Abstract
Malaria is a vector-borne disease that is a great burden on the poorest and most marginalized communities of the tropical and subtropical world. Approximately 41 species of Anopheline mosquitoes can effectively spread species of Plasmodium parasites that cause human malaria. Proposing a natural classification for the subfamily Anophelinae has been a continuous effort, addressed using both morphology and DNA sequence data. The monophyly of the genus Anopheles, and phylogenetic placement of the genus Bironella, subgenera Kerteszia, Lophopodomyia and Stethomyia within the subfamily Anophelinae, remain in question. To understand the classification of Anophelinae, we inferred the phylogeny of all three genera (Anopheles, Bironella, Chagasia) and major subgenera by analysing the amino acid sequences of the 13 protein coding genes of 150 newly sequenced mitochondrial genomes of Anophelinae and 18 newly sequenced Culex species as outgroup taxa, supplemented with 23 mitogenomes from GenBank. Our analyses generally place genus Bironella within the genus Anopheles, which implies that the latter as it is currently defined is not monophyletic. With some inconsistencies, Bironella was placed within the major clade that includes Anopheles, Cellia, Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia, which were found to be monophyletic groups within Anophelinae. Our findings provided robust evidence for elevating the monophyletic groupings Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia to genus level; genus Anopheles to include subgenera Anopheles, Baimaia, Cellia and Christya; Anopheles parvus to be placed into a new genus; Nyssorhynchus to be elevated to genus level; the genus Nyssorhynchus to include subgenera Myzorhynchella and Nyssorhynchus; Anopheles atacamensis and Anopheles pictipennis to be transferred from subgenus Nyssorhynchus to subgenus Myzorhynchella; and subgenus Nyssorhynchus to encompass the remaining species of Argyritarsis and Albimanus Sections.
Keywords: Anopheles, mitochondrial genomes, phylogenetics
1. Introduction
Malaria transmission is endemic in 99 countries and territories of tropical and subtropical areas of the world. Globally, approximately 3 billion people are at risk of becoming infected with Plasmodium parasites. The risk is variable, with some regions at high risk, whereas other areas are progressing towards elimination of malaria, or have succeeded in eliminating it [1,2]. In 2013, about 198 million cases of malaria occurred worldwide (estimates ranged from 124 to 283 million), with approximately 584 000 deaths (estimates ranged from 367 000 to 755 000), accounting for 78% of all deaths in children aged under 5 years. Even considering the uncertainties in the latest estimates of cases and deaths, malaria is a huge burden on the poorest and most marginalized communities living in endemic countries [3]. In the Americas, there were 389 390 malaria cases in 2014. Brazil accounted for 36.8% of these, followed by the Bolivarian Republic of Venezuela with 23.3% and Peru with 16.6%. These three countries together accounted for 76.7% of malaria cases in 2014; however, the highest annual parasite index (API) per 1000 people was registered in Suriname (17.4), Guyana (16.5) and Venezuela (15.3) [4]. In 2015, the Bolivarian Republic of Venezuela accounted for 30%, Brazil 24%, Peru 19% and Colombia 10% of estimated malaria cases [3]. The numbers of cases have increased because of economic conditions, mining activities and decreased vector control strategies. For instance, Venezuela reported more cases in 2014 than in any year in the previous 50 years [4].
Approximately, 41 species of the genus Anopheles (subfamily Anophelinae) can effectively transmit six species of the genus Plasmodium, P. falciparum (Welch), P. vivax Grassi & Feletti, P. malariae Feletti & Grassi, P. ovale curtisi Sutherland et al., P. ovale wallikeri Sutherland et al. and P. knowlesi Sinton and Mulligan, from an infected to a susceptible person [5]. Anopheles mosquitoes also transmit the filarial parasite Wuchereria bancrofti Cobbold, and Brugia malayi Brug, as well as various arboviruses, to humans [6].
Mosquitoes belong to Culicidae, a nematocerous family of Diptera. They are subdivided into two subfamilies, Culicinae and Anophelinae. Culicinae is distributed worldwide and has 3067 species in 38 genera, including Aedes and Culex. Anophelinae has a cosmopolitan distribution with 485 formally recognized species and several unnamed members of species complexes that have not been formally described (WRBU 2016, http://wrbu.org/VecID_MQ.html). The current scheme of classification of the Anophelinae subdivides it into three genera, Anopheles (472 species in addition to several unnamed members of species complexes, cosmopolitan), Bironella (eight species, Australasian) and Chagasia (five species, Neotropical).
The genus Anopheles has eight subgenera and various informal groups as sections, series, groups and subgroups (table 1), which were defined based on morphological traits of adults, fourth-instar larvae and pupae [7,8]. Most of the sections, series, groups and subgroups are based on non-monophyletic groups (figs 4–6 in [8]). The genus Bironella is subdivided into three subgenera, Bironella, Brugella and Neobironella [9], and Chagasia has no subgeneric classification [10].
Table 1.
Present scheme of internal classification of the subfamily Anophelinae (genus, subgenus, section and series), type species and number of species in each subgenus.
| genus | subgenus | section | series | type species | no. species |
|---|---|---|---|---|---|
| Anopheles | Anopheles | Anopheles maculipennis Meigen | 183 | ||
| Angusticorn | Anopheles | ||||
| Cycloleppteron | |||||
| Lophoscelomyia | |||||
| Laticorn | Arribalzagia | ||||
| Myzorhynchus | |||||
| Baimaia | Anopheles kyondawensis Abraham | 1 | |||
| Cellia | Anopheles pharoensis Theobald | 224 | |||
| Cellia | |||||
| Myzomyia | |||||
| Neocellia | |||||
| Neomyzomyia | |||||
| Paramyzomyia | |||||
| Pyretophorus | |||||
| Christya | Anopheles implexus (Theobald) | 2 | |||
| Kerteszia | Anopheles boliviensis (Theobald) | 12 | |||
| Lophopodomyia | Anopheles squamifemur Antunes | 6 | |||
| Nyssorhynchus | Anopheles argyritarsis Robineau Desvoidy | 39 | |||
| Albimanus | Albimanus | ||||
| Oswaldoi | |||||
| Argyritarsis | Albitarsis | ||||
| Argyritarsis | |||||
| Myzorhynchella | |||||
| Stethomyia | Anopheles nimbus Theobald | 5 | |||
| Bironella | Bironella | Bironella gracilis Theobald | 2 | ||
| Brugella | Bironella travestita (Brug) | 3 | |||
| Neobironella | Bironella confusa Bonne-Wepster | 3 | |||
| Chagasia | Chagasia neivae Cruz | 5 |
Despite several efforts, a stable classification for the subfamily Anophelinae remains elusive. For example, relationships among the genera Anopheles, Bironella and Chagasia were addressed using both morphological traits [8,11,12] and DNA sequence data [13,14]. However, both morphology and molecular data failed to yield a unified consensus of the relationships among these genera. Relationships remain unresolved with contradictory hypotheses regarding the monophyly of the genus Anopheles and the placement of Bironella within the subfamily [8,13–15]. The genus Bironella was found either within the genus Anopheles as the sister group of the subgenus Stethomyia [11] or outside the genus Anopheles [14]. Recently, Harbach & Kitching [8] found Bironella clustered with species of Anopheles, without considering the possibility of the former being a subgenus of the latter. Both morphology [8,11] and DNA sequences [13,14] confirmed Chagasia to be a sister group to the clade composed of Anopheles and Bironella within Anophelinae. By contrast, monophyly of the genus Anopheles is subject to a certain degree of taxonomic instability. For instance, Krzywinski et al. [16] corroborated the monophyly of the genus Anopheles as well as of the nominal subgenus Anopheles, using DNA sequences of two protein-coding nuclear genes (white and G6PD), one protein-coding mitochondrial gene (ND5) and the D2 region of the ribosomal gene. Additionally, when the white gene DNA sequences were analysed separately, Bironella was the sister taxon of Anopheles. However, Sallum et al. [11,13], and Collucci & Sallum [17] found the genus Anopheles paraphyletic relative to Bironella. In addition, Harbach & Kitching [8,12] proposed two new subgenera (Baimaia and Christya) within Anopheles, but maintained Bironella as a valid genus even though Bironella and the subgenera Stethomyia and Baimaia had been placed nested within the subgenus Anopheles.
Currently, the genus Anopheles is subdivided into eight subgenera (table 1). The subgenus Anopheles, being cosmopolitan, has the largest geographical distribution; Cellia occurs in the Afrotropical, Australasian and Oriental regions; Kerteszia, Lophopodomyia, Stethomyia and Nyssorhynchus are restricted to the Neotropics, with Anopheles (Nyssorhynchus) albimanus reaching southern parts of the Nearctic. Little information exists concerning the distributions of subgenera Baimaia and Christya, proposed by Harbach & Kitching [8,12]. The former occurs in limited areas of Southeast Asia and was nominated to include a unique species that uses crabholes as larval habitat [18]. The subgenus Christya occurs in the sub-Sahara [8], and includes two sylvatic species, Anopheles implexus (Theobald) and Anopheles okuensis Brunhes, Le Goff and Geoffroy.
Phylogenetic relationships among subgenera of the genus Anopheles remain unresolved. Foley et al. [19], Sallum et al. [11] and Freitas et al. [15] found some indication that the subgenus Anopheles is paraphyletic. Collucci & Sallum [17] used 111 morphological characters and 36 species of Anopheles (Anopheles) with five outgroup taxa, and showed that Anopheles (Anopheles) was a monophyletic group and that Bironella was a sister lineage. In addition, Krzywinski et al. [20], based on the results of phylogenetic analysis of the DNA sequences of the white gene, found evidence supporting monophyly of the subgenus Anopheles, a sister taxa relationship of subgenera Nyssorhynchus and Kerteszia, and monophyly of a group composed of Cellia and Anopheles. Furthermore, the subgenus Lophopodomyia was found as sister taxon to the clade formed of Nyssorhynchus and Kerteszia, whereas the subgenus Stethomyia was placed outside the clade composed of other Anopheles subgenera. Results of a phylogenetic analysis carried out for 12 species of Anopheles (Kerteszia) confirmed the monophyly of the subgenus Kerteszia, and the close relationship between Nyssorhynchus and Kerteszia [21].
