Abstract
The adult mammalian heart possesses only limited capacity for innate regeneration and the response to severe injury is dominated by the formation of scar tissue. Current therapy to replace damaged cardiac tissue is limited to cardiac transplantation and thus many patients suffer progressive decay in the heart’s pumping capacity to the point of heart failure. Nanostructured systems have the potential to revolutionize both preventive and therapeutic approaches for treating cardiovascular disease. Here, we outline recent advancements in nanotechnology that could be exploited to overcome the major obstacles in the prevention of and therapy for heart disease. We also discuss emerging trends in nanotechnology affecting the cardiovascular field that may offer new hope for patients suffering massive heart attacks.
Heart failure is a leading cause of death worldwide and its prognosis remains poor1,2. Atherosclerosis is the most common cause of myocardial infarction (MI; heart attack)3 and many patients who suffer an MI will lose a substantial portion of their myocardium. By 2030, coronary atherosclerosis is expected to cause ~12 million deaths annually through its life-threatening complications, including acute coronary syndromes such as ST-segment elevation MI and non-ST-segment elevation MI4,5. Cardiac ischemia, if not reversed promptly, initiates a cascade of irreversible events leading to cell death, regional contractile dysfunction and muscle tissue replacement by scar tissue3. The repair process after cardiac injury includes modest cardiomyocyte (CM) generation, probably mediated by the proliferation of pre-existing differentiated CMs6. However, the innate proliferative capacity of CMs is very low, and this capacity also declines significantly with age, further reducing the likelihood of regenerating the dead tissue.
Despite advances in our scientific and clinical understanding, the mortality rate among heart failure patients remains high, with 50% of those diagnosed with severe heart failure dying within five years7. Heart transplants, while effective, are limited by donor heart availability (for example, only 2,000 annual transplants versus 550,000 new cases of congestive heart failure per year in the USA)8. Current modalities to treat systolic heart failure include pharmaceuticals, surgical reconstruction and implantable devices9. However, although these conventional treatments have improved quality of life and outcomes for many patients, systolic heart failure remains a progressive disease. Thus, there is a growing need for alternative therapeutic approaches.
Given the unique physical and chemical properties of nanostructured systems, nanoscience and nanotechnology have recently demonstrated the potential to overcome many of the limitations of cardiovascular medicine through the development of new pharmaceuticals, imaging reagents and modalities, and biomedical devices. As coronary atherosclerosis is the most common cause of death globally, in this Review we cover the current state of the art in employing nanoparticulate systems either to inhibit or treat ischemic heart injuries caused by the stenosis or occlusion of coronary arteries (Fig. 1). We provide a brief overview of recent advances in the use of nanoplatforms for early detection and treatment of coronary atherosclerosis to inhibit MI. We also introduce new therapeutic opportunities in the regeneration/repair of ischemic myocardium using both nanoparticles and nanostructured biomaterials that can deliver therapeutic molecules and/or (stem) cells into hibernating myocardium. We also offer an overview of recent advances in precise in vivo imaging of transplanted cells using bacterially developed nanoparticles and explain how these findings address crucial issues in in vivo cell monitoring and facilitate the clinical translation of cell therapies. Finally, we examine the strengths and limitations of current approaches and discuss likely future trends in the application of nanotechnology to cardiovascular nanomedicine.
Figure 1. Applications of various nanoplatforms in the prevention and treatment of cardiovascular disease.
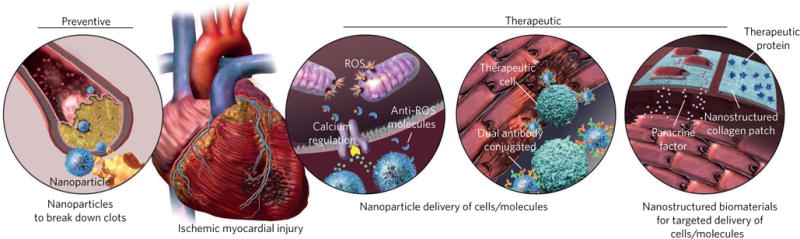
Nanoplatforms can target and break down coronary artery plaques and prevent injuries caused by stenosis or occlusion of arteries. Nanoparticulate systems can also reduce the adverse effects of reperfusion injuries and regenerate/salvage myocardium after MI, through sustained and targeted delivery of cells, biomolecules and paracrine factors.
