Abstract
Importance
Delirium is defined as an acute disorder of attention and cognition. It is a common, serious, and often fatal condition among older patients. Although often underrecognized, delirium has serious adverse effects on the individual’s function and quality of life, as well as broad societal effect with substantial health care costs.
Objective
To summarize the current state of the art in diagnosis and treatment of delirium and to highlight critical areas for future research to advance the field.
Evidence Review
We searched Ovid MEDLINE, Embase, and the Cochrane Library for the past 6 years, from January 1, 2011 until March 16, 2017 using a combination of controlled vocabulary and keyword terms. Since delirium is more prevalent in older adults, the focus was on studies in elderly populations; studies based solely in the intensive care unit (ICU) and non-English-language articles were excluded.
Findings
Of 127 articles included, 25 were clinical trials, 42 cohort studies, 5 systematic reviews and meta-analyses, and 55 were other categories. A total of 11 616 patients were represented in the treatment studies. Advances in diagnosis have included the development of brief screening tools with high sensitivity and specificity, such as the 3-Minute Diagnostic Assessment, 4 A’s test, and proxy-based measures such as the Family Confusion Assessment Method. Measures of severity, such as the Confusion Assessment Method-Severity Score, can aid in monitoring response to treatment, risk stratification, and assessing prognosis. Nonpharmacologic approaches focused on risk factors such as immobility, functional decline, visual or hearing impairment, dehydration, and sleep deprivation are effective for delirium prevention and also are recommended for delirium treatment. Current recommendations for pharmacologic treatment of delirium, based on recent reviews of the evidence, recommend reserving use of antipsychotics and other sedating medications for treatment of severe agitation that poses risk to patient or staff safety or threatens interruption of essential medical therapies.
Conclusion and Relevance
Advances in diagnosis can improve recognition and risk stratification of delirium. Prevention of delirium using nonpharmacologic approaches is documented to be effective, while pharmacologic prevention and treatment of delirium remains controversial.
INTRODUCTION
Delirium, defined as an acute disorder of attention and cognition, is a common, life-threatening, and often preventable clinical syndrome in older persons. Often occurring after acute illness, surgery, or hospitalization, the development of delirium initiates a cascade of events culminating in loss of independence, increased morbidity and mortality, institutionalization, and high health care costs. In the United States, more than 2.6 million adults 65 years and older each year develop delirium and account for an estimated more than $164 billion in annual healthcare expenditures.1 Given its adverse effect on function and quality of life, delirium holds significant societal implications for the individual, family, community, and the entire health care system.
Delirium remains underrecognized and rates of identification have not improved significantly over time. Rates of unrecognized delirium, defined as delirium diagnosed by an expert assessor after the diagnosis was not made by the patient’s treating physicians and nurses, ranged from 55% to 70% in 2000–20012, 3 and still remain around 60% in 2015.4 Delirium is a complex and challenging condition, and a synthesis of current evidence should optimize clinical care. The goals of this review were (1) to summarize the current approaches to diagnosis and treatment of delirium, (2) to highlight recent advances, and (3) to underscore critical gaps in knowledge where future research is needed to advance the field.
CURRENT APPROACH TO DIAGNOSIS AND TREATMENT OF DELIRIUM
Delirium remains a clinical diagnosis, and the condition is easily overlooked.1 Recognition is based on brief cognitive screening and careful bedside observation of key features. The current reference standard diagnostic criteria are the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5) from the American Psychiatric Association5 and the International Classification of Diseases and Related Health Problems, Tenth Revision from the World Health Organization.6 Key diagnostic features, derived from DSM-5 and the widely used Confusion Assessment Method (CAM),7, 8 include an acute onset and fluctuating course of symptoms, inattention, impaired level of consciousness, and disturbance of cognition indicating disorganization of thought (eg, disorientation, memory impairment, or alteration in language) (CAM algorithm in eFigure 2 in the Supplement). Other features supportive of the delirium diagnosis include alterations in sleep-wake cycle, perceptual disturbances (eg, hallucinations or misperceptions), delusions, inappropriate or unsafe behavior, and emotional lability.7 Delirium includes both hypoactive and hyperactive forms. The hypoactive form is more common among older persons, often goes unrecognized, and is associated with higher rates of complications and mortality.9, 10
The cornerstone of diagnosis is determining the patient’s baseline mental status, and the acuity of any changes; with delirium, the changes typically occur over hours to days. This step is critical and requires obtaining the history from a knowledgeable informant. Neglecting the baseline mental status assessment is a leading reason for a missed diagnosis, since the acute change might otherwise be missed. Once the baseline mental status is determined, delirium is diagnosed by using brief cognitive screening tests such as the Mini-Cog11 or the Short Portable Mental Status Questionnaire12 and rating with a validated delirium instrument.
Conditions which may mimic delirium include dementia, depression, and psychosis (Table 1). As described above, an acute change in mental status from baseline may distinguish delirium from other conditions. Furthermore, inattention, while common in delirium, tends to occur in later stages of dementia. For accurate differential diagnosis, knowledge of the patient’s baseline is essential to make the diagnosis. Alteration in the level of consciousness is another feature unique to delirium that is less common with dementia, depression, or psychosis.
Table 1.
Clinical Features of Diseases that Mimic Delirium
| Features | Conditiona | |||
|---|---|---|---|---|
| Delirium | Dementia | Depression | Psychosis | |
| Acute change in mental status | + | − | − | ± |
| Inattention | + | ± | ± | ± |
| Altered consciousness | + | − | − | − |
| Disorganized thinking | + | ± | − | + |
| Altered psychomotor activity | + | ± | + | + |
| Chronic duration | ± | + | + | ± |
“±” indicates that the feature may be present
The next step is a careful physical and neurological examination, searching for possible causes. Because delirium can signify an acute medical emergency, all patients presenting with delirium need rapid, targeted evaluation for electrolyte or metabolic derangements, infection, or organ failure. The specific selection of tests should be based on information obtained from the history and physical examination, keeping in mind that delirium is often multifactorial in etiology and can be influenced by a number of predisposing (eg, older age, cognitive impairment, multiple comorbidities), precipitating factors (eg, infections, metabolic derangement, drugs), or both.. Some conditions presenting with symptoms of delirium, such as hepatic or uremic encephalopathy, acute drug intoxication, alcohol withdrawal delirium (delirium tremens), or Wernicke-Korsakoff syndrome (WKS), have specific treatments (eg, thiamine supplementation for WKS) and therefore should not be overlooked in the evaluation.
Examination of cerebrospinal fluid is not required for most older patients presenting with delirium and fever; however, lumbar puncture13 should be strongly considered in patients presenting with fever, headache, signs suspicious for meningitis14 or encephalitis,15 or when a specific neurological cause of acute mental status change (such as vasculitis or herpes encephalitis) must be excluded. Neuroimaging can be useful in identifying the etiology of delirium if the history suggests recent falls or examination reveals deteriorating mental status or focal neurologic findings.16
Delirium and dementia commonly coexist. It is important not only to distinguish between delirium and dementia diagnostically but also to recognize when delirium is superimposed on a preexisting dementia, which has important prognostic implications, including accelerated rate of cognitive and functional decline,17 increased length of hospital stay,18 and higher rates of rehospitalization,17 institutionalization,19 and death,19 compared with dementia alone. Interview with a caregiver for baseline mental status, prior diagnosis of mild cognitive impairment or dementia, and time course of cognitive changes (typically over months for dementia), plus administration of proxy-rated tools, such as the Informant Questionnaire on Cognitive Decline of the Elderly,20 can help establish the presence of an underlying dementia. The presence of depression should also be ruled out in the interview with the patient and family, using brief depression screening tools such as the Geriatric Depression Scale.21
Primary prevention of delirium with nonpharmacologic multicomponent approaches has been shown to be effective and have gained widespread acceptance as the most effective strategy for delirium.22 While many pharmacologic approaches have been evaluated in clinical trials, at present there is no convincing, reproducible evidence that any of these treatments are effective for either prevention or treatment of delirium.1, 23
METHODS
Search was conducted in Ovid MEDLINE, Embase, and the Cochrane Library from January 1, 2011 through March 16, 2017, using a combination of controlled vocabulary and keyword terms. Concepts were created for the topics of (1) delirium or confusion, (2) diagnosis or prevention or therapy, (3) randomized trials (using the Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE, sensitivity- and precision-maximizing version, 2008 revision), and (4) elderly adults. The search was limited to articles published in English. In addition to randomized trials, the overall search strategy was also designed to find other types of studies (eAppendix 1 in the Supplement). We identified 2303 titles and abstracts from the electronic search and also found an additional 37 eligible articles from the reference lists of relevant studies. Two hundred fifty-four full-text articles were retrieved for manual review. One hundred twenty-seven articles were used for this review, of which 25 were clinical trials, 42 were cohort studies, 5 were systematic reviews and meta-analyses, and 55 were other categories including methodological papers, clinical guidelines, and biomarker studies that were not cohort studies. A total of 11 616 patients were represented in the treatment studies. The complete list of search strategies and a search flow diagram are provided in eAppendix 1 and eFigure 1 in the Supplement/.