Foster et al. [22] looked at relationships within Anopheles (Nyssorhynchus), and noted that recovery of known higher-level relationships benefited from more sequence data, and by extrapolation proposed using complete mitochondrial genomes for such problems in future. The mitochondrial genome is a rich source of information and has been used in several studies [23–27]. Analysis of complete mitochondrial genomes of Anopheles species has provided new evidence for species complexes and a new understanding of the phylogenetic relationships among them [27–31]. Similarly, promising results have been obtained for the classification of the Culex coronator species complex [26]. It is remarkable that results of phylogenetic analyses, which included the mitochondrial genomes of 12 species of the lepidopteran superfamily Noctuoidea, found robust support for the monophyly of each noctuoid family [32]. It is appreciated that using complete mitochondrial genomes in phylogenetics can be problematic [24], but here the authors suggest that if gene order rearrangements, nucleotide frequency and strand bias do not vary greatly among taxa then mitochondrial genomes still have value.
Compositional bias in DNA sequences can distort the results of phylogenetic analysis, so analysis using the protein sequences derived from DNA sequences of protein-coding genes are often preferred. DNA sequences suffer from saturation more than protein sequences, partly because there are fewer character states in DNA sequences than in protein. In addition, because selection acts directly on protein sequences, but indirectly on DNA sequences, the proteins evolve more slowly than DNA. Saturation and biases such as compositional heterogeneity tend to manifest most strongly in rapidly evolving sequences, and so DNA tends to be more biased than amino acids [33–35]. In this study, we used the protein sequences of mitochondrial genomes.
In this study, family- and genus-level relationships were inferred using phylogenomic analyses of the amino acid sequences of the 13 protein coding genes of 168 newly sequenced mitochondrial genomes of Anophelinae and Culex species, supplemented with 23 RefSeq mitogenomes from GenBank, in order: (i) to address the monophyly of family Culicidae, and subfamilies Anophelinae and Culicinae; (ii) to define the phylogenetic position of Bironella and Chagasia within the subfamily; (iii) to establish major monophyletic groups within Anophelinae; (iv) to verify the monophyly of the subgenera Anopheles, Cellia, Lophopodomyia, Kerteszia, Nyssorhynchus and Stethomyia; and (v) to test the current classification of the subfamily Anophelinae. In this study, we provide evidence that supports an alternative hypothesis for the classification of Anophelinae based on monophyly of inferred groups drawn from mitogenomic data.
2. Material and methods
2.1. Taxon sampling
In the study, representatives of all three current genera of Anophelinae and six subgenera of the genus Anopheles were included in the ingroup. The species sampled for this study and the sources of specimens are listed in electronic supplementary material, table S1; the current classification of the species is in table 2. Larvae and pupae were either collected from field habitats or obtained from link-reared offspring of blood-fed females collected in the field. Both larvae and pupae were maintained in the laboratory to obtain adult males and females associated with larval and pupal exuviae. Freshly emerged mosquitoes were quickly anaesthetized with ethyl acetate, and either kept separate in minute plastic vials in silica gel or individually frozen at −80°C. One individual of Anopheles atacamensis was collected as an adult male in the Atacama Desert, Chile. An entire fourth-instar larva of Bironella hollandi was employed for the study. Species identifications were based on either adult male genitalia or fourth-instar larval morphological features. For some taxa, identification was also based on the external morphology of the eggs observed in a Jeol JSM-6460 scanning electron microscope (SEM, Jeol Ltd., Akishima, Japan) as described by Sallum et al. [36] and Nagaki et al. [37].
Table 2.
List of species of the genus Anopheles employed in the present study according to current classification. Mitochondrial genome sequences of species of the subgenus Cellia and Anopheles quadrimaculatus were obtained from GenBank, and the rest were newly sequenced in this study.
| subgenus | section | series | specific epithet |
|---|---|---|---|
| Anopheles | Angusticorn | Anopheles | quadrimaculatus |
| eiseni geometricus | |||
| Laticorn | Arribalzagia | costai | |
| near costai | |||
| fluminensis | |||
| forattinii | |||
| intermedius | |||
| minor | |||
| peryassui | |||
| Cellia | Neomyzomyia | cracens | |
| farauti 4 | |||
| hinesorum | |||
| Pyretophorus | gambiae | ||
| Kerteszia | cruzii | ||
| bellator | |||
| homunculus | |||
| leneanus | |||
| Lophopodomyia | gilesi | ||
| pseudotibiamaculatus | |||
| Nyssorhynchus | Albimanus | Oswaldoi | evansae |
| noroestensisa | |||
| galvaoi | |||
| konderi A | |||
| konderi B | |||
| konderi C | |||
| oswaldoi | |||
| oswaldoi A | |||
| oswaldoi SP Form | |||
| rangeli | |||
| dunhami | |||
| goeldii | |||
| nuneztovari A | |||
| albertoi | |||
| arthuri | |||
| arthuri B | |||
| arthuri C | |||
| arthuri D | |||
| rondoni | |||
| strodei | |||
| striatus | |||
| benarrochi | |||
| triannulatus | |||
| Argyritarsis | Albitarsis | albitarsis | |
| albitarsis H | |||
| deaneorum | |||
| marajoara | |||
| oryzalimnetes | |||
| braziliensis | |||
| near braziliensis | |||
| Argyritarsis | argyritarsis | ||
| sawyeri | |||
| darlingi | |||
| paulistensisb | |||
| lanei | |||
| atacamensis | |||
| Myzorhynchella | antunesi | ||
| guarani | |||
| lutzii | |||
| lutzii A | |||
| lutzii B | |||
| parvus | |||
| pristinus | |||
| Stethomyia | kompi | ||
| nimbus |
aAnopheles noroestensis is currently in synonymy with Anopheles evansae. Specimens employed in this study are from the type locality. bAnopheles paulistensis is currently in synonymy with Anopheles darlingi. Specimens employed in this study are from the type locality.
2.2. Genomic DNA isolation
DNA was extracted from each specimen individually following the animal tissue DNA extraction protocol provided by the QIAgen DNeasy® Blood and Tissue Kit (QIAgen Ltd, Crawley, UK). DNA was eluted to a volume of 200 l with Buffer AE (10 mM Tris–Cl; 0.5 mM EDTA; pH 9.0) and stored at −80°C as part of the frozen entomological collection of the Faculdade de Saúde Pública, Universidade de São Paulo, Brazil. Genomic DNA extracts were used for PCR amplifications.
2.3. PCR amplification and sequencing
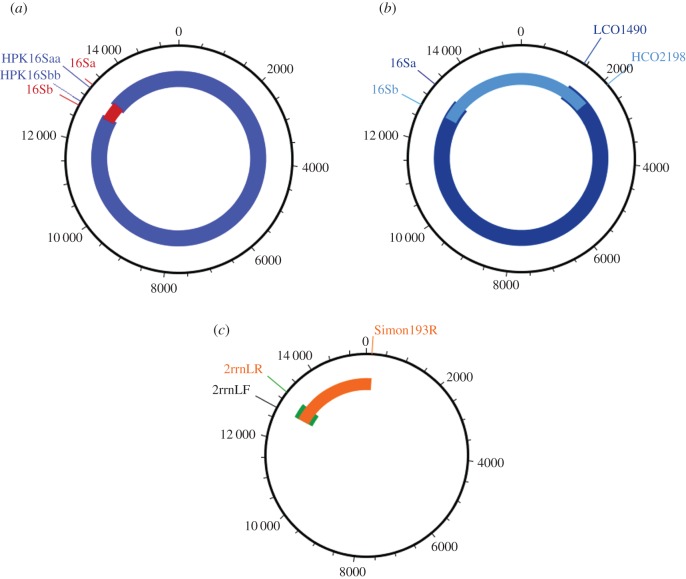
Mitochondrial genomes of Anophelinae and Culex species were amplified either in a single long-range PCR or two overlapping long PCR fragments using GoTaq® Long PCR Master Mix (Promega, Wisconsin, USA). The PCR primers employed were conserved either across all arthropods or designed from Anopheles sequences (electronic supplementary material, table S3). The remaining portions of the mitochondrial genome were amplified with several primers designed for specific regions based on alignments of newly sequenced Anopheles DNA sequences and used for internal PCRs (electronic supplementary material, table S3). The position of the primers in the mitochondrial genome is in figure 1. The primer pairs HPK16Saa and HPK16Sbb were employed to amplify approximately 15 300 base pairs (bp), whereas the primer pairs LCO1490-16Sa amplified approximately 12 000 bp and HCO2198-16Sb approximately 4800 bp. Because PCR success varied between specimens, the amplification strategy varied according to species and specimen; for details of full amplification strategy, primers employed for varied PCRs and thermal cycling conditions, see electronic supplementary material, table S4.
Figure 1.
Scheme of amplifications performed in this study. Blue bars show amplified fragments sequenced by Illumina technology, while remaining colours show Sanger-sequenced fragments. (a,b) The two methods of amplification of the mitochondrial genome used in this study. In both (a) and (b), the complete mitochondrial DNA was amplified in two steps. In (a), fragments of about 15.058 kbp and about 655 bp were amplified and sequenced. In (b), the fragments about 11.857 kbp and about 4.785 kbp were amplified and sequenced in both directions using the same set of primers employed for PCR amplification. (c) The problematic regions after assembly of the mitochondrial genome. Some samples showed low coverage in these regions (green and orange) and then new amplifications and sequencing were carried out to complete the genome sequence. The green region in Anopheles evansae and Anopheles eiseni was amplified employing species specific primers F.
The long-range PCR amplicons were purified using DNA Clean & ConcentradorTM (Zymo Research, California, USA) and quantified using the Qubit 2.0 fluorometer (LifeTechnologies, Oregon, USA). Certain regions of the mitochondrial genome were amplified by PCR using Platinum® Taq DNA polymerase (Invitrogen, California, USA), and a series of primers designed for specific portions of the genome (electronic supplementary material, table S3). The PCR amplicons were purified by PEG precipitation (20% polyethylene glycol 8000/2.5 M NaCl).
2.4. Nextera DNA sample preparation and Illumina sequencing
Next-generation sequencing and Sanger sequencing were employed to obtain DNA sequences from 168 individuals of both Anophelinae and Culex species (electronic supplementary material, table S4). Long PCR products were employed to obtain barcode libraries using Nextera® XT DNA Sample Preparation Kit (Illumina, Illinois, USA), and sequenced on the Illumina MiSeq platform with paired-end 250 bp chemistry.