Diagnosis and treatment of coronary atherosclerosis
Nanoparticles have demonstrated potential in both detection and removal of atherosclerotic plaques10–15. The initial stage of plaque formation is the activation of endothelial cells of artery walls (due to genetic disorders and many environmental risk factors, including biochemical stimuli and inflammation10), which attract monocytes and facilitate their migration into the intima11. The monocytes undergo maturation towards macrophages and form foam cells by substantial uptake of lipids11. In the progressive stage, extracellular matrix (ECM) macromolecules are synthesized by proliferation of several cells, including smooth muscle cells12. This newly developed matrix provides a fertile ground for lipids, cholesterol crystals and micro-vessels to form a necrotic central region and a fibrous cap on the plaque12; decreasing the thickness of the fibrous cap and increasing the size of the necrotic central core can enhance the risk of thrombosis, which may lead to MI. Therefore, the atherosclerotic plaque is a complex environment containing lipids, cholesterol crystals and many inflammatory cells such as monocytes, macrophages and foam cells, and their secreted cytokines10. Nanoparticles of different types (for example, inorganic, organic and polymers)13 designed to target these cells are promising candidates for both imaging and treatment of atherosclerotic plaques through increasing the fibrous cap thickness and decreasing the necrotic core area. With regard to imaging, such nanoparticles contain either an intrinsic imaging modality (for example, superparamagnetic iron oxide nanoparticles that enhance magnetic resonance imaging (MRI) contrast14) or imaging tags15/molecules16 (for example, isotope reporters). These nanoparticles are internalized by macrophages and/or monocytes, rendering the plaques visible to clinicians17. However, caution is necessary when imaging macrophages in plaques, as it was very recently revealed that superparamagnetic iron oxide nanoparticles change macrophages’ functionality from normal (M2) to pro-inflammatory (M1)18. As atherosclerosis is recognized as a chronic inflammatory disease, these nanoparticles may exacerbate inflammation, further damaging vessels and increasing the risk of MI. More studies are needed to confirm the potential inflammatory effect of superparamagnetic iron oxide nanoparticles.
In addition to diagnosis, nanoparticles can also deliver therapeutic biomolecules to the site of coronary atherosclerosis and shrink plaques by reducing inflammation (for example, by activation of pro-resolving pathways), and removing lipids and cholesterol crystals19,20. Polymeric materials such as poly(d-lactic acid), polyethylene glycol (PEG) and poly lactic-co-glycolic acid (PLGA) have emerged as a major class of biodegradable and controlled-release systems for delivering biomolecules/proteins to the plaque site21–23. To help resolve inflammation, nanoparticles can target and release anti-inflammatory biomolecules (for example, Ac2-26 peptide23 and interlukin-10 cytokine24) and drugs (for example, statin25). As vascular inflammation and injury can expose collagen IV21,26, anti-collagen IV ligands can be used to target moieties for nanoparticles23,24. For instance, in vivo delivery of functional interlukin-10 to atherosclerotic plaques, using anti-collagen IV-targeted PLGA–PEG nanoparticles substantially improved (increased) fibrous cap thickness while decreasing the necrotic core area in a mouse model of atherosclerosis22. Reconstituted high-density lipoprotein25 and sugar-based amphiphilic macromolecules27 can also be used on the surface of nanoparticles for targeting plaques. For example, because their charge and hydrophobicity are similar to those of oxidized lipoproteins, sugar-based amphiphilic macromolecules have become promising candidates for fabrication of nanoparticles to target scavenger receptors on the surface of macrophages27. Suppression of scavenger receptors (for example, MSR1 and CD36) on the surface of macrophages at the plaque site can block their oxidized lipid uptake and reduce inflammation by inhibiting foam-cell formation28,29.
