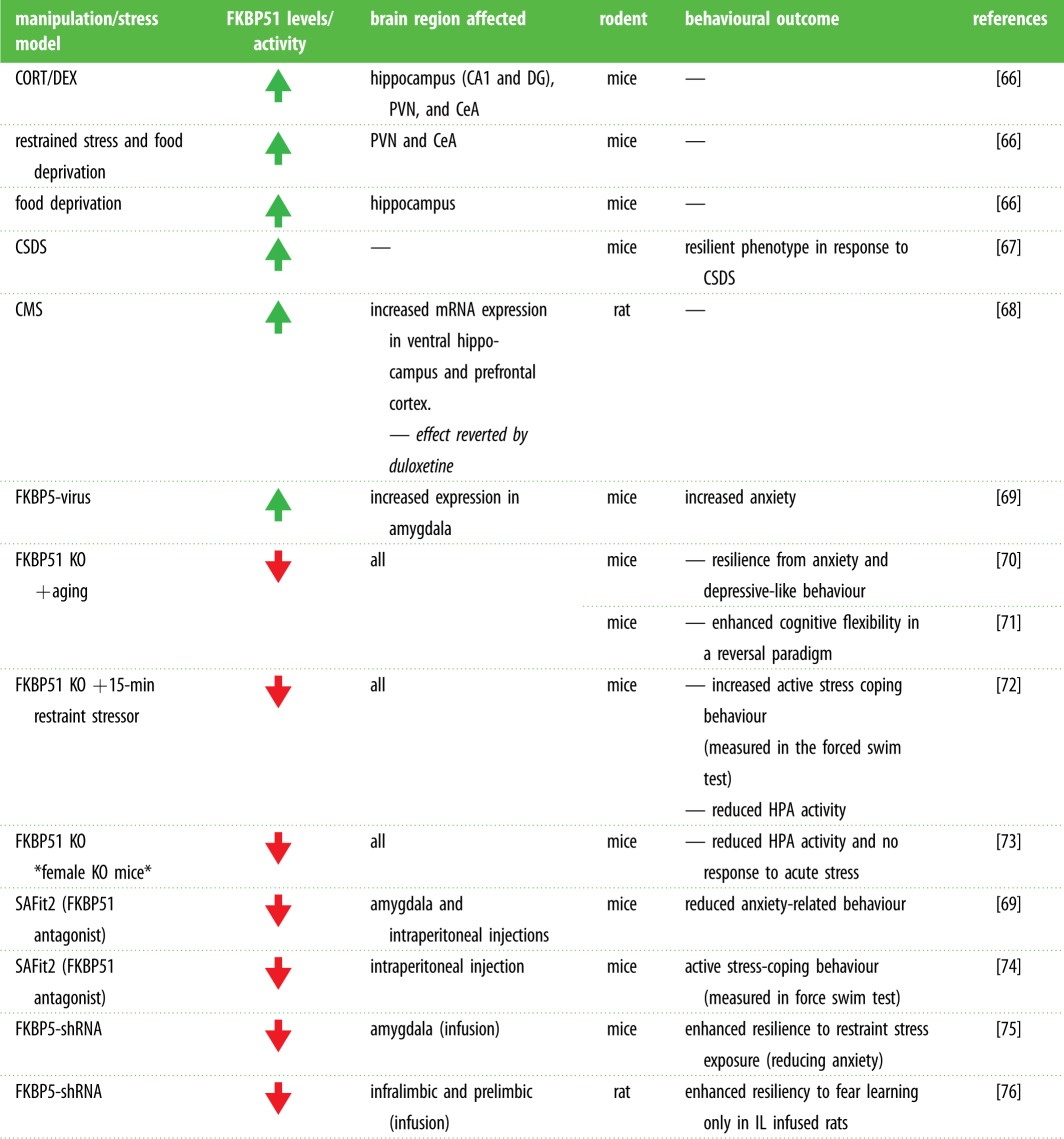 |
(d). Other FKBP5 risk SNPs and altered protein expression
Common FKBP5 SNPs have also been shown to increase risk for schizophrenia [28], bipolar disorder [83], suicide attempt [84] and psychosis [85]. Many of these SNPs alter FKBP5 expression and are risk factors for more than one of these disorders (figure 2). Increased FKBP5 expression was found in the prefrontal cortex of patients diagnosed with schizophrenia [28] and bipolar disorder [86]. Additionally, FKBP5 expression was found to be increased in the medial temporal and frontal gyrus in patients with Alzheimer's disease [32] and autism spectrum disorders [87]. Inversely, FKBP5 expression was shown to be reduced in the amygdala of post-mortem brains from suicide victims [88]. More studies need to be done to understand the mechanism underlying these FKBP5 expression changes and how this change in FKBP51 levels effects disease onset and/or progression.
