Abstract
The absorption of poorly water-soluble drugs is influenced by the luminal gastrointestinal fluid content and composition, which control solubility. Simulated intestinal fluids have been introduced into dissolution testing including endogenous amphiphiles and digested lipids at physiological levels; however, in vivo individual variation exists in the concentrations of these components, which will alter drug absorption through an effect on solubility. The use of a factorial design of experiment and varying media by introducing different levels of bile, lecithin, and digested lipids has been previously reported, but here we investigate the solubility variation of poorly soluble drugs through more complex biorelevant amphiphile interactions. A four-component mixture design was conducted to understand the solubilization capacity and interactions of bile salt, lecithin, oleate, and monoglyceride with a constant total concentration (11.7 mM) but varying molar ratios. The equilibrium solubility of seven low solubility acidic (zafirlukast), basic (aprepitant, carvedilol), and neutral (fenofibrate, felodipine, griseofulvin, and spironolactone) drugs was investigated. Solubility results are comparable with literature values and also our own previously published design of experiment studies. Results indicate that solubilization is not a sum accumulation of individual amphiphile concentrations, but a drug specific effect through interactions of mixed amphiphile compositions with the drug. This is probably due to a combined interaction of drug characteristics; for example, lipophilicity, molecular shape, and ionization with amphiphile components, which can generate specific drug–micelle affinities. The proportion of each component can have a remarkable influence on solubility with, in some cases, the highest and lowest points close to each other. A single-point solubility measurement in a fixed composition simulated media or human intestinal fluid sample will therefore provide a value without knowledge of the surrounding solubility topography meaning that variability may be overlooked. This study has demonstrated how the amphiphile ratios influence drug solubility and highlights the importance of the envelope of physiological variation when simulating in vivo drug behavior.
Keywords: Biopharmaceutics Classification System, design of experiment, FaSSIF, FeSSIF, IVIVC, 4MD
1. Introduction
The in vivo bioavailability of orally administered drugs is influenced by the gastrointestinal tract (GIT) through several factors including solubility, dissolution, intestinal permeability, and metabolism.1 For poorly soluble drugs, an important factor is solubility, which in the gastrointestinal tract is influenced by the variable composition of the intestinal fluid2 either due to natural biological variation3,4 or changes from a fasted to a fed state after eating.5
1.1. Development of Simulated Intestinal Fluid
To imitate the in vivo solubility behavior in vitro, simulated intestinal fluids representing the fasted (FaSSIF) and fed (FeSSIF) states were developed by employing known physiologically relevant conditions (e.g., pH) and concentrations of components (e.g., bile salt and lecithin).1,6 Over time several studies have modified the original recipes to more closely mimic the available physiological data; for example, pH, osmolality, surface tension, and the in vitro behavior of poorly soluble drugs in sampled human intestinal fluid. In fasted simulated media, for example (Table 1), the biorelevant surfactants bile salt and lecithin can affect the solubility and dissolution of poorly soluble drugs,7 and changes to the bile salt and phospholipid concentrations were introduced. Further studies examined the impact of pure bile salts and osmolality adjusting agents8 along with different concentrations of components9 at the extremes of the known ranges. An updated fasted recipe (FaSSIF-V2) was introduced reducing the concentration of lecithin to 0.2 mM, which increased the bile salt/lecithin ratio to 15:1, along with adjustment to mimic the in vivo osmolality.10 Further data from the analysis of human intestinal fluids suggested the presence and influence of free fatty acids and cholesterol,11,12 and a modification to include these (FaSSIF-V2-plus) was developed. The bile salt/lecithin ratio can be quite variable among individuals,13 with ranges from 2.5:1 to 15:1 in fasted and fed states, although the fed state variation was generally smaller.4,14,15 A further updated version of FaSSIF media has recently been proposed and investigated (FASSIF-V3) with various prototypes of this media being constructed with the addition or exclusion of different bile salts and cholesterol.16
Table 1. Literature Composition of Fasted Simulated Intestinal Fluid.
| Sunesen
et al., 20059 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| component/parameter and concentration (mM) | Dressman et al., 19981 | Galia e al., 19986 | Pedersen et al., 20007 | Vertzoni et al., 20048 | low | high | Jantratid et al., 200810 V2 | Psachoulias et al., 201211 V2-plus | Fuchs et al., 201516 V3 GC/TC-Chol |
| sodium TC | 5 | 3 | 3 | 2.5 | 6.3 | 3 | 3 | 1.4 | |
| sodium GC | 3.7 | 1.4 | |||||||
| lecithin | 1.5 | 0.75 | 0.9 | 0.75 | 0.5 | 1.25 | 0.2 | 0.2 | 0.035 |
| lysolecithin | 0.315 | ||||||||
| sodium oleate | 0.56 | 0.315 | |||||||
| cholesterol | 0.2 | 0.2 | |||||||
| NaH2PO4 | (K) 29 | (K) 28.6 | 50 | 28.66 | (K) 29 | (K) 29 | 13.51 | ||
| NaOH | qs | qs | ∼13.8 | 34.8 | 3.19 | ||||
| NaCl | (K) 220 | (K) 103.3 | 150 (total Na) | 106 | no salt (KCl) | no salt (KCl) | 68.62 | 91.62 | |
| pancreatin | 100 U/mL | ||||||||
| tris/maleic acid | 19.12 | ||||||||
| osmolality (mOsmol) | 280–310 | 270 ± 10 | 270 ± 10 | 180 ± 10 | 220 ± 10 | ||||
| pH | 6.8 | 6.5 | 6.5 | 6.5 | 6.8 | 6.8 | 6.5 | 6.7 ± 0.05 | |
1.2. Human Intestinal Fluid
Research into human intestinal fluid is hampered by the anatomical difficulty of sampling via a nasal or oral catheter; however, concomitant research to the development of the simulated fluids has continuously expanded and refined knowledge around the composition and properties of this complex biological material. This has permitted an ever expanding analysis of the physicochemical properties of the fluid,3,4 providing compositional data on both the fasted and fed states5,15 and samples for solubility determinations.17 The solubility behavior of poorly water-soluble drugs is dependent upon fluid composition especially the biorelevant surfactants such as bile salt, lecithin, free fatty acid, and monolgyceride.18,19 Recent ultrastructural characterization indicates that intestinal fluid is composed of a range of micellar, vesicular, colloidal, and lipid droplet systems with the ratio of each system dependent upon the composition and fed or fasted state.20 Human intestinal fluid therefore represents a complex multicomponent media where any one component or combination of components has the possibility to influence drug solubility.
1.3. Simulated Intestinal Media Solubility
The application in vitro of fixed recipe simulated intestinal media only provides a single-point solubility measurement and cannot represent in vivo variability of intestinal fluid composition and solubility throughout this complex space. A number of studies have approached the impact of the variation of intestinal media component concentrations on solubility, for example, the solubility of danazol has been linearly related to bile salt concentration7 in sampled human intestinal fluid and solubility of multiple poorly soluble drugs linked to a “complex interplay” of factors.5 The solubility of poorly soluble drugs is also known to vary between the different simulated media recipes sometimes by almost an order of magnitude.16,21 A statistical exploration using a fractional factorial design of experiment (DoE) of the solubility effect of the measured human intestinal fluid ranges of various factors in fasted, and fed media22,23 indicates that a two or three log variation in solubility is possible. The DoE studies highlighted that media pH is a major driver for the solubility of ionizable drugs, especially acidic, while for all drugs, buffer, salt, and pancreatin had no or limited impact. For poorly soluble basic or nonionizable drugs, biorelevant surfactants and complex drug specific interactions between combinations of factors were important. A similar hypothesis was presented by Clarysse and colleagues that shows the amphiphilic contents play a major role in nonionized drugs (danazol, nifedipine), while pH has a greater influence on the solubility range for ionizable drugs (diazepam).5 The DoE studies also suggested interesting drug specific solubility variations induced by media components;22,23 however, the experimental approach did not visualize the subtle interplay of component concentrations and ratios on solubility.