2.5. Sanger DNA sequencing
For some specimens, it was problematic to obtain the entire mitochondrial DNA using Illumina sequencing technology only. Consequently, we obtained small fragments of certain portions of the mitochondrial genome to complete the circular DNA molecule. In these situations, we amplified the target DNA using primers that were developed for specific regions (electronic supplementary material, table S5). PCR products were electrophoresed in 1.0% TBE agarose gels stained with GelRed Nucleic Acid Gel Stain (Biotium Inc., Hayward, USA). Sanger sequencing reactions [38] were carried out in one direction using ABI Big Dye Terminator Kit v.3.1 (PE Applied Biosystems, Warrington, England). Sequencing reactions were purified in Sephadex G50® columns (GE Healthcare), analysed on an ABI Prism 3130—Avant Genetic Analyser (Applied Biosystems, Foster City, CA, USA), and edited using Sequencher® for Windows v. 5.1. Sanger DNA fragments were assembled to the mitochondrial genome obtained using Illumina sequencing technology to complete the circular molecule.
2.6. Sequence assembly and annotation
De novo assembly used MIRA v. 4.9 [39] and IDBA-UD v. 1.1.2 [40], aided by CAP3 [41] and visualized using Tablet [42]. MIRA was also used for assembly by mapping against very similar sequences and for mapping with extension. Blastn [43] was used to identify artefactual sequence repeats, which were excised, and for identifying overlapping ends for circularizing. Circularizing some assemblies required bridging with Sanger sequences as mentioned above.
When the sequences had been circularized, annotation began with the MITOS website http://mitos.bioinf.uni-leipzig.de [44]. Sequences were then circularly permuted so that they started with the trnI(gat) gene. Protein-coding genes were then checked with GeneWise (part of the Wise2 package v. 2.4 http://www.ebi.ac.uk/birney/wise2) using an HMM model (HMMER v.2, http://hmmer.org/) based on alignments of NCBI RefSeq mosquito mitochondrial translations. GenBank format files were made using tbl2asn (https://www.ncbi.nlm.nih.gov/genbank/tbl2asn2/), which were then read and manipulated using Biopython ([45], http://biopython.org/) and p4 ([46], http://p4.nhm.ac.uk). Boundaries of all genes were further checked by eye using alignments as a guide.
2.7. Phylogenetic analysis
The χ2-test for compositional homogeneity used p4 ([46], http://p4.nhm.ac.uk). Alignments were made using Clustal Omega [47]. Alignments were masked for reliable sites using GBlocks [48] with default settings except that parameter ‘Allowed Gap Positions’ was set to half. Duplicate sequences were removed before phylogenetic analysis, and restored with branch lengths of zero for presentation. Phylogenetic analysis used Phylobayes-MPI v. 1.5 [49], PAUP v. 4.0b10 [50], Phyml v. 20120412 [51], RAxML v. 8.1.3 [52] and IQ-Tree v. 1.5.4 [53–56]
3. Results
3.1. Newly sequenced mitogenomes, compositional heterogeneity
We sequenced 168 mosquito mitogenomes, including 148 Anopheles, 1 Bironella, 1 Chagasia and 18 Culex. The mitochondrial genomes of five species of Anopheles were obtained from GenBank (table 2). There were 64 Anopheles species, 55 of which were sequenced for the first time. Mitochondrial genomes of four species of Anopheles (Kerteszia) from the Atlantic Forest of Brazil have been described in Oliveira et al. [27], including Anopheles bellator, An. cruzii, An. homunculus and An. laneanus. Demari-Silva et al. [26] described mitochondrial genomes of four species: Culex coronator (two specimens), Cu. usquatus (one specimen), Cu. camposi (one specimen) and Cu. usquatissimus (two specimens), of the Coronator Group of Culex (Culex). Other Culex (Culex) species newly sequenced and included in the analyses as outgroup taxa were Culex lygrus, Cu. nigripalpus, Cu. chidesteri, Cu. mollis, Cu. declarator, Cu. bidens, Cu. brami, Cu. dolosus CJForm, Cu. bilineatus and Cu. surinamensis. Culex pipiens pipiens (NC_015079.1), Cu. quinquefasciatus (NC_014574.1), Ae. notoscriptus (NC_025473.1), Ae. aegypti (NC_010241.1) and Ae. albopictus (NC_006817.1) have been previously sequenced and were obtained from GenBank. All the mitochondrial genomes had 37 genes, and all were in the same order and on the same strand (electronic supplementary material, table S7). The 168 genomes ranged in size from 15 322 to 16 052 bp, with Anopheles from 15 322 to 15 739, Culex from 15 568 to 16 052, Bironella 15 772 and Chagasia 15 717.
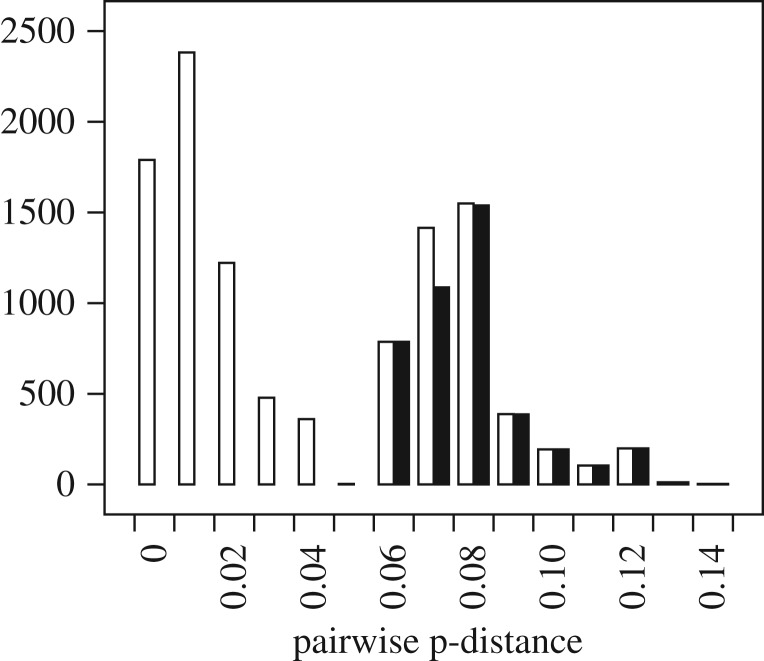
The translations of the 13 protein-coding genes of the 150 newly sequenced mitogenomes of Anophelinae were similar, and after alignment GBlocks identified only one site to be masked. The translations were aligned and then concatenated to make a supermatrix of length 3735 (after masking the GBlocks site), and then compared with the corresponding DNA sequences (length 11 205). A χ2-test for compositional homogeneity did not show significant heterogeneity in the amino acid sequences (χ2=138.8; d.f.=2831; p=1.0) but showed substantial heterogeneity in the DNA (χ2=539.8; d.f.=447; p=0.0017). This test suffers from a high probability of type-II error, and so although a better test may show compositional heterogeneity in the translations, we can be sure that the DNA sequences are compositionally heterogeneous. This favours using protein sequences in subsequent phylogenetic analyses. This amino acid alignment had 772 parsimony informative sites (21% of the 3735 sites), while the corresponding DNA alignment had 4418 parsimony informative sites (39% of the 11 205 sites). Pairwise divergences between sequences in the protein alignment were examined using p-distances (figure 2), and as we were interested in relationships between genera and subgenera the protein sequences were deemed sufficiently diverged for our purpose.
Figure 2.
p-Distances between pairs of aligned, concatenated protein sequences, length 3735 aa. Empty bars show all p-distances except between pairs of sequences from the same species (the smallest distance in this set is 0.0005, representing two differences over the sequence pair), and filled bars show distances between taxa from different genera or subgenera (the smallest distance in this set is 0.064, representing 238 differences over the sequence pair).
3.2. Phylogenetic analyses
The results shown here address questions of relationships among genera in the Anophelinae and relationships among subgenera in the genus Anopheles. Most phylogenetic analyses were carried out using the amino acid sequences of the protein coding genes of the 168 newly sequenced mitochondrial genomes, supplemented with 23 RefSeq mitogenomes from GenBank.
Phylogenetic analysis of the Culicidae using protein sequences from mitogenomes available in GenBank with and without the new Bironella and Chagasia mitogenome sequences showed in both cases that the root of the Culicidae (mosquitoes) was between the two subfamilies (electronic supplementary material, figure S1). The rooting between the two subfamilies of the mosquitoes appears to be uncontradicted. Bironella was clustered within Anopheles (0.73 PP; electronic supplementary material, figure S1), but with Kerteszia sister to the clade composed of Bironella and Anopheles. The posterior probability for the branch leading to the clade composed of Kerteszia with Bironella and Anopheles was 1.0 (electronic supplementary material, figure S1). Examination of split supports that were not in the consensus tree shows that support for genus Anopheles (excluding Bironella and Chagasia) is 0.26 PP.
3.3. Genus-level relationships in Anophelinae rooted by Culicidae
The current generic classification of the Anophelinae includes Chagasia, Bironella and Anopheles, and so we would expect to see them as separate groups. However, although Chagasia is sister to the other groups, Bironella is nested within Anopheles (table 3; electronic supplementary material, figures S2–S7).
Table 3.
Summary of analyses using Culex and Aedes as outgroup. Support values for Bironella within Anopheles versus monophyletic genus Anopheles are shown. Support for ‘Bironella within Anopheles’ is defined here as the best supported split that separates Bironella and some Anopheles taxa with the outgroup.
| with RefSeq | model | Bironella within Anopheles | monophyletic Anopheles | electronic supplementary material, figure |
|---|---|---|---|---|
| + | CAT-Poisson | 0.86 | 0.07 | S2 |
| − | CAT-Poisson | 0.51 | 0.16 | S3 |
| + | CAT60-mtart | 0.94 | 0.0 | S4 |
| − | CAT60-mtart | 0.82 | <0.01 | S5 |
| + | CAT-GTR | 0.80 | 0.07 | S6 |
| − | CAT-GTR | 0.45 | 0.29 | S7 |
| + | CAT-GTR | 0.67 | 0.07 | S8 |
| − | CAT-GTR | 0.52 | 0.19 | S9 |
In order to see whether long branch effects were affecting the placement of Bironella, in analyses shown in electronic supplementary material, figures S8 and S9, several of the longest branches (except Bironella itself) were removed. However, the strongest support was found for Bironella within Anopheles, with little support for monophyletic Anopheles, suggesting that long branch effects did not affect placement of Bironella (table 3, last two lines).