Studies based solely on the intensive care unit (ICU) were excluded, since this setting was considered outside the scope of the current review and has been examined in comprehensive reviews.24, 25 In addition, since delirium is more prevalent in older adults, we focused on studies in populations 65 years and older. For selected studies on pharmacologic prevention and treatment, article quality was rated with the Cochrane Collaboration tool for assessing risk of bias.26
RESULTS
Clinical Diagnosis
Since 2011, the following new information has become available, and these sections highlights key advances in diagnosis during the past 6 years.
Screening Instruments
The CAM,7 published in 1990, continues to be the most widely used delirium instrument worldwide, used in more than 4500 original published studies to date and translated into 19 languages. The CAM algorithm is based on the presence of 4 core features of delirium (acute onset and fluctuating course of symptoms, inattention, and either disorganized thinking or altered level of consciousness7) and has high sensitivity (94%–100%), specificity (90%–95%), and interrater reliability (κ = 0.92).8,27 More recently, more than 20 delirium screening tools have been introduced, many of which have been developed in the past 6 years (Table 2). These screening tools are used to alert the clinicians to the presence of possible delirium. Since screening tools have varying sensitivity and specificity, a positive screening test should lead to further investigation for more definitive diagnosis of delirium.
Table 2.
Characteristics of Delirium Screening Tools (last 6 years)a
| Screening Tool | Setting | No. (% male) | Age, mean (SD), y | Assessment Time | Scoring | Sensitivity (Cognitively Impaired), %b | Specificity (Cognitively Impaired), %b | Inter-rater reliability (95% CI) | Description (No. Of Question Items) |
|---|---|---|---|---|---|---|---|---|---|
| 3D-CAM28 | Hospital | 201 (38) | 84 (5.4) | 3 min (median) | Possible delirium if (1) acute onset or fluctuation AND (2) inattention AND EITHER (3) disorganized thinking OR (4) altered level of consciousness | 95 (96) | 94 (86) | 95% | Screening tool derived from the CAM (10 PQI,10 ORI)c |
| CAM-S29,d | Hospital | 1219 (41) | 77–80e | Long form, 10–15 minutes; Short form, NR | Maximum score: 19 (long form), 7 (short form) | NAf | N/A | Long form ICC = 0.88; Short form ICC = 0.92 | Delirium assessment tool derived from the CAM (long form: 4 PQI, 2 CQI, 4 ORI; short form 1 PQI, 1 CQI, 2 ORI) |
| 4AT30,g | Hospital | 234 (36) | 84 (5.9) | < 2 min | Maximum score =12; possible delirium when score ≥ 4 | 90 (94) | 84 (91) | NR | Screening tool for delirium and cognitive impairment (5 PQI, 2 ORI) |
| DelApp31 | Hospital | 156 | 85 (delirium group) 87 (dementia group) 75 (control group) |
< 5 min | Maximum score =10; median, 6 (IQR, 4–7) in delirium group, 10 (IQR, 10–10) in control group | 98 | 93 | NR | Software for objective measurement of attention (9 ORI, 1 PQI)h |
| FAM-CAM32 | Homei | 52j (33) | 82 (8) | NR | Possible delirium if (1) acute onset or fluctuation AND (2) inattention AND EITHER (3) disorganized thinking OR (4) altered level of consciousness | (88) | (98) | κ = 0.85 (0.65–1.0) | Screening tool for caregivers (11 CQI) |
| I-AGeD33 | Hospital | 88k (27) | 86.4 (8.5) | NR | Maximum score = 10; possible delirium when score > 4 | 77.4l | 63.2 | NR | Caregiver-based questionnaire (10 CQI) |
| Inter-RAI34,m | Hospital | 239 (49) | 82 (6.4) | NR | Possible delirium if (1) acute change in mental status and (2) mental function varies over the course of the day | 82 (90) | 91 (69) | κ = 0.65–0.76 | Screening tool for acute care (4 ORI) |
| MOTYB + Signs of confusion35 | Hospital | 265 (51.1) | 69 (27) | NR | Possible delirium if the patient failed MOTYB or was confused (subjective or objectively) | 93.8 (87.5) | 84.7 (71.4) | NR | Screening tool for acute care (2 PQI, 6 ORI) |
| RADAR36 | Hospital, long-term care | 193 (40) | 80.8 (7.8) | 7 s (average) | Maximum score = 3; possible delirium when score ≥ 1 | 73 (71.4) | 67 (42.9) | κ = 0.34–0.79 | Tool for nursing staff (3 ORI) |
| SQeeC37 | Hospital | 100 (40) | 87 | 30 s to 3 min | Possible delirium if unable to answer first question or wrong answer to second question | 83 (83) | 81 (59) | NR | Tool for evaluating level of consciousness (2 PQI) |
| Sour Seven38 | Hospital | 80 (36) | 81.3 (8.9) | 1–2 min (nurses); 2–5 min (caregivers) | Maximum score = 18; possible delirium when score ≥ 4 | 89.5 | 90 | 64.3%–92.8%n | Tool for informal caregivers and untrained nurses (7 ORI) |
Abbreviations: CAM, Confusion Assessment Method; CAM-S, CAM-Severity Score; ; CI, confidence interval; CQI, caregiver questionnaire items; FAM-CAM, Family-CAM; κ, Kappa; ICC, intra-class correlation coefficient; Inter-RAI, Inter-Resident Assessment Instrument; IQR, interquartile range; I-AGeD, Informant Assessment of Geriatric Delirium scale;; MOTYB, Months Of The Year Backwards; NA, not applicable; No, number; NR, not reported; ORI, observer rating items; PQI, patient questionnaire items; RADAR, Recognizing Acute Delirium As part of your Routine; SQeeC, Simple Query for Easy Evaluation of Consciousness; 3D-CAM, 3 minute diagnostic assessment; 4AT, 4 A’s Test. ;
Inclusion criteria: (1) study published during the defined search period, (2) reference standard delirium assessment was completed using Diagnostic and Statistical Manual of Mental Disorders (DSM) IV or 5, or DSM-derived criteria such as CAM, Delirium Rating Scale, or Neelon and Champagne Confusion Scale. Exclusion criteria: (1) study of delirium in the critically ill (intensive care unit); (2) validation study of a non-English version of an existing delirium screening tool.
Sensitivity and specificity of the screening tool to detect delirium against the gold standard such the Diagnostics and Statistical Manual of Mental Disorders criteria. Some studies also determined the sensitivity and specificity of the screening tool for detecting delirium in individuals with cognitive impairment, and those numbers are reported in parentheses.
There is a skip pattern option for which if any item in a section is answered incorrectly or endorsed as yes, then the rest of the PQI in that section and corresponding ORI can be skipped, allowing administration of fewer questions (as few as 3). However, the 3D-CAM was validated with administration of all items.
The CAM-S is intended to be used in addition to the original CAM algorithm; it will not yield a delirium diagnosis but only to quantify the intensity of delirium.
CAM-S was validated in 2 different cohorts; mean age was 77 years in the SAGES Study and 80 years in the Project Recovery sample.
CAM-S is a tool to quantify the intensity of delirium; therefore, sensitivity and specificity as a delirium screening tool does not apply.
Information available at http://www.the4at.com.
DelApp is a visual sustained attention counting task and is still in research phase.
All patients enrolled in the study had preexisting cognitive impairment.
52 dyads (patient and caregiver).
Construction cohort N = 88, 2 validation cohorts of N = 59 and N = 33.
The sensitivity and specificity of 77.4 % and 63.2% were derived from a cut-off score of greater than 4 on I-AGeD; sensitivity and specificity in patients without dementia were 100% and 65.2% respectively. Sensitivity of 2 validation cohorts ranged from 70.0% to 88.9%; specificity ranged from 66.7% to100%.
Delirium screening tool consists of 4 observational delirium items from the Inter-RAI acute care comprehensive assessment system: acute change mental status from baseline, mental function varies over the course of the day, episode of disorganized speech, and easily distracted.
Comparison of agreement between untrained nurses and geriatric psychiatrist calculated by each question; comparison of agreement between caregivers and geriatric psychiatrist ranged from 44% to 84% in agreement.