1.4. Four Component Mixture Design
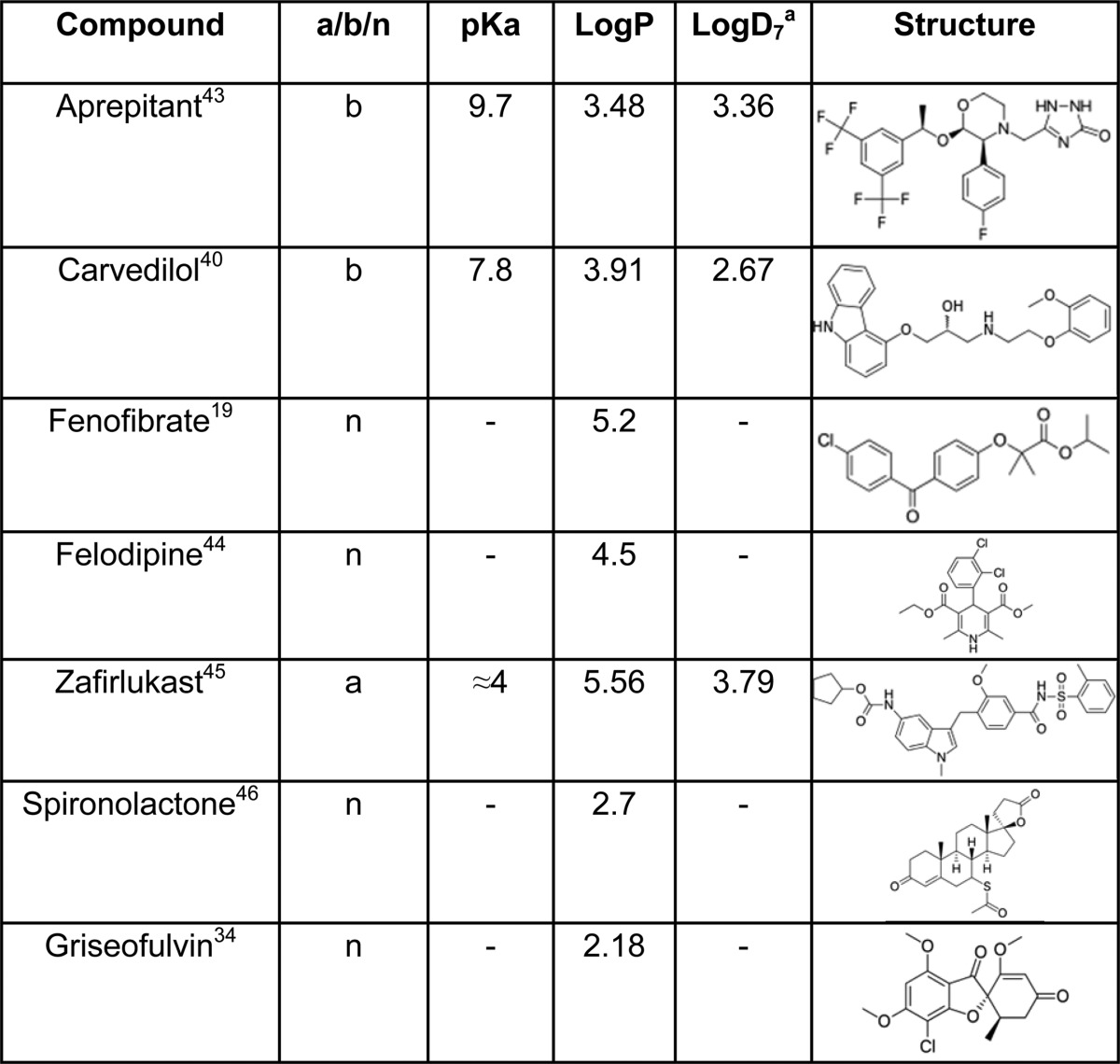
To investigate and visualize potential interactions between the biorelevant amphiphiles bile salt, lecithin, oleate, and monoglyceride on the solubilization capacity of simulated intestinal fluids, we have conducted a solubility study in which the total amphiphile concentration is maintained at a physiological concentration equal to the fasted simulated intestinal media. This was performed as a four-component mixture design (4MD) equilibrium solubility study consisting of the four aforementioned amphiphiles at various molar ratios, but with a constant total molar concentration (11.7 mM) representing the fasted state.22 4MD has been previously applied to investigate the phase behavior of phospholipids24 in cell membranes but have not been applied to intestinal fluid mixtures. Only Biopharmaceutics Classification System (BCS) Class II drugs, which exhibited remarkably high magnitudes of solubility in the presence of oleate, lecithin, and bile salt, were selected from the previous studies,22,23 with representative acid, base, and neutral drugs (Table 2). Based on published DoE studies,22,23 the behavior of oleate in the 4MD will depend on solution pH, and in order to eliminate this effect, the 4MD was only conducted at pH 7 where oleate will be predominantly ionized. The buffer and salt concentrations were also kept at biorelevant levels identical to the previous DoE, which demonstrated that they do not influence solubility. The 4MD has not previously been applied to this problem and will provide a direct visualization of the solubility profile within the media component space investigated.
Table 2. Physicochemical Properties and Molecular Structures of Drugs Used in 4MD43,44,45,46.
ACD classic calculated values: www.acdlabs.com.
2. Materials and Methods
2.1. Materials
Hydrochloric acid (HCl), potassium hydroxide (KOH), acetic acid, sodium dihydrogen orthophosphate (NaH2PO4), sodium chloride (NaCl), chloroform, griseofulvin, spironolactone, and fenofibrate were sourced from Sigma-Aldrich, U.K. Aprepitant, carvedilol, felodipine, and zafirlukast were kindly provided through the OrBiTo initiative through Dr R. Holm, Head of Preformulation, Lundbeck, Denmark. The four amphiphiles in this particular study will be denoted as BS, PL, OA, and MG. Sodium taurocholate (BS) was purchased from Sigma-Aldrich; Lecithin S PC (phosphatidylcholine from Soybean “98%”, PL) was purchased from Lipoid, Germany; sodium oleate (OA) was obtained from BDH Chemical Ltd. Poole England; monoglyceride (MG) was a gift from Croda International. All water used was ultrapure Milli-Q water. Methanol and acetonitrile were HPLC grade (VWR, U.K.), and ammonium acetate was obtained from Merck, Germany.
2.2. Mixture Design and Equilibrium Solubility Measurements
From the previous DoE, the combinations of BS, PL, and OA are displayed in Table 3. A medium total amphiphile concentration (11.7 mM) was chosen for the 4MD study. 4MD contained 39 compositions in the tetrahedron contour plot, including four compositions inside the tetrahedron and 35 compositions on the four surfaces of the tetrahedron. Each face represented a phase with the absence of one of the four amphiphiles, and therefore, the four faces are represented as BS/PL/OA, BS/PL/MG, BS/OA/MG, and PL/OA/MG. Each face had 15 combinations, with five points on the side shared by two faces (Figure 1). The concentrations were given in mol % of the total molar concentration. The water content was more than 99 wt % for all the compositions, indicating this was a dilute system.
Table 3. Concentration (mM) of Three Amphiphiles Used in the Previous DoE Based on Physiological Levels.