3.4. Genus-level relationships in Anophelinae rooted by Chagasia
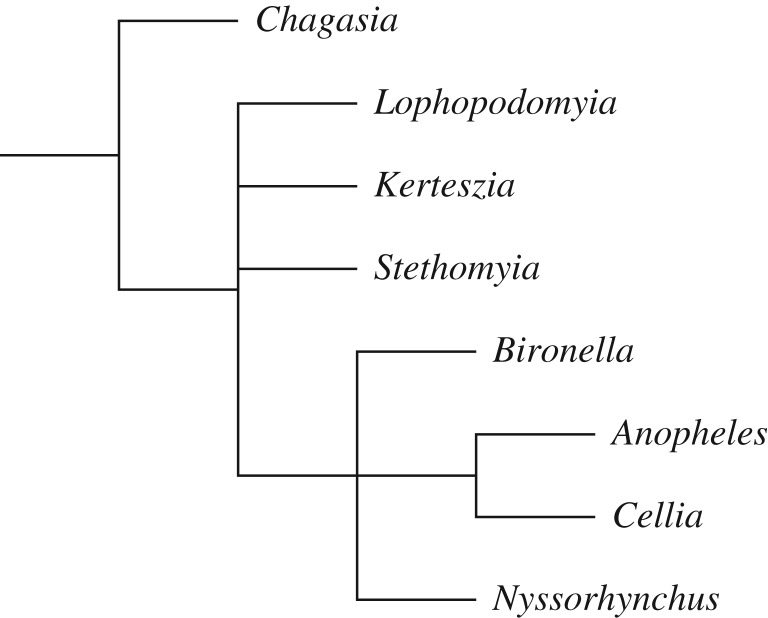
It is evident in electronic supplementary material, figures S2–S7 that Chagasia was the earliest branching genus in the Anophelinae, and so we will use Chagasia as a valid root for the rest of the Anophelinae. The series of analyses shown in electronic supplementary material, figures S10–S19 and summarized in table 4 were rooted by Chagasia, and used all the new Anopheles sequences together with Bironella, both with and without the nine Anopheles RefSeq sequences. Culex sequences were not used here to remove the possibility that the presence of that outgroup could distort the ingroup relationships. In many cases, there was stronger support for Bironella within Anopheles than there was for monophyletic Anopheles (table 4). Results of the analyses using the CAT-GTR model was an exception that showed moderate (0.72, 0.71 BPP) support for Bironella with Chagasia (electronic supplementary material, figures S10 and S11); this is counter to the CAT-GTR analyses rooted by Culex as shown in figures S6–S9, where support for monophyletic genus Anopheles was low (0.07–0.265 BPP) with this model. Placement of Bironella was often sister to Anopheles subgenera Cellia, Anopheles and Nyssorhynchus (figure 3) and this was equivalent to support for Chagasia together with Anopheles subgenera Lophopodomyia, Kerteszia and Stethomyia (LKS, not including Bironella), which was highest with the CAT60-MtArt model and lowest with CAT-GTR.
Table 4.
Support for monophyletic genus Anopheles within Anophelinae, rooted by Chagasia. LKS is Lophopodomyia, Kerteszia and Stethomyia, subgenera of Anopheles.
| software | model/method | RefSeq | monophyletic Anopheles | Chagasia + LKS | electronic supplementary material, figure |
|---|---|---|---|---|---|
| PB | CAT-GTR | + | 0.72 | 0.20 | S10 |
| PB | CAT-GTR | − | 0.71 | 0.12 | S11 |
| PB | CAT60-MtArt | + | 0.01 | 0.905 | S12 |
| PB | CAT60-MtArt | − | 0.0 | 0.90 | S13 |
| PB | CAT-Poisson | + | 0.105 | 0.785 | S14 |
| PB | CAT-Poisson | − | 0.045 | 0.84 | S15 |
| RAxML | JTT+F, RAxML-CATa | + | 0.22 | 0.55 | S16 |
| RAxML | JTT+F, RAxML-CAT | − | 0.31 | 0.39 | S17 |
| PAUP* | maximum parsimony | + | 0.53 | 0.24 | S18 |
| PAUP* | maximum parsimony | − | 0.56 | 0.21 | S19 |
aProttest was used for model choice for the RAxML analysis. Prottest recommended JTT+G+F with an AICw of 1.0, and so that model was used, although for the RAxML rapid bootstrap the RAxML-CAT ASRV (among-site rate variation) was used, with only the final optimization evaluated with Gamma ASRV.
Figure 3.
Most analyses described in this study place Bironella within genus Anopheles.
3.5. Anophelinae with fast sites removed
In this set of analyses fewer Nyssorhynchus sequences were used, and we looked at fast site removal to examine reliability of monophyletic Anopheles. The removal of fast sites was conducted in two ways, neither of which uses a tree:
Using diversity, that is, the number of different kinds of amino acid characters in a site [57]. It is assumed that the higher the diversity the higher will be the site rate. Data were prepared by discarding sites with a diversity higher than 3, as well as constant sites, leaving 793 of the original 1128 sites.
Using TIGER software [58], which identifies fast sites using compatibility. TIGER bins sites into 10 bins, and the sites in the fastest bin were removed, as well as constant sites, leaving 774 of the original 1128 sites.
The results (table 5; electronic supplementary material, figures S20–S28) of the analyses using all sites (with fewer Nyssorhynchus) agreed with results of previously described analyses, where the CAT-GTR model showed some small (47% and 50% in replicate analyses) support for monophyletic Anopheles, and the JTT analyses with RAxML and Phyml showed little (28% and 4%) support for monophyletic Anopheles. Using only the slow sites in the data can make the analysis more reliable, because biases in the data that may cause a lack of model fit would generally manifest in the fast sites and so their removal would be beneficial [58,59]. When this was done (table 5), support for monophyletic Anopheles was eroded, which seems to argue that the high support for monophyletic Anopheles by the CAT-GTR model was unreliable. Oddly, using JTT with RAxML and Phyml, support for monophyletic Anopheles increased when fast sites were removed, which appears to argue the opposite. However, there was still poor support (less than 50%) for monophyletic Anopheles after fast site stripping, and in spite of the ambiguity and contradictions, the tree shown in figure 3 appears to be the best summary.
Table 5.
Summary of support values for monophyletic genus Anopheles using fast site stripping.
| sites | n sites | program, model | monophyletic Anopheles | electronic supplementary material, figure |
|---|---|---|---|---|
| all | 1128 | Phylobayes, CAT-GTR | 0.47 | S20 |
| all | 1128 | Phylobayes, CAT-GTR | 0.50 | S21 |
| all | 1128 | RAxML, PROTCATJTTF | 0.28 | S22 |
| all | 1128 | Phyml, JTT+G+F | 0.04 | S23 |
| slow only, by diversity | 793 | Phylobayes, CAT-GTR | 0.205 | S24 |
| slow only, by diversity | 793 | RAxML, PROTCATJTTF | 0.31 | S25 |
| slow only, by diversity | 793 | Phyml, JTT+G+F | 0.31 | S26 |
| slow only, by TIGER | 774 | Phylobayes, CAT-GTR | 0.245 | 4 |
| slow only, by TIGER | 774 | RAxML, PROTCATJTTF | 0.48a | S27 |
| slow only, by TIGER | 774 | Phyml, JTT+G+F | 0.33 | S28 |
aNote that a consensus tree made from bootstraps of the RAxML analysis of TIGER sites shows monophyletic Anopheles (with 48% bootstrap support) while the RAxML tree for the same analysis, which had undergone more ML rearrangements, does not.
3.6. Phylogenetic analysis with DNA sequences
While this study has focused on amino acid data, for comparison DNA alignments corresponding to the Anophelinae amino acid alignments including RefSeq sequences were prepared, and analysed with a partitioned ML model, and with the CAT-GTR model of PhyloBayes (electronic supplementary material, figures S29–S34). Results were broadly similar, with mostly well-supported clades of Stethomyia, Lophopodomyia, Kerteszia, Anopheles, Cellia and Nyssorhynchus. However, in contrast with the phylogenies based on translations (generally as in figure 3), support for backbone arrangements of these groups was generally poor and inconsistent using DNA. Support for Bironella within Anopheles was higher with the PhyloBayes CAT-GTR analyses, and lower for the ML analyses (table 6).
Table 6.
Summary of support values for monophyletic genus Anopheles using DNA sequences.
| sites | n sites | n taxa | program, model | monophyletic Anopheles | electronic supplementary material, figure |
|---|---|---|---|---|---|
| positions 1, 2, 3 | 11 202 | 156 | IQ-Tree, partitioned | 0.80 | S29 |
| positions 1, 2 | 7468 | 156 | IQ-Tree, partitioned | 0.91 | S30 |
| positions 1, 2, 3 | 11 202 | 156 | PhyloBayes, CAT-GTR | 0.76 | S31 |
| positions 1, 2 | 7468 | 156 | PhyloBayes, CAT-GTR | 0.40 | S32 |
| fewer taxa, positions 1, 2a | 1727 | 61 | PhyloBayes, CAT-GTR | 0.25 | S33 |
| fewer taxa, positions 1, 2, slow sitesa | 1244 | 60 | PhyloBayes, CAT-GTR | 0.43 | S34 |
aConstant sites removed.
3.7. Results summary
With some inconsistencies, it is most likely that Bironella is placed within Anopheles.
Placement of Lophopodomyia, Stethomyia and the clade composed of Cellia with Anopheles, were not consistent in the analyses.
The current subgenera—Stethomyia, Lophopodomyia, Kerteszia, Anopheles and Cellia—were consistently found to be monophyletic groups.
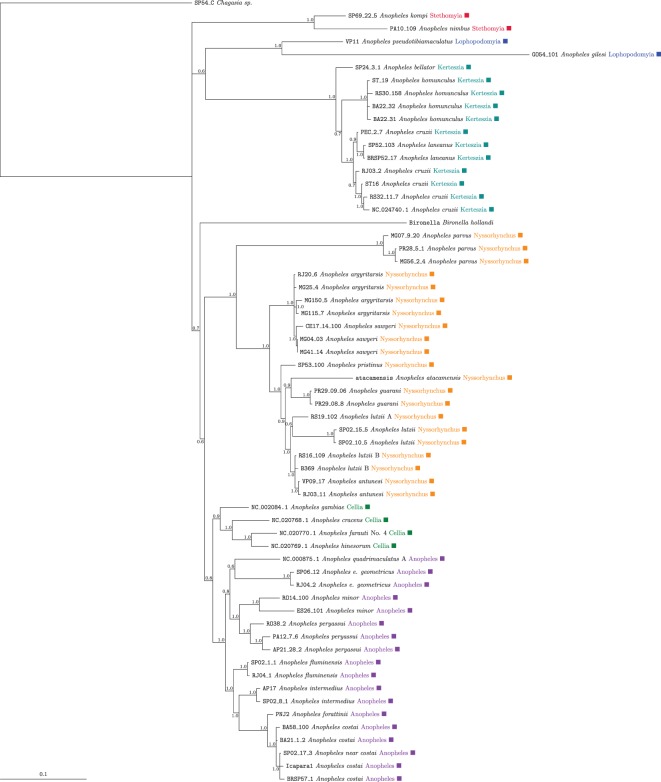
The subgenus Nyssorhynchus was unambiguously subdivided into two strongly supported groups that were found in all analyses independent of the approach and model adopted. The Nyssorhynchus was subdivided into two major monophyletic groups (BPP=1.0) (electronic supplementary material, figures S10–S15, S20, S21, S24 and figure 4). One group was composed of Anopheles parvus and the second group included the remaining species of the Myzorhynchella Section plus An. atacamensis of the Argyritarsis Section that was recovered as sister to the group (An. argyritarsis plus An. sawyeri). Monophyly of the Argyritarsis and Albimanus Sections was not corroborated by any of the analysis and partition schemes.