The main limiting issue for design of safe and efficient nanoparticles for both prognosis and treatment of coronary atherosclerosis is our lack of a deep understanding of the biological identity of nanoparticles. More specifically, nanoparticles in contact with biological fluids are quickly surrounded by a layer of proteins that form what is called the protein corona, which has not yet been adequately addressed in the field of cardiac nanotechnology. The biological identity of nanoparticles is thus defined by the precise type, amount and conformation of the proteins in the corona14,30. Through nanoparticles’ interaction with biological systems including cells, their biological fate (for example, biodistribution, targeting efficacy and therapeutic efficacy) is substantially affected by the composition of the corona31,32. For example, the protein corona can shield the targeting species on the surface of nanoparticles33,34, cause mistargeting and significantly reduce efficacy in the treatment of atherosclerosis. In addition, the protein corona can change the drug-release profile of nanocarriers35 and complicate their therapeutic efficacy. Therefore, to accelerate the clinical translation of nanoparticles and nanostructured materials for use in cardiac nanotechnology, their biological identities must be precisely assessed and reported. Furthermore, new strategies (for example, use of zwitterionic coatings36 and controlling corona structure37) should be developed to diminish any obstructive effect of the protein corona in targeting and drug release. Last but not least, careful attention should be given to the possible role of nanoparticles in inducing conformational changes in some important proteins with inflammatory roles, such as fibrinogen, which have the capacity to activate the integrin receptor and increase the activity of the nuclear factor (NF)-κB signalling pathway, which leads to the release of inflammatory cytokines38. Such phenomena may further increase the immune complexity of atherosclerotic plaques, substantially increasing the risk of plaque rupture and consequent MI.
Cell therapy for salvage and regeneration of heart tissue
Over the past decade, the majority of efforts in myocardial regeneration have been centred on cell-based cardiac repair39–42. Multiple cell types including bone-marrow-derived mononuclear stem cells, mesenchymal stem cells (MSCs), endothelial progenitors, induced pluripotent stem cells (iPSCs) and iPSC-derived CMs have been employed to remuscularize the injured heart43. However, clinical translation of such approaches suffers from four major limitations: (1) poor engraftment and electromechanical coupling of the therapeutic cells in heart tissue39; (2) lack of robust, accurate and safe in vivo monitoring of the therapeutic cells44; (3) potential arrhythmic complications45; and (4) lack of methodologies to produce large numbers of patient-specific mature and functional CMs for replacing cells lost through infarction. It is noteworthy that the lack of control over the cells following transplantation is another substantial issue in the field that completely depends on the biological milieu. One theory is that most cells currently being used do not differentiate to new heart cells but instead elicit paracrine effects. Even if cells do not generate new CMs, engraftment for some period of time may be useful for their secretion of paracrine factors. In addition, accurate methods are necessary to assess the engraftment of the therapeutic cells and assess their in vivo therapeutic efficacy and side effects (for example, teratoma formation)46.
Poor engraftment of therapeutic cells into the myocardium is caused at least partially by the immune reaction to the exogenously transplanted cells39. To resolve this issue, the use of autologous CMs has been proposed. Patient-specific CMs could be made by reprogramming patient cells (for example, monocytes and fibroblasts) to iPSCs, followed by their differentiation to CMs using a well-defined chemical approach47. Although patient-specific CMs could help salvage the pre-infarcted area, their therapeutic effects might still be limited by their poor contact with endogenous CMs, limiting their electromechanical integration48–50. Another reason for the poor engraftment of therapeutic cells could be the lack of proper space in the dense myocardial tissue, which is a fairly toxic environment for the therapeutic cells. In the rest of this section, we discuss how nanoparticles might address the four major limitations in the clinical translation of cell therapy identified above.
Nanoparticles could substantially help overcome the limitations of patient-specific therapeutic cells by targeting the injured portion of the myocardium. Besides engraftment and electromagnetic coupling of the therapeutic cells in heart tissue, it is now well accepted that the release of paracrine factors by therapeutic cells is a factor in improving ventricular function after cell therapy51,52. Therefore, targeted delivery of transplanted therapeutic cells to injured CMs could significantly enhance their therapeutic efficacy; dual-antibody-conjugated superparamagnetic iron oxide nanoparticles have been proposed (Fig. 2)48.
Figure 2. Dual-antibody-conjugated magnetic nanoparticles target therapeutic cells and regenerate the injured myocardium.