| DOE | BS | PL | FA | total concentration |
|---|---|---|---|---|
| 1 | 1.5 | 0.2 | 0.5 | 2.2 |
| 2 | 1.5 | 1 | 0.5 | 3 |
| 3 | 5.9 | 0.2 | 0.5 | 6.6 |
| 4 | 5.9 | 1 | 0.5 | 7.4 |
| 5 | 1.5 | 0.2 | 10 | 11.7 |
| 6 | 1.5 | 1 | 10 | 12.5 |
| 7 | 5.9 | 0.2 | 10 | 16.1 |
| 8 | 5.9 | 1 | 10 | 16.9 |
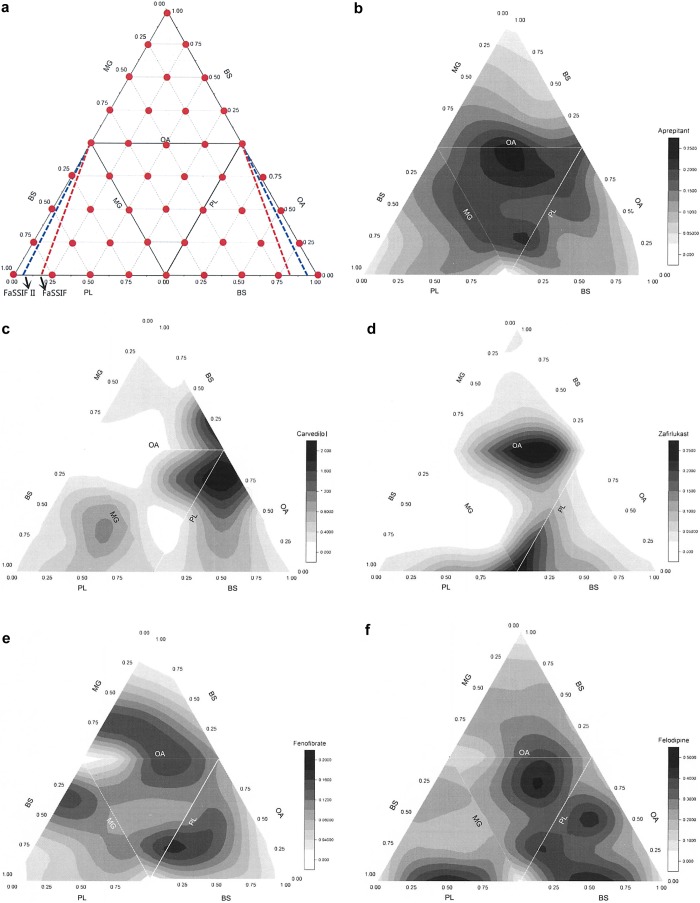
Figure 1.
Solubility contour plot determined by the 4-component mixture design (4MD) experiment. The main triangle consists of four smaller triangles representing the four surfaces of the tetrahedron when open from the top vertex, which is 100 mol % BS. The color shades attached to individual figures represent the solubility contour concentration (mM) values for each drug, note solubility ranges vary. In panel a, 4MD measurement points, red line indicates media containing BS/PL ratio of 4/1, representing FaSSIF, and the blue line indicates media with ratio of 15/1, representing FaSSIF II (see Table 1). BS, bile salt; OA, sodium oleate; MG, glyceryl monooleate; PL, soya phosphatidyl choline.
The 4MD was constructed using Minitab 16.0 and simplex lattice with four component input. The lattice incorporated four variables, and the design was augmented with axial points, which included points in the center of the tetrahedron rather than just the surface. The solubility data were analyzed in Minitab 16.0 to fit into quadratic and special cubic models. Solubility data was also fit into multiple ternary contour plots with smoothing.
Phosphate buffer containing 68 mM NaCl and 45 mM NaH2PO4 was prepared with deionized water and pH adjusted to 7. Stock solutions at 11.7 mM of BS and OA were freshly prepared from solids by dissolving in the phosphate buffer. The PL stock solution was prepared by dissolving lipid in chloroform, removing the chloroform by evaporation under nitrogen, and dispersing the dried PL film into phosphate buffer. MG cannot dissolve in buffer, so the stock MG was prepared by mixing BS (1 mM) and MG (10.7 mM) making a total concentration of 11.7 mM, and for practical experimental reasons, this solution was employed as 100 mol % MG. The required 4MD media was prepared from the stock solutions and equilibrium solubility determined in duplicate.
An excess amount of solid drug was then added to 4 mL of each mixed lipid media in Corning 15 mL centrifuge tubes and then placed on a rotating wheel mixer for 1 h. If required the pH was adjusted back to 7. Tubes were then placed on the mixer and equilibrated at 37 °C for 24 h, pH being checked after incubation. This time frame and procedures have previously been shown to provide equilibrium solubility.22 The saturated supernatant was separated by centrifugation at 13,000 rpm for 5 min and transferred for HPLC analysis using an Agilent Technologies 1260 Series Liquid Chromatography system with Clarity Chromatography software. Individual HPLC conditions are presented in Table 4.
Table 4. HPLC Assay Conditions15,31a.
| drug | mobile phase | column | flow rate (mL/min) | injection volume (μL) | detection (nm) | retention time (min) | r2 |
|---|---|---|---|---|---|---|---|
| aprepitant | ACN/0.05 M ammonium acetate (60:40) pH 4.5 | 2 | 1 | 10 | 220 | 2.2 | 1.0000 |
| carvedilol | ACN/0.05 M ammonium acetate (55:45) pH 4 | 2 | 1 | 10 | 243 | 2.2 | 0.9993 |
| felodipine | methanol/water(75:25, v/v) | 2 | 1 | 20 | 260 | 5 | 0.9999 |
| fenofibrate | ACN/water (70:30 v/v) | 1 | 1 | 100 | 291 | 3 | 1.0000 |
| griseofulvin | ACN/water (50:50 v/v) | 2 | 0.5 | 10 | 291 | 3.7 | 0.9970 |
| spironolactone | ACN/water (50:50 v/v) | 1 | 1 | 10 | 238 | 3 | 1.0000 |
| zafirlukast | ACN/10 mM phosphate buffer pH 6 50:50 | 1 | 1 | 10 | 245 | 2.2 | 0.9989 |
Column 1: Speck and Burke ODS-H optimal 150 × 30 mm id 5 μm. Column 2: Agilent Polaris 5 C18-A 150 × 4.6 MM id 5 μm. ACN, acetonitrile; TFA, trifluoroacetic acid.
For the BS/PL/OA mixture design surface only, the zeta potential of each of the mixed samples was measured by using Malvern clear disposable zeta cell (DTS 1060C) on a Malvern Zetasizer Nano instrument. Results indicate that drug loading did not markedly affect the measured zeta potentials of the mixed solutions (data not shown).
3. Results
3.1. Equilibrium Solubility
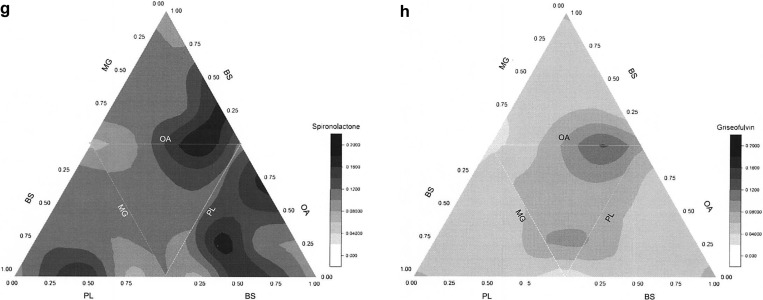
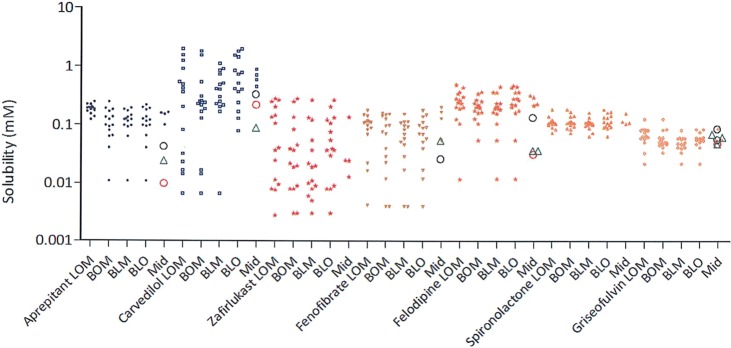
Figure 1b–h presents the solubility contour plots determined by the 4MD experiment for each drug (excluding the data points inside the tetrahedron) for an aqueous system containing a total amphiphile concentration at 11.7 mM. The main triangle consists of four smaller triangles representing the four faces (where each face is a ternary contour plot with the absence of one of the four amphiphiles) of the tetrahedron when open from the top vertex, which is 100 mol % BS. The color shades indicate solubility differences, with black representing the highest solubility and white the lowest. From Figure 1b–h, it is evident that each of the seven drugs has a unique profile, none have identical high solubility zones, and that the extent and variability of solubility is different for each drug. The individual solubility data points on the tetrahedron’s faces for each drug in the 4MD analysis are presented in Figure 2; the fifth column displays the additional four points inside the tetrahedron, which involve all amphiphiles. These data show interesting differences; for example, the solubility of spironolactone and griseofulvin varies in a narrow range, while others (i.e., carvedilol) show a 250-fold variation. This demonstrates that for each drug the four faces almost reflect the same range of drug solubility, with a few exceptions that did not cover the lower solubility points. Specifically, the BS/PL/OA combination for carvedilol did not cover solubility below 0.1 mM, while the other three faces have. Examination of the points inside the tetrahedron, which include all four amphiphiles, did not reveal a greater extent of solubility variability. Additionally, it is noted that each drug shows a different behavior with regard to the contribution of the four biorelevant amphiphiles, for example, the solubility of zafirlukast only increases when the proportion of PL increases, while carvedilol solubility is sensitive to OA concentration. In the case of fenofibrate and felodipine, the four amphiphiles in combination would provide better solubility (Figure 1 and Table 5).
