The evolution of the Metazoa is one of the most important and consequential events having taken place in our biosphere. Although Cavalier-Smith [1] has propounded a likely means by which animals evolved through an archetypal pre-sponge with choanocytes, an alternative route via immotile, non-suspension-feeding Ediacaran diploblastic organisms with a pre-placozoan grade of organization such as the Rangeomorpha [2] should also be considered (figure 1). Pre-placozoans differ from pre-sponges by their lack of basal pinacocytes and suspension-feeding capabilities, and by a feeding mode that relies on establishing symbiosis with, or directly phagocytosing chemoautotrophic bacteria [2]. Here we discuss how pre-sponges and pre-placozoans could have coexisted during early stages of metazoan evolution, resulting from planktonic, multicellular craspedid-like stem choanoflagellates settling on hard or soft substrates respectively. The conceptual approach to exploring the evolution of metazoans is corroborated by Ediacaran fossil evidence [2] and milestones in the fossil record that support the pre-placozoan route to animal diversification.
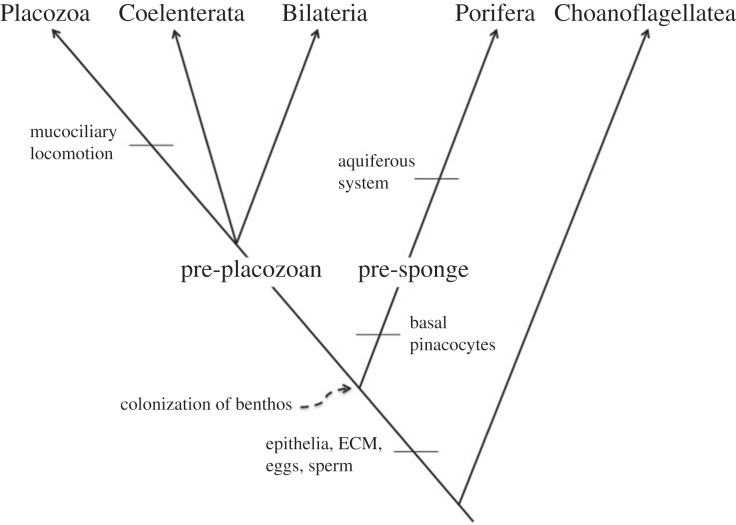
Figure 1.
Potential scenario for metazoan evolution, representing the pre-placozoan and pre-sponge routes to the diversification of major metazoan groups. Pre-placozoans and pre-sponges develop following benthic colonization by a planktonic, swimming ball of cells on hard (pre-sponge) or soft (pre-placozoan) substrates. Relationships between non-poriferan metazoans, proposed to be derived from a pre-placozoan ancestor, are purposefully unresolved in this tree.
Given the comparative ease with which multicellular organisms can evolve [1], and the variety of benthic substrates available for colonization, all routes to the evolution of the Metazoa should be considered. The pre-sponges described by Cavalier-Smith evolved on hard substrates, and consisted of two cell types (flagellated choanocytes that retained their suspension-feeding function when they became benthic, and basal pinacocytes that developed as an adaptation for attachment to hard substrates, but which are not involved in feeding) forming a simple epithelium that enveloped an acellular mesohyl [1]. The pre-placozoan model presents a similar body plan (simple epithelium surrounding mesohyl), but with choanocyte-like cells being involved in feeding and water circulation [2], since epibenthic animals on soft substrates have no requirement for specialized basal attachment cells. Hard rocky substrates ideal for pre-sponges form only a small portion of Ediacaran marine benthic settings, largely restricted to high-energy coastlines and submarine canyons. The majority of the Ediacaran seafloor consisted of soft sediment, which would have been unsuitable for pre-sponges, but ideal for pre-placozoans [2]. We therefore consider that both pre-placozoans and pre-sponges could have evolved during the Ediacaran, but are likely to have done so in very different benthic habitats (figure 1).
We agree entirely with Cavalier-Smith's assertion that the first benthos should show little to no reduction in feeding capacity in association with their adoption of the benthic mode of life [2]. We note however that a pre-placozoan model, which invokes a chemosymbiotic or phagocytotic lower surface, actually shows no reduction in feeding capacity [2], which is in contrast to the slight loss of feeding capacity invoked in the evolution of non-feeding basal pinacocytes [1]. The development of pre-sponges with basal pinacocytes makes sense for organisms settling on a hard substrate, but for an organism settling on porous soft sediment, the lower cells need not be specialized for attachment, but rather can be specialized for feeding upon the sedimentary microbiota (particularly the thiobios).