Closely linked to FKBP5 expression, FKBP5 DNA methylation has been shown to be altered following prenatal and postnatal stress. In fact, a recent study showed that prenatal maternal stress induced methylation changes in genes regulating the HPA axis [89]. Although they assessed a small number of participants (24 newborn–mother dyads), FKBP5 cg03546163 methylation was higher in placenta and maternal blood in the traumatized group. In line with this study, Paquette and colleagues investigated whether placental FKBP5 methylation changes could alter fetal neurodevelopment related to HPA dysregulation. They found that higher methylation in FKBP5 intron 7 was associated with greater infant motor activity [90]. These results are consistent with the hyperarousal associated in PTSD [91] and suggest FKBP5 may regulate vigilance. Methylation changes were also observed in a recent study including 174 ethnically diverse children who were exposed to physical or emotional maltreatment. In comparison to controls, children who reported moderate or severe maltreatment also showed lower levels of FKBP5 methylation at the two regulatory regions in intron 7 [92]. Similar alterations in FKBP5 DNA methylation have also been associated with preterm birth. FKBP5 DNA methylation was lower in preterm infants compared to term equivalent-age infants [93]. This decreased methylation was restored to normal levels by 1 year of age. Additional studies are necessary to evaluate if these alterations increase any risk long-term. Just recently, the interaction of FKBP5 risk SNPs with early life stress in preterm infants was investigated. This study revealed that risk SNPs, combined with the stress in the neonatal intensive care unit, increased risk for neurodevelopmental impairments [94]. Additional studies are needed to both confirm these associations and to evaluate the longitudinal effects of this interaction.
4. FKBP51: a potential biomarker and therapeutic target for mental health disorders
The use of FKBP51 levels as a potential biomarker to predict clinical outcomes and risk for neuropsychiatric disorders is currently being investigated. Since expression of FKBP5 in neural tissues is practically impossible to determine in living patients, peripheral correlates are being examined. In mice, FKBP5 methylation in whole blood and the brain was positively correlated following chronic stress [30,31], but whether this correlation translates to humans with peripheral expression or epigenetic changes has not been determined. However, in human studies, FKBP51 levels were found to be significantly higher in lymphocytes in depressed individuals with common FKBP5 SNPs. These changes correlated with faster antidepressant responses, but more frequent depressive episodes [21]. Another study showed that FKBP5 expression was significantly decreased in whole blood from patients with PTSD [34]. So, while alterations in FKBP5 have been identified in peripheral tissue of humans with mood disorders, to understand the relevance of FKBP5 as a biomarker, more studies need to be performed to understand the relationship between FKBP51 levels in the periphery and the brain and how this relates to risk, progression and treatment outcomes of neuropsychiatric disease.
Since high expression of FKBP51 has been linked to increased susceptibility to develop mood disorders, decreasing FKBP51 levels or activity may be beneficial in preventing or treating these neuropsychiatric disorders. This has been supported in several independent preclinical studies. FKBP5−/− mice are not only viable, they are resilient to depressive-like behaviours when compared to wild-type littermates, without affecting cognition or locomotor functions [70,72,80]. Thus, it has been proposed that inhibition of FKBP51 activity or expression could be advantageous in stress-related and depressive disorders. However, since FKBP51 is highly homologous to other FKBP proteins, like FKBP52, developing treatments with selectivity for FKBP51 has been difficult [74]. Targeting FKBP52 could be detrimental, since ablating FKBP52 in mice resulted in male infertility, increased stress sensitivity and affected neuroendocrine response [95]. Recently, a selective FKBP51 antagonist was developed by induced fit. This antagonist, SAFit2, has higher selectivity for FKBP51 than FKBP52 and was shown to enhance neuronal cell and stress-coping behaviour [69,74]. While these developments are promising, additional studies are needed to better characterize SAFit2 and to identify alternative therapeutic interventions that can specifically regulate FKBP51 activity or levels.
