Abstract
Objective: Leakage of monomers from dental fillings due to incomplete curing is very common. The objective of the present study was to examine the cytokine profile in cells exposed to triethyleneglycol dimethacrylate (TEGDMA) and the adjuvant properties of TEGDMA.
Materials and methods: Human peripheral blood mononuclear cells were exposed to TEGDMA (500 and 1000 μM) for 24 h in vitro. Bio-Plex Pro™ assays were used for analysis and detection of cytokines. In vivo, BALB/c mice were immunized subcutaneously in the base of the tail with TEGDMA in combination with ovalbumin (OVA).
Results: The cytokine levels of IL-8, IL-18, GRO-α and MCP-1 were significantly increased for both concentrations. IL-1β, IL-6 and TNF-α was only significantly increased in cultures exposed to 500 μM TEGDMA. The concentration of TNF-α was significantly decreased in cultures exposed to 1000 μM TEGDMA. Animals immunized with OVA co-administrated with TEGDMA had a significantly higher IgE and IgG anti-OVA antibody levels in blood than animals immunized with OVA only.
Conclusions: TEGDMA affects production of proinflammatory cytokines IL-1β, IL-6, IL-8, IL-18 and TNF-α. This inflammatogenic capacity renders TEGDMAs adjuvant properties, which may interfere with the homeostasis between the immune system and the indigenous microflora in the oral cavity.
Keywords: Dentin-bonding agents, methacrylates, cytokines, antibodies
Introduction
Dental composite resins, which are biomaterials that are used to replace biological tissues in both appearance and function, consist of inorganic filler particles and an organic matrix of acrylate/methacrylate polymers [1]. The organic component may contain any of several different acrylate/methacrylate monomers, although one of the most commonly used monomer is triethylene glycol dimethacrylate (TEGDMA) [1–3]. TEGDMA improves the bonding strength and viscosity of the resin composite [4]. After curing, the composite material contains unreacted monomers, which may be released into the oral cavity and/or diffuse through the dentin into the pulp [5–7]. Previous studies have shown that the concentration of TEGDMA that reaches the pulp could be about 4 mmol/L [8]. The leakage of the monomers is time-dependent, and about 90% of the unreacted monomers are released in the first 24 h post-polymerization [9]. The monomers may come in contact with leukocytes that are present in the pulp and oral cavity. The population of white blood cells found in the dental pulp consists of CD4+ and CD8+ T lymphocytes, as well as dendritic cells (DCs), neutrophils and macrophages [10]. Gingival crevicular fluid (GCF) flows into the gingival crevice through the junctional epithelium thereby transporting cells into the oral cavity. The population of cells in the GCF comprises 95–97% neutrophils, 2–3% monocytes 1–2% and lymphocytes, with fewer T cells than B lymphocytes [11]. Since many different cells are present in the oral cavity, pulp and epithelium, a variety of cells may encounter free monomers. It is therefore important to study the different effects that the monomers may have on the cells.
Previous studies have suggested that co-exposure with TEGDMA and lipopolysaccharide (LPS) attenuates LPS-induced cytokine responses in the mouse macrophage cell line RAW264.7 [12–14]. In contrast, another study has reported that TEGDMA exposure leads to increased levels of IL-8 mRNA, while the IL-6 and IL-10 mRNA levels were reduced in human dental pulp cells [15]. TEGDMA does also induce production of MCP-1 and IL-1β in vitro in exposed human gingival cells, pulp fibroblasts and in an oral mucosa model [16–18]. TEGDMA may also interfere with cellular signal transduction pathways that control the response of eukaryotic cells to environmental stimuli by activating mitogen-activated protein kinases in human pulp-derived cells [12]. Another study conducted by Krifka et al. [19] has shown that TEGDMA interferes with the regulation of cellular pathways through transcription factors that are activated as a consequence of DNA damage, e.g. p53, or initiated downstream of MAPK (mitogen-activated protein kinases), e.g. c-Jun, ATF-2 and ATF-3. Moreover, significantly increased production of IL-1β occurred after TEGDMA exposure in an oral mucosa model, which in turn led to significant mucosal damage [18]. The production of IL-1β and IL-18 is dependent on the assembly of the NLRP3 inflammasome, which comprised the NOD-like receptor NLRP3 (also known as NALP3, CIAS1, PYPAF1 or Cryopyrin), the apoptotic speck protein that contains a C-terminal caspase recruitment domain (ASC) and the protease caspase-1. Formation of the NLRP3 inflammasome leads to the processing of pro-IL-1β and pro-IL-18, resulting in the secretion of IL-1β and IL-18, respectively. The proinflammatory cytokines initiate an autocrine cascade, promoting the secretion of additional proinflammatory products, including TNF-α and IL-8 [20]. These alternations of the immune system are most probably the underlying cause of the inflammatory reactions, such as mucosal irritation, epithelial proliferation and hypersensitivity that have previously been reported after placement of resin-based composites and adhesives [21].
The objective of the present observational study was to investigate the potential inflammatogenic/adjuvant properties of TEGDMA monomers and to study the cytokine profile using human mononuclear white blood cells exposed to different concentrations of TEGDMA in vitro. TEGDMA exposure led to increased production of certain cytokines while production of others was unaffected or decreased. In vivo, mice were immunized with ovalbumin (OVA) in combination with TEGDMA or without TEGDMA. Inclusion of TEGDMA in the inoculum led to a significantly increased IgG and IgE anti-OVA antibody levels in blood indicating that TEGDMA has adjuvant properties.