Figure 2.
Equilibrium solubility measurements of 4MD. (green) Reported solubility values for each drug in HIF, (red) reported solubility values for each drugs in FaSSIF II media, and (black) reported solubility values for each drug in FaSSIF I media; all values are from refs (2) and (18). Equilibrium solubility of each drug in media with the absence of one amphiphile (four surfaces of the tetrahedron, named after the amphiphile initials; for example, BPO represents media containing BS, PL, and OA, with no addition of MG), and “mid” represents media in combination of four amphiphiles, which are therefore inside the tetrahedron.
Table 5. Composition (Molar Ratio of Amphiphiles) of Media with the Highest and Lowest Three Solubilities of Each Drug.
| aprepitant | griseofulvin | felodipine | fenofibrate | spironolactone | zafirlukast | carvedilol | |
|---|---|---|---|---|---|---|---|
| high solubility zone | 75% PL, 25% OA | 25% MG, 75% OA | 25% MG and PL, 50% OA | 12.5% BS, MG, and PL, 62.5% OA | 25% MG, 75% OA | 25% MG, 75% OA | 25% PL, 75% OA |
| 25% MG and PL, 50% OA | 12.5% BS, MG, and PL, 62.5% OA | 25% BS and PL, 50% OA | 75% PL, 25% OA | 25% BS and OA, 50% PL | 100% PL | 25% BS, 75% OA | |
| 50% MG, 50% OA | 75% PL, 25% OA | 50% BS, 50% PL | 12.5% BS, PL, and OA, 62.5% MG | 25% BS, 75% OA | 50% MG, 50% OA | 100% OA | |
| low solubility zone | 100% PL | 100% PL | 100% PL | 100% BS | 25% BS, 75% PL | 100% MG | 100% MG |
| 100% BS | 100% MG | 100% BS | 100% MG | 100% MG | 25% BS, 75% OA | 75% MG, 25% OA | |
| 75% BS, 25% OA | 25% BS and PL, 50% MG | 100% MG | 75% BS, 25% OA | 75% MG, 25% OA | 25% BS and MG, 50% PL | 50% MG, 50% OA |
3.2. Statistical Analysis
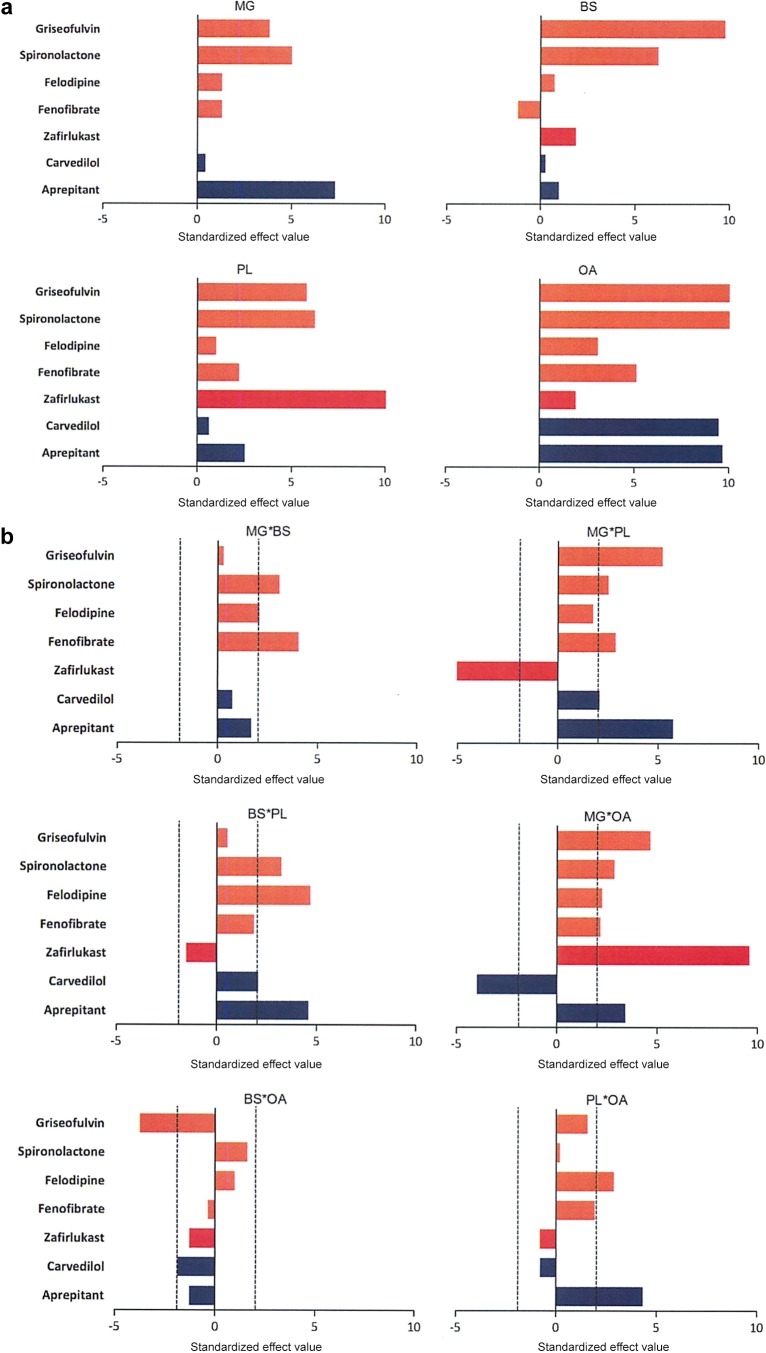
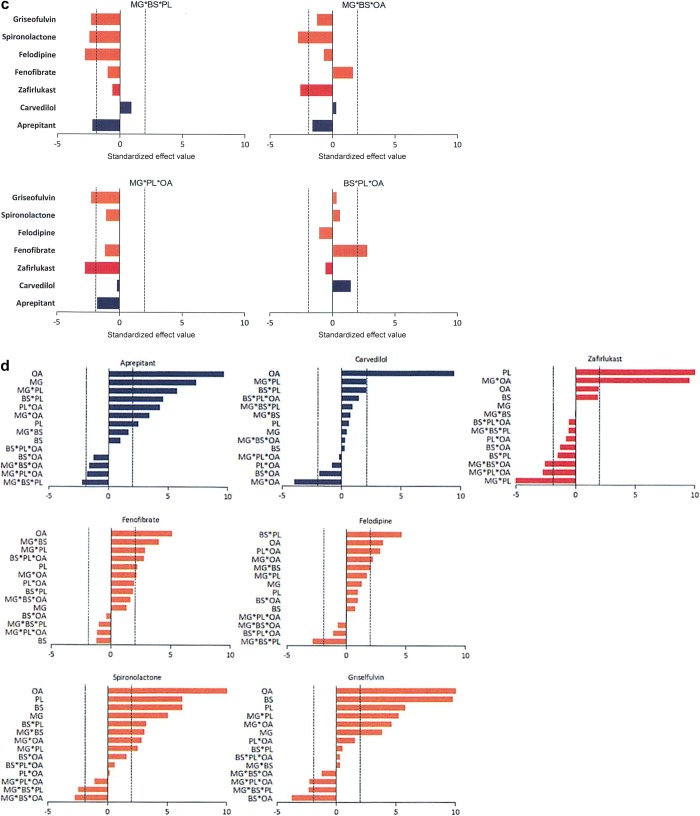
The standardized effect value for each amphiphile can be calculated by dividing coefficient with standard error, which is similar to a p-value and thus indicates the effects in the model that are statistically significant (Figure 3a-c). There is no p-value generated for each 100% amphiphile (single terms), but only standardized effect values to show the magnitude. The standardized effect values of each single amphiphile are closely related with the solubility in the media that has 100 mol % of that amphiphile, thus the higher the value, the higher the solubility within the pure amphiphile. OA exhibits the highest influence on solubility for six out of seven drugs, while BS and MG have the least solubilization effect on their own. For two amphiphile interactions (Figure 3b), 17 out of a possible 40 two combinations had a statistically significant positive standardized effect on solubility and three had a statistically significant negative standardized effect on solubility, with more than half of the possible amphiphile interactions not significantly influencing solubility. For example, MG with OA negatively affects carvedilol solubility, MG with PL negatively impacts zafirlukast solubility and BS with OA negatively impacts griseofulvin solubility. The three amphiphile interactions (Figure 3c) are only statistically significant in nine out of 20 eight possible occurrences and eight of these have a negative effect on solubility with the combination of MG/BS/PL negatively impacting four out of the seven drugs. Except for zafirlukast, OA exhibited a dominantly significant positive effect for all drugs. Aprepitant is positively affected by MG while the other drugs, zafirlukast and carvedilol are not and the PL/MG negatively impacts zafirlukast in contrast to carvedilol and aprepitant. OA has a marked effect on carvedilol, and the presence of MG in the media reduces its solubility.