5. Limitation of studies and concluding remarks
Despite the increasing number of studies examining the role of glucocorticoids and FKBP51 in various CNS disorders, we should be aware of the limitations that each study presents. Some limitations are: (i) lack of longitudinal assessments; (ii) inclusion of patients with multiple medications; (iii) limited number of patients causing low-powered statistical analyses; (iv) limited information about environmental factors through lifespan; (v) variations in type of assessments (e.g. self-reported versus clinician administrated); (vi) absence of formal meta-analysis; (vii) not including sex as a biological variable. In the case of PTSD, some studies are limited by variations in the years after trauma, type of trauma (war veterans versus childhood abuse) and type of controls (trauma-exposed versus general population).
One of the major limitations in current studies is the high comorbidity presented among patients. For example, at least half of the patients suffering from PTSD have also been diagnosed with depression [96]. This is because both stress-related diseases have overlapping symptoms and risk factors (childhood adversity and abuse) [44,97]. Different approaches like functional neuroimaging, HPA function assays and identification of genetic markers may allow us to distinguish differences. In line with this, GR signalling and FKBP5 genotypes also differ between these disorders.
Although more studies are needed to understand the mechanism by which FKBP5 alters the risk of numerous disorders, FKBP5 may be beneficial as a biomarker for increased risk to stress and trauma exposure. By combining genetic, epigenetic and transcriptional measures, we may gain a more thorough understanding of the role of FKBP5 in affective disorders. This knowledge may be beneficial for diagnosing and treating patients suffering from these disorders.
Acknowledgements
We would like to acknowledge Dr Chad A. Dickey whose inspirational brilliance and determination will always be remembered.
Data accessibility
This article has no additional data.
Competing interests
The authors have no competing interests to declare.
Funding
L.J.B. was supported by NIH R01 MH103848. M.C.-M. was supported by NIH R01NS073899S1. J.T.P. was supported by RCMI BRAIN and MAGIC Cores (NIMHHD G12 MD007579-27) and R15 MH101700 from the National Institutes of Health.
References
- 1.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. 2003. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22, 1158–1167. ( 10.1093/emboj/cdg108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. 2015. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl Acad. Sci. USA 112, E2497–E2506. ( 10.1073/pnas.1412651112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisitkun T, Shen R-F, Knepper MA. 2004. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13 368–13 373. ( 10.1073/pnas.0403453101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E. 2004. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J. Biol. Chem. 279, 45 379–45 388. ( 10.1074/jbc.M405486200) [DOI] [PubMed] [Google Scholar]
- 5.Evans CG, Wisén S, Gestwicki JE. 2006. Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1-42) aggregation in vitro. J. Biol. Chem. 281, 33 182–33 191. ( 10.1074/jbc.M606192200) [DOI] [PubMed] [Google Scholar]
- 6.Wong DS, Jay DG. 2016. Emerging roles of extracellular Hsp90 in cancer. In Advances in cancer research (eds Isaacs J, Whitesell L), pp. 141–163. San Diego, CA: Elsevier. [DOI] [PubMed] [Google Scholar]
- 7.Kadeba PI, Vasauskas AA, Chen H, Wu S, Scammell JG, Cioffi DL. 2013. Regulation of store-operated calcium entry by FK506-Binding immunophilins. Cell Calcium 53, 275–285. ( 10.1016/j.ceca.2012.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Harlan RE. 1990. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39, 579–604. ( 10.1016/0306-4522(90)90244-X) [DOI] [PubMed] [Google Scholar]
- 9.Bremner JD, et al. 2003. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28, 733–750. ( 10.1016/S0306-4530(02)00067-7) [DOI] [PubMed] [Google Scholar]
- 10.Guy NC, Garcia YA, Sivils JC, Galigniana MD, Cox MB. 2015. Functions of the Hsp90-Binding FKBP immunophilins. pp. 35–68. Berlin, Germany: Springer International Publishing; [DOI] [PubMed] [Google Scholar]
- 11.Sanchez ER. 2012. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim. Biophys. Acta 1823, 722–729. ( 10.1016/j.bbamcr.2011.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB. 1998. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 12, 1903–1913. ( 10.1210/mend.12.12.0204) [DOI] [PubMed] [Google Scholar]