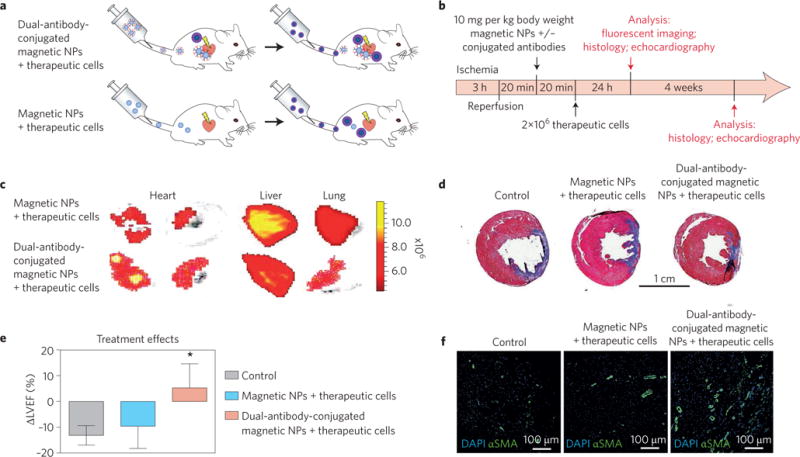
a,b, Schematic showing the injection of the magnetic nanoparticles (NPs) with and without conjugated antibodies in vivo. c, Fluorescent imaging of the rat’s organs after injection of the therapeutic cells (rat bone marrow mononuclear cells labelled with a fluorescent dye) showing their targeted accumulation in the hearts of animals that received the dual-antibody-conjugated magnetic nanoparticles, unlike those that received regular magnetic nanoparticles. There are significantly fewer off-targeted therapeutic cells (for example, in lung) in animals that received dual-antibody-conjugated magnetic nanoparticles compared with the regular magnetic nanoparticles. d, Image of the whole heart section (trichrome staining) showing the significant reduction in scar size (blue) and substantial enhancement of viable (red) tissues in animals that received dual-antibody-conjugated magnetic nanoparticles compared with the regular magnetic nanoparticles and control sample (no therapeutic cells), 4 weeks post reperfusion. e, Echocardiography results, 4 weeks post reperfusion, revealed significant improvement in left ventricular ejection fraction (LVEF) in the targeted-nanoparticle group compared with the regular nanoparticle and control groups. f, Confocal microscopic images showing a significant effect of nanoparticle-targeted cell therapies on angiogenesis compared with the regular nanoparticle and control groups. Blue represents DAPI for cell nuclei and green is alpha smooth muscle actin (αSMA) for smooth muscle cells. Figure adapted from ref.48, Macmillan Publishers Ltd.
Each magnetic nanoparticle is conjugated to an antibody (anti-CD45) specific to therapeutic cells (that is, bone-marrow-derived stem cells expressing CD45) and to an antibody (anti-CD34) to injured CMs (CD34-positive cells). This theranostic technology may have the capacity to track the delivery of nanoparticle-labelled therapeutic cells to injured CMs in the pre-infarcted area to evaluate their therapeutic potential in vivo using MRI. This nanoplatform has demonstrated promising therapeutic efficacy in not only delivery of cells to the desired portion of the heart but also enhancement of heart function including left ventricular ejection fraction (Fig. 2)48. However, the main drawback of this approach in a clinical set-up may be the substantially low percentage (around 1%) of the injected cells that reach the target site.
Another strategy for site-specific delivery of therapeutic cells is the use of nanoplatforms to create cells with improved therapeutic potential. As an example, magnetic nanoparticles were shown to accelerate expression of critical gap junction proteins (for example, connexin 43) in cardiomyoblasts53. Co-culturing of these cardiomyoblasts (which have more gap junction proteins than regular cardiomyoblasts) with MSCs substantially increased their crosstalk, leading to the formation of cells with improved therapeutic potential. These new cells demonstrated higher levels of both engraftment capacity and desirable paracrine factors (for salvaging the hibernated cells in the pre-infarcted area of myocardium) compared with conventional therapeutic cells53. Injection of the improved therapeutic cells into the pre-infarcted area in rats substantially enhanced heart function and significantly reduced scar size compared with rats that received conventional therapeutic cells.
In addition to the targeted delivery of cells using dual-antibody-labelled nanoparticles, another strategy is to label the therapeutic cells with magnetic nanoparticles and retain them at the injection site using external magnetic force54,55. As an example, human cardiosphere-derived stem cells were safely labelled with magnetic nanoparticles (that is, ferumoxytol), injected into the pre-infarcted lesions of rat myocardium, and their restorative and therapeutic effects on myocardium were monitored in the presence and absence of an external magnetic field54. The magnetic field significantly increased cardiac retention and engraftment of transplanted therapeutic cells, leading to the improvement of left ventricular ejection fraction without any trace of cardiac inflammation or iron overload. There is therefore reason to hope that the local delivery of therapeutic cells via dual-antibody-conjugated iron oxide nanoparticles to the injured CMs and enhancement of their restoration with an external magnetic field might become an optimal treatment platform for cardiac injury.