Definitive diagnosis of delirium should be conducted by a trained, experienced clinician and would entail cognitive testing and neurologic examination for fulfillment of key diagnostic features, including disturbance in mental status that represents a change from baseline and fluctuates in severity during the day; inattention (reduced ability to sustain attention and follow conversations); disorganization of thought, such as problems with memory, orientation, or language; and impaired consciousness, such as hypervigilance, drowsiness or stupor. The presence of an underlying organic etiology or multiple etiologies is also required. The 3-Minute Diagnostic Assessment (3D-CAM) provides a brief assessment (3 orientation items, 4 attention items, 3 symptom probes, and 10 observational items) that facilitates rating of the 4 core CAM features and demonstrated a sensitivity of 95% and specificity of 94% when compared to a clinical reference standard rating in a prospective validation study in hospitalized patients.28 Another screening tool is the 4A’s Test (4AT), which has been validated in various clinical settings.30 This tool is also brief and easy to administer and has a sensitivity of 89.7% and specificity of 84.1%. The 4AT provides a score range suggestive of cognitive impairment for which more detailed cognitive testing is advised.30 Both 3D-CAM and 4AT validation studies have high ratings by the Standards for Reporting of Diagnostic Accuracy criteria.39
In recent years, many well-established delirium screening tools have been adapted or used in various clinical and research applications. For instance, the CAM7 is often used as a reference standard in studies of more newly developed delirium screening tools.40 The Short CAM has been more recently adapted and validated across a large range of patient populations including medical, surgical, ICU (CAM-ICU), emergency department, nursing home, and palliative care.40 Other screening tools with more recent validation studies include the Nursing Delirium Symptom checklist (Nu-DESC), which includes assessment of disorientation, inappropriate behavior, inappropriate communication, illusions or hallucinations, and psychomotor retardation. The checklist has sensitivity of 72% and specificity of 80%41: however, limitations include the potential for overweighting of hyperactive or agitation symptoms and the risk of missing hypoactive delirium. The Modified Richmond Agitation and Sedation Scale (mRASS), which measures arousal, sedation and level of consciousness, has been advocated as a screening tool for delirium. However, the mRASS has a low sensitivity of 64% to70%,42, 43 and the usefulness of the scale depends on the prevalence of decreased mental status in the population. In settings with high prevalence of sedation and depressed sensorium, such as the postoperative recovery room and ICU, this approach may be valuable; however, routine use of the mRASS is not recommended outside of these settings, since many cases of delirium will be missed.
Assessment of Delirium Severity
The measurement of delirium severity has assumed increased importance for tracking clinical course and recovery, monitoring response to treatment, and evaluating pathophysiologic mechanisms. Widely used delirium severity measures have included the Delirium Rating Scale-Revised-98 (DRS-R-98)44 and the Memorial Delirium Assessment Scale.45 The DRS-R-98 has scale items covering language, thought processes, motor symptoms, and cognition that are designed to capture gradations of symptom intensity.44 The Memorial Delirium Assessment Scale was designed for use in clinical intervention trials and has scale items for assessing disturbance in arousal, level of consciousness, as well as cognitive function and psychomotor activity.45
A recent advance is the development of the CAM-Severity Scale (CAM-S), a new scoring system based on either the short or long version of the CAM. A high-quality validation study involving 2 cohorts totaling more than 1219 patients showed that the CAM-S has strong psychometric properties and high predictive validity for important clinical outcomes related to delirium, including length of stay, hospital costs, nursing home placement, and death.29 A subsequent study examined the severity of an episode of delirium over the entire hospital stay and compared 9 different measures reflecting intensity, duration, cognitive change, or a combination of these measures. This study demonstrated that episode severity measures including both intensity and duration, such as the sum of all CAM-S scores across the hospitalization, had the strongest association with posthospital outcomes at 30 and 90 days.46 The Delirium Observation Screening scale is a new nurse-based delirium measure47 that correlates strongly with DRS-R98 scores, but validation studies have not yet been completed.
Approaches to Maximize Detection of Delirium
Because of its fluctuating nature and frequent hypoactive presentation, the detection of delirium can be especially challenging. Interview-based methods are sometimes conducted during brief encounters and need to be applied multiple times a day to improve the detection of delirium; however, this may not be feasible in many settings. Standardized chart-based methods,48 based on identification of keywords (eg, mental status change, disoriented/reoriented) by trained clinician abstractors, can be used in combination with interviews to maximize detection of delirium, particularly episodes occurring during night shifts. These methods have been validated to show sensitivity of 74% and specificity of 83% in comparison with a reference standard rating or clinical consensus panel. Therefore, the combined method of interview plus chart review48 is the recommended approach when complete and highly sensitive detection of delirium is needed.
Refinement of Approaches for Definitive Diagnosis
One of the problems in comparing different screening tools is that there is no uniform approach to delirium diagnosis by a clinical reference standard. In a recent review,49 the reference standard was found to range from a single physician’s clinical evaluation to consensus diagnosis based on comprehensive assessment using information gathered from patients, nurses, family members, and medical records. Since sensitivity and specificity determinations for each screening tool can vary depending on the reference standard used, more standardization will improve the ability to cross-validate and to directly compare different screening tools.
Biomarkers for Delirium
Biomarkers have assumed increasing importance, since they may be useful for identifying patients at higher risk for developing delirium and yield clues to potential underlying pathophysiologic mechanisms. Because delirium can be due to different etiologies, various biomarkers, including inflammatory, neurodegenerative, metabolic, and neurotransmitter-based, have been examined in the past 6 years. Inflammation is thought to play an important role in the pathogenesis of delirium, and recent studies have focused on inflammatory markers, including interleukins, and C-Reactive Protein50 (eTable 1 in the Supplement). Although numerous biomarkers have been studied, none have yet been validated for clinical application, such as diagnosis or monitoring of delirium.
Novel Uses of Electroencephalography
The current role for electroencephalography (EEG) in the diagnosis of delirium is to aid in differentiating delirium from nonconvulsive status epilepticus, focal dyscognitive seizures, or psychiatric conditions. Recent studies support the use of EEG in patients with a known history of seizures, findings suggestive of seizures (eg, gaze deviation), history of brain trauma or stroke, or treatment with medications that lower seizure threshold (eg, fluoroquinolones, bupropion).51,52 In a recent innovation, bispectral EEG monitoring and adjustment of anesthetic depth has been shown to be associated with a marked reduction in postoperative delirium53,54 and is currently under investigation in a large clinical trial.55
Advances in Prevention and Treatment
Development of systematic reviews and guidelines have served to facilitate application of more evidence-based approaches. In 2014, the American Geriatrics Society and the American College of Surgeons jointly released clinical practice guidelines for the prevention and treatment of postoperative delirium.23 The guidelines, developed in accordance with Institute of Medicine standards, highlight the importance of multicomponent nonpharmacologic prevention strategies, education of healthcare professionals, medical evaluation of delirium etiology, optimizing pain management with nonopioids, and avoiding high-risk medications (Table 3). New recommendations included avoidance of drug treatment for hypoactive delirium and avoidance of benzodiazepines for treatment of delirium, except in cases of alcohol or benzodiazepine withdrawal.
Table 3.
American Geriatrics Society Clinical Practice Guidelines for the Prevention and Treatment of Postoperative Deliriuma
| Recommendation | Description |
|---|---|
| Multicomponent nonpharmacologic interventions (for prevention) | Delivered by interdisciplinary team for at-risk older adults Includes mobility and walking, avoiding physical restraints, orienting to surroundings, sleep hygiene, adequate oxygen, fluids, and nutrition |
| Educational programs | Ongoing, provided for healthcare professionals |
| Medical evaluation | Identify and manage underlying organic contributors to delirium |
| Pain management | Should be optimized, preferably with nonopioid medications |
| Medications to avoid | Any medications associated with precipitating delirium (eg, high-dose opioids, benzodiazepines, antihistamines, dihydropyridines) Cholinesterase inhibitors should not be newly prescribed to prevent or treat postoperative delirium Benzodiazepines should not be used as first-line treatment of delirium-associated agitation Benzodiazepines and antipsychotics should be avoided for treatment of hypoactive delirium |
| Weak: Evidence in Favor of These Interventions, But Level of Evidence or Potential Risks Limit Strength of Recommendation | |
| Multicomponent nonpharmacologic interventions (for treatment) | Delivered by interdisciplinary team when older adults are diagnosed with postoperative delirium to improve clinical outcomes |
| Pain management | Injection of regional anesthetic at the time of surgery and postoperatively to improve pain control with the goal of preventing delirium |
| Antipsychotics | The use of antipsychotics (haloperidol, risperidone, olanzapine, quetiapine, or ziprasidone) at the lowest effective dose for shortest possible duration may be considered to treat delirious patients who are severely agitated, distressed, or threatening substantial harm to self, others, or both |
Adapted from American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults best practice statement 56 and abstracted clinical practice guideline.23 Full guideline available at http://www.geriatricscareonline.org
Prevention
Multicomponent Nonpharmacologic Interventions
Primary prevention with multicomponent nonpharmacologic approaches has been consistently demonstrated to be the most effective strategy for delirium prevention among hospitalized, non-ICU medical and surgical patients. These prevention strategies include early mobilization, adequate hydration, sleep enhancement, orientation to time and place, therapeutic activities such as reminiscence (for cognitive stimulation), and hearing and vision optimization by using hearing and vision aids as needed. Table 4 provides details on these specific approaches to guide clinicians in how to implement delirium prevention strategies.
Table 4.