- 13.Pirkl F, Buchner J. 2001. Functional analysis of the Hsp90-associated human peptidyl propyl Cis/Trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol. 308, 795–806. ( 10.1006/jmbi.2001.4595) [DOI] [PubMed] [Google Scholar]
- 14.Scammell JG, Denny WB, Valentine DL, Smith DF. 2001. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three new world primates. Gen. Comp. Endocrinol. 124, 152–165. ( 10.1006/gcen.2001.7696) [DOI] [PubMed] [Google Scholar]
- 15.Wochnik GM, Lle J, Egg R, Abel GA, Schmidt U, Holsboer F, Rein T. 2004. FK506-binding Proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 280, 4609–4616. ( 10.1074/jbc.M407498200) [DOI] [PubMed] [Google Scholar]
- 16.Harrell JM, Murphy PJM, Morishima Y, Chen H, Mansfield JF, Galigniana MD, Pratt WB. 2004. Evidence for glucocorticoid receptor transport on microtubules by dynein. J. Biol. Chem. 279, 54 647–54 654. ( 10.1074/jbc.M406863200) [DOI] [PubMed] [Google Scholar]
- 17.Baughman G, Harrigan MT, Campbell NF, Nurrishf SJ, Bourgeois S. 1991. Genes newly identified as regulated by glucocorticoids in murine thymocytes. Mol. Endocrinol. 5, 637–644. ( 10.1210/mend-5-5-637) [DOI] [PubMed] [Google Scholar]
- 18.Reynolds PD, Ruan Y, Smith DF, Scammell JG. 1999. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab. 84, 663–669. ( 10.1210/jc.84.2.663) [DOI] [PubMed] [Google Scholar]
- 19.Zannas AS, Wiechmann T, Gassen NC, Binder EB. 2016. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology 41, 261–274. ( 10.1038/npp.2015.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klengel T, et al. 2013. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 16, 33–41. ( 10.1038/nn.3275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder EB, et al. 2004. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36, 1319–1325. ( 10.1038/ng1479) [DOI] [PubMed] [Google Scholar]
- 22.Menke A, Klengel T, Rubel J, Brückl T, Pfister H, Lucae S, Uhr M, Holsboer F, Binder EB. 2013. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav. 12, 289–296. ( 10.1111/gbb.12026) [DOI] [PubMed] [Google Scholar]
- 23.Stamm TJ, et al. 2016. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 30, 40–47. ( 10.1177/0269881115620459) [DOI] [PubMed] [Google Scholar]
- 24.Fujii T, et al. 2014. The common functional FKBP5 variant rs1360780 is associated with altered cognitive function in aged individuals. Sci. Rep. 4, 6696 ( 10.1038/srep06696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczepankiewicz A, Leszczyńska-Rodziewicz A, Pawlak J, Narozna B, Rajewska-Rager A, Wilkosc M, Zaremba D, Maciukiewicz M, Twarowska-Hauser J. 2014. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J. Affect. Disord. 164, 33–37. ( 10.1016/j.jad.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 26.Binder EB, et al. 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–1305. ( 10.1001/jama.299.11.1291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihaljevic M, et al. 2016. The emerging role of the FKBP5 gene polymorphisms in vulnerability–stress model of schizophrenia: further evidence from a Serbian population. Eur. Arch. Psychiatry Clin. Neurosci. 1–13. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair D, Fillman SG, Webster MJ, Weickert CS. 2013. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci. Rep. 3, 3539 ( 10.1038/srep03539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddox SA, Schafe GE, Ressler KJ. 2013. Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Front. Psychiatry 4, 62 ( 10.3389/fpsyt.2013.00062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewald ER, Wand GS, Seifuddin F, Yang X, Tamashiro KL, Potash JB, Zandi P, Lee RS. Alterations in DNA methylation of Fkbp5 as a determinant of blood–brain correlation of glucocorticoid exposure. Psychoneuroendocrinology 2014. 44, 112–122. ( 10.1016/j.psyneuen.2014.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee RS, Tamashiro KLK, Purcell RH, Huo Y, Rongione M, Potash JB, Wand GS. 2011. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology 218, 303–312. ( 10.1007/s00213-011-2307-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair LJ, Zhang B, Dickey CA. 2013. Potential synergy between tau aggregation inhibitors and tau chaperone modulators. Alzheimers Res. Ther. 5, 41 ( 10.1186/alzrt207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schülke J-P, Wochnik GM, Lang-Rollin I, Gassen NC, Knapp RT, Berning B, Yassouridis A, Rein T, Bridger JM. 2010. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS ONE 5, e11717 ( 10.1371/journal.pone.0011717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yehuda R, et al. 2009. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol. Psychiatry 66, 708–711. ( 10.1016/j.biopsych.2009.02.034) [DOI] [PubMed] [Google Scholar]