Materials and methods
TEGDMA treatment in vitro of mononuclear cells from human blood
Fresh blood cells from eight healthy blood donors were obtained from Sahlgrenska University Hospital in Gothenburg, Sweden. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Bio-Sciences, Uppsala, Sweden). The cells were resuspended in PBS, centrifuged, and resuspended in Dulbecco’s Modified Eagle’s Medium (D-MEM) (Invitrogen, Lidingö, Sweden) that was supplemented with 5% heat-inactivated human AB serum (Sigma Chemical Co., St. Louis, MO), 100 U/mL of penicillin and 100 μg/mL of streptomycin (Invitrogen). Cell viability was determined by staining with 0.4% Trypan Blue (Sigma-Aldrich, Steinheim, Germany) and counted under a microscope using a Bürker chamber.
PBMCs (2 × 106) were cultured at 37 °C in a humidified atmosphere with 5% CO2 in the presence or absence of 500 μM and 1000 μM TEGDMA in 24-well plates in duplicates. After 24 h of culturing, the cell viability was estimated in range of 90–95%, the cells were stored frozen at −20 °C in order to lyse the cells for subsequent cytokine analysis, thereafter they were centrifuged and the supernatants were used for analysis.
Cytokine assays
The 21plex Group II and 27plex Group I cytokine panels (Bio-Plex Pro™ Human Cytokine Assay; Bio-Rad Laboratories, Hemel Hempstead, UK) were used to measure the cytokines, chemokines, and growth factor levels in accordance with manufacturer’s instructions. Briefly, supernatants (n = 8) were incubated with color-coded beads conjugated with antibodies directed to the desired biomarker, e.g. the cytokine TNF-α. Antibodies directed against different biomarkers have different colored beads to which they are attached. The sample was added, and the beads reacted with the biomarker of interest present in the sample. After each step, a washing series was performed to remove unbound protein and thereafter a biotinylated detection antibody (used to create a sandwich complex) is added. The final detection complex is formed when streptavidin-phycoerythrin (SA-PE) conjugate is added to bind to the biotinylated antibody. Phycoerythrin acts as a fluorescent indicator, or reporter. The concentration of the cytokines were measured using a BioPlex 200 instrument equipped with BioManager analysis software (BioRad, Hercules, CA), using red and green lasers to detect the different colors on the beads while measuring the fluorescence intensity using a standard curve. The red (635 nm) laser and the green (532 nm) laser measure concentration (pg/ml) and median fluorescence intensity (MFI). The concentration of the analyte bound to each bead is proportional to the MFI of the reporter signal. The detection limit differed for each cytokine (depending on the standard curve for each one).
Heat map
To simplify the interpretation of the structures and patterns in the dataset, Hierarchical Clustering Explorer (HCE) 3.0 (University of Maryland, College Park) was used. A heat map was created on the expression patterns of selected cytokines after exposure of human PBMCs to TEGDMA (n = 8). The median value was calculated for each cytokine manually and the values were normalized and transformed into color codes representing higher (black), intermediate (grey) and lower (white) expression of each cytokine using HCE.
Animals
Female, 6-week-old BALB/c mice (Charles River Laboratories, Sulzfeld, Germany) were used throughout the study and were kept in the animal facility according to governmental rules. The Ethical Committee for Animal Experimentation in Gothenburg, Sweden approved the protocols.
Immunization
A primary injection (immunization) of 50 μL of the test solution was administered subcutaneously at the base of the tail to two groups of mice (n = 8/group). The first group received a solution that contained OVA at 50 μg/animal. The second group received OVA at 50 μg/animal plus TEGDMA (Sigma-Aldrich, Steinheim, Germany) at 20 μmol/animal. In order to obtain a substantial immune response the animals were given an identical booster injection (a re-exposure to the immunizing antigen) three weeks later. The mice were sacrificed 2 weeks after the booster injection.
IgG anti-OVA antibodies
To measure the IgG anti-OVA antibody levels in serum samples, plates (MaxiSorp Immuno plate; Nunc, Kamstrup, Denmark) were coated overnight at 4 °C with OVA (10 μg/mL) dissolved in PBS. The following day, the plates were washed with PBS that contained Tween 20 (Sigma-Aldrich) and blocked with 0.1% BSA and 0.05% Tween in PBS. The plates were washed again before the addition of serum and incubated at 37 °C for 2 h. The plates were then washed and incubated with an ALP-labeled rabbit anti-mouse IgG antibody (Mabtech, Nacka Strand, Sweden) in the dark at room temperature for 1 h. Next, 4-Nitrophenyl phosphate disodium salt hexahydrate (Sigma-Aldrich) at 1 mg/mL was added and the plates were incubated at 37 °C for 1 h. The resulting color intensity was read spectrophotometrically at 405 nm. A standard, composed of a pool of serum from all samples used as a positive control for anti-OVA-IgG antibodies, was run on each plate.