Multicomponent Nonpharmacologic Approaches to Delirium Prevention
| Approach | Description |
|---|---|
| Orientation and therapeutic activities | Provide lighting, signs, calendars, clocks Reorient the patient to time, place, person, your role Introduce cognitively stimulating activities (eg, reminiscing) Facilitate regular visits from family, friends |
| Fluid repletion | Encourage patients to drink; consider parenteral fluids if necessary Seek advice regarding fluid balance in patients with comorbidities (heart failure, renal disease) |
| Early mobilization | Encourage early postoperative mobilization, regular ambulation. Keep walking aides (canes, walkers) nearby at all times Encourage all patients to engage in active, range-of-motion exercises |
| Feeding assistance | Follow general nutrition guidelines and seek advice from dietician as needed Ensure proper fit of dentures |
| Vision and hearing | Resolve reversible cause of the impairment Ensure working hearing and visual aids are available and used by those who need them |
| Sleep enhancement | Avoid medical or nursing procedures during sleep if possible Schedule medications to avoid disturbing sleep Reduce noise at night |
| Infection prevention | Look for and treat infections Avoid unnecessary catheterization Implement infection-control procedures |
| Pain management | Assess for pain, especially in patients with communication difficulties Begin and monitor pain management in those with known or suspected pain |
| Hypoxia protocol | Assess for hypoxia and oxygen saturation |
| Psychoactive medication protocol | Review medication list for both types and number of medications |
Because delirium is usually precipitated by multiple factors, effective prevention strategies should be implemented together (typically 3 or more at a time) by a multidisciplinary team. In a meta-analysis of 14 interventional studies based on the Hospital Elder Life Program,57,58 these approaches significantly reduced the risk of incident delirium by 53% (odds ratio, 0.47 [95% CI, 0.38–0.58]), and the risk of falls by 62% (odds ratio, 0.38 [95% CI, 0.25–0.60]) among hospitalized, non-ICU patients 65 years and older.22
Multicomponent nonpharmacologic approaches are cost-effective, with 1 study demonstrating an incremental net monetary benefit of £8180 (US $12 852 in 2014), using a cost-effectiveness threshold of £20 000 per quality-adjusted life year.59 This study took the novel approach of statistical modeling for patients undergoing surgical hip fracture repair, using decision tree analysis to explore deterministic and probabilistic sensitivity analyses. A Cochrane review of delirium prevention examined 39 trials involving 16 082 patients60 and found moderate-quality evidence that multicomponent nonpharmacologic interventions are effective for prevention of incident delirium but less robust for decreasing delirium severity or duration.60 Educating nursing aides and caregivers, specialized geriatric units, and music and psychotherapy have been examined for delirium prevention but results are not definitive.61,62 Multicomponent nonpharmacologic approaches for delirium prevention have been examined in specific patient populations. In 1 study of hospitalized patients with dementia, these approaches resulted in noticeable decreases in delirium incidence.63 Prior to implementation of nonpharmacologic delirium prevention approaches, approximately 20% of patients developed postoperative delirium, whereas after implementation, only 4.9% of patients became delirious.63
However in long-term care, cancer patients, and terminal illness, the effect of these interventions on delirium incidence has been more limited.64–66 Geriatric consultative approaches have been applied in different settings, but their success is dependent on adherence of the health care staff to recommendations made by the consultants.1
Pharmacologic Approaches
Selected pharmacologic delirium prevention studies from the past 6 years are summarized in Table 5. In a recent Cochrane review that examined prophylactic antipsychotics compared with control for preventing delirium in hospitalized non-ICU medical and surgical adult patients 16 years or older, there was no clear benefit of antipsychotics as a group.60 Some studies suggest that prophylaxis with antipsychotics can prevent postoperative delirium; however, methodologic limitations preclude a definitive recommendation at this time (Table 5). The same meta-analysis also found minimal evidence to support the use of medications to prevent delirium, including cholinesterase inhibitors, melatonin and melatonin-receptor agonist (ramelteon) based on meta-analysis.60
Table 5.
Selected Delirium Prevention and Treatment Studies, last 6 yearsa
| Sources by Category |
Study Design |
Setting (Study Duration) |
Sample Size (Intervention/ Control) |
Intervention | Control | Outcome | Results, Intervention vs Control, No.(%) |
Overall Quality Scoreb |
|---|---|---|---|---|---|---|---|---|
| Antipsychotics (Typical and Atypical) | ||||||||
| Fukata et al,67 2014 | Randomized, open-label, prospective | Elective GI/orthopedic surgery (2007–2012) | 119 (59/60) | Haloperidol, intravenous (2.5 mg/d, postoperative days 1–3) | No haloperidol | Delirium incidence | 25/59 (42.4) vs 20/60 (33.3) P = .31 | 5 |
| Hakim et al,68 2012 | RCT | Cardiac surgery (2007–2010) | 101 (51/50) | Risperidone, oral (0.5 mg /12 h postoperative day) | Placebo | Delirium incidence among those with subsyndromal delirium | 7/51 (13.7) vs 17/50 (34) P = .03 | 6 |
| Vochteloo et al,69 2011 | PCT | Hip fracture surgery (2008–2009) | 378 (173/205) | Haloperidol, oral (1 mg/twice daily in high-risk patients) | No haloperidol in lower-risk patients | Delirium incidence | 73/173 (42.4) vs 29/205 (14.1) P < 0.001c | 3 |
| Wang et al, 70 2012 | RCT | Noncardiac surgery (2009–2010) | 457 (229/228) | Haloperidol, intravenous (1.7 mg/d, postoperative day 1) | Placebo | Delirium incidence | 35/229 (15.3) vs 53/228 (23.4) P = .03 | 4 |
| Melatonin or Ramelteon | ||||||||
| Al-Aama et al,71 2011 | RCT | Hospital, medical (2007–2008) | 122 (61/61) | Melatonin, oral (0.5 mg/d) | Placebo | Delirium incidence | 2/56 (3.6) vs 10/52 (19.2) P < .02d | 5 |
| de Jonghe et al,72 2014 | RCT | Hip fracture surgery (2008–2012) | 378 (186/192) | Melatonin, oral (3 mg/d) | Placebo | Delirium incidence | 55/186 (29.6) vs 49/192 (25.5) P = .40 | 6 |
| Hatta et al,73 2014 | RCT | Hospital, medical (2011–2012) | 67 (33/34) | Ramelteon, oral (8 mg/d) | Placebo | Delirium incidence | 1/33 (3) vs 11/34 (32) P = .003 | 6 |
| Perioperative Interventionsd | ||||||||
| Ashraf et al,74 2015 | RCT | Elective cardiac cathertizationf | 93 (47/46) | Premedication with diphenhydramine (25 mg) and diazepam (5 mg) | No premedication | Delirium incidence | 0/47 (0) vs 0/46 (0) (NS) | 4 |
| Lurati et al,75 2012 | RCT | Noncardiac surgery (2006–2010) | 385 (184/201) | Sevoflurane | Propofol | Delirium incidence | 21/184 (11.4) vs 29/201 (14.4) P = .38 | 6 |
| Oh et al,76 2016 | Retrospective | Hip fracture surgery (2012–2014) | 174 (78/96) | Sugammadex, intravenous (2 mg/kg) | Neostigmine, intravenous (0.05 mg/kg) + glycopyrrolate, intravenous (0.01 mg/kg) | Delirium incidence | 26/78 (33.3) vs 35/96 (36.5) P = .75 | 4 |
| Stoppe et al,77 2013 | RCT | Cardiac surgery (2011) | 30 (15/15) | Xenon | Sevoflurane | Delirium incidence | 3/15 (20) vs 4/15 (27) P = .67 | 4 |
| Whitlock et al,78 2015 | RCT | Cardiac surgery (2007–2013) | 7507 (3755/3752) | Methylprednisolone, intravenous (250 mg at induction and at initiation of cardiopulmonary bypass) | Placebo | Delirium incidence | 295/3755 (8) vs 289/3752 (8) P = .80 | 6 |
| Djainai et al,79 2016 | RCT | Cardiac surgery (2011–2014) | 183 (91/92) | Dexmedetomidine (0.4μg/kg bolus, then 0.2–0.7 μg/kg/h) | Propofol (25–50 μg/kg/min) | Delirium incidence | 16/91(17.5) vs 29/92 (31.5) P = .03 | 6 |
| Liu et al80 2016 | RCT | Orthopedic surgery (2014–2015) | 197 (99/98) | Dexmedetomidine (0.2–0.4 μg/kg/h) | Placebo | Delirium incidence | In 65- to 75-year-olds,7/31 (22.6) vs 16/30 (43.3) P < .01g | 5 |
| Li et al,81 2017 | RCT | Cardiac surgery (2014–2015) | 285 (142/143) | Dexmedetomidine (0.6 μg/kg bolus, then 0.4 μg/kg/h) | Placebo | Delirium incidence | 7/142 (4.9) vs 11/143 (7.7) P = .30 | 6 |
| Dighe et al,82 2014 | RCT, post hoc analysis | Orthopedic surgery (2007–2011) | 161 (83/78) | Gabapentin (200 mg 3 times daily x4 d) | Placebo | Delirium incidence | 10/83 (12) vs 7/78 (9) P = .53 | 4 |
| Marcantonio et al,83 2011 | RCT | Hip fracture surgery (2007–2008) | 16 (7/9) | Donepezil, oral (5 mg/d) | Placebo | Delirium presence over time | 7/11 (64) vs placebo 9/14 (64) P = .94h | 6 |
| Papado-poulos et al,84 2014 | RCT | Orthopedic surgeryf | 106 (51/55) | Ondansetron, intravenous (8 mg/d x5 d) | Placebo | Delirium incidence | 18/51 (35.3) vs 29/55 (52.7) on postoperative day 2 P = .07i | 5 |
| Pesonen et al,85 2011 | RCT | Cardiac surgery (2008–2009) | 70 (35/35) | Pregabalin, oral (150 mg/d on day 1;75 mg/12 h on postoperative days 1–5) | Placebo | CAM-ICU scorej | Mean score, postoperative day 1: 24/25 vs 21/25 P = .04 | 5 |
Abbreviations: CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; GI, gastrointestinal; NS, nonsignificant; RCT, randomized clinical trial.