It is important to note that the colonization of soft-bottom marine sediments by immotile organisms is challenging, partly because their bodies reduce the supply of oxygen to the underlying sediment–water interface, and cause the build-up of toxic hydrogen sulfide [2]. There are a number of means by which organisms protect themselves from sulfide build-up:
(1) Isolation of body tissues using an impermeable barrier such as is produced by the collagen rich basal pinacocytes of sponges [3]. Such cells are a pre-requisite for the development of a sessile pre-sponge [1].
(2) Isolation of body tissues using a mucus barrier, such as observed in bivalve gills [4] and annelids at hydrothermal vents [5]. Such mucus layers are typically colonized by bacteria, which may both play a role in detoxification, and act as a food source.
(3) Active or passive oxygenation of the lower surface of the body, by diffusion through body tissues or by transport of seawater using cilia [2] or flagella (the two being variants of the same organelle [6]), thereby oxidizing pore-water sulfides. An important consequence of this oxygenation is that it enhances chemolithoautotrophic microbial production along the lower surface [2].
(4) Locomotion, which is a characteristic of most Metazoa—and therefore likely to be preceded by either or all of 1–3 above—the earliest evidence for which is associated with the purported placozoans Dickinsonia and Yorgia [7–9] and their trails [10].
(5) Movement by directional growth, which is generally slow and an ineffective means of avoiding sulfide build-up. It is a common trait of organisms like amoebozoans [11], but may have been employed by small pre-sponges or sponges.
There is no doubt that choanocytes were important to the pre-sponge lineage as a means of collecting food particles for nutrition [1]. Cavalier-Smith [1] emphasizes that it is the rare ability of choanoflagellate cells to aggregate without loss of feeding capacity that made stem choanoflagellates our ancestors. Our recently proposed model [2] for a pre-placozoan grade of organization for Ediacaran rangeomorph macrofossils provides an alternative feeding hypothesis for the early multicellular epibenthic animals that derived from aggregated flagellated cells, and highlights the likely importance of sulfur-oxidizing bacteria as a food source, and the possible development of chemosymbiosis/phagotrophy in early animals. As discussed above, one means by which animals might avoid the build-up of sulfide below their bodies is by oxygenating the sediment, thereby stimulating microbial productivity, particularly among the sulfur oxidizing bacteria.
The Ediacaran Rangeomorpha characteristically have high surface area to volume ratios [12]. It has been noted that immotile sediment-reclining rangeomorph taxa such as Fractofusus (figure 2d) have that extensive, almost fractal, surface permanently in contact with the sediment. Concomitantly, it is likely that the surface was irrigated through ciliary/flagellar action, or that oxygen reached that surface via diffusion through the animal's body. A by-product of oxygenating the ventral epithelium of the Fractofusus animal, to mitigate cell damage from porewater sulfides, is stimulation of chemolithoautotrophic biomass that would have been available for phagocytotic ingestion since phagotrophy is a fundamental property of eukaryotes [13]. Slightly more derived extracellular symbioses are extremely simple to evolve [14,15], and provide another non-suspensivorous means to power large simple organisms, constituting an alternative to the pre-sponge model for the early epibenthos [1] (figure 1).
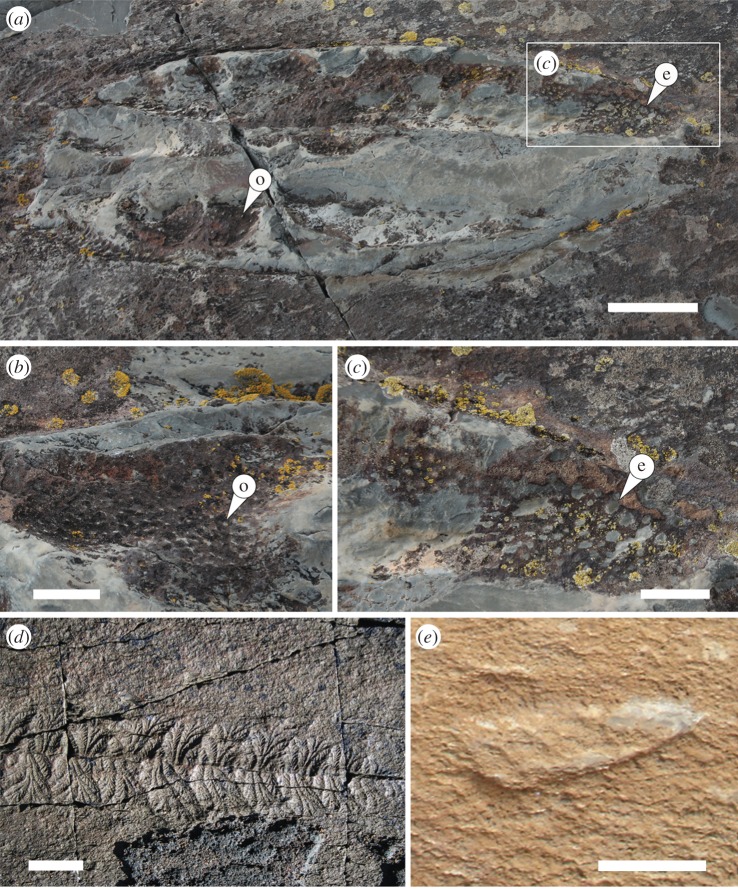
Figure 2.