The majority of the current literature on the use of nanotechnologies for enhancing the engraftment of therapeutic cells in heart tissue is focused on superparamagnetic iron oxide nanoparticles. This is mainly because of their biocompatibility and capacity for simultaneous imaging and targeting, using both an external magnetic field and targeting moieties on their surface. However, as one recent report revealed that these nanoparticles increase tumour-associated macrophage activation18, more studies are needed to investigate their potential role in exacerbating myocardial inflammation, which might increase the risk of therapeutic cell rejection by the activated immune system.
Although magnetic nanoparticles (and particularly superparamagnetic iron oxide nanoparticles) demonstrate great promise in both delivery of cells into the desired part of the myocardium and imaging of viable therapeutic cells14,56, recent reports reveal that their signal persists in the myocardium even weeks after the disappearance of the therapeutic cells, causing errors in the assessment of therapeutic function57,58. For instance, ferumoxide (commercially available superparamagnetic iron oxide nanoparticles) was used to label stem cells but remained in the myocardium weeks after disappearance of the cells, as confirmed by bioluminescence imaging58–60. To overcome this major issue, the use of a living contrast agent, magneto-endosymbionts (MEs) derived from magnetotactic bacteria, has been proposed to safely and effectively label and precisely monitor CMs obtained through the chemically defined approach (Fig. 3)44. To assess the possible toxicity of the MEs to other organs, the highest doses (5.0 × 109) were injected by both intravascular and intramyocardial routes in mouse models, and the concentrations of multiple markers together with the histological examination of vital organs (for example, liver, kidney, spleen, heart and pancreas) and key indices of infection and toxicity in blood (white blood cells, red blood cells and platelet count) were carefully probed (examples are shown in Fig. 3d,e). The results suggest that the live contrast agents are unlikely to trigger an in vivo immune response. Using MRI and bioluminescence imaging to track the viability of genetically modified bioluminescence cells with a firefly luciferase (FLuc) reporter gene, it was found that the MEs cleared within one week of cell death, while the superparamagnetic iron oxide nanoparticles remained for more than two weeks after cell death (Fig. 3f–h)57. These findings suggest that MEs can be considered as robust and effective biological contrast agents to track viable therapeutic cells in an in vivo model and can accelerate the clinical translation of in vivo MRI monitoring.
Figure 3. The use of a living contrast agent, MEs derived from magnetotactic bacteria, for safe labelling and precise monitoring of CMs.
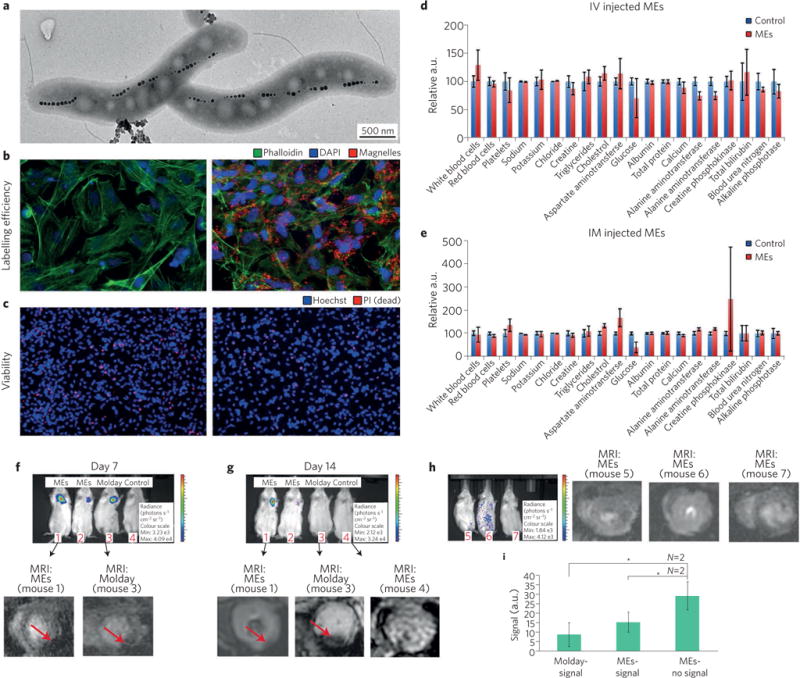
a, Transmission electron microscopy image of the magnetosome structure. b, Fluorescent images of the unlabelled (left panel) and ME-labelled (right panel) iPSC-derived chemically defined CMs demonstrating internalization of MEs in the CMs; red, green and blue colours show the MEs, cell membrane and cell nucleus, respectively. c, Propidium iodide (PI) viability assay demonstrates the safe labelling of the MEs (left and right panels show unlabelled and ME-labelled CMs, respectively). Blue colour shows the cell nucleus; red colour denotes dead cells. d,e, Intravascular (IV; d) and intramyocardial (IM; e) injection of the MEs showing no signs of infection or blood toxicity, as there were no substantial changes in the relative concentrations of liver and spleen enzymes. a.u., arbitrary units. f, Representative whole-body bioluminescence images of mice that