Some studies were excluded if studies were conducted exclusively in the intensive care unit or the duration of intensive care unit stay could not be determined. Studies with overall quality score less than 2 were also excluded.
The quality rating was based on the Cochrane risk of bias overall quality score, with 1 point assigned for each of 6 domains found to be at low risk of bias. Low risk of bias, 6 (low risk of bias in all domains), unclear risk of bias = not enough information to make a clear judgment (high or unclear risk of bias on 1 or more domains); high risk of bias, less than 4 (high risk of bias on ≥2 domains).
In this study, the patients received prophylactic haloperidol if they were determined to be at high risk based on the Risk Model for Delirium score. Because of protocol violation, there were 26 patients in the high-risk group who did not receive haloperidol, but the delirium incidences in tehse patients were not significantly different from those of patients who received prophylaxis.
Numerator and denominators calculated after the prevalent delirium cases were subtracted (melatonin n = 5, placebo n = 9).
Delirium incidence was not the primary outcome for some of the perioperative intervention studies (Lurati et al75 [tertiary end point], Whitlock et al78 [secondary outcome]). Tgere were included because fo the study quality, intervention type, and large study population.
Study duration not reported.
This study examined the effect of dexmedetomidine on patients with amnestic mild cognitive impairment and in individuals with normal cognition, stratified bv age. Patients with amnestic mild cognitive impairment: dexmedetomidine vs placebo (normal saline), 22.6% vs 43.3% (P < .01) for those aged 65 to 75 years and 37.5% vs 90% (P < .01) for those 75 years or older. Patients with normal cognition: dexmedetomidine vs placebo (normal saline) 11.9% vs 30.8% (P < .01) for those aged 65 to 75 years and 16.7% vs 36.8% (P < .01) for those 75 years or older.
Denominator was number of interview, more than 1 interview per patient.
Study reports statistical significance on postoperative days 3 to 5, but the total number of individuals who experienced delirium in each group are not reported.
Modified Finnish CAM-ICU score (highest 25 points).
Delirium Prevention for the Surgical Patient
Most perioperative measures involving the use of different types of sedation or anesthesia have not effectively reduced the incidence of delirium (Table 5). One study showed that dexmedetomidine may be effective in reducing delirium incidence in patients with mild cognitive impairment, but this finding will need to be replicated in larger studies.80 Other strategies, including tight control of glucose levels and blood transfusions for delirium prevention in the perioperative setting, have shown varying degrees of benefit.86,87 Moderate quality evidence suggests that adjusting the depth of anesthesia according to bispectral index monitoring can decrease the incidence of delirium.60,88
Treatment
Nonpharmacologic Approaches
Few recent studies have examined nonpharmacologic approaches for the treatment of delirium. One pilot study involving 143 nursing home patients examined a modified Hospital Elder Life Program in the long-term care setting for prevention and treatment of delirium and found that it was feasible, with high satisfaction rates and decreased hospitalization rates. However, further testing of the intervention will be needed in a clinical trial.89 A recent clinical trial using daily therapeutic activities such as reminiscence activities for cognitive stimulation in the postacute care setting for delirium superimposed on dementia found no benefit on delirium duration or severity but did demonstrate significantly improved executive function and decreased length of stay.90 Other studies have focused on specialized delirium rooms or improving sleep to treat delirium with use of earplugs, bright light therapy, and sleep protocols – but with varying and limited results.91,92
Pharmacologic Treatment Approaches
Selected pharmacologic delirium treatment studies from the past 6 years are summarized in Table 6. Most studies do not show benefit of antipsychotics in decreasing the duration or severity of delirium. A recent comprehensive, systematic review examined antipsychotic drugs including oral risperidone, oral olanzapine, oral seroquel, intramuscular ziprasidone, and oral, intravenous and intramuscular haloperidol100 and concluded that the current evidence does not support the use of antipsychotics for treatment (or prevention) of delirium in hospitalized older adults. There was no significant decrease in delirium incidence among 19 studies and no change in delirium duration, severity, hospital or intensive care length of stay, or reduction in mortality.
Table 6.
Selected Delirium Treatment (Typical and Atypical Antipsychotics) Studies, Last 6 Years2
| Sources by Category |
Study Design |
Setting (Study Duration) |
Sample Size (Intervention/ Control) |
Intervention | Control | Outcome | Results, Intervention vs Control, No. (%) |
Overall Quality Scoreb |
|---|---|---|---|---|---|---|---|---|
| Atalan et al,93 2013 | RCT | Cardiac surgery (2010–2012) | 53 (27/26) | Morphine sulfate, intramuscular (5 mg, up to 20 mg/d) | Haloperidol, intramuscular (5 mg, up to 20 mg/d) | Delirium duration in those with hyperactive delirium | 31.56 h vs 33.9 h P = .61 | 4 |
| Boettger et al,94 2015 | Open-label, matched | Hospital, oncology (2000–2006) | 84 (21 haloperidol/21 risperidone, 21 aripiprazole, 21 olanzapine) | Haloperidol (5.5 mg) | Risperidone (1.3 mg) Aripiprazole (18.3 mg) Olanzapine (7.1mg) (mean doses 4–7 d) | Delirium resolution and adverse-effect profiles (typical vs atypical) | 16/21 (76.2) vs 18/21 (85.7) [risperidone], 16/21 (76.2) [aripiprazole], and 13/21 (61.9) [olanzapine] P = .42c | 3 |
| Kishi et al,95 2012 | Case series | Hospital, cancerd | 29 (intervention) | Risperidone, oral (0.5–1 mg to start, then titrated) | No control group | Delirium severity (responder, 25% reduction in the DRS-R-98 from baseline to day 7) | No significant differences in the number of treatment responders vs nonresponders, 14/29 (48) vs 15/29 (51) | 2 |
| Maneeton et al,96 2013 | RCT | Hospital, medical (2009–2011) | 52 (24/28) | Quetiapine, oral (25–100 mg/d for 1–7 d) | Haloperidol, oral (0.5–2 mg/d for 1–7 d) | Delirium severity by DRS-R-98 (higher score = more severe) | 22.9 (6.9) vs 21.7 (6.7) P = .59 | 5 |
| Schrøder Pedersen et al,97 2013 | Prospective cohort | Cardiac surgery (2012) | 240 (123/117) | Standardized treatment with haloperidol, oral (2.5–5 mg 3 times daily x1.5 d, then taper) | No standardized treatment protocol | Delirium duration | 3 (range, 1–5) d vs 1 (range, 1–4) d P = .23 | 3 |
| Yoon et al,98 2013 | Prospective observa-tional | Hospital, medical surgicald | 80 (23 haloperidol/21 risperidone, 18 olanzapine, 18 quetiapine) | Haloperidol, oral (0.5–10 mg/d) | Risperidone, oral (0.25–4 mg/d) Olanzapine, oral (1–20 mg/d) Quetiapine, oral (25–200 mg/d) |
Delirium severity ≥50% reduction from baseline by DRS-K | 15/23 (65.2) vs 14/21 (66.6) [risperidone], 12/18 (66.6) [olanzapine], and 13/18 (72.2) [quetiapine] P = .97 | 2 |
| Agar et al,99 2017 | RCT | Inpatient hospice, hospital palliative care (2008–2014) | 249 (81 haloperidol, 82 risperidone/86 placebo) | Haloperidol, risperidone, oral (0.25 mg every 12 h, up to 2 mg/d)e | Placebo | Delirium symptom scores on day 3 (higher score = more severe) | Haloperidol vs placebo: 0.24 U higher in haloperidol group, P = .009 Risperidone vs placebo: 0.48 U higher in risperidone group, P = .02 |
6 |
Abbreviations: DRS-K, Korean version of the Delirium Rating Scale-Revised-98 (DRS-R-98); RCT, randomized clinical trial.
Some studies were excluded if studies were conducted exclusively in the intensive care unit or the duration of intensive care unit stay could not be determined. Studies with overall quality score less than 2 were also excluded.
The quality rating was based on the Cochrane risk of bias overall quality score, with 1 point assigned for each of 6 domains found to be at low risk of bias. Low risk of bias, 6 (low risk of bias in all domains), unclear risk of bias = not enough information to make a clear judgment (high or unclear risk of bias on 1 or more domains); high risk of bias, less than 4 (high risk of bias on ≥2 domains).