Figure 4.
Anophelinae with reduced taxa, rooted by Chagasia. Mitochondrial protein sequences, slow sites only using TIGER, analysed with PhyloBayes using the CAT-GTR model.
4. Discussion
The systematic treatment of the Anophelinae has undergone extensive changes since Theobald [60], who proposed several genera based on characteristics of abdominal and thoracic scales. Subsequently, Christophers [61] named three genera based on characteristics of the male genitalia. Later, Edwards [62] and Root [63] recognized the three genera—Anopheles, Myzomyia (equivalent to Cellia) and Nyssorhynchus—as subgenera. Edwards [64] added Stethomyia as a subgenus of Anopheles, with Kerteszia as an informal group within the subgenus Nyssorhynchus. Then, Antunes [65] proposed the Lophopodomyia subgenus, and Komp [66] elevated Kerteszia to subgenus level. More recently, Harbach et al. [18] described the subgenus Baimaia, and Harbach & Kitching [8] resurrected Christya from synonymy with Anopheles. Currently, Anophelinae encompasses three genera, Anopheles, Bironella and Chagasia, with the genus Anopheles encompassing eight subgenera, of which four—Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia—are primarily limited to the Neotropical Region [8,67] (table 1). The subdivision of the genus Anopheles into subgenera is based primarily on characters of the male genitalia, especially the number and placement of setae in the gonocoxite, characteristics of the ventral and dorsal claspette, aedeagus, proctiger and the ninth segment [11]. The largest subgenera in number of species are Anopheles, Cellia and Nyssorhynchus, and each subgenus is subdivided into several informal groups, subgroups and complexes [8,67].
Several previous studies have attempted to recover internal relationships among Anophelinae genera and among the Anopheles subgenera using morphology [8,11,12,21], nuclear and mitochondrial protein-coding genes [20,22], mitochondrial and ribosomal genes, among others [13,16], but the results have been unclear. That most studies were done with few taxa and few genes are among the reasons for the unsettled results, and this motivated the use of complete mitochondrial genomes and a wide taxon sampling in this study. Using mitochondrial genomes has been considered a positive advance over previously used molecular datasets for recovering interfamily relationships and for increasing support for deep nodes in phylogenies of termites [23]. However, it has also become evident that the mitochondrial genome may fail to reconstruct deep phylogenetic relationships [25,68]. Model choice can have a crucial role when using mitochondrial genomes, as in the recent study of paraneopteran orders by Li et al. [69]. They found big differences in substitution rates in different lineages, leading to apparent long branch attraction using site-homogeneous empirical models, which, however, was ameliorated using the site-heterogeneous CAT and CAT-GTR models as implemented in PhyloBayes. They also noted extreme saturation, and that also appeared to be accommodated well by CAT and CAT-GTR. They described the tree-heterogeneous composition of the DNA sequences, but they did not prefer use of AA sequences (which would have decreased the tree-heterogeneous composition) because using protein sequences decreased support for some groupings recovered by DNA sequences. However, a previous study using mitochondrial genomes for deep insect phylogenetics by Talavera & Vila [70] recommended using amino acid sequences to avoid long branch attraction, in addition to use of the PhyloBayes CAT model. For example it was only by using amino acid sequences with the CAT model that the Strepsiptera lineage was released from long branch attraction to the Hymenoptera, allowing it to be placed as sister to Coleoptera in agreement with current morphological and nuclear gene phylogenies. Although they had some success in avoiding long branch attraction using the CAT model with amino acid data, going deeper they were not able to recover super-order relationships reliably in insects.
In this study, we noted that the DNA sequences of our mitochondrial genomes were heterogeneous in composition, while the amino acid translations were not, as measured using a χ2-test for compositional heterogeneity (see Results, paragraph 2), and this was a major reason for us to use the amino acid sequences of the protein-coding genes. We mainly used the CAT-GTR model in PhyloBayes, but we compared this model with others. We also used long-branch taxa exclusion, fast site exclusion, and different outgroup rooting levels in order to test our results. Although there were limitations of the mitochondrial genome for inferring deep branch relationships within Anophelinae, the results of our phylogenetic analyses provided support for groups that have been previously defined based on morphological differences and similarities [60,64], and results of cladistics analyses [8,11,12], among other taxonomic studies. An analysis rooted using other nematocerous Diptera confirmed monophyly of the Culicinae family, and monophyly of the Anophelinae and Culicinae subfamilies (electronic supplementary material, figure S1).
Our analysis of relationships in Anophelinae partly contradict the current scheme of classification proposed by Harbach & Kitching [8] at the genus and subgenus levels. There is no contradiction regarding the phylogenetic systematization of the genus Chagasia as a monophyletic group that is sister to the clade composed of Bironella and Anopheles genera within Anophelinae. This is in agreement with other studies using either morphological characters [11,71] or different sources of DNA sequence [13,15,16,20]. However, the single representative of the genus Bironella included in the study, Bironella hollandi, was found either within the genus Anopheles or as its sister, depending on the analytical approach adopted and data partitioning schemes. Placement of Bironella nested within a more inclusive monophyletic group consisting of species of the genus Anopheles does not seem to be attributable to long branch attraction (table 3; electronic supplementary material, figures S8, S9, S24–S28). Consequently, the current status of Bironella as a genus within the Anophelinae and the monophyly of the genus Anopheles sensu lato are arguable. The limited sampling of some groups such as Bironella (one species), Stethomyia and Lophopodomyia (two species each, see below) may have contributed to the inconsistent deep relationships within Anophelinae. Thus, in order to resolve the phylogenetic position of Bironella, one strategy would be to use better taxon sampling; along with species from the other two Bironella subgenera—Neobironella and Brugella—the taxon sample should also include Anopheles and Cellia species from the Afrotropical, Indo-Malay, Australasian and Palearctic biodiversity regions. Another strategy would be to use nuclear sequences of single-copy genes and transcriptomes to overcome the problems that seem to be inherent in deep phylogenetics using mitogenomes [72–74].
Within Anophelinae, our estimated phylogenetic trees recovered relationships that are congruent with those suggested in the current classification proposed by Harbach & Kitching [8]. Species of the genus Anopheles consistently clustered into six major strongly supported monophyletic groups, coincident with current named subgenera: Anopheles, Cellia, Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia (electronic supplementary material, figures S1–S28). However, in our study phylogenetic relationships among Lophopodomyia, Kerteszia and Stethomyia were unstable, varying depending on the method and taxon sampling. There are two major sources of instability in the classification of Anophelinae: (i) the genus Anopheles is probably not monophyletic because the genus Bironella probably lies within it (figure 3; electronic supplementary material, figure S1) and (ii) the current internal classification of the subgenus Nyssorhynchus is primarily based on non-monophyletic lineages (electronic supplementary material, figures S2–S28). Further, considering the presence of non-monophyletic groups within Anophelinae, we feel confident in proposing a new scheme of classification for the subfamily, mainly focused on rearrangements of subgenera of the genus Anopheles (table 7). Elevation of Neotropical subgenera of Anopheles to genus level can be justified and supported if we consider that the primary aim of any biological classification is the systematization of monophyletic supraspecific taxa, and name them formally or demonstrate their presence in nature [76,77]. Recently, Wilkerson et al. [78] restored Aedini classification to a generic designation that has been applied worldwide by medical entomologists. The main reasons for the decision, in the name of classification stability, were the community consensus and hall of fame criteria that are important considerations for Aedes aegypti and Aedes albopictus, among other medically important species of the genus Aedes. In addition, Wilkerson and colleagues also reversed the classification summarized in Reinert et al. [79] to allow taxonomists to accurately assign new species to a genus and to obtain additional knowledge about strongly supported monophyletic groups of species that will orient further nomenclature changes and taxon naming within Aedini.
Table 7.
New classification proposal for the subfamily Anophelinae.
| Genus sensu Harbach [75]* | Newly proposed genus systematization | Subgenus sensu Harbach [75]* | Newly proposed subgenus systematization | Type species |
|---|---|---|---|---|
| Anopheles Meigen, 1818 | Anopheles Meigen, 1818 | Anopheles maculipennis Meigen, 1818 | ||
| Anopheles Meigen, 1818 | Anopheles Meigen, 1818 | Anopheles maculipennis Meigen, 1818 | ||
| Baimaia Harbach, Rattanarithikul & Harrison | Baimaia Harbach, Rattanarithikul & Harrison | Anopheles kyondawensis Abraham, 1947 | ||
| Christya Theobald, 1903 | Christya Theobald, 1903 | Anopheles implexus (Theobald, 1903) | ||
| Cellia Theobald, 1902 | Cellia Theobald, 1902 | Cellia pharoensis (Theobald, 1901) | ||
| Kerteszia Theobald, 1905 | Kerteszia Theobald, 1905 | — | Kerteszia boliviensis Theobald 1905 | |
| Lophopodomyia Antunes, 1937 | Lophopodomyia Antunes, 1937 | — | Lophopodomyia squamifemur (Antunes, 1937) | |
| Nyssorhynchus Blanchard, 1902 | Nyssorhynchus Blanchard, 1902 | Nyssorhynchus Blanchard, 1902 | Nyssorhynchus argyritarsis (Robineau-Desvoidy, 1827) | |
| Myzorhynchella Theobald, 1907 | Nyssorhynchus niger [60]; Currently, synonym of Ny. lutzii | |||
| To be described | — | Nyssorhynchus parvus (Chagas, 1907) | ||
| Stethomyia Theobald, 1902 | Stethomyia Theobald, 1902 | — | Stethomyia nimbus Theobald, 1902 | |
| Bironella Theobald, 1905 | Bironella Theobald, 1905 | Bironella gracilis Theobald, 1905 | ||
| Bironella Theobald, 1905 | Bironella Theobald, 1905 | Bironella gracilis Theobald, 1905 | ||
| Brugella Edwards, 1930 | Brugella Edwards, 1930 | Bironella travestita (Brug, 1928) | ||
| Neobioronella Tenorio, 1977 | Neobioronella Tenorio, 1977 | Bironella confusa Bonne-Wepster, 1951 | ||
| Chagasia Cruz, 1906 | Chagasia Cruz, 1906 | — | — | Chagasia neivae Cruz, 1906; Currently, synonym of Chagasia fajardi |
*Reference: [75]
The classification proposed herein is supported by results of phylogenetic studies and the presence of natural groups that have been accepted by most medical entomologists. We find support for our decision when we consider the taxon naming criteria (TNC) suggested by Vences et al. [76]. According to these authors, taxonomists should provide a universal and stable system of classification for supraspecific taxa, and they proposed three major groups of criteria—priority, secondary and accessory, that should be considered prior to any decision about naming monophyletic supraspecific taxa and consequent nomenclature changes. The priority group includes: (i) mandatory monophyly of the taxon in an inferred species tree, (ii) clade stability derived from analyses that included various methods of tree inference, clade robustness, corroborated by a distinct set of characters and (iii) phenotypic diagnosability. The secondary and accessory groups include four criteria each, among them biogeography, manageability, hall of fame, nomenclature stability and community consensus.