Ediacaran fossils from the Mistaken Point Formation, Newfoundland. (a–c) Blackbrookia s. l., a possible sponge, commonly co-occurring with rangeomorphs. Note presence of pyrite over the inferred epidermis (e) of the sponge, which has multiple small, rounded features that may be casts of ostia (o). Scale bar, 5 cm (a); 1 cm (b and c). (d) Fractofusus misrai, a reclining, pre-placozoan rangeomorph. Scale bar, 1 cm. (e) Thectardis avalonensis, a possible pre-sponge. Scale bar, 1 cm. (Online version in colour.)
Immotile, potentially chemosymbiotic, Fractofusus are similar to the (arguably) simplest known extant animal, the placozoan Trichoplax, which uses its mucociliary sole for locomotion, having evolved exoenzymes to digest extracellular food rather than relying on the phagocytosis of microbes. It is interesting to note that Trichoplax is considered to have a simple chemical nervous system, which is sensitive to the build-up of sulfide below its body [2,16]. The mode of life of the pre-sponges and sponges as suspensivores/osmotrophs on hard substrates is simple, and has few selection pressures requiring the evolution of a nervous system. We consider that animals with pre-placozoan body plans are much closer in their mode of life to the Placozoa and other non-sponge metazoans. The evolution of nematocysts, electrical synapses, muscular locomotion and the gut have been well covered in the review of Cavalier-Smith [1], but we see no logical reason why a pre-sponge should be a more likely ancestor to the Metazoa than a phagotrophic or chemosymbiotic pre-placozoan diploblastic organism (figure 1). Other selective forces favouring multicellularity include aspects of reproduction (development of germline and soma) and immunity, which should have been important to both pre-sponge and pre-placozoan lineages.
1. Fossil milestones
The fossil record provides compelling evidence for the presence of pre-sponges, pre-placozoans and some crown taxa prior to the Cambrian explosion. Notwithstanding recent reviews that have concluded that there is insufficient evidence to be sure of Ediacaran sponges [17], fossil pre-sponges are probably present, but—in the absence of the aquiferous system, spicules and ostia/oscula used to diagnose true water-pumping sponges—difficult to recognize. Some fossil organisms from the Ediacaran of Newfoundland (approx. 565 Ma) that lived on soft sediment in deep water have surficial structures comparable to the choanocyte-lined ostia of the Homoscleromorpha (figure 2a–c), implying the evolution of the aquiferous system by this time. It may be inferred that pre-sponges are likely to have been present in older rocks, but would have lacked any distinctive preservable surface morphology [1]. The most likely pre-sponge candidate in the Ediacaran of Newfoundland (approx. 580 Ma) is the anomalously featureless, and therefore enigmatic, Thectardis which has been widely compared to sponges [18], but fails to meet the criteria for being a true sponge [17] (figure 2e; [18,19]). The oldest fossiliferous strata (approx. 580 Ma) have a rich biota of multicellular macroorganisms, particularly pre-placozoan rangeomorph animals such as Fractofusus (figure 2d) [2,20]. Finally, there is a growing consensus that some of the late Ediacaran (approx. 550 Ma) taxa (e.g. Dickinsonia, Yorgia and Kimberella) were bilaterians [9,10,21].
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rstb.2017.0336; http://dx.doi.org/10.1098/rstb.2015.0476.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
D.M. and S.C.D. are funded by Natural Sciences and Engineering Research Council of Canada Discovery Grants.