IgE anti-OVA antibodies
The levels of OVA-specific IgE were assayed using an ELISA kit (BioLegend, San Diego, CA) according to the manufacturer’s instructions. In brief, sera from mice were diluted 1:2 and the standard was diluted 1:2 in seven steps. The samples were added to the OVA pre-coated microtiter plate and washed before the mouse IgE detection antibody was added. The resulting color intensity was read spectrophotometrically at 405 nm. The detectable concentration range of the ELISA was 0.313–20 ng/mL.
Statistical analyses
All analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). For all tests, a p value of <.05 was considered as statistically significant. Statistical comparisons between paired samples were made using the Wilcoxon matched-pairs signed-rank test. For unpaired samples, the Mann–Whitney U-test was used.
Results
Cytokine production in cultures of human mononuclear leucocytes
All the cytokines produced from cultures exposed to various concentrations of TEGDMA that had median level >10 pg ml−1 (total of 20 different cytokines) were included in a heat map based on the median values of the measured concentrations from eight donors. Also, two cytokines involved in activation of the NLRP3 inflammasome (IL-1β and IL-18) were included (Figure 1). After 24 h exposure of two different concentrations of TEGDMA (500 μM and 1000 μM), to the cultured PBMCs, the levels of cytokines in the culture supernatants were elevated in comparison to unexposed cells. There was a tendency toward higher levels of cytokines in the PBMC cultures exposed to the lower concentration of TEGDMA (500 μM) (Figure 1).
Figure 1.
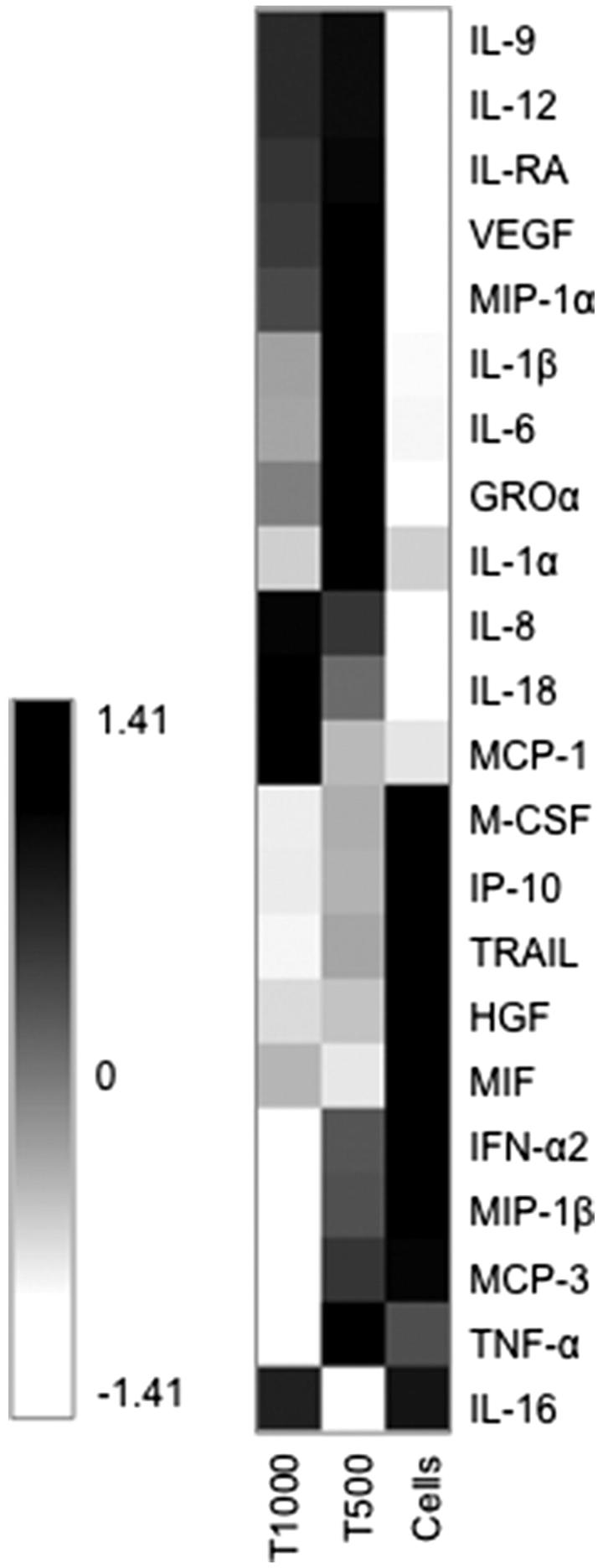
Heat map of cytokine levels in culture supernatants of human peripheral blood mononuclear cells (PBMCs) (n = 8) that were exposed to 0 μM (Cells), 500 μM (T500) or 1000 μM (T1000) of triethylene glycol dimethacrylate (TEGDMA). The cytokine levels in the culture supernatants were measured with a multiplexed bead-based cytokine immunoassay. The median value combined from the eight different individuals were calculated for each cytokine and the values were normalized and transformed using Hierarchical Clustering Explorer into color codes representing higher (black), intermediate (grey), and lower (white) expression of each cytokine.