Figure 3.
Standardized effect values of (a) individual amphiphiles for each drug; (b) two-amphiphile interactions; (c) three-amphiphile interactions; and (d) each drug. Factors/interactions are in decreasing order of magnitude. Bars over the dashed line show statistical significance.
Minitab fits the data generated from the design of mixtures into quadratic and special cubic models; however, the determinant coefficient r2 (<0.8) is not significant for any of the drugs. This indicates that the predictors (amphiphile ratio/concentration) in the model are not sufficient to explain the solubility variation of the drugs. Drug induced drug–micelle interaction is also a possible factor,14 which was not included in the model due to the limited drug property diversities. A simple linear correlation to fit the total ratio (x, expressed as mol proportion of the total 11.7 mol) of one to two of the amphiphiles and the drug solubility (y, mM) was attempted. However, only the BS ratio has a negative linear relationship with aprepitant (r2 = 0.7164) and fenofibrate (r2 = 0.716). Only two out of 28 possibilities showed linearity, which indicates the nonlinear nature of the analysis, and these results suggest that the systems undergo a more complicated interaction between the amphiphiles and the drugs rather than simply a sum of solubilization capacity of individual components.
Vertices, which represent single amphiphile dominated media, tend to show poor solubilization capabilities (Table 5 and Figure 1). For example, the vertex representing 100 mol % BS generally is light in color indicating lower drug solubility, implying that a high ratio of BS alone did not show any solubilization advantages for these drugs. Similarly, according to Table 5, 100 mol % PL is in the low solubility zone of fenofibrate, aprepitant, and felodipine, and 100 mol % MG is in the low solubility zone of fenofibrate, zafirlukast and carvedilol. The only exception is that 100 mol % lecithin is in the high solubility zone of zafirlukast. Interestingly, apart from high solubility zones, carvedilol and zafirlukast illustrate extensive low solubility zones (Figure 1 and Table 5) where the amphiphile composition exhibits unfavorable solubilization.
4. Discussion
4.1. Equilibrium Solubility
The equilibrium solubility data of each drug’s 4MD correspond with literature solubility values in fasted human or simulated intestinal fluids (Figure 2) and is comparable to the published fasted DoE.22 The results also indicate that two (spironolactone and griseofulvin) out of seven drugs tested have comparatively constant solubility across the tetrahedron in agreement with their relative insensitivity to changes in the fasted DoE media.22 For the other five drugs, Figures 1 and 2 indicate that with the same total amphiphile concentration the proportion of each component can have a remarkable influence on solubility with, in some cases, the highest and lowest solubility points close to each other (Table 5). The low solubility of carvedilol in BS rich systems also agrees with literature reports that BS has a negative impact on carvedilol solubility behavior.22,25 PL and MG are both poorly dispersible in aqueous buffer without the other solubilization agents such as BS,26 and Table 5 shows that generally 100 mol % PL and 100 mol % MG media provide very poor solubilization. However, PL exhibited excellent solubilization for aprepitant, felodipine, and fenofibrate, provided that an appropriate ratio of BS or OA (see Table 5) was present. These similarities to literature data indicate that the 4MD is exploring a relevant solubility space for fasted simulated media and exhibiting properties similar to previously published studies. However, the current 4MD study utilized a series of media containing the same total concentration of biorelevant amphiphiles but encompassing changing ratios, a situation that has not previously been systematically examined.
Several papers have reported the concentration-dependent solubilizing capacity of different biorelevant amphiphiles or combinations of amphiphiles for drugs in simulated intestinal fluids. For danazol and probucol, a linear relationship between solubility and total media concentration of BS, OA, and MG was determined.27 Sunesen et al. reported the solubility of danazol shows positive linearity in the presence of four amphiphiles (BS, PL, OA, MG) irrespective of the type of amphiphile used;14 a similar relationship was presented for danazol, griseofulvin, fenofibrate, cinnarazine,19 and estradiol.28
Other studies have examined the impact of various simulated fasted media on solubility;13,16,29 however, these studies are not fully comparable since there are amphiphile composition, concentration, and ratio changes (Table 1) (FaSSIF6 vs FaSSIF-V210 vs FASSIF-V2plus12 vs FaSSIF-V316) between the systems. Nevertheless, in these systems, cyclosporine,16 nifedipine,16,19 dipyridamole,16,19 danazol,16,19 ketoconazole,16,19 celecoxib,16 felodipine16 and fenofibrate16 all show varying degrees of media-dependent solubility variation. Indicating a similar although not directly comparable behavior to that presented in the current study.
Studies into the structures present within intestinal media indicate that these consist of colloidal micelles, vesicles, and lipid droplets;20,30 however, comparable studies of micelle structure to the current system are not available. One study on simulated fasted fluids indicates that these only consist of mixed micelles,20 an observation that is likely to apply to the current 4MD experiment. Figures 1 and 2 along with Table 4 indicate that the ratio of biorelevant amphiphiles can influence drug solubility. Since the 4MD maintains a constant pH and salt concentration, this variation has to be due to changing interactions between the amphiphiles altering “micellar” behavior or surface tension16 and subsequent interactions with the drug to influence solubilization. However, the statistical output for the 4MD was equilibrium solubility, and micellar characteristics were not studied.
The lipophilicity of a drug can affect how much it engages with the lipid-rich micelles.27,29,31 Fagerberg et al. discovered drug compounds with a log D(oct) greater than 3 that showed increased solubilization in colloidal structures within fasted and fed intestinal media.32 Therefore, it is not surprising to find that the solubility of spironolactone (log P = 2.7)33 and griseofulvin (log P = 2.18)34 was not significantly affected by amphiphiles at this concentration since both of them have a comparatively lower log P than the other drugs tested (Table 2). However, their low degree of interaction with BS–PL might also be conformationally related to their relatively planar molecular structure, or the lack of nonpolar motif may reduce the hydrophobic interaction with micelles. Previous studies also indicated that griseofulvin solubility was not heavily influenced by the concentration of BS, PL, and OA.22 For the steroidal drug spironolactone, Hammad and Muller (1998)35 reported a similar phenomenon with three other steroidal drugs, prednisolone, progesterone, and estradiol. Although carvedilol has a similar log D, the specific interaction with bile salt (see above) appears to dominate other solubility characteristics.