received the phosphate-buffered saline control and CMs labelled either with ME or the magnetic nanoparticles (Molday) after 7 d. g, Bioluminescence images of the mice 14 d after cell injection (top) and corresponding in vivo MRI images of the murine hearts (bottom); the signal from the injected labelled cells is denoted by red arrows. h, Representative bioluminescence images of mice with dead CMs at day 14 of treatment with ME-labelled cells and the corresponding in vivo MRI images. i, Comparison of signal intensity for Molday (dark signal) and ME (no signal), showing significantly lower signal of ME*-labelled dead cells compared with other samples with positive signal (P < 0.05). Figure adapted from ref. 44, Macmillan Publishers Ltd.
One of the other important clinical challenges of cell therapy, even if therapeutic cells integrate with the host tissue, is the potential for arrhythmic complications, which has been observed in non-human primate hearts45. Local delivery of antiarrhythmic drugs, using nanocarriers, can be an effective strategy to overcome the potential arrhythmic issue. The nanocarriers may also substantially diminish the toxicity of antiarrhythmic drugs (for example, amiodarone, which demonstrated pulmonary toxicity). For example, amiodarone-loaded liposomes were shown to substantially overcome lethal arrhythmia issues in a rat model and substantially reduce the negative haemodynamic changes elicited by free amiodarone61. As the role of antiarrhythmic drugs in non-human primate hearts has not yet been reported, the field will benefit from future studies in this area.
Large numbers of patient-specific mature and functional CMs will be needed not only for replacing cells lost through infarction but also for cardiotoxicity screening of drugs. The importance of using patient-specific mature CMs for cardiotoxicity screening is that personalized medicine has firmly established that a particular cytotoxic agent can cause cardiotoxicity in one patient but not another62,63. As extracting large numbers of cardiac cells from a patient’s heart tissue is generally unfeasible, scientists have searched for other approaches to create mature patient-specific CMs, and many laboratories are developing such approaches. Culturing such CMs is of great interest not only because of their applications in cell therapy but also owing to their considerable potential in the patient-specific study of heart disease64,65. Human-iPSCs (hiPSCs) have created unique opportunities in cardiology; specifically, CMs differentiated from these stem cells have excellent potential in the study of heart disease and can model patient-specific myocardial physiology in vitro47,66,67. However, the substantial structural and functional differences between hiPSC/MSC-derived CMs (derived from existing chemically defined approaches) and adult mature CMs pose major challenges for precise drug screening and highly efficient therapeutic applications65,68–71.
To overcome these major hurdles, scientists have employed several techniques (for example, microfabrication, long-term culture, three-dimensional (3D) tissue engineering, mechanical loading, electrical stimulation, modulation of substrate stiffness and treatment with neurohormonal factors) to engineer hiPSC-derived CMs whose properties and functions match those of fully matured CMs72. In addition, nanopatterned substrates have been widely used to encourage maturation of chemically defined CMs73–76. However, new methods proposed thus far have not yielded highly mature, patient-specific CMs on a scale sufficient to meet clinical needs77. Moreover, compared with immature cells, functional and mature CMs release more therapeutic paracrine factors, which are believed to salvage hibernating cells in the pre-infarcted area of myocardium39,78.