Adverse effects: extramyramidal symptoms (haloperidol, 4 of 21 [19%]; risperidone, 1 of 21 [4.8%]), sedation (olanzapine, 6 of 21 [28.6%]).
Study duration not reported.
The study had different loading, initial, and maximum doses for participants 65 years or younger and for participants older than 65 years. Participants 65 years or younger received a 0.5-mg loading dose with the first dose of 0.5 mg, then 0.5-mg maintenance dose every 12 hours. Doses could be titrated by 0.25 to 0.5 mg, with maximum dose of 4 mg. For participants older than 65 years, the loading, initial, and maximum doses were halved.
Potential harm was demonstrated in 2 studies in which more patients required institutionalization after treatment with antipsychotics. Moreover, in a randomized control trial of atypical antipsychotic drugs in palliative care settings, participants receiving oral risperidone or haloperidol had higher delirium symptom scores and were more likely to require breakthrough treatment compared with participants receiving placebo. Participants in the placebo/nonpharmacologic management group also had better overall survival compared to those in the haloperidol group.99 Only a few limited studies have considered pharmacologic approaches other than antipsychotics for the treatment of delirium, and no definitive recommendations can be made at this time. More research is needed to establish safe and effective pharmacologic treatment approaches.
DISCUSSION
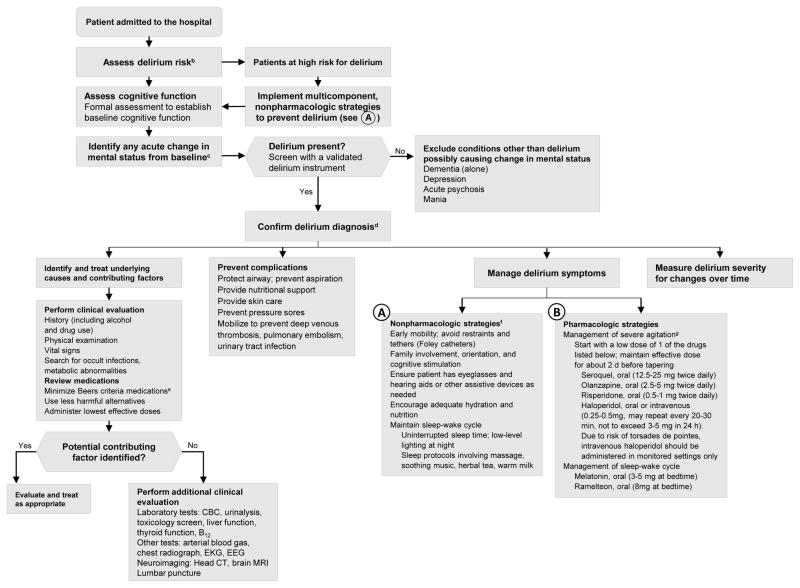
To assist clinicians with the evaluation and treatment of delirium, a detailed suggested algorithm is presented in the Figure, which synthesizes recent evidence gleaned from this comprehensive review with all prior evidence. The algorithm entails assessing delirium risk, instituting delirium prevention measures, evaluating and managing delirium once its presence is confirmed, and treating delirium using both nonpharmacologic and, in appropriate cases, pharmacologic strategies. While such an approach has not been validated, it is based on the best available evidence from prior studies and incorporates relevant recent evidence, such as current evidence against the use of antipsychotic medications in the treatment of delirium because of lack of efficacy and increased risk of adverse events and poor outcomes. Advances in diagnosis have included the development of new brief screening tools (Short-CAM adaptations, 3D-CAM, and 4AT) to improve delirium identification. Delirium severity, such as measured with the new CAM-S scoring, has been recognized as increasingly important for tracking clinical course, prognosis, and response to treatment. Measures that capture both intensity and duration of an episode of delirium (such as the sum of all CAM-S scores) correlate best with clinical outcomes in a direct, graded relationship. For complete capture of delirium episodes, a combined approach including interview and chart review is recommended. Intraoperative EEG monitoring and bispectral monitoring are emerging strategies that identify delirium risk and help to adjust depth of anesthesia, which may decrease risk.
Figure. Suggested Algorithm for Delirium Evaluation and Treatmenta.
CBC indicates complete blood cell count; CT, computed tomography; EEG, electroencephalogram; EKG, electrocardiogram; MRI, magnetic resonance imaging.
aAlthough the algorithm is evidence-based, it has not been validated.
bCommon delirium risk factors include dementia or cognitive impairment, functional or mobility impairment, visual or hearing impairment, dehydration, sleep deprivation, history of alcohol misuse, advanced age (> 70 years), multiple coexisting medical illnesses, and presence of specific comorbidities (eg, stroke, depression).
cDelirium should be considered a life-threatening medical emergency until proven otherswise; therefore, the presence of an acute change in mental status should trigger a rapid evaluation. Increasingly, many hospitals are incorporating delirium pathways (standing order sets for evaluation and treatment of delirium), implementation of delirium screening tools into the electronic medical record, and dedicated delirium wards/services.
dDelirium is diagnosed in the presence of the following core features: (1) acute and fluctuating mental status change from baseline; (2) inattention PLUS (3) disorganized thinking OR (4) altered level of consciousness.10
eThe Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (Beers Criteria) can help identify medications that should be avoided or used at lowest possible dose. This includes tricyclic antidepressants, anticholinergics, antihistamines (eg, diphenhydramine), benzodiazepines, corticosteroids, H2-receptor antagonists, meperidine, sedative-hypnotics, thioridazine, and chlorpromazine.
fMulticomponent, nonpharmacologic strategies should be used for both delirium prevention and treatment.
gReserve antipsychotic medications for use only when behvaiors (ie, agitation, hallucinations) pose a serios safety hazard to patient, staff, or both or when there is risk of interrupting essential medical care.
Primary prevention with multicomponent nonpharmacologic approaches such as reorientation, early mobilization, therapeutic activities, hydration, nutrition, sleep strategies, and hearing and vision adaptations are effective and cost-effective and remain the cornerstone of delirium management. However, these approaches can be labor intensive, and streamlined approaches include the use of volunteers, aides, or nonlicensed professionals to enhance feasibility and reduce costs of implementation. Development of effective treatments have been hindered by multiple challenges, including the multifactorial contributors, diagnostic complexity, multimorbidity, heightened risk of adverse effects (ie, drug interactions), and need for multicomponent approaches. Although promising approaches are emerging, safe and highly effective pharmacologic treatments for delirium have not yet been identified. Antipsychotics are often used for patients with delirium and with severe agitation and safety risks but may contribute to heightened adverse effects and poorer long-term outcomes. Therefore, similar to the initiative by the Centers for Medicare and Medicaid Services to reduce the use of antipsychotics for improved dementia care, a concerted effort to reduce the use of antipsychotics and focus on nonpharmacologic management may improve delirium care.
Several limitations of this review must be acknowledged. The literature search was restricted to the past 6 years; however, inclusion of recent systematic reviews allowed incorporation of many additional years of evidence. Studies based solely in the ICU were excluded, because they were considered outside the scope of this review and already covered in recent comprehensive reviews. Moreover, only studies published in English were included. Last, for many areas explored, we found weak to insufficient evidence, which limited our recommendations. High quality, adequately powered randomized clinical trials represent an important priority for the field.
Advances in the pathophysiologic understanding of delirium will be critical to advance the diagnosis and treatment of delirium. High priority areas for future investigation are outlined in eTable2 in the Supplement. Biomarkers are likely to play an increasing role in confirming diagnosis, stratifying risk, monitoring severity, and providing mechanistic understanding of delirium. Because inflammation is thought to play an important role in the pathogenesis of delirium,101 inflammatory markers are widely studied for delirium risk stratification and monitoring (eTable1 in the Supplement). Although several studies have shown the association of elevated levels of inflammatory biomarker levels including interleukins and C-reactive protein, with delirium, the results are not always consistent and not yet ready for clinical application.50,102 Similar to biomarker studies in other fields, standardization of assay platforms across laboratories and validation across different clinical populations will facilitate incorporation of biomarkers into clinical practice.
Innovative treatment approaches may include identifying pathophysiologically targeted approaches, boosting cognitive reserve, providing neuroprotection, enhancing sleep, and multipronged combination approaches. Given the complex and multifactorial etiology of delirium, innovative approaches are greatly needed to break the escalating cycle of brain dysfunction that is the hallmark of the disorder and thereby effectively treat this condition, which is common and a highly morbid condition among older adults.
CONCLUSIONS
Delirium is a common, serious condition associated with increased morbidity and mortality in older patients as well as enormous societal costs. Advances in diagnosis can improve recognition and risk stratification of delirium, and many brief delirium screening tools have been developed in the past 6 years to allow improvement in recognition and risk stratification. Along with thorough clinical examination and laboratory testing, additional tools such as imaging and fluid biomarkers are being studied to enhance clinical risk stratification and diagnosis. Pharmacologic prevention and treatment of delirium remains controversial, and nonpharmacologic management of delirium remains the cornerstone of delirium prevention and treatment. Prevention of delirium using nonpharmacologic approaches is documented to be effective, whereas pharmacologic prevention and treatment of delirium remains controversial.