For the remaining drugs (aprepitant, fenofibrate, felodipine, zafirlukast), solubility behavior is difficult to attribute to any specific drug property due to the low number of test drugs and limited molecular diversity or congruity. Zafirlukast has a comparatively high log P (log P = 5.56), and on the BS/PL/OA surface, its solubility is dominantly affected by the concentration of PL, which might be attributable to its planar and rotatable structure, which could fit between the hydrophobic chains of PL. Additional components in the 4MD reveal another high solubility zone for zafirlukast with equal blend of MG and OA, both of which have long alkyl chain tails. Warren and colleagues showed in a simulation using molecular dynamics that more lipophilic molecules tend to interact with the amphiphile’s lipid alkane chain region, which again indicates interaction of the drug with the lipid system is highly dependent on the polarity of both the drug and lipid molecules.36 Nevertheless, apart from the multiple phase forms and micelle sizes observed across different ratios, no standardized rules and clear correlation have been identified between the composition of the media and different drug solubility.19
The results indicate that with the same total biorelevant amphiphile concentration the proportion of each component can have a remarkable influence on solubility indicating that not only the amphiphile concentration but also the ratio of the agents can influence drug solubility. Some of this behavior can be ascribed to the drugs’ physicochemical properties (log P), but the variation in profile between the drugs indicates that the specific molecular interaction or interactions between the drug and the amphiphile system play an important role with high log P compounds. A principal component analysis on solubility in human and simulated intestinal fluids29 indicated that a range of structural molecular factors can be important. Results with a larger range of drug properties and structures will be required to provide a meaningful analysis.
4.2. Amphiphile Solubility Effects
The 4MD statistical analysis provides a standardized effect value for the contribution to solubility of each individual amphiphile and combinations of amphiphiles (Figure 3a–d). For five of the drugs (aprepitant, carvedilol, fenofibrate, spironolactone, and griseofulvin), the solubilizing effect of oleic acid is dominant, a result in agreement with the previous fasted simulated media DoE.22 For zafirlukast, PL, and for felodipine, an interaction between BS and PL are the dominant solubilizing factors at variance with the DoE where BS and OA, respectively, were the most significant, although the 4MD factors for each drug were statistically significant in the DoE. There is a total of 98 possible significant results (four for each amphiphile, six for dual combinations, and four for three-way combinations for each of the seven drugs), and 33 of these exhibit a positive effect on solubility (Figure 3d).
The 4MD also for each drug (with the exception of fenofibrate where no significant negative effects are present) determines amphiphile combinations that significantly negatively impact solubility. This occurs in 11 of the possible 98 results, and interestingly, this is always a combination of two or three amphiphiles with MG contributing to 10 results, BS and PL, 7, and OA, 6. Due to statistical limitations of the previous DoE, three-way interactions were not studied and MG was not a factor; therefore, a complete comparison is not possible.
The remaining possible effects (54 out of 98) do not significantly impact solubility, indicating that in the majority of cases mixtures of amphiphiles do not interact. An effect that was also evident in the fasted DoE.22
These findings are very similar to those of the reported fasted DoE indicating that the 4MD is examining a system that is comparable to simulated media with a focus on the solubility effects due to the biorelevant amphiphiles. The y-axes in Figure 3d, which rank the individual and combination effects, are different for each drug, again emphasizing the individuality of each drug’s behavior in this system.
4.3. 4MD Advantages and Limitations
The difficulty of fitting 4MD data into linear models based on individual amphiphile concentrations suggests a complicated interaction among the amphiphiles, which is in agreement with DoE studies in which the amphiphile component interactions were significant for basic and neutral compounds. For example, significant interactions of BS and OA affected felodipine, griseofulvin, fenofibrate, zafirlukast, aprepitant, and carvedilol, while interactions with PL affected fewer drugs, which might be due to the narrow range of PL concentration utilized (0.2–1 mM).22 However, the roles and interactions of PL became more evident in 4MD, as the range was expanded (0–11.7 mM). The dashed line in Figure 1 indicates a possible space by providing BS/PL in a 4:1 and 15:1 ratio, with multiple levels of either MG or OA, which best resembles the possible scenarios in FaSSIF media.6,10 This clearly covers a set of limited solubility variations that could happen in the intestine, and although some of the combination ratios in the 4MD are not physiologically relevant in the fasted state, they provide a larger range and indicate potential risks of solubility sensitivity issues.
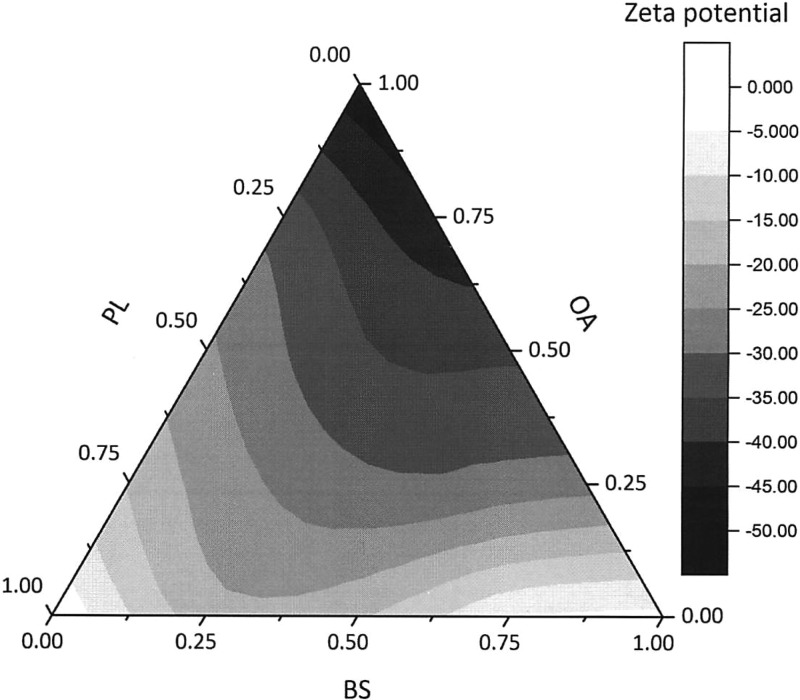
Although combinations of amphiphiles exhibit better solubilization, our study incorporated four different amphiphiles, and the highest solubility was shown to vary from drug to drug. BS and OA are two ionizable amphiphiles, the carboxylate group of OA has a pKa of about 5; however, pKa could increase considerably to above 7 in PL vesicles or other mixed aggregates.37,38 Temperature and ionic strength can also affect the apparent pKa. These ionization changes may affect solubilization capacities of the amphiphiles and the charge interaction with ionizable drugs. Carvedilol (pKa = 7.8, log P = 3.91)39,40 is a moderately hydrophobic and ionic compound, which is protonated at pH 7. Our data shows solubilization is aided by a more negatively charged system with higher OA (75%–100% OA). The solubility distribution within the BS/PL/OA surface of carvedilol resembles the zeta potential contour plot (Figure 4), which implies that electrostatic attraction becomes a predominant factor for carvedilol–micelle interaction.
Figure 4.
Zeta potential (mV) distribution in BS/PL/OA mixture (0% MG), based on 15 different compositions. Each point is an average number from two measurements (processed in OriginPro 9.0.0 SR1).
One advantage of the 4MD versus the DoE is that the levels of amphiphiles in the previous DoE were selected based on the physiological level, which can over emphasize one amphiphile relative to another if the selected concentration range is intrinsically much higher (OA, BS much higher than PL, MG). However, in the 4MD, all four components are positioned in an equal molar range. This approach gives more insight into the existence of complex phase boundaries, which change dynamically as the fluids are altered down their journey from duodenum to colon. Some of the ranges used in this approach may exceed the physiological level in the fasted state. However, only one total concentration was investigated in this study, which did not reach the fed state level where even more diverse drug behavior is to be expected. For example, griseofulvin absorption from GIT has been shown to increase with the administration of a high fat meal.41 This was not shown in our study, which might be due to the limited range of this 4MD envelope.
5. Conclusions
The current 4MD study design generates an increase in information over the previous literature and focuses on the influence of biorelevant amphiphiles on the equilibrium solubility of BCS II drugs. Drugs from previous DOE studies22,23 with a statistically significant standardized effect value indicating biorelevant amphiphiles increase solubility were selected, and the results provide comparable solubility data to literature results with both human and simulated intestinal fluids. This approach generates information that differs from previous studies, where drug solubility has a linear relationship with the total concentration of amphiphiles regardless of the type of amphiphiles used.9,27,28
Two out of seven drugs (griseofulvin, spironolactone) in this study with log P values less than 3 show marginal solubility influence induced by changing the amphiphile ratios, which adds additional information that the concentration and also the ratio of surfactants would not affect their solubility significantly. For the other five drugs (felodipine, fenofibrate, zafirlukast, carvedilol, and aprepitant) with log P values greater than 3, the results imply that the media solubilization capacities are not a simple sum of the effects of the four biorelevant amphiphiles with drug lipophilicity and that more complicated interactions and drug specific effects are important. It also suggests some insights into the drug–micelle interactions, and some of the effects observed are in good agreement with reported studies,14,35,42 indicating that drug solubility is related to lipophilicity with other factors, for example, molecular shape and charge contributing. However, the unique behavior of each drug in this study and the limited number tested does not permit detailed conclusions to be drawn.