Recent developments in the field include new approaches to physically differentiate stem cells into a wide variety of fully matured cell types79. For example, bioinspired cell-imprinted substrates have shown success in reliable and efficient control of MSCs’ differentiation toward chondrocytes80 and keratinocytes81. It has also been demonstrated that cell-patterned substrates modulate the differentiation, re-differentiation and trans-differentiation of a variety of cells82. Therefore, one exciting opportunity in the field is to develop nanopatterned substrates (for example, cell-imprinted substrates with the exact shape and topography of mature CMs) to physically direct the differentiation and maturation of patient-specific CMs. The success of such an approach could signal the advent of a sustainable source of functional cells for both precise drug screening and cell-therapy applications. We believe that nano-patterned, pseudo-3D tissue-culture substratum with cell shapes could lead to the eventual production of scalable quantities of highly mature patient-specific CMs, which could be used in many applications including cardiotoxicity screening and myocardial salvage/regeneration.
The main clinical translation challenge with mass-produced mature functional CMs is high immune rejection, mainly due to the allogeneic nature of these cells to recipient patients39. The use of patient-specific cells and persistent systemic immune suppression have been proposed to delay rejection of therapeutic cells39,83. In addition, mimicking evolutionary changes in cancer cells for evading the immune system, using immune checkpoints (for example, cytotoxic T lymphocyte antigen 4 (CTLA4), programmed death ligand-1 (PD-L1) and indoleamine 2,3-dioxygenase (IDO))84, has recently been proposed to overcome immunogenicity issues in therapy for myocardial regeneration85. For example, it was shown that human embryonic stem cells with the capability to express CTLA4 and PD-L1 before and after differentiation (for example, towards CMs) are immune protected in humanized mice. IDO, another important immune checkpoint, permits tumour cells to escape the immune system by depletion of L-tryptophan in the microenvironment of cells86. In this case, transfection of the CMs to produce IDO on the surface of CMs may also help therapeutic cells to evade the immune system and substantially increase their therapeutic efficacy. In addition to the transfection approach, development of new nano-platforms that facilitate depletion of L-tryptophan in the microenvironment of the therapeutic cells may substantially enhance cell survival. The proposed approaches could accelerate the clinical translation of mass-produced therapeutic cells, optimizing their efficacy.
Delivery of therapeutic molecules to CMs
Current approaches to repair the myocardium after stenosis of coronary arteries (for example, angioplasty) or MI (for example, coronary artery bypass grafting) can induce a complex of reperfusion injuries, including production of reactive oxygen species (ROS), alterations in intracellular calcium trafficking, dysfunction of both microvascular and endothelial cells, alteration of myocardial metabolism, and activation of the immune system87,88. Reducing such injuries could substantially enhance the therapeutic efficacy of current approaches. To salvage heart tissue after reperfusion injury, a biomolecular therapy approach was proposed. Sustained and precise delivery of biomolecules (for example, drugs, growth factors, short interfering RNA, antioxidants and immunosuppressants) to the myocardium can diminish the catastrophic effects of re-perfusion injury. However, successful delivery to the injured part of the myocardium remains elusive.
Nanoparticles demonstrate great potential for delivering therapeutic agents specifically to the ischemic injured heart, although they accumulate mainly at pre-infarcted areas rather than the diseased tissue. Recognition of specific receptors on the surface of injured CMs (which are located mainly in pre-infarcted areas) is the first essential step for designing highly efficient actively targeted nanoparticles. The overexpression of CD34 and angiotensin II type 1 (AT1) receptors on the surface of injured CMs has become a promising target in the design of nanoparticles. For example, anti-CD34-conjugated superparamagnetic iron oxide nanoparticles48 or targeted liposomes (with a ligand specific to AT1)89 demonstrated a strong ability to target the injured part of the myocardium in vivo. As ischemic injury causes disruption of endothelial barriers, nanoparticles with long blood circulation time can more easily cross the endothelial barrier and accumulate in the infarcted area. The mechanism is similar to the enhanced permeability and retention effect90–93, which is the foundation of nanoparticle delivery to several types of tissue including solid cancer tumours and inflammation sites24,94. Therefore, for passive delivery of biomolecules, nanoparticles with highly beneficial pharmacokinetics (for example, polymeric nanoparticles95 and liposomes96) have superior therapeutic efficacy, mainly due to their long blood circulation compared with other nanoparticles. There are two