- 35.Kang JI, Chung HC, Jeung H-C, Kim SJ, An SK, Namkoong K. 2012. FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology 37, 1569–1576. ( 10.1016/j.psyneuen.2012.02.017) [DOI] [PubMed] [Google Scholar]
- 36.O'Leary JC, Zhang B, Koren J, Blair LJ, Dickey CA, Dickey CA. 2013. The role of FKBP5 in mood disorders: action of FKBP5 on steroid hormone receptors leads to questions about its evolutionary importance. CNS Neurol. Disord. Drug Targets 12, 1157–1162. [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, Brockmöller J. 2008. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics 9, 841–846. ( 10.2217/14622416.9.7.841) [DOI] [PubMed] [Google Scholar]
- 38.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. 2001. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J. Abnorm. Psychol. 110, 585–599. ( 10.1037/0021-843X.110.4.585) [DOI] [PubMed] [Google Scholar]
- 39.Den Hollander-Gijsman ME, De Beurs E, Van Der Wee NJA, Van Rood YR, Zitman FG. 2010. Distinguishing between depression and anxiety: a proposal for an extension of the tripartite model. Eur. Psychiatry 25, 197–205. ( 10.1016/j.eurpsy.2009.09.005) [DOI] [PubMed] [Google Scholar]
- 40.Burke HM, Davis MC, Otte C, Mohr DC. 2005. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30, 846–856. ( 10.1016/j.psyneuen.2005.02.010) [DOI] [PubMed] [Google Scholar]
- 41.Spijker AT, van Rossum EFC. 2012. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology 95, 179–186. ( 10.1159/000329846) [DOI] [PubMed] [Google Scholar]
- 42.Tozzi L, et al. 2016. Single-nucleotide polymorphism of the FKBP5 gene and childhood maltreatment as predictors of structural changes in brain areas involved in emotional processing in depression. Neuropsychopharmacology 41, 487–497. ( 10.1038/npp.2015.170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appel K, et al. 2011. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology 36, 1982–1991. ( 10.1038/npp.2011.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohrt BA, Worthman CM, Ressler KJ, Mercer KB, Upadhaya N, Koirala S, Nepal MK, Sharma VD, Binder EB. 2015. Cross-cultural gene–environment interactions in depression, post-traumatic stress disorder, and the cortisol awakening response: FKBP5 polymorphisms and childhood trauma in South Asia. Int. Rev. Psychiatry 27, 180–196. ( 10.3109/09540261.2015.1020052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han K-M, et al. 2017. Influence of FKBP5 polymorphism and DNA methylation on structural changes of the brain in major depressive disorder. Sci. Rep. 7, 42621 ( 10.1038/srep42621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou Y, Wang F, Feng X, Li W, Tao J, Pan F. 2010. Neuroscience letters meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neurosci. Lett. 484, 56–61. ( 10.1016/j.neulet.2010.08.019) [DOI] [PubMed] [Google Scholar]
- 47.Cattaneo A, et al. 2012. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets.’ Neuropsychopharmacology 38, 377–385. ( 10.1038/npp.2012.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gassen NC, et al. 2015. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 8, 119 ( 10.1126/scisignal.aac7695) [DOI] [PubMed] [Google Scholar]
- 49.Gassen NC, et al. 2014. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Med. 11, e1001755 ( 10.1371/journal.pmed.1001755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. ( 10.1001/archpsyc.62.6.593) [DOI] [PubMed] [Google Scholar]
- 51.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM. 2006. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 40, 550–567. ( 10.1016/j.jpsychires.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 52.Yehuda R, Golier JA, Yang R-K, Tischler L. 2004. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol. Psychiatry 55, 1110–1116. ( 10.1016/j.biopsych.2004.02.010) [DOI] [PubMed] [Google Scholar]
- 53.Matić G, et al. 2014. Mineralocorticoid receptor and heat shock protein expression levels in peripheral lymphocytes from war trauma-exposed men with and without PTSD. Psychiatry Res. 215, 379–385. ( 10.1016/j.psychres.2013.11.022) [DOI] [PubMed] [Google Scholar]
- 54.Matić G, et al. 2013. Lymphocyte glucocorticoid receptor expression level and hormone-binding properties differ between war trauma-exposed men with and without PTSD. Prog. Neuropsychopharmacol. Biol. Psychiatry 43, 238–245. ( 10.1016/j.pnpbp.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 55.Yehuda R, Ledoux J. 2007. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56, 19–32. ( 10.1016/j.neuron.2007.09.006) [DOI] [PubMed] [Google Scholar]