This result indicates that not only the amphiphile concentration but also the ratio of the agents can influence drug solubility and that the resulting solubility topography is drug dependent. Therefore, a single-point solubility measurement in a fixed composition media will provide a value that might be situated in a valley, plateau, slope, or peak. In simulated media systems or in human intestinal fluid samples with a constant ratio of components, without knowledge of the surrounding solubility topography, some variability may be overlooked. Reproducible solubility determinations might indicate a consistent solubility throughout the entire space (griseofulvin, spironolactone) or a measurement on a plateau (aprepitant), while variability may relate to measurement on a slope. Therefore, targeting simulated media analysis to a physiological related and compacted ratio range of bile, phospholipids, and digested lipids while covering the concentration of fasted and fed states would provide information on the variation and sensitivity of drug solubility to specific combinations of biorelevant components.
There may be opportunities to incorporate the solubility information from the 4MD into PBPK models, which would be useful for predicting the individual variability or disease-related changes in the gastrointestinal fluids that can affect drug bioavailability. Future studies could employ a higher concentration of mixed amphiphiles that would be generated in the fed state and pH could be altered, which is likely to provide even more complicated systems through ionization changes to the drug,5 the amphiphiles,37,38 and the interactions between drugs and amphiphiles.22,23 Further studies with a larger samples of drugs with varying molecular properties (not simply log P) or a homologous series will be required in order to dissect the relationship of drug properties to amphiphile-specific solubilization.
Acknowledgments
The authors gratefully acknowledge the financial support of the Oral Biopharmaceutical Tools (OrBiTo) (115369), European Union Innovative Medicines Initiative Program and the assistance and input of the multiple colleagues associated with this project. G.W.H. is funded by Cancer Research UK (C149/A20740, and C149/A20496).
Glossary
Abbreviations
- BCS
Biopharmaceutics Classification System
- DoE
design of experiment
- FaSSIF
fasted simulated intestinal fluid
- FeSSIF
fed simulated intestinal fluid
- IVIVC
in vitro–in vivo correlation
- 4MD
4-component mixture design
The authors declare no competing financial interest.
References
- Dressman J. B.; Amidon G. L.; Reppas C.; Shah V. P. Dissolution testing as a prognostic tool for oral drug absorption: Immediate release dosage forms. Pharm. Res. 1998, 15 (1), 11–22. 10.1023/A:1011984216775. [DOI] [PubMed] [Google Scholar]
- Augustijns P.; Wuyts B.; Hens B.; Annaert P.; Butler J.; Brouwers J. A review of drug solubility in human intestinal fluids: Implications for the prediction of oral absorption. Eur. J. Pharm. Sci. 2014, 57C, 322–332. 10.1016/j.ejps.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Bergstrom C. A.; Holm R.; Jorgensen S. A.; Andersson S. B.; Artursson P.; Beato S.; Borde A.; Box K.; Brewster M.; Dressman J.; Feng K. I.; Halbert G.; Kostewicz E.; McAllister M.; Muenster U.; Thinnes J.; Taylor R.; Mullertz A. Early pharmaceutical profiling to predict oral drug absorption: current status and unmet needs. Eur. J. Pharm. Sci. 2014, 57, 173–99. 10.1016/j.ejps.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Fuchs A.; Dressman J. B. Composition and Physicochemical Properties of Fasted-State Human Duodenal and Jejunal Fluid: A Critical Evaluation of the Available Data. J. Pharm. Sci. 2014, 103 (11), 3398–3411. 10.1002/jps.24183. [DOI] [PubMed] [Google Scholar]
- Clarysse S.; Psachoulias D.; Brouwers J.; Tack J.; Annaert P.; Duchateau G.; Reppas C.; Augustijns P. Postprandial changes in solubilizing capacity of human intestinal fluids for BCS class II drugs. Pharm. Res. 2009, 26 (6), 1456–66. 10.1007/s11095-009-9857-7. [DOI] [PubMed] [Google Scholar]
- Galia E.; Nicolaides E.; Horter D.; Lobenberg R.; Reppas C.; Dressman J. B. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 1998, 15 (5), 698–705. 10.1023/A:1011910801212. [DOI] [PubMed] [Google Scholar]
- Pedersen B. L.; Mullertz A.; Brondsted H.; Kristensen H. G. A comparison of the solubility of danazol in human and simulated gastrointestinal fluids. Pharm. Res. 2000, 17 (7), 891–894. 10.1023/A:1007576713216. [DOI] [PubMed] [Google Scholar]
- Vertzoni M.; Fotaki N.; Kostewicz E.; Stippler E.; Leuner C.; Nicolaides E.; Dressman J.; Reppas C. Dissolution media simulating the intralumenal composition of the small intestine: physiological issues and practical aspects. J. Pharm. Pharmacol. 2004, 56 (4), 453–62. 10.1211/0022357022935. [DOI] [PubMed] [Google Scholar]
- Sunesen V. H.; Pedersen B. L.; Kristensen H. G.; Mullertz A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur. J. Pharm. Sci. 2005, 24 (4), 305–13. 10.1016/j.ejps.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Jantratid E.; Janssen N.; Reppas C.; Dressman J. B. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm. Res. 2008, 25 (7), 1663–76. 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- Psachoulias D.; Vertzoni M.; Goumas K.; Kalioras V.; Beato S.; Butler J.; Reppas C. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm. Res. 2011, 28 (12), 3145–58. 10.1007/s11095-011-0506-6. [DOI] [PubMed] [Google Scholar]
- Psachoulias D.; Vertzoni M.; Butler J.; Busby D.; Symillides M.; Dressman J.; Reppas C. An in vitro methodology for forecasting luminal concentrations and precipitation of highly permeable lipophilic weak bases in the fasted upper small intestine. Pharm. Res. 2012, 29 (12), 3486–98. 10.1007/s11095-012-0844-z. [DOI] [PubMed] [Google Scholar]
- Kleberg K.; Jacobsen F.; Fatouros D. G.; Mullertz A. Biorelevant media simulating fed state intestinal fluids: colloid phase characterization and impact on solubilization capacity. J. Pharm. Sci. 2010, 99 (8), 3522–32. 10.1002/jps.22122. [DOI] [PubMed] [Google Scholar]
- Persson E. M.; Gustafsson A. S.; Carlsson A. S.; Nilsson R. G.; Knutson L.; Forsell P.; Hanisch G.; Lennernas H.; Abrahamsson B. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm. Res. 2005, 22 (12), 2141–2151. 10.1007/s11095-005-8192-x. [DOI] [PubMed] [Google Scholar]
- Riethorst D.; Mols R.; Duchateau G.; Tack J.; Brouwers J.; Augustijns P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2015, 105, 673. 10.1002/jps.24603. [DOI] [PubMed] [Google Scholar]
- Fuchs A.; Leigh M.; Kloefer B.; Dressman J. B. Advances in the design of fasted state simulating intestinal fluids: FaSSIF-V3. Eur. J. Pharm. Biopharm. 2015, 94, 229–40. 10.1016/j.ejpb.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Clarysse S.; Tack J.; Lammert F.; Duchateau G.; Reppas C.; Augustijns P. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J. Pharm. Sci. 2009, 98 (3), 1177–92. 10.1002/jps.21502. [DOI] [PubMed] [Google Scholar]
- Soderlind E.; Karlsson E.; Carlsson A.; Kong R.; Lenz A.; Lindborg S.; Sheng J. J. Simulating Fasted Human Intestinal Fluids: Understanding the Roles of Lecithin and Bile Acids. Mol. Pharmaceutics 2010, 7 (5), 1498–1507. 10.1021/mp100144v. [DOI] [PubMed] [Google Scholar]