In this study, we invoke the priority recommendations of Vences et al. as unambiguous support for elevating Neotropical Nyssorhynchus, Kerteszia, Lophopodomyia and Stethomyia subgenera to genus level. The monophyly of these taxa were always robust, independent of the analytical phylogenetic approach, taxon sampling strategy, or source of data employed for the analyses, such as morphology [11,12,17,21], nuclear and mitochondrial DNA sequence data [13,15,16] and mitogenome data as shown in this study. In addition, Nyssorhynchus, Kerteszia, Lophopodomyia and Stethomyia can be easily distinguished from the clade composed of Anopheles and Cellia based on autapomorphies of female and male genitalia or a set of morphological characters that together can be employed to distinguish these taxa from other Anophelinae genera [11,12,17,21]. The secondary TNC criteria, such as time banding, adaptive zone, hybrid viability of taxa and biogeography, cannot be used because there is not enough available information in the published literature.
The accessory TNC criteria include the manageability of a higher taxon that should contain a number of lower taxa manageable for the human mind, avoiding oversplitting and creating monotypic groups. Thus, the criteria of manageability provide extra strength to elevate Nyssorhynchus, Kerteszia, Lophopodomyia and Stethomyia monophyletic lineages to genus level. These Neotropical taxa contain few (a ‘manageable’ number) of species, and each of them can be recognized by their morphological distinctiveness from other Anophelinae genera. The genus Anopheles (410 species, table 1) that encompasses the species-rich subgenera Cellia (224 species) and Anopheles (183 species) is more problematic in terms of manageability and morphological diagnosability because they are not phenotypically homogeneous [11,12,17,21]. Characters of the male genitalia, whose homology has not been clearly defined, can distinguish these genera. As argued by Vences and colleagues, over-splitting a supraspecific taxon is a way to favour the principle of stability. However, this extreme situation should be avoided because it would have an undesirable impact on the evolutionary classification of organisms. The hall of fame accessory taxon naming criterion that urges taxonomists to consider the economy of change when proposing reclassification of organisms justifies our decision for not splitting the monophyletic clade composed of Anopheles and Cellia into smaller monophyletic subunits. The major reason for not splitting is that both the phylogeny and the phenotypic diagnosability are incomplete for these subgenera and thus require further study. On the other hand, the highly stable monophyly of the Neotropical Nyssorhynchus, Kerteszia, Lophopodomyia and Stethomyia subgenera justify elevating them to genus level.
Taking all the results together, we challenge the current classification of Anophelinae by proposing a revision at the genus and subgenus ranks that is consistent with our interpretation of the phylogenetic trees. Our revision preserves the six monophyletic groups that were recovered regardless of the analytical approaches adopted in the study. These are the six subgenera of Anopheles, the monophyly of which has been previously corroborated by morphology [8,11,12] and nuclear gene datasets [13,15,16,20,22,80]. Accordingly, the subgenera Nyssorhynchus, Kerteszia, Lophopodomyia and Stethomyia are elevated to genus rank, and the genus Anopheles will include the subgenera Anopheles, Baimaia, Christya and Cellia (table 7). Therefore, species assigned originally to a particular subgenus will be moved from the genus Anopheles to their respective newly proposed genus.
Focusing on the Nyssorhynchus clade, we propose adjustments in the current classification. The Nyssorhynchus clade is composed of two major monophyletic sister groups (electronic supplementary material, figures S1–S28). One group includes specimens of Anopheles parvus from the Myzorhynchella Section [81], and the second group is composed of remaining species assigned originally to the Albimanus [82], Argyritarsis [83] and Myzorhynchella [81] Sections of Nyssorhynchus (electronic supplementary material, figures S2–S28). Although Anopheles parvus had been placed in the Myzorhynchella Section on the basis of morphological similarities with other species of the section [84,85], Bourke et al. [86], in a phylogenetic analysis of the Myzorhynchella Section employing DNA sequences of the nuclear white gene, showed that Anopheles parvus is placed outside a more inclusive group consisting of most Myzorhynchella species. Then, Foster et al. [22] proposed that the species should be placed into a separate subgenus of Anopheles because Anopheles parvus is phenotypically distinguishable by unique morphological features in the egg [87] and male genitalia [84,85] in addition to the large K2P COI barcode distances compared with other Nyssorhynchus species. Our results here show that the Myzorhynchella Section [81] is not a monophyletic group because Anopheles parvus is consistently placed as a sister group to all the other Nyssorhynchus, separate from the other Myzorhynchella. In addition, Anopheles atacamensis of the Argyritarsis Section nests within the Myzorhynchella Section (see table 2, which lists the other members of the Myzorhynchella Section in the current classification). The Myzorhynchella were described as a genus of Anophelinae by Theobald [60] to include Myzorhynchella nigra Theobald. Then, the genus Myzorhynchella was synonomysed with Anopheles by Howard et al. [88], and redefined as a species group within the subgenus Nyssorhynchus by Christophers [89]. Later, Galvão [85] elevated Myzorhynchella to subgenus rank, which was accepted by Lane [90]. More recently, Peyton et al. [81] redefined Myzorhynchella as a section of the subgenus Nyssorhynchus. Considering that the type species of the Myzorhynchella is Anopheles nigra currently in synonomy with Anopheles lutzii Cruz, the name Myzorhynchella is preserved to be the clade that contains Anopheles lutzii. Elevating the Myzorhynchella to a subgenus of the genus Nyssorhynchus implies that it will encompasses Anopheles antunesi, An. atacamensis, An. guarani, An. lutzii, An. nigritarsis, An. pictipennis and An. pristinus. Consequently, Anopheles parvus will be placed into a new genus, as yet unnamed, that will be described in a further study. The second major monophyletic group of the Nyssorhynchus clade includes Anopheles argyritarsis, the type species of Nyssorhynchus, and thus preserves the name Nyssorhynchus at the genus rank. Species of the Nyssorhynchus genus are placed into two monophyletic groups here defined as subgenera. One subgenus contains species of the Albimanus [82] and Argyritarsis Series [83] (sensu [67]), except for Anopheles atacamensis. As Anopheles argyritarsis belongs to this clade, we consider it as the Nyssorhynchus subgenus.
5. Summary
With this study, we provided phylogenetic support for monophyly of Culicidae, and the subfamilies Anophelinae and Culicinae. The genus Chagasia is consistently the earliest branching group within Anophelinae. The phylogenetic position of Bironella, while not conclusive, is most likely within the current genus Anopheles, which implies that the latter as currently defined is not monophyletic. The subgenus Nyssorhynchus is sister to the clade containing Anopheles parvus, a species that belongs to a yet unnamed genus. Cellia, Anopheles, Kerteszia, Lophopodomyia and Stethomyia are monophyletic groups of the Anophelinae.
With the results of this study, we suggest modifications to the Anophelinae classification as follows:
Elevate the monophyletic groupings Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia to genus level.
Genus Anopheles to include subgenera Anopheles, Baimaia, Cellia and Christya.
Anopheles parvus to be removed from Nyssorhynchus and to be placed into a new genus to be described in the near future.
Genus Nyssorhynchus to include two subgenera—Myzorhynchella and Nyssorhynchus.
Myzorhynchella to be elevated from a Section to subgenus rank of the newly proposed genus Nyssorhynchus—subgenus Myzorhynchella.
Both Anopheles atacamensis and Anopheles pictipennis to be transferred from subgenus Nyssorhynchus to the newly proposed subgenus Myzorhynchella.
Subgenus Nyssorhynchus to include species of the Argyritarsis and Albimanus Sections, except for those transferred to the Myzorhynchella subgenus.
We provide this alternative hypothesis for classification of Anophelinae in table 7.
Acknowledgements
We are in debt to Monique Albuquerque Motta (IOC—Rio de Janeiro), Luiz Carlos Oliveira (FSP-USP), Allan Silva Martins and other colleagues that kindly helped us in the field collections.
Ethics
All necessary permits were obtained for the described study. Mosquito collections were carried out under permit no. 16938-1 from Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) to M.A.S.M. Specific permission was not required for these locations as permission to collect was granted under the permanent permit. The collection locations were not privately owned or protected in any way, and the field collections did not involve protected or endangered species.
Data accessibility
DNA sequences are available at the NCBI GenBank under the following accession codes: KU551283–KU551289, MF040161–MF040165 and MF381582–MF381737. All specimen vouchers are deposited in the Universidade de São Paulo, Faculdade de Saúde Pública Collection. Data related to this paper can be found on the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1d8th [91].
Authors' contributions
M.A.M.S., P.G.F. and E.S.B. devised the study. E.S.B., D.C.S’A., S.S.N. and C.G. made field collections. D.C.S’A. and S.S.N. did specimen acquisition. D.C.S’A. and C.C.M. did specimen curation. T.M.P.d.O. did laboratory manipulations for sequencing. P.G.F. did sequence assembly, annotation and curation, phylogenetic analysis and made the phylogenetic figures. S.N. and C.E.L. reviewed and edited the first draft of the manuscript. P.G.F., M.A.M.S. and J.E.C. wrote the manuscript. M.A.M.S. did species identification, got financial support and oversaw the project. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grant nos. 2014/26229-7 and 2011/20397-7) was provided to M.A.M.S. and E.S.B.; additionally, NIH AI R01110112 provided partial financial support to J.E.C. and M.A.M.S.; CNPq grant no. 301877/2016-5 to M.A.M.S.