- 56.Hensel-Dittmann D, Schauer M, Ruf M, Catani C, Odenwald M, Elbert T, Neuner F. 2011. Treatment of traumatized victims of war and torture: a randomized controlled comparison of narrative exposure therapy and stress inoculation training. Psychother. Psychosom. 80, 345–352. ( 10.1159/000327253) [DOI] [PubMed] [Google Scholar]
- 57.Gillespie CF, Phifer J, Bradley B, Ressler KJ. 2009. Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety 26, 984–992. ( 10.1002/da.20605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarapas C, et al. 2011. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis. Markers. 30, 101–110. ( 10.1155/2011/328054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta D. 2011. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder. Arch. Gen. Psychiatry 68, 901 ( 10.1001/archgenpsychiatry.2011.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boscarino JA, Erlich PM, Hoffman SN, Rukstalis M, Stewart WF. 2011. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 188, 173–174. ( 10.1016/j.psychres.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watkins LE, Han S, Harpaz-Rotem I, Mota NP, Southwick SM, Krystal JH, Gelernter J, Pietrzak RH. 2016. FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: results from the national health and resilience in veterans study. Psychoneuroendocrinology 69, 98–105. ( 10.1016/j.psyneuen.2016.04.001) [DOI] [PubMed] [Google Scholar]
- 62.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF. 2010. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 35, 1684–1692. ( 10.1038/npp.2010.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauls DL, Abramovitch A, Rauch SL, Geller DA. 2014. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 15, 410–424. ( 10.1038/nrn3746) [DOI] [PubMed] [Google Scholar]
- 64.Howe AS, et al. 2016. Candidate genes in panic disorder: meta-analyses of 23 common variants in major anxiogenic pathways. Mol. Psychiatry 21, 665–679. ( 10.1038/mp.2015.138) [DOI] [PubMed] [Google Scholar]
- 65.Bortoluzzi A, Blaya C, da Rosa ED, Paim M, Rosa V, Leistner-Segal S, Gus Manfro G. 2015. What can HPA axis-linked genes tell us about anxiety disorders in adolescents? Trends Psychiatry Psychother. 37, 232–237. ( 10.1590/2237-6089-2015-0035) [DOI] [PubMed] [Google Scholar]
- 66.Scharf SH, Liebl C, Binder EB, Schmidt MV, Müller MB. 2011. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 6, e16883 ( 10.1371/journal.pone.0016883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner KV, et al. 2012. Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: influence of paroxetine treatment. Neuropsychopharmacology 37, 2797–2808. ( 10.1038/npp.2012.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. 2013. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology 38, 616–627. ( 10.1038/npp.2012.225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartmann J, et al. 2015. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J. Neurosci. 35, 9007–9016. ( 10.1523/JNEUROSCI.4024-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Leary JC, et al. 2011. A new anti-depressive strategy for the elderly: ablation of FKBP5/FKBP51. PLoS ONE 6, e24840 ( 10.1371/journal.pone.0024840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabbagh JJ, O'Leary JC, Blair LJ, Klengel T, Nordhues BA, Fontaine SN, Binder EB, Dickey CA, Yoshikawa T. 2014. Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PLoS ONE 9, e107241 ( 10.1371/journal.pone.0107241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Touma C, et al. 2011. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 70, 928–936. ( 10.1016/j.biopsych.2011.07.023) [DOI] [PubMed] [Google Scholar]
- 73.Hoeijmakers L, Harbich D, Schmid B, Lucassen PJ, Wagner KV, Schmidt MV, Hartmann J, Campolongo P. 2014. Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS ONE 9, e95796 ( 10.1371/journal.pone.0095796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaali S, et al. 2015. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 11, 33–37. ( 10.1038/nchembio.1699) [DOI] [PubMed] [Google Scholar]