- Kleberg K.; Jacobsen J.; Mullertz A. Characterising the behaviour of poorly water soluble drugs in the intestine: application of biorelevant media for solubility, dissolution and transport studies. J. Pharm. Pharmacol. 2010, 62 (11), 1656–68. 10.1111/j.2042-7158.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- Riethorst D.; Baatsen P.; Remijn C.; Mitra A.; Tack J.; Brouwers J.; Augustijns P. An In-Depth View into Human Intestinal Fluid Colloids: Intersubject Variability in Relation to Composition. Mol. Pharmaceutics 2016, 13 (10), 3484–3493. 10.1021/acs.molpharmaceut.6b00496. [DOI] [PubMed] [Google Scholar]
- Bevernage J.; Brouwers J.; Clarysse S.; Vertzoni M.; Tack J.; Annaert P.; Augustijns P. Drug supersaturation in simulated and human intestinal fluids representing different nutritional states. J. Pharm. Sci. 2010, 99 (11), 4525–34. 10.1002/jps.22154. [DOI] [PubMed] [Google Scholar]
- Khadra I.; Zhou Z.; Dunn C.; Wilson C. G.; Halbert G. Statistical investigation of simulated intestinal fluid composition on the equilibrium solubility of biopharmaceutics classification system class II drugs. Eur. J. Pharm. Sci. 2015, 67, 65–75. 10.1016/j.ejps.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Dunn C.; Khadra I.; Wilson C. G.; Halbert G. W. Statistical investigation of simulated fed intestinal media composition on the equilibrium solubility of oral drugs. Eur. J. Pharm. Sci. 2017, 99, 95–104. 10.1016/j.ejps.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konyakhina T. M.; Wu J.; Mastroianni J. D.; Heberle F. A.; Feigenson G. W. Phase diagram of a 4-component lipid mixture: DSPC/DOPC/POPC/chol. Biochim. Biophys. Acta, Biomembr. 2013, 1828 (9), 2204–14. 10.1016/j.bbamem.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S.; Shukla D.; Jain A.; Mishra B.; Singh S. Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J. Colloid Interface Sci. 2009, 335 (2), 242–9. 10.1016/j.jcis.2009.03.047. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F. BEHAVIOR AND SOLUBILITY OF MONOGLYCERIDES IN DILUTE, MICELLAR BILE-SALT SOLUTION. Biochim. Biophys. Acta 1963, 70 (3), 306–316. 10.1016/0006-3002(63)90754-6. [DOI] [PubMed] [Google Scholar]
- Zangenberg N. H.; Mullertz A.; Kristensen H. G.; Hovgaard L. A dynamic in vitro lipolysis model II: Evaluation of the model. Eur. J. Pharm. Sci. 2001, 14 (3), 237–244. 10.1016/S0928-0987(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Ilardia-Arana D.; Kristensen H. G.; Mullertz A. Biorelevant dissolution media: aggregation of amphiphiles and solubility of estradiol. J. Pharm. Sci. 2006, 95 (2), 248–55. 10.1002/jps.20494. [DOI] [PubMed] [Google Scholar]
- Clarysse S.; Brouwers J.; Tack J.; Annaert P.; Augustijns P. Intestinal drug solubility estimation based on simulated intestinal fluids: comparison with solubility in human intestinal fluids. Eur. J. Pharm. Sci. 2011, 43 (4), 260–9. 10.1016/j.ejps.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Mullertz A.; Reppas C.; Psachoulias D.; Vertzoni M.; Fatouros D. G. Structural features of colloidal species in the human fasted upper small intestine. J. Pharm. Pharmacol. 2015, 67 (4), 486–92. 10.1111/jphp.12336. [DOI] [PubMed] [Google Scholar]
- Kossena G. A.; Boyd B. J.; Porter C. J. H.; Charman W. N. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J. Pharm. Sci. 2003, 92 (3), 634–648. 10.1002/jps.10329. [DOI] [PubMed] [Google Scholar]
- Fagerberg J. H.; Karlsson E.; Ulander J.; Hanisch G.; Bergstrom C. A. Computational prediction of drug solubility in fasted simulated and aspirated human intestinal fluid. Pharm. Res. 2015, 32 (2), 578–89. 10.1007/s11095-014-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora D. I.; Udrescu S.; Albu F.; David V.; Medvedovici A. Analytical issues in HPLC/MS/MS simultaneous assay of furosemide, spironolactone and canrenone in human plasma samples. J. Pharm. Biomed. Anal. 2010, 52 (5), 734–740. 10.1016/j.jpba.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Mithani S. D.; Bakatselou V.; TenHoor C. N.; Dressman J. B. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 1996, 13 (1), 163–167. 10.1023/A:1016062224568. [DOI] [PubMed] [Google Scholar]
- Hammad M. A.; Muller B. W. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998, 46 (3), 361–367. 10.1016/S0939-6411(98)00037-X. [DOI] [PubMed] [Google Scholar]
- Warren D. B.; King D.; Benameur H.; Pouton C. W.; Chalmers D. K. Glyceride lipid formulations: molecular dynamics modeling of phase behavior during dispersion and molecular interactions between drugs and excipients. Pharm. Res. 2013, 30 (12), 3238–53. 10.1007/s11095-013-1206-1. [DOI] [PubMed] [Google Scholar]
- Small D. M.; Cabral D. J.; Cistola D. P.; Parks J. S.; Hamilton J. A. THE IONIZATION BEHAVIOR OF FATTY-ACIDS AND BILE-ACIDS IN MICELLES AND MEMBRANES. Hepatology 1984, 4 (5), S77–S79. 10.1002/hep.1840040814. [DOI] [PubMed] [Google Scholar]
- Edwards K.; Silvander M.; Karlsson G. AGGREGATE STRUCTURE IN DILUTE AQUEOUS DISPERSIONS OF OLEIC-ACID SODIUM OLEATE AND OLEIC-ACID SODIUM OLEATE EGG PHOSPHATIDYLCHOLINE. Langmuir 1995, 11 (7), 2429–2434. 10.1021/la00007a020. [DOI] [Google Scholar]
- Mannhold R. The impact of lipophilicity in drug research: A case report on beta-blockers. Mini-Rev. Med. Chem. 2005, 5 (2), 197–205. 10.2174/1389557053402701. [DOI] [PubMed] [Google Scholar]
- Loftsson T.; Vogensen S. B.; Desbos C.; Jansook P. Carvedilol: Solubilization and cyclodextrin complexation: A technical note. AAPS PharmSciTech 2008, 9 (2), 425–430. 10.1208/s12249-008-9055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crounse R. G. EFFECTIVE USE OF GRISEOFULVIN. Arch. Dermatol. 1963, 87 (2), 176. 10.1001/archderm.1963.01590140038006. [DOI] [PubMed] [Google Scholar]
- Kossena G. A.; Charman W. N.; Boyd B. J.; Dunstan D. E.; Porter C. J. H. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: A phase diagram approach. J. Pharm. Sci. 2004, 93 (2), 332–348. 10.1002/jps.10554. [DOI] [PubMed] [Google Scholar]
- Ridhurkar D. N.; Ansari K. A.; Kumar D.; Kaul N. S.; Krishnamurthy T.; Dhawan S.; Pillai R. Inclusion complex of aprepitant with cyclodextrin: evaluation of physico-chemical and pharmacokinetic properties. Drug Dev. Ind. Pharm. 2013, 39 (11), 1783–92. 10.3109/03639045.2012.737331. [DOI] [PubMed] [Google Scholar]
- Scholz A.; Abrahamsson B.; Diebold S. M.; Kostewicz E.; Polentarutti B. I.; Ungell A. L.; Dressman J. B. Influence of hydrodynamics and particle size on the absorption of felodipine in labradors. Pharm. Res. 2002, 19 (1), 42–46. 10.1023/A:1013651215061. [DOI] [PubMed] [Google Scholar]
- Madsen C. M.; Boyd B.; Rades T.; Mullertz A. Supersaturation of zafirlukast in fasted and fed state intestinal media with and without precipitation inhibitors. Eur. J. Pharm. Sci. 2016, 91, 31–9. 10.1016/j.ejps.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Berthod A.; Carda-Broch S.; Garcia-Alvarez-Coque M. C. Hydrophobicity of ionizable compounds. A theoretical study and measurements of diuretic octanol-water partition coefficients by countercurrent chromatography. Anal. Chem. 1999, 71 (4), 879–888. 10.1021/ac9810563. [DOI] [Google Scholar]