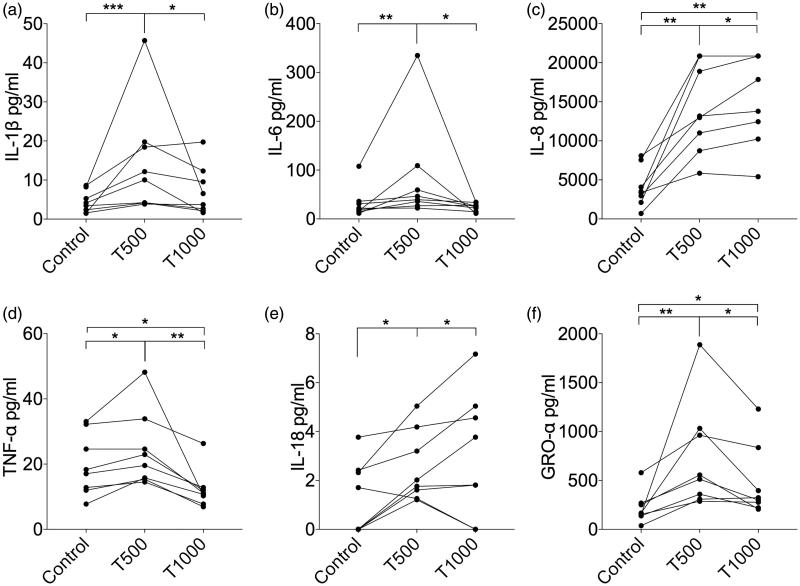
From the initial screening of the 22 human cytokines, 6 representative proinflammatory cytokines (IL-1β, IL-6, IL-8, IL-18, GRO-α and TNF-α) and a chemokine, MCP-1 were selected for further analysis.
The levels of IL-18, GRO-α and MCP-1 in the culture supernatants of PBMCs exposed to 500 μM or 1000 μM of TEGDMA were significantly higher than those in the supernatants of unexposed control cells (Figures 2 and 3). The levels of IL-1β, IL-6 and TNF-α were significantly increased only in PBMC cultures exposed to 500 μM TEGDMA (Figure 2). The concentration of TNF-α was significantly decreased in cultures where the cells were exposed to TEGDMA 1000 μM (Figure 2). The level of IL-8 was the highest in the PBMC cultures exposed to 1000 μM TEGDMA (Figure 2).
Figure 2.
Cytokine production by PBMCs exposed to two different concentrations of TEGDMA. The concentrations of the proinflammatory cytokines (a) IL-1β, (b) IL-6, (c) IL-8, (d) TNF-α, (e) IL-18 and (f) GRO-α in the supernatants from cultures of PBMCs (n = 8) cultured with or without TEGDMA for 24 h. Statistical analysis was carried out using Wilcoxon matched-pairs signed-rank test; *p < .05; **p < .01; ***p < .005.
Figure 3.
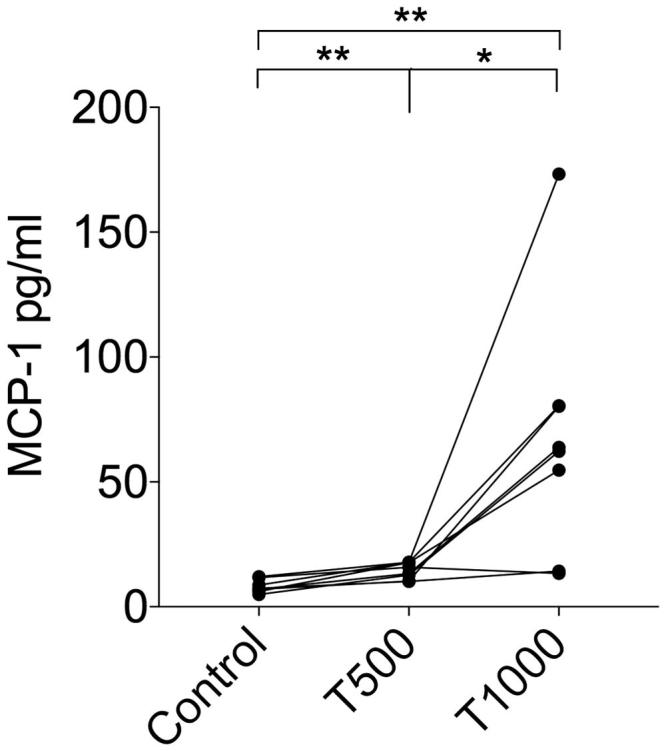
MCP-1 levels in culture supernatants after TEGDMA treatment of human PBMCs (n = 8) for 24 h. Statistical evaluation was performed using the Wilcoxon matched-pairs signed-rank test; *p < .05; **p < .01.
IgG and IgE anti-OVA antibody levels
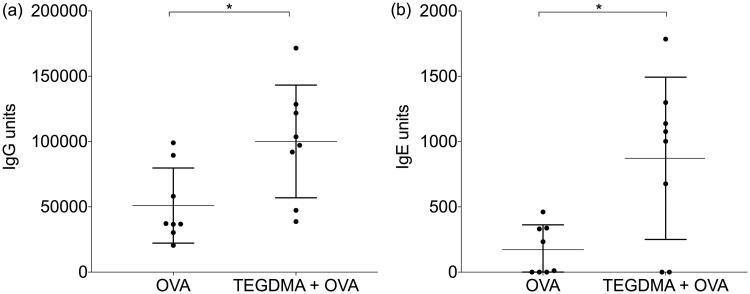
Mice were immunized subcutaneously at the base of the tail with OVA or OVA together with TEGDMA. The animals that were immunized with OVA plus TEGDMA had significantly higher levels of IgG anti-OVA antibodies in the blood than the animals immunized with OVA alone (p = .0104) (Figure 4(a)).