Future high-quality, adequately powered studies of pharmacologic treatment are a priority to identify approaches that are effective and safe.
Supplementary Material
KEY POINTS.
Question
What advances in diagnosis, prevention, and management of delirium in older adults have been introduced in the last 6 years?
Findings
Brief screening tools and improved delirium severity measurement tools have been developed for recognition and risk stratification of delirium. Delirium prevention with nonpharmacologic multicomponent strategies is effective. For pharmacologic management of delirium, the benefits do not outweigh the harms, and recommendations are to reserve treatment for patients with severe agitation that poses safety risks.
Meaning
Advances in screening and diagnosis of delirium can improve recognition and risk stratification, while implementation of nonpharmacologic delirium prevention strategies can substantially improve outcomes among older patients.
Acknowledgments
Funding/Support: This work was supported in part by K23AG043504 from the National Institutes of Health (NIH)/National Institute on Aging (NIA) (ESO), the Roberts Fund (ESO), 3UL1TR001102 from the National Center for Advancing Translational Sciences (TGF); P01AG031720 (SKI), R24AG054259 (SKI), R01AG044518 (SKI), and K07AG041835 (SKI) from the NIA and by the Milton and Shirley F. Levy Family Chair (SKI). The funding sources had no role in the design and conduct of the study; collection, analysis and interpretation of the data; writing of the manuscript or decision to submit the paper for publication.
The authors gratefully acknowledge the assistance of Asha Albuquerque and Alexandra Pletnikova for the literature review in this study, and the assistance of Dr. Eyal Kimchi for his critical review of an earlier draft of this manuscript. We are also grateful to Carrie Price, Clinical Informationist at the William H. Welch Medical Library, the Johns Hopkins University School of Medicine, for her assistance with literature search. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Lynne Morishita.
References
- 1.Inouye S, Westendrop R, Saczynski J. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hustey F, Meldon S, Palmer R. Prevalence and documentation of impaired mental status in elderly emergency department patients. Acad Emerge Med. 2000;7(10):1166–1166. [PubMed] [Google Scholar]
- 3.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 4.de la Cruz M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in comprehensive cancer center. Support Care Cancer. 2015;23(8):2427–2433. doi: 10.1007/s00520-015-2610-3. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Society; 2013. [Google Scholar]
- 6.World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. World Health Organization; 1993. [Google Scholar]
- 7.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 8.Wei L, Fearing M, Sternberg E, Inouye S. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson T, Raeburn C, Tran Z, Brenner L, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146(3):295–300. doi: 10.1001/archsurg.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Kim S, Kim J, et al. Differential associations between delirium and mortality according to delirium subtype and age: a prospective cohort study. Psychosom Med. 2015;77(8):903–910. doi: 10.1097/PSY.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 11.Borson S, Scanlan J, Benedict L, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1108–1113. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296(16):2012–2022. doi: 10.1001/jama.296.16.2012. [DOI] [PubMed] [Google Scholar]
- 14.Attia J, Hatala R, Cook DJ, Wong JG. Does this adult patient have acute meningitis? JAMA. 1999;282(2):175–181. doi: 10.1001/jama.282.2.175. [DOI] [PubMed] [Google Scholar]
- 15.Metersky ML, Williams A, Rafanan AL. Retrospective analysis: are fever and altered mental status indications for lumbar puncture in a hospitalized patient who has not undergone neurosurgery? Clin Infect Dis. 1997;25(2):285–288. doi: 10.1086/514531. [DOI] [PubMed] [Google Scholar]
- 16.Lai M, Wong TND. Intracranial cause of delirium: computed tomography yield and predictive factors. Int Med J. 2012;42(4):422–427. doi: 10.1111/j.1445-5994.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 17.Gross A, Jones R, Habtemariam D, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fick D, Steis M, Waller J, Inouye S. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8(9):500–505. doi: 10.1002/jhm.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morandi A, Davis D, Fick D, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation in patients. J Am Med Dir Assoc. 2014;15(5):349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorm A. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;(24):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage J, Brink T, Rose T, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. J Psychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Hshieh T, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reade M, Finfer S. Sedation and delirium in the intensive care unit. New England Journal of Medicine. 2014;370(5):444–454. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]
- 25.Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive Care Unit Delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125(6):1229–1241. doi: 10.1097/ALN.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman DG, Gotzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcantonio E, Ngo L, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Int Med. 2014;161(8):554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Me. 2014;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellelli G, Morandi A, Davis D, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43(4):496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tieges Z, Stiobhairt A, Scott K, et al. Development of a smartphone application for the objective detection of attentional deficits in delirium. Int Psychogeriatr. 2015;27(8):1251–1262. doi: 10.1017/S1041610215000186. [DOI] [PubMed] [Google Scholar]
- 32.Steis MR, Evans L, Hirschman KB, et al. Screening for delirium using family caregivers: convergent calidity of the Family Conufusion Assessment Method and interviewer-rated Confusion Assessment Method. J Am Geriatr Soc. 2012;60(11):2121–2126. doi: 10.1111/j.1532-5415.2012.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodius-Meester HF, van Campen JP, Fung W, Meagher DJ, van Munster BC, de Jonghe JF. Development and validation of the Informant Assessment of Geriatric Delirium Scale (I-AGeD): recognition of delirium in geriatric patients [in Dutch] Tijdschr Gerontol Geriatr. 2013;44(5):206–214. doi: 10.1007/s12439-013-0028-2. [DOI] [PubMed] [Google Scholar]
- 34.Salih SA, Paul S, Klein K, Lakhan P, Gray L. Screening for delirium within the interRAI acute care assessment system. J Nutr Health Aging. 2012;16(8):695–700. doi: 10.1007/s12603-012-0074-4. [DOI] [PubMed] [Google Scholar]
- 35.O’Regan NA, Ryan DJ, Boland E, et al. Attention! a good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85(10):1122–1131. doi: 10.1136/jnnp-2013-307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voyer P, Champoux N, Desrosiers J, et al. Recognizing acute delirium as part of your routine [RADAR]: a validation study. BMC Nurs. 2015;14(19):19. doi: 10.1186/s12912-015-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin HS, Eeles E, Pandy S, Pinsker D, Brasch C, Yerkovich S. Screening in delirium: a pilot study oftwo screening tools, the Simple Query for Easy Evaluation of Consciousness and Simple Question inDelirium. Australas J Ageing. 2015;34(4):259–264. doi: 10.1111/ajag.12216. [DOI] [PubMed] [Google Scholar]
- 38.Shulman RW, Kalra S, Jiang JZ. Validation of theSour Seven Questionnaire for screening delirium inhospitalized seniors by informal caregivers and untrained nurses. BMC Geriatr. 2016;16(1):44. doi: 10.1186/s12877-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD Group. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Chem Lab Med. 2003;41(1):68–73. doi: 10.1515/CCLM.2003.012. [DOI] [PubMed] [Google Scholar]
- 40.De J, Wand AP. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist. 2015;55(6):1079–1099. doi: 10.1093/geront/gnv100. [DOI] [PubMed] [Google Scholar]
- 41.Neufeld KJ, Leoutsakos JS, Sieber FE, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br JAnaesth. 2013;111(4):612–618. doi: 10.1093/bja/aet167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chester JG, Beth Harrington M, Rudolph JL VA DeliriumWorking Group. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J HospMed. 2012;7(5):450–453. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morandi A, Han JH, Meagher D, et al. Detecting delirium superimposed on dementia: evaluation of the diagnostic performance of the Richmond Agitation and Sedation Scale. J Am Med Dir Assoc. 2016;17(9):828–833. doi: 10.1016/j.jamda.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-Revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 45.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain SymptomManage. 1997;13(3):128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 46.Vasunilashorn SM, Marcantonio ER, Gou Y, et al. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. J Gen Intern Med. 2016;31(10):1164–1171. doi: 10.1007/s11606-016-3671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheffer AC, van Munster BC, Schuurmans MJ, de Rooij SE. Assessing severity of delirium by the Delirium Observation Screening Scale. Int J Geriatr Psychiatry. 2011;26(3):284–291. doi: 10.1002/gps.2526. [DOI] [PubMed] [Google Scholar]
- 48.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J AmGeriatr Soc. 2014;62(3):518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neufeld KJ, Nelliot A, Inouye SK, et al. Delirium diagnosis methodology used in research: a survey-based study. Am J Geriatr Psychiatry. 2014;22(12):1513–1521. doi: 10.1016/j.jagp.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: a longitudinal nested case-control study. Biol Psychiatry. 2017;81(2):145–153. doi: 10.1016/j.biopsych.2016.03.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu TM, Loh NK, Tan NC. Clinical risk factors for non-convulsive status epilepticus during emergent electroencephalogram. Seizure. 2013;22(9):794–797. doi: 10.1016/j.seizure.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Sutter R, Ruegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332–1341. doi: 10.1212/WNL.0000000000002023. [DOI] [PubMed] [Google Scholar]