References
- 1.Feachem RGA, et al. 2010. Shrinking the malaria map: progress and prospects. Lancet 376, 1566–1578. (doi:10.1016/S0140-6736(10)61270-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Modrek S, Gosling RD, Feachem RGA. 2013. Malaria eradication: is it possible? Is it worth it? Should we do it? Lancet Glob. Health 1, e2–e3. (doi:10.1016/S2214-109X(13)70002-0) [DOI] [PubMed] [Google Scholar]
- 3.WHO. 2016. World malaria report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.PAHO. 2016. Report on the situation of malaria in the Americas 2014. Washington DC: Pan American Health Organization. [Google Scholar]
- 5.Calderaro A, et al. 2013. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar. J. 12, 321 (doi:10.1186/1475-2875-12-321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forattini OP. 2002. Culicidologia Médica II. São Paulo, Brazil: Editora da Universidade de São Paulo. [Google Scholar]
- 7.Harbach RE. 2015. Mosquito taxonomic inventory. See http://www.mosquito-taxonomic-inventory.info.
- 8.Harbach RE, Kitching IJ. 2016. The phylogeny of Anophelinae revisited: inferences about the origin and classification of Anopheles (Diptera: Culicidae). Zool. Scr. 45, 34–47. (doi:10.1111/zsc.12137) [Google Scholar]
- 9.Tenorio JA. 1977. Revision of the genus Bironella (Diptera: Culicidae). J. Med. Entomol. 14, 317–361. (doi:10.1093/jmedent/14.3.317) [Google Scholar]
- 10.Harbach RE, Howard TM. 2009. Review of the genus Chagasia (Diptera: Culicidae: Anophelinae). Zootaxa 2210, 1–25. [Google Scholar]
- 11.Sallum MAM, Schultz TR, Wilkerson RC. 2000. Phylogeny of Anophelinae (Diptera: Culicidae) based on morphological characters. Ann. Entomol. Soc. Am. 93, 745–775. (doi:10.1603/0013-8746(2000)093[0745:POADCB]2.0.CO;2) [Google Scholar]
- 12.Harbach RE, Kitching IJ. 2005. Reconsideration of anopheline mosquito phylogeny (Diptera: Culicidae: Anophelinae) based on morphological data. Syst. Biodiversity 3, 345–374. (doi:10.1017/S147720000500174X) [Google Scholar]
- 13.Sallum MAM, Schultz TR, Foster PG, Aronstein K, Wirtz RA, Wilkerson RC. 2002. Phylogeny of Anophelinae (Diptera: Culicidae) based on nuclear ribosomal and mitochondrial DNA sequences. Syst. Entomol. 27, 361–382. (doi:10.1046/j.1365-3113.2002.00182.x) [Google Scholar]
- 14.Krzywinski J, Besansky NJ. 2003. Molecular systematics of Anopheles: from subgenera to subpopulations. Ann. Rev. Entomol. 48, 111–139. (doi:10.1146/annurev.ento.48.091801.112647) [DOI] [PubMed] [Google Scholar]
- 15.Freitas LA, Russo CAM, Voloch CM, Mutaquiha OCF, Marques LP, Schrago CG. 2015. Diversification of the genus Anopheles and a Neotropical clade from the Late Cretaceous. PLoS. ONE 10, e0134462 (doi:10.1371/journal.pone.0134462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzywinski J, Wilkerson RC, Besansky NJ. 2001. Evolution of mitochondrial and ribosomal gene sequences in Anophelinae (Diptera: Culicidae): implications for phylogeny reconstruction. Mol. Phylogenet. Evol. 18, 479–487. (doi:10.1006/mpev.2000.0894) [DOI] [PubMed] [Google Scholar]
- 17.Collucci E, Sallum MAM. 2007. Cladistic analysis of the subgenus Anopheles (Anopheles) Meigen (Diptera: Culicidae) based on morphological characters. Mem. Inst. Oswaldo Cruz 102, 277–292. (doi:10.1590/S0074-02762007005000026) [DOI] [PubMed] [Google Scholar]
- 18.Harbach RE, Rattanarithikul R, Harrision [sic] BA. 2005. Baimaia, a new subgenus for Anopheles kyondawensis Abraham, a unique crabhole-breeding anopheline in southeastern Asia. Proc. Entomol. Soc. Wash. 107, 750–761. [Google Scholar]
- 19.Foley DH, Bryan JH, Yeates D, Saul A. 1998. Evolution and systematics of Anopheles: insights from a molecular phylogeny of Australasian mosquitoes. Mol. Phylogenet. Evol. 9, 262–275. (doi:10.1006/mpev.1997.0457) [DOI] [PubMed] [Google Scholar]
- 20.Krzywinski J, Wilkerson RC, Besansky NJ. 2001. Toward understanding Anophelinae (Diptera, Culicidae) phylogeny: insights from nuclear single-copy genes and the weight of evidence. Syst. Biol. 50, 540–556. (doi:10.1080/10635150119931) [PubMed] [Google Scholar]
- 21.Collucci E, Sallum MAM. 2003. Phylogenetic analysis of the subgenus Kerteszia of Anopheles (Diptera: Culicidae: Anophelinae) based on morphological characters. Insect Syst. Evol. 34, iii–372. (doi:10.1163/187631203X00018) [Google Scholar]
- 22.Foster PG, Bergo ES, Bourke BP, Oliveira TMP, Nagaki SS, Sant’Ana DC, Sallum MAM. 2013. Phylogenetic analysis and DNA-based species confirmation in Anopheles (Nyssorhynchus). PLoS ONE 8, e54063 (doi:10.1371/journal.pone.0054063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron SL, Lo N, Bourguignon T, Svenson GJ, Evans TA. 2012. A mitochondrial genome phylogeny of termites (Blattodea: Termitoidae): robust support for interfamilial relationships and molecular synapomorphies define major clades. Mol. Phylogenet. Evol. 65, 163–173. (doi:10.1016/j.ympev.2012.05.034) [DOI] [PubMed] [Google Scholar]
- 24.Bernt M, et al. 2013. A comprehensive analysis of bilaterian mitochondrial genomes and phylogeny. Mol. Phylogenet. Evol. 69, 352–364. (doi:10.1016/j.ympev.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 25.Cameron SL. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Ann. Rev. Entomol. 59, 95–117. (doi:10.1146/annurev-ento-011613-162007) [DOI] [PubMed] [Google Scholar]
- 26.Demari-Silva B, Foster PG, de Oliveira TMP, Bergo ES, Sanabani SS, Pessôa R, Sallum MAM. 2015. Mitochondrial genomes and comparative analyses of Culex camposi, Culex coronator, Culex usquatus and Culex usquatissimus (Diptera: Culicidae), members of the coronator group. BMC Genomics 16, 831 (doi:10.1186/s12864-015-1951-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira TMP, Foster PG, Bergo ES, Nagaki SS, Sanabani SS, Marinotti O, Marinotti PN, Sallum MAM. 2016. Mitochondrial genomes of Anopheles (Kerteszia) (Diptera: Culicidae) from the Atlantic forest, Brazil. J. Med. Entomol. 53, 790–797. (doi:10.1093/jme/tjw001) [DOI] [PubMed] [Google Scholar]
- 28.Krzywinski J, Grushko OG, Besansky NJ. 2006. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol. Phylogenet. Evol. 39, 417–423. (doi:10.1016/j.ympev.2006.01.006) [DOI] [PubMed] [Google Scholar]
- 29.Krzywinski J, Li C, Morris M, Conn JE, Lima JB, Povoa MM, Wilkerson RC. 2011. Analysis of the evolutionary forces shaping mitochondrial genomes of a Neotropical malaria vector complex. Mol. Phylogenet. Evol. 58, 469–477. (doi:10.1016/j.ympev.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno M, Marinotti O, Krzywinski J, Tadei WP, James AA, Achee NL, Conn JE. 2010. Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time. Malar. J. 9, 127 (doi:10.1186/1475-2875-9-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue K, et al. 2013. Mitochondrial genome sequences reveal deep divergences among Anopheles punctulatus sibling species in Papua New Guinea. Malar. J. 12, 64 (doi:10.1186/1475-2875-12-64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Cameron SL, Lees DC, Xue D, Han H. 2015. A mitochondrial genome phylogeny of owlet moths (Lepidoptera: Noctuoidea), and examination of the utility of mitochondrial genomes for lepidopteran phylogenetics. Mol. Phylogenet. Evol. 85, 230–237. (doi:10.1016/j.ympev.2015.02.005) [DOI] [PubMed] [Google Scholar]
- 33.Foster PG, Hickey DA. 1999. Compositional bias may affect both DNA-based and protein-based phylogenetic reconstructions. J. Mol. Evol. 48, 284–290. (doi:10.1007/PL00006471) [DOI] [PubMed] [Google Scholar]
- 34.Rota-Stabelli O, Lartillot N, Philippe H, Pisani D. 2013. Serine codon-usage bias in deep phylogenomics: pancrustacean relationships as a case study. Syst. Biol. 62, 121–133. (doi:10.1093/sysbio/sys077) [DOI] [PubMed] [Google Scholar]
- 35.Cox CJ, Li B, Foster PG, Embley TM, Civáň P. 2014. Conflicting phylogenies for early land plants are caused by composition biases among synonymous substitutions. Syst. Biol. 63, 272–279. (doi:10.1093/sysbio/syt109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallum MAM, Foster PG, Dos Santos CLS, Flores DC, Motoki MT, Bergo ES. 2010. Resurrection of two species from synonymy of Anopheles (Nyssorhynchus) strodei Root, and characterization of a distinct morphological form from the Strodei Complex (Diptera: Culicidae). J. Med. Entomol. 47, 504–526. (doi:10.1093/jmedent/47.4.504) [DOI] [PubMed] [Google Scholar]
- 37.Nagaki SS, Motta MdA, Sallum MAM. 2010. Redescription of Anopheles (Nyssorhynchus) antunesi Galvão & Amaral and description of a new species of the Myzorhynchella Section (Diptera: Culicidae) from Serra da Mantiqueira, Brazil. Mem. Inst. Oswaldo Cruz 105, 278–285. (doi:10.1590/S0074-02762010000300006) [DOI] [PubMed] [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA 74, 5463–5467. (doi:10.1073/pnas.74.12.5463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chevreux B, Wetter T, Suhai S. 1999. Genome sequence assembly using trace signals and additional sequence information. In Computer Science and Biology: Proc. of the German conf. on bioinformatics (GCB) 99, Heidelberg, Germany, pp. 45–56.