References
- 1.Cavalier-Smith T. 2017. Origin of animal multicellularity: precursors, causes, consequences—the choanoflagellate/sponge transition, neurogenesis and the Cambrian explosion. Phil. Trans. R. Soc. B 372, 20150476 ( 10.1098/rstb.2015.0476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufour SC, McIlroy D. 2016. Ediacaran pre-placozoan diploblasts in the Avalonian biota: the role of chemosynthesis in the evolution of early animal life. In Earth system evolution and early life: a celebration of the work of Martin Brasier (eds Brasier AT, McIlroy D, McLoughlin N), vol. 448. London, UK: Geological Society of London. [Google Scholar]
- 3.Maldonado M. 2004. Choanoflagellates, choanocytes and animal multicellularity. Invert. Biol. 123, 1–22. ( 10.1111/j.1744-7410.2004.tb00138) [DOI] [Google Scholar]
- 4.Liberge M, Gros O, Frenkiel L. 2001. Lysosomes and sulfide-oxidizing bodies in the bacteriocytes of Lucina pectinata, a cytochemical and microanalysis approach. Mar. Biol. 139, 401–409. ( 10.1007/s002270000526) [DOI] [Google Scholar]
- 5.Juniper SK, Martineu P. 1995. Alvinellids and sulfides at hydrothermal vents of the eastern Pacific: a review. Integr. Comp. Biol. 35, 174–185. ( 10.1093/icb/35.2.174) [DOI] [Google Scholar]
- 6.Moran J, McKean PG, Ginger ML. 2014. Eukaryotic flagella: variations in form, function and composition during evolution. BioScience 64, 1103–1114. ( 10.1093/biosci/biu175) [DOI] [Google Scholar]
- 7.Dzik J. 2003. Anatomical information content in the Ediacaran fossils and their possible zoological affinities. Integr. Comp. Biol. 32, 114–126. ( 10.1093/icb/43.1.114) [DOI] [PubMed] [Google Scholar]
- 8.Ivantsov AY, Malakovskaya YE. 2002. Gigantski sledy vendskikh zhivotnykh. Dokl. Akad. Nauk. 385, 383–386. [Google Scholar]
- 9.Sperling EA, Vinther J. 2010. A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes. Evol. Dev. 12, 201–209. ( 10.1111/j.1525-142X.2010.00404.x) [DOI] [PubMed] [Google Scholar]
- 10.Ivantsov AY. 2013. Trace fossils of Precambrian metazoans ‘Vendobionta’ and ‘Mollusks’. Stratigr. Geol. Correl. 21, 252–264. ( 10.1134/S0869593813030039) [DOI] [Google Scholar]
- 11.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasier MD, Antcliffe JB, Liu AG. 2012. The architecture of Ediacaran fronds. Palaeontology 55, 1105–1124. ( 10.1111/j/1457-4983.2012.01164.x) [DOI] [Google Scholar]
- 13.Fenchel T. 2012. Anaerobic eukaryotes. In Anoxia: evidence for eukaryote survival and paleontological strategies. Cellular origin, life in extreme habitats and astrobiology, vol. 21 (eds Altenbach AV, Bernhard JM, Seckbach J), pp. 3–16. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 14.Smith DC. 1979. From extracellular to intracellular: the establishment of a symbiosis. Proc. R. Soc. Lond. B. 204, 115–130. ( 10.1098/rspb.1979.0017) [DOI] [PubMed] [Google Scholar]
- 15.Rosati G. 2004. Ectosymbiosis in ciliated protozoa. In Symbiosis: mechanisms and model systems (ed. Seckbach J.), pp. 475–488. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 16.Arendt D, Benito-Gutierrez E, Brunet T, Marlow H. 2015. Gastric pouches and their mucociliary sole: setting the stage for nervous system evolution. Phil. Trans. R. Soc. B 370, 20150286 ( 10.1098/rstb.2015.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antcliffe JB, Callow RH, Brasier MD. 2014. Giving the early fossil record of sponges a squeeze. Bio. Rev. Camb. Phil. Soc. 89, 972–1004. ( 10.1111/brv.12090) [DOI] [PubMed] [Google Scholar]
- 18.Sperling EA, Peterson KJ, Laflamme M. 2011. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology 9, 24–33. ( 10.1111/j.1472-4669.2010.00259.x) [DOI] [PubMed] [Google Scholar]
- 19.Clapham ME, Narbonne GM, Gehling JG, Greentree C, Anderson MM. 2004. Thectardis avalonensis: a new ediacaran fossil from the Mistaken Point Biota, Newfoundland. J. Paleontol. 78, 1031–1036. ( 10.1666/0022-3360(2004)078%3C1031:TAANEF%3E2.0.CO;2) [DOI] [Google Scholar]
- 20.Liu AG, McIlroy D, Matthews JJ, Brasier MD. 2011. A new assemblage of juvenile Ediacaran fronds from the Drook Formation, Newfoundland. J. Geol. Soc. Lond. 169, 395–403. ( 10.1144/0016-76492011-094) [DOI] [Google Scholar]
- 21.Gold DA, Runnegar B, Gehling JG, Jacobs DK. 2015. Ancestral state reconstruction of ontogeny supports a bilaterian affinity for Dickinsonia. Evol. Dev. 17, 315–324. ( 10.1111/ede.12168) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.