Figure 4.
IgG and IgE anti-ovalbumin (OVA) antibody levels in mice. Mice (n = 8/group) were immunized with OVA with or without TEGDMA. The mice received an identical booster injection 3 weeks after the first immunization and were sacrificed 2 weeks after the booster injection. The IgG (a) and IgE (b) anti-OVA antibody levels in sera were determined by ELISA. The Mann–Whitney U test was used for statistical comparison; *p < .05.
Mice that were immunized with OVA plus TEGDMA had also significantly higher levels of IgE anti-OVA antibodies in the blood than the animals immunized with OVA alone (p = .0468) (Figure 4(b)).
Discussion
TEGDMA and HEMA are two major components of dentin-bonding agents (DBAs) and dental resin composites used currently in dentistry [2,3]. We have previously shown that HEMA exerts various effects on the immune system, both in vitro and in vivo, in mice. Some of them are increased activity in the draining lymph nodes [22], dermatitis at the site of injection, adjuvant properties [23] and impaired growth rate [24]. Since TEGDMA is present in many dental restoration materials and is commonly used as a bonding agent, we considered it of interest to investigate whether this methacrylate also interacts with the immune system.
We have studied the adjuvant properties of TEGDMA in vivo in mice. We have also studied the effect of TEGDMA on the production of a group of cytokines that are involved in inflammation and a chemokine in vitro in cultures of human white blood cells.
We have previously shown that the methacrylate monomer HEMA has adjuvant properties. Thus, mice immunized with OVA in combination with HEMA had significantly higher levels of IgG1 and IgE anti-OVA antibodies in the blood than animals immunized with OVA without HEMA [23]. In the present study, mice were immunized subcutaneously with TEGDMA plus OVA, a common model antigen [25,26]. We found that mice immunized with OVA in combination with TEGDMA had higher levels of IgG and IgE anti-OVA antibodies in the blood than control mice immunized with OVA alone. Therefore, TEGDMA, similar to HEMA, seems to have the capacity to act as an adjuvant in vivo and to enhance the antibody response to an antigen. Consequently, there is a possibility that exposure of the oral mucosa to TEGDMA may contribute to the initiation of allergic reactions or mucosal inflammation. These findings are of interest, since allergic contact stomatitis due to residual monomers has been observed in some dental patients after restorative treatment [27–30]. Contact stomatitis is an inflammatory reaction of the oral mucosa caused by contact with irritants or allergens. It might be possible that due to the adjuvant properties of the residual TEGDMA monomers, a hypersensitivity reaction is induced. However, further studies are required in order to confirm these theories.
Furthermore, TEGDMA may contribute to the initiation of allergy to other substances, such as latex proteins from gloves, which may affect dental personnel. A study conducted among Swedish dentists showed that natural rubber latex glove-related hand eczema is associated with IgE-mediated allergy [31]. A theory is that the TEGDMA, in a similar way to the increased IgE anti-OVA antibody levels, also enhance the IgE anti-latex antibody levels.
Another interesting speculation is that, the adjuvant properties of TEGDMA may be linked to the previously reported abilities of the monomer to cause allergic contact dermatitis (ACD) [32–34]. ACD is a cutaneous delayed-type hypersensitivity (DTH) reaction that appears 24–72 h after antigen exposure, as compared to immediate hypersensitivity response, which usually appears within seconds of an antigen challenge [35]. Cutaneous hypersensitivity response is divided into two phases, a sensitization and elicitation. During the sensitization phase, cutaneous Langerhans' cells take up and process antigen, and migrate to regional lymph nodes where T cells get activated and memory T cells are produced. The memory T cells end up in the dermis, and during the elicitation phase the antigens are presented to them, which lead to release of cytokines such as IFN-γ and IL-17. This stimulates the keratinocytes of the epidermis to release proinflammatory cytokines such as IL-1 (IL-1β and IL-18), IL-6, TNF-α and the chemokine IL-8. These cytokines/chemokine enhance the inflammatory response by inducing the migration of monocytes into the lesion and by attracting more T cells [35].
Leucocytes secrete many different types of cytokines with different functions. One group of cytokines is the chemokines, such as MCP-1, which recruit different types of white blood cells to sites of infection and/or inflammation. Furthermore, there are proinflammatory cytokines, such as TNF-α, GRO-α, IL-1β, IL-6, IL-8, and IL-18, and anti-inflammatory cytokines, such as IL-1Ra and IL-10. Another group of cytokines are the growth factors, which include VEGF. TEGDMA-exposure of human PBMCs affected the production of cytokines from all these various cytokines groups.
The increased production of proinflammatory cytokines by TEGDMA-treated cells may be due to the formation of the NLRP3 inflammasome, resulting in the production of IL-1β and IL-18. This leads to the initiation of an autocrine cascade, involving the secretion of additional proinflammatory cytokines, including IL-8 [20]. However, further studies are required to confirm the involvement of the NLRP3 inflammasome in the TEGDMA-induced increase in IL-1β and IL-18 production.