- 40.Peng Y, Leung HCM, Yiu S-M, Chin FYL. 2012. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28, 1420–1428. (doi:10.1093/bioinformatics/bts174) [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Madan A. 1999. CAP3: A DNA sequence assembly program. Genome Res. 9, 868–877. (doi:10.1101/gr.9.9.868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milne I, Stephen G, Bayer M, Cock PJ, Pritchard L, Cardle L, Shaw PD, Marshall D. 2013. Using Tablet for visual exploration of second-generation sequencing data. Brief. Bioinf. 14, 193–202. (doi:10.1093/bib/bbs012) [DOI] [PubMed] [Google Scholar]
- 43.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 25, 3389–3402. (doi:10.1093/nar/25.17.3389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 69, 313–319. (doi:10.1016/j.ympev.2012.08.023) [DOI] [PubMed] [Google Scholar]
- 45.Cock PJA, et al. 2009. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423. (doi:10.1093/bioinformatics/btp163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster PG. 2004. Modeling compositional heterogeneity. Syst. Biol. 53, 485–495. (doi:10.1080/10635150490445779) [DOI] [PubMed] [Google Scholar]
- 47.Sievers F, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (doi:10.1038/msb.2011.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552. (doi:10.1093/oxfordjournals.molbev.a026334) [DOI] [PubMed] [Google Scholar]
- 49.Lartillot N, Philippe H. 2004. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol. Biol. Evol. 21, 1095–1109. (doi:10.1093/molbev/msh112) [DOI] [PubMed] [Google Scholar]
- 50.Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony (* and other methods). Version 4.
- 51.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. (doi:10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 52.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. (doi:10.1093/bioinformatics/btu033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. (doi:10.1093/molbev/msu300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minh BQ, Nguyen MAT, von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30, 1188–1195. (doi:10.1093/molbev/mst024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 65, 997–1008. (doi:10.1093/sysbio/syw037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. (doi:10.1038/nmeth.4285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansmann S, Martin W. 2000. Phylogeny of 33 ribosomal and six other proteins encoded in an ancient gene cluster that is conserved across prokaryotic genomes: influence of excluding poorly alignable sites from analysis. Int. J. Syst. Evol. Microbiol. 50, 1655–1663. (doi:10.1099/00207713-50-4-1655) [DOI] [PubMed] [Google Scholar]
- 58.Cummins CA, McInerney JO. 2011. A method for inferring the rate of evolution of homologous characters that can potentially improve phylogenetic inference, resolve deep divergence and correct systematic biases. Syst. Biol. 60, 833–844. (doi:10.1093/sysbio/syr064) [DOI] [PubMed] [Google Scholar]
- 59.Rodríguez-Ezpeleta N, Brinkmann H, Roure B, Lartillot N, Lang BF, Philippe H. 2007. Detecting and overcoming systematic errors in genome-scale phylogenies. Syst. Biol. 56, 389–399. (doi:10.1080/10635150701397643) [DOI] [PubMed] [Google Scholar]
- 60.Theobald FV. 1907. A monograph of the Culicidae or mosquitoes, vol. 4 London, UK: British Museum (Natural History). [Google Scholar]
- 61.Christophers S. 1915. The male genetalia of Anopheles. Indian J. Med. Res. 3, 371–394. [Google Scholar]
- 62.Edwards F. 1921. A revision of the mosquitos [sic] of the Palaearctic Region. Bull. Entomol. Res. 12, 263–351. (doi:10.1017/S0007485300040207) [Google Scholar]
- 63.Root F. 1923. The male genitalia of some American Anopheles mosquitoes. Am. J. Hyg. 3, 264–279. (doi:10.1093/oxfordjournals.aje.a118933) [Google Scholar]
- 64.Edwards F. 1932. Genera Insectorum. Diptera, Pam. Culicidae, vol. Fascicle 194. Brussels, Belgium: Desmet-Verteneuil. [Google Scholar]
- 65.Antunes P. 1937. A new Anopheles and a new Goeldia from Colombia (Dipt. Culic.). Bull. Entomol. Res. 28, 69–73. (doi:10.1017/S000748530003830X) [Google Scholar]
- 66.Komp W. 1937. The species of the subgenus Kerteszia of Anopheles (Diptera, Culicidae). Ann. Entomol. Soc. Am. 30, 492–529. (doi:10.1093/aesa/30.3.492) [Google Scholar]
- 67.Harbach RE. 2013. The phylogeny and classification of Anopheles. In Anopheles mosquitoes: new insights into malaria vectors (ed. MS Rijeka), pp. 3–55. Rijeka, Croatia: Intech. (doi:10.5772/54695)
- 68.Kjer KM, Simon C, Yavorskaya M, Beutel RG. 2016. Progress, pitfalls and parallel universes: a history of insect phylogenetics. J. R. Soc. Interface 13, 20160363 (doi:10.1098/rsif.2016.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Shao R, Song N, Song F, Jiang P, Li Z, Cai W. 2015. Higher-level phylogeny of paraneopteran insects inferred from mitochondrial genome sequences. Sci. Rep. 5, 8527 (doi:10.1038/srep08527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talavera G, Vila R. 2011. What is the phylogenetic signal limit from mitogenomes? The reconciliation between mitochondrial and nuclear data in the Insecta class phylogeny. BMC Evol. Biol. 11, 315 (doi:10.1186/1471-2148-11-315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harbach RE, Kitching IJ. 1998. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 23, 327–370. (doi:10.1046/j.1365-3113.1998.00072.x) [Google Scholar]
- 72.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. (doi:10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 73.Peters RS, et al. 2017. Evolutionary history of the hymenoptera. Curr. Biol. 27, 1013–1018. (doi:10.1016/j.cub.2017.01.027) [DOI] [PubMed] [Google Scholar]
- 74.Branstetter MG, Danforth BN, Pitts JP, Faircloth BC, Ward PS, Buffington ML, Gates MW, Kula RR, Brady SG. 2017. Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr. Biol. 27, 1019–1025. (doi:10.1016/j.cub.2017.03.027) [DOI] [PubMed] [Google Scholar]
- 75.Harbach RE. 2016. Mosquito taxonomic inventory. See www.mosquito-taxonomic-inventory.info/. Updated 22 April 2016 by Ralph E. Harbach.
- 76.Vences M, Guayasamin JM, Miralles A, De La Riva I. 2013. To name or not to name: criteria to promote economy of change in Linnaean classification schemes. Zootaxa 3636, 201–244. (doi:10.11646/zootaxa.3636.2.1) [DOI] [PubMed] [Google Scholar]
- 77.Wiley EO, Lieberman BS. 2011. Phylogenetics: theory and practice of phylogenetic systematics. New York, NY: John Wiley & Sons. [Google Scholar]
- 78.Wilkerson RC, Linton Y-M, Fonseca DM, Schultz TR, Price DC, Strickman DA. 2015. Making mosquito taxonomy useful: a stable classification of tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS ONE 10, e0133602 (doi:10.1371/journal.pone.0133602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reinert JF, Harbach RE, Kitching IJ. 2009. Phylogeny and classification of tribe Aedini (Diptera: Culicidae). Zool. J. Linn. Soc. 157, 700–794. (doi:10.1111/j.1096-3642.2009.00570.x) [Google Scholar]
- 80.Bourke BP, Foster PG, Bergo ES, Calado DC, Sallum MAM. 2010. Phylogenetic relationships among species of Anopheles (Nyssorhynchus)(Diptera, Culicidae) based on nuclear and mitochondrial gene sequences. Acta. Trop. 114, 88–96. (doi:10.1016/j.actatropica.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 81.Peyton E, Wilkerson RC, Harbach RE. 1992. Comparative analysis of the subgenera Kerteszia and Nyssorhynchus of Anopheles (Diptera: Culicidae). Mosq. Syst. 24, 51–69. (doi:10.21236/ADA259117) [Google Scholar]
- 82.Faran M. 1980. Mosquito studies (Diptera: Culicidae) XXXIV. A revision of the Albimanus Section of the subgenus Nyssorhynchus of Anopheles. Contrib. Am. Entomol. Inst. 15, 1–215. [Google Scholar]
- 83.Linthicum K. 1988. A revision of the Argyritarsis section of the subgenus Nyssorhynchus of Anopheles (Diptera: Culicidae). Mosq. Syst. 20, 98–271. [Google Scholar]
- 84.Root F. 1927. Studies on Brazilian mosquitoes. IV. Notes on some Brazilian species of Anopheles. Am. J. Hyg. 7, 599–605. (doi:10.1093/oxfordjournals.aje.a120432) [Google Scholar]
- 85.Galvão A. 1941. Contribuição ao conhecimento das espécies de Myzorhynchella (Diptera: Culicidae). Arch. Zool. S. Paulo 2, 505–576. [Google Scholar]
- 86.Bourke BP, Nagaki SS, Bergo ES, Cardoso JdC, Sallum MAM. 2011. Molecular phylogeny of the Myzorhynchella Section of Anopheles (Nyssorhynchus) (Diptera: Culicidae): genetic support for recently described and resurrected species. Mem. Inst. Oswaldo Cruz 106, 705–715. (doi:10.1590/80074-02762011000600010) [DOI] [PubMed] [Google Scholar]
- 87.Forattini O, Sallum MAM, Bergo E, Flores D. 1998. Ultrastructure of eggs of Anopheles rondoni, Anopheles lutzii and Anopheles parvus, three species of the subgenus Nyssorhynchus. J. Am. Mosq. Control Assoc. 14, 256–265. [PubMed] [Google Scholar]
- 88.Howard LO, Dyar HG, Knab F. 1917. The mosquitoes of North and Central America and the West Indies, part II. vol. 4. Washington DC: Carnegie Institution. [Google Scholar]
- 89.Christophers S. 1924. Provisional list and reference catalogue of the Anophelini. Indian J. Med. Res. 3, 1–105. [Google Scholar]
- 90.Lane J. 1953. Neotropical culicidae, vol. 2 São Paulo, Brazil: University of São Paulo. [Google Scholar]
- 91.Foster PG. et al. 2017. Data from: Phylogeny of Anophelinae using mitochondrial protein coding genes Dryad Digital Repository. (doi:10.5061/dryad.1d8th) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Foster PG. et al. 2017. Data from: Phylogeny of Anophelinae using mitochondrial protein coding genes Dryad Digital Repository. (doi:10.5061/dryad.1d8th) [DOI] [PMC free article] [PubMed]
Data Availability Statement
DNA sequences are available at the NCBI GenBank under the following accession codes: KU551283–KU551289, MF040161–MF040165 and MF381582–MF381737. All specimen vouchers are deposited in the Universidade de São Paulo, Faculdade de Saúde Pública Collection. Data related to this paper can be found on the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1d8th [91].