- 75.Attwood B, Bourgognon J, Patel S, Mucha M. 2011. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature 473, 372–375. ( 10.1038/nature09938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Criado-Marrero M, Morales Silva RJ, Velazquez B, Hernández A, Colon M, Cruz E, Soler-Cedeño O, Porter JT. 2017. Dynamic expression of FKBP5 in the medial prefrontal cortex regulates resiliency to conditioned fear. Learn. Mem. 24, 145–152. ( 10.1101/lm.043000.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tovote P, Fadok JP, Lüthi A. 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331. ( 10.1038/nrn3945) [DOI] [PubMed] [Google Scholar]
- 78.Tye KM, et al. 2011. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362. ( 10.1038/nature09820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. 2016. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. ( 10.1016/j.neuroscience.2015.07.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann J, et al. 2012. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62, 332–339. ( 10.1016/j.neuropharm.2011.07.041) [DOI] [PubMed] [Google Scholar]
- 81.Volk N, Pape JC, Engel M, Zannas AS, Cattane N, Cattaneo A, Binder EB, Chen A. 2016. Amygdalar MicroRNA-15a is essential for coping with chronic stress. Cell Rep. 17, 1882–1891. ( 10.1016/j.celrep.2016.10.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albu S, et al. 2014. Deficiency of FK506-binding protein (FKBP) 51 alters sleep architecture and recovery sleep responses to stress in mice. J. Sleep Res. 23, 176–185. ( 10.1111/jsr.12112) [DOI] [PubMed] [Google Scholar]
- 83.Willour V, et al. 2009. Family-based association of FKBP5 in bipolar disorder. Mol. Psychiatry 14, 261–268. ( 10.1038/sj.mp.4002141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch M-A. 2010. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology 35, 1674–1683. ( 10.1038/npp.2009.236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ajnakina O, et al. 2014. Role of environmental confounding in the association between FKBP5 and first-episode psychosis. Front. Psychiatry 5, 84 ( 10.3389/fpsyt.2014.00084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seifuddin F, Pirooznia M, Judy JT, Goes FS, Potash JB, Zandi PP. 2013. Systematic review of genome-wide gene expression studies of bipolar disorder. BMC Psychiatry 13, 213 ( 10.1186/1471-244X-13-213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel N, Crider A, Pandya CD, Ahmed AO, Pillai A. 2016. Altered mRNA levels of glucocorticoid receptor, mineralocorticoid receptor, and co-chaperones (FKBP5 and PTGES3) in the middle frontal gyrus of autism spectrum disorder subjects. Mol. Neurobiol. 53, 2090–2099. ( 10.1007/s12035-015-9178-2) [DOI] [PubMed] [Google Scholar]
- 88.Pérez-Ortiz JM, García-Gutiérrez MS, Navarrete F, Giner S, Manzanares J. 2013. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology 38, 1251–1258. ( 10.1016/j.psyneuen.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 89.Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. 2016. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic–pituitary–adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev. 87, 61–72. ( 10.1111/cdev.12487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ. 2014. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS ONE 9, e104913 ( 10.1371/journal.pone.0104913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Kamkwalala A, Jovanovic T. 2012. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 42, 533–543. ( 10.1017/S0033291711001565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tyrka AR, Ridout KK, Parade SH, Paquette AG, Marsit CJ, Seifer R. 2015. Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Dev. Psychopathol. 27, 1637–1645. ( 10.1017/S0954579415000991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piyasena C, et al. 2016. Dynamic changes in DNA methylation occur during the first year of life in preterm infants. Front. Endocrinol. 7, 158 ( 10.3389/fendo.2016.00158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D'Agata AL, Walsh S, Vittner D, Cong X, McGrath JM, Young EE. 2017. FKBP5 genotype and early life stress exposure predict neurobehavioral outcomes for preterm infants. Dev. Psychobiol. 59, 410–418. ( 10.1002/dev.21507) [DOI] [PubMed] [Google Scholar]
- 95.Hartmann J, et al. 2012. Fkbp52 heterozygosity alters behavioral, endocrine and neurogenetic parameters under basal and chronic stress conditions in mice. Psychoneuroendocrinology 37, 2009–2021. ( 10.1016/j.psyneuen.2012.04.017) [DOI] [PubMed] [Google Scholar]
- 96.Flory JD, Yehuda R. 2015. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 17, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. 2004. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol. Psychiatry 55, 745–751. ( 10.1016/j.biopsych.2003.11.018) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.