In order to examine the production of cytokines that follows exposure to TEGDMA in vitro, human mononuclear leucocytes from healthy blood donors were exposed to two different concentrations of TEGDMA (500 μM and 1000 μM). Depending on the dental bonding resin used, different amount of TEGDMA may be present (up to 50%). Pure TEGDMA has a concentration of 3.8 mol/l, whereas a dental bonding resin may contain TEGDMA concentration of up to 1.9 mol/l. The dilution of TEGDMA across 0.45 mm of dentin has been shown to be approximately 571 times [36]. Thus, the concentration of residual TEGDMA-monomers that reaches the pulp can be estimated to be around 3.3 mmol/L, the concentrations used in this study are in the range of concentrations that could be found clinically. However, it is important to have in consideration that there is not a constant amount of monomers in a restorative material that reaches pulp tissue. Some of the important factors that may affect the amount are; concentration of the methacrylate monomers used in the dentin adhesive, the polymerization extent and the thickness of the dentin.
The cultured PBMCs that were exposed to the lower concentration of TEGDMA (500 μM) secreted significantly higher levels of the proinflammatory cytokines GRO-α, IL-1β, IL-6, IL-8, IL-18 and TNF-α than the PBMCs cultured in medium only. All these cytokines have an important role in different inflammatory reactions. The amount of TEGDMA inducing peak cytokine production seemed to be 500 μM. Since cell viability was similar in cultures with 500 μM and 1000 μM TEGDMA, the decreased cytokine production at the higher concentration of TEGDMA indicates a disturbed function of the cells which may be due to TEGDMAs ability to cause formation of reactive oxygen species (ROS) [37]. Increased levels of ROS may induce modifications that attenuate the activity of cellular components. The production of some of the cytokines may be inhibited due to the disturbed function of the cells while others can still be produced. Another aspect to have in mind that may have had an impact on the different levels of cytokine production at the different concentrations of TEGDMA may be the fact that different cytokines have different half-lives where some may have already been degraded before the measurements. However, this is an interesting finding that needs further investigation.
Elevated levels of IL-1, IL-6 and IL-8 in inflamed dental pulps compared to healthy pulp tissues have also been reported [38,39]. Dose-dependent increases in the levels of MCP-1 and IL-8 were observed after TEGDMA treatment. Some of these results are in concordance with the results of previous studies in which cells that were exposed to TEGDMA produced IL-1β [17,18], IL-6 [40], MCP-1 [16] and IL-8 [15]. In contrast to our study, earlier findings indicate that TEGDMA led to decreased levels in IL-6 mRNA and LPS-induced IL-1β production [14,15]. Thus, the responses to TEGDMA may vary between different concentrations and cells used for the studies.
There appears to be nexus between the cytokine responses (mainly involvement of the innate immune system) of the PBMCs, and the antigen-specific antibody responses (adaptive immune response) induced by the methacrylates. Previous studies on the mechanisms of adjuvants have shown the importance of recruitment of cells of the innate immune system at the site of injection. Most active substances in adjuvants are ligands for pattern recognition receptor, whereas others act by providing a key component of the innate response i.e. cytokines that are critical in the regulation of the immune response. Important cytokines that have been observed in conjunction with the widely used adjuvants Aluminum hydroxide (alum) and the oil-in-water emulsion MF59 are CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β) and CXCL8 (IL-8), all involved in cell recruitment from blood into peripheral tissue [41]. Another study has suggested that, it is possible that alum promotes Th2 immune response through the induction of IL-25 and/or IL-6 [42]. Another cytokine involved in adjuvanticity is IL-1, which has been shown to enhance in vivo secondary antibody responses of mice to a protein antigen (bovine serum albumin) [43]. Staruch et al. also purposed a mechanism behind this phenomenon, where IL-1 could activate B-cells directly as well as enhance the release of IL-2 from T cells. The IL-2 would in turn enhance the proliferation of T helper cells that recognize the antigen [43]. It has been purposed that many agents may exert their adjuvant effects by inducing release of IL-1 from macrophages. The production of IL-1β is due to formation of the NLRP3 inflammasome. This is consistent with the previously discussed mechanism in association with the adjuvant properties of alum, which is due to the formation of the NLRP3 inflammasome. Eisenbarth et al. [44] showed that the NLRP3 inflammasome is a crucial component in the adjuvant activity of alum, in NLRP3, ASC and caspase-1 knockout mice. Combining all these proposals together, there might be different mechanisms behind the adjuvant properties of TEGDMA, both involving the different cytokines that are secreted due to TEGDMA exposure (IL-1β, IL-6, IL-8, IL-18, GRO-α, MCP-1 and TNF-α), and due to the possibility that TEGDMA might cause formation of the NLRP3 inflammasome. However, further studies are required in order to confirm this.
The current study has some limitations, so interpretation of the results should take these into account. The concentrations of the methacrylate/acrylate monomers used in the in vivo studies were higher than those usually found in a clinical setting. Higher in vivo concentrations had to be used in order to establish the maximum effects induced by the methacrylate on the immune responses. Nevertheless, similar concentrations have been used previously in other studies [45]. However, it is important to have in consideration that amounts of residual monomers vary.