- 53.Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118(4):809–817. doi: 10.1213/ANE.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan MT, Cheng BC, Lee TM, Gin T CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 55.Wildes TS, Winter AC, Maybrier HR, et al. Protocol for the Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open. 2016;6(6):e011505. doi: 10.1136/bmjopen-2016-011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J AmColl Surg. 2015;220(2):136–148. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 58.Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? a systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196–204. doi: 10.1093/ageing/afu173. [DOI] [PubMed] [Google Scholar]
- 59.Akunne A, Davis S, Westby M, Young J. The cost-effectiveness of multi-component interventions to prevent delirium in older people undergoing surgical repair of hip fracture. Eur J Orthop Surg Traumatol. 2014;24(2):187–195. doi: 10.1007/s00590-013-1170-9. [DOI] [PubMed] [Google Scholar]
- 60.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldberg S, Bradshaw L, Whittamore K, Gladman J, Harwood R. Comparison of a specialist Medical and Mental Health Unit with standard care for older people with delirium and dementia admitted to a general hospital: a randomized controlled trial (NIHR TEAM trial) Eur Geriatr Med. 2013;4(suppl 1):S173. [Google Scholar]
- 62.Cheong CY, Tan JA, Foong YL, et al. Creative music therapy in an acute care setting for older patients with delirium and dementia. Dement Geriatr Cogn Dis Extra. 2016;6(2):268–275. doi: 10.1159/000445883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kratz T, Heinrich M, Schlaus E, Diefenbacher A. Preventing postoperative delirium. Dtsch Arztebl Int. 2015;112(17):289–296. doi: 10.3238/arztebl.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clegg A, Siddiqi N, Heaven A, Young J, Holt R. Interventions for preventing delirium in older people in institutional long-term care. Cochrane Database Syst Rev. 2014;(1):CD009537. doi: 10.1002/14651858.CD009537.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Gagnon P, Allard P, Gagnon B, Merette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187–194. doi: 10.1002/pon.1881. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Gil T. Interventions for preventing delirium in older people in institutional long-term care. Int J Nurs Stud. 2016;55:133–134. doi: 10.1016/j.ijnurstu.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Fukata S, Kawabata Y, Fujisiro K, et al. Haloperidol prophylaxis does not prevent postoperative delirium in elderly patients: a randomized, open-label prospective trial. Surg Today. 2014;44(12):2305–2313. doi: 10.1007/s00595-014-0859-7. [DOI] [PubMed] [Google Scholar]
- 68.Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987–997. doi: 10.1097/ALN.0b013e31825153cc. [DOI] [PubMed] [Google Scholar]
- 69.Vochteloo AJ, Moerman S, van der Burg BL, et al. Delirium risk screening and haloperidol prophylaxis program in hip fracture patients is a helpful tool in identifying high-risk patients, but does not reduce the incidence of delirium. BMC Geriatr. 2011;11:39. doi: 10.1186/1471-2318-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 71.Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694. doi: 10.1002/gps.2582. [DOI] [PubMed] [Google Scholar]
- 72.de Jonghe A, van Munster BC, Goslings JC, et al. Amsterdam Delirium Study Group. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547–E556. doi: 10.1503/cmaj.140495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatta K, Kishi Y, Wada K, et al. DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 74.Ashraf JM, Schweiger M, Vallurupalli N, Bellantonio S, Cook JR. Effects of oral premedication on cognitive status of elderly patients undergoing cardiac catheterization. J Geriatr Cardiol. 2015;12(3):257–262. doi: 10.11909/j.issn.1671-5411.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lurati Buse GA, Schumacher P, Seeberger E, et al. Randomized comparison of sevoflurane versus propofol to reduce perioperativemyocardial ischemia in patients undergoing noncardiac surgery. Circulation. 2012;126(23):2696–2704. doi: 10.1161/CIRCULATIONAHA.112.126144. [DOI] [PubMed] [Google Scholar]
- 76.Oh CS, Rhee KY, Yoon TG, Woo NS, Hong SW, Kim SH. Postoperative delirium in elderly patients undergoing hip fracture surgery in the sugammadex era: a retrospective study. Biomed Res Int. 2016;2016:1054597. doi: 10.1155/2016/1054597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoppe C, Fahlenkamp AV, Rex S, et al. Feasibility and safety of xenon compared with sevoflurane anaesthesia in coronary surgical patients: a randomized controlled pilot study. Br J Anaesth. 2013;111(3):406–416. doi: 10.1093/bja/aet072. [DOI] [PubMed] [Google Scholar]
- 78.Whitlock RP, Devereaux PJ, Teoh KH, et al. SIRS Investigators. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10000):1243–1253. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 79.Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Ma L, Gao M, Guo W, Ma Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin Exp Res. 2016;28(4):729–736. doi: 10.1007/s40520-015-0492-3. [DOI] [PubMed] [Google Scholar]
- 81.Li X, Yang J, Nie XL, et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS One. 2017;12(2):e0170757. doi: 10.1371/journal.pone.0170757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dighe K, Clarke H, McCartney CJ, Wong CL. Perioperative gabapentin and delirium following total knee arthroplasty: a post-hoc analysis of a double-blind randomized placebo-controlled trial. Can J Anaesth. 2014;61(12):1136–1137. doi: 10.1007/s12630-014-0235-5. [DOI] [PubMed] [Google Scholar]
- 83.Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59(suppl 2):S282–S288. doi: 10.1111/j.1532-5415.2011.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Papadopoulos G, Pouangare M, Papathanakos G, Arnaoutoglou E, Petrou A, Tzimas P. The effect of ondansetron on postoperative delirium and cognitive function in aged orthopedic patients. Minerva Anestesiol. 2014;80(4):444–451. [PubMed] [Google Scholar]
- 85.Pesonen A, Suojaranta-Ylinen R, Hammaren E, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. Br J Anaesth. 2011;106(6):873–881. doi: 10.1093/bja/aer083. [DOI] [PubMed] [Google Scholar]
- 86.Saager L, Duncan AE, Yared JP, et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemia increases delirium after cardiac surgery. Anesthesiology. 2015;122(6):1214–1223. doi: 10.1097/ALN.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gruber-Baldini AL, Marcantonio E, Orwig D, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J AmGeriatr Soc. 2013;61(8):1286–1295. doi: 10.1111/jgs.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(suppl1):i98–i105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 89.Boockvar KS, Teresi JA, Inouye SK. Preliminary data: an adapted hospital elder life program to prevent delirium and reduce complications of acute illness in long-term care delivered by certified nursing assistants. J AmGeriatr Soc. 2016;64(5):1108–1113. doi: 10.1111/jgs.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kolanowski A, Fick D, Litaker M, et al. Effect of cognitively stimulating activities for the symptom management of delirium superimposed on dementia: a randomized controlled trial. J Am Geriatr Soc. 2016;64(12):2424–2432. doi: 10.1111/jgs.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Rompaey B, Elseviers MM, Van Drom W, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16(3):R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang J, Choi W, Ko YH, Joe SH, Han C, Kim YK. Bright light therapy as an adjunctive treatment with risperidone in patients with delirium: a randomized, open, parallel group study. Gen Hosp Psychiatry. 2012;34(5):546–551. doi: 10.1016/j.genhosppsych.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Atalan N, Efe Sevim M, Akgun S, Fazlıoğulları O, Başaran C. Morphine is a reasonable alternative to haloperidol in the treatment of postoperative hyperactive-type delirium after cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(5):933–938. doi: 10.1053/j.jvca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 94.Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care. 2015;13(4):1079–1085. doi: 10.1017/S1478951514001059. [DOI] [PubMed] [Google Scholar]
- 95.Kishi Y, Kato M, Okuyama T, Thurber S. Treatment of delirium with risperidone in cancer patients. Psychiatry Clin Neurosci. 2012;66(5):411–417. doi: 10.1111/j.1440-1819.2012.02346.x. [DOI] [PubMed] [Google Scholar]
- 96.Maneeton B, Maneeton N, Srisurapanont M, Chittawatanarat K. Quetiapine versus haloperidol in the treatment of delirium: a double-blind, randomized, controlled trial. Drug Des Devel Ther. 2013;7:657–667. doi: 10.2147/DDDT.S45575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroder Pedersen S, Kirkegaard T, Balslev Jorgensen M, Lind Jorgensen V. Effects of a screening and treatment protocol with haloperidol on post-cardiotomy delirium: a prospective cohort study. Interact Cardiovasc Thorac Surg. 2014;18(4):438–445. doi: 10.1093/icvts/ivt501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry. 2013;13:240. doi: 10.1186/1471-244X-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34–42. doi: 10.1001/jamainternmed.2016.7491. [DOI] [PubMed] [Google Scholar]
- 100.Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J AmGeriatr Soc. 2016;64(4):705–714. doi: 10.1111/jgs.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Westhoff D, Witlox J, Koenderman L, et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation. 2013;10(1):122. doi: 10.1186/1742-2094-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.