Another aspect worth considering is the possibility that responses in mice may not occur in precisely the same way in humans. However, mice are still the main in vivo model used for studying human immunology and are crucial for progress in our understanding of the immune system [46].
In conclusion, the properties of TEGDMA to modulate cytokine production and the capacity to cause inflammation (due to the production of proinflammatory cytokines) may lead to interference with the normal regulation of the immune response to harmless oral antigens, e.g. food proteins. This capacity may also affect the homeostasis between the immune system and the indigenous oral microflora.
We cannot give a description of the exact mechanisms behind the effect of TEGDMA on the immune system but the results suggest involvement of the NLRP3 inflammasome. However, further studies are required in order to confirm this.
Funding Statement
This work was supported by the TUAGBG-365041 grant from the Faculty of Odontology (TUA), University of Gothenburg.
Disclosure statement
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Ethical Committee for Animal Experimentation in Gothenburg, Sweden (N186/15) approved this study.
References
- 1. Chen MH. Update on dental nanocomposites. J Dent Res. 2010;89:549–560. [DOI] [PubMed] [Google Scholar]
- 2. Moharamzadeh K, Van Noort R, Brook IM, et al. . Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater. 2007;23:40–44. [DOI] [PubMed] [Google Scholar]
- 3. Stanislawski L, Lefeuvre M, Bourd K, et al. . TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res A. 2003;66:476–482. [DOI] [PubMed] [Google Scholar]
- 4. Goncalves F, Kawano Y, Pfeifer C, et al. . Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur J Oral Sci. 2009;117:442–446. [DOI] [PubMed] [Google Scholar]
- 5. Gerzina TM, Hume WR.. Diffusion of monomers from bonding resin-resin composite combinations through dentine in vitro. J Dent. 1996;24:125–128. [DOI] [PubMed] [Google Scholar]
- 6. Spahl W, Budzikiewicz H, Geurtsen W.. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent. 1998;26:137–145. [DOI] [PubMed] [Google Scholar]
- 7. Bationo R, Jordana F, Boileau MJ, et al. . Release of monomers from orthodontic adhesives. Am J Orthod Dentofacial Orthop. 2016;150:491–498. [DOI] [PubMed] [Google Scholar]
- 8. Noda M, Wataha JC, Kaga M, et al. . Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J Dent Res. 2002;81:265–269. [DOI] [PubMed] [Google Scholar]
- 9. Ferracane JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–452. [DOI] [PubMed] [Google Scholar]
- 10. Jontell M, Okiji T, Dahlgren U, et al. . Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998;9:179–200. [DOI] [PubMed] [Google Scholar]
- 11. Attstrom R. Presence of leukocytes in crevices of healthy and chronically inflamed gingivae. J Periodontal Res. 1970;5:42–47. [DOI] [PubMed] [Google Scholar]
- 12. Krifka S, Petzel C, Hiller KA, et al. . Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials 2010;31:2964–2975. [DOI] [PubMed] [Google Scholar]
- 13. Mathisen GH, Ansteinsson V, Samuelsen JT, et al. . TEGDMA and filler particles from dental composites additively attenuate LPS-induced cytokine release from the macrophage cell line RAW 264.7. Clin Oral Invest. 2015;19:61–69. [DOI] [PubMed] [Google Scholar]
- 14. Bolling AK, Samuelsen JT, Morisbak E, et al. . Dental monomers inhibit LPS-induced cytokine release from the macrophage cell line RAW264.7. Toxicol Lett. 2013;216:130–138. [DOI] [PubMed] [Google Scholar]
- 15. Golz L, Simonis RA, Reichelt J, et al. . In vitro biocompatibility of ICON(®) and TEGDMA on human dental pulp stem cells. Dent Mater. 2016;32:1052–1064. [DOI] [PubMed] [Google Scholar]
- 16. Gregson KS, Terrence O'Neill J, Platt JA, et al. . In vitro induction of hydrolytic activity in human gingival and pulp fibroblasts by triethylene glycol dimethacrylate and monocyte chemotatic protein-1. Dent Mater. 2008;24:1461–1467. [DOI] [PubMed] [Google Scholar]
- 17. Moharamzadeh K, Brook IM, Scutt AM, et al. . Mucotoxicity of dental composite resins on a tissue-engineered human oral mucosal model. J Dent. 2008;36:331–336. [DOI] [PubMed] [Google Scholar]
- 18. Moharamzadeh K, Franklin KL, Brook IM, et al. . Biologic assessment of antiseptic mouthwashes using a three-dimensional human oral mucosal model. J Periodontol. 2009;80:769–775. [DOI] [PubMed] [Google Scholar]
- 19. Krifka S, Petzel C, Bolay C, et al. . Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials. 2011;32:1787–1795. [DOI] [PubMed] [Google Scholar]
- 20. Burton L, Paget D, Binder NB, et al. . Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31:73–80. [DOI] [PubMed] [Google Scholar]
- 21. Hensten-Pettersen A. Skin and mucosal reactions associated with dental materials. Eur J Oral Sci. 1998;106:707–712. [PubMed] [Google Scholar]
- 22. Sandberg E, Dahlgren UI. Application of HEMA on intact mouse skin-effects on the immune system. Contact Derm. 2006;54:186–191. [DOI] [PubMed] [Google Scholar]
- 23. Sandberg E, Kahu H, Dahlgren UI.. Inflammatogenic and adjuvant properties of HEMA in mice. Eur J Oral Sci. 2005;113:410–416. [DOI] [PubMed] [Google Scholar]
- 24. Andersson J, Dahlgren U.. Effects on mouse immunity of long-term exposure in vivo to minute amounts of HEMA. Eur J Oral Sci. 2011;119:109–114. [DOI] [PubMed] [Google Scholar]
- 25. Basto AP, Badenes M, Almeida SC, et al. . Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein. Mol Immunol. 2015;64:36–45. [DOI] [PubMed] [Google Scholar]
- 26. Larsen ST, Lund RM, Thygesen P, et al. . Investigation of the adjuvant and immuno-suppressive effects of benzyl butyl phthalate, phthalic acid and benzyl alcohol in a murine injection model. Food Chem Toxicol. 2003;41:439–446. [DOI] [PubMed] [Google Scholar]
- 27. Fisher AA. Allergic sensitization of the skin and oral mucosa to acrylic denture materials. J Am Med Assoc. 1954;156:238–242. [DOI] [PubMed] [Google Scholar]
- 28. Giunta JL, Grauer I, Zablotsky N.. Allergic contact stomatitis caused by acrylic resin. J Prosthet Dent. 1979;42:188–190. [DOI] [PubMed] [Google Scholar]
- 29. Koutis D, Freeman S.. Allergic contact stomatitis caused by acrylic monomer in a denture. Australas J Dermatol. 2001;42:203–206. [DOI] [PubMed] [Google Scholar]
- 30. Venables ZC, Narayana K, Johnston GA.. Two unusual cases of allergic contact stomatitis caused by methacrylates. Contact Dermatitis. 2016;74:126–127. [DOI] [PubMed] [Google Scholar]
- 31. Wrangsjo K, Wallenhammar LM, Ortengren U, et al. . Protective gloves in Swedish dentistry: use and side-effects. Br J Dermatol. 2001;145:32–37. [DOI] [PubMed] [Google Scholar]
- 32. Wrangsjo K, Swartling C, Meding B.. Occupational dermatitis in dental personnel: contact dermatitis with special reference to (meth)acrylates in 174 patients. Contact Dermatitis. 2001;45:158–163. [DOI] [PubMed] [Google Scholar]
- 33. Kiec-Swierczynska MK. Occupational allergic contact dermatitis due to acrylates in Lodz. Contact Derm. 1996;34:419–422. [DOI] [PubMed] [Google Scholar]
- 34. Aalto-Korte K, Henriks-Eckerman ML, Kuuliala O, et al. . Occupational methacrylate and acrylate allergy–cross-reactions and possible screening allergens. Contact Dermatitis. 2010;63:301–312. [DOI] [PubMed] [Google Scholar]
- 35. Janeway CA TP Jr, Walport M.. Immunobiology. 5th ed. New York: Garland Science; 2001. [Google Scholar]
- 36. Hanks CT, Wataha JC, Parsell RR, et al. . Permeability of biological and synthetic molecules through dentine. J Oral Rehabil. 1994;21:475–487. [DOI] [PubMed] [Google Scholar]
- 37. Samuelsen JT, Dahl JE, Karlsson S, et al. . Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007;23:34–39. [DOI] [PubMed] [Google Scholar]
- 38. Barkhordar RA, Hayashi C, Hussain MZ.. Detection of interleukin-6 in human dental pulp and periapical lesions. Endod Dent Traumatol. 1999;15:26–27. [DOI] [PubMed] [Google Scholar]
- 39. Silva AC, Faria MR, Fontes A, et al. . Interleukin-1 beta and interleukin-8 in healthy and inflamed dental pulps. J Appl Oral Sci. 2009;17:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmalz G, Schweikl H, Hiller KA.. Release of prostaglandin E2, IL-6 and IL-8 from human oral epithelial culture models after exposure to compounds of dental materials. Eur J Oral Sci. 2000;108:442–448. [DOI] [PubMed] [Google Scholar]
- 41. Seubert A, Monaci E, Pizza M, et al. . The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. [DOI] [PubMed] [Google Scholar]
- 42. Serre K, Mohr E, Toellner KM, et al. . Molecular differences between the divergent responses of ovalbumin-specific CD4 T cells to alum-precipitated ovalbumin compared to ovalbumin expressed by Salmonella. Mol Immunol. 2008;45:3558–3566. [DOI] [PubMed] [Google Scholar]
- 43. Staruch MJ, Wood DD.. The adjuvanticity of interleukin 1 in vivo. J Immunol. 1983;130:2191–2194. [PubMed] [Google Scholar]
- 44. Eisenbarth SC, Colegio OR, O'Connor W, et al. . Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rustemeyer T, de Groot J, von Blomberg BM, Frosch PJ, Scheper RJ. Cross-reactivity patterns of contact-sensitizing methacrylates. Toxicol Appl Pharmacol. 1998;148:83–90. [DOI] [PubMed] [Google Scholar]
- 46. Mestas J, Hughes C. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004. [DOI] [PubMed] [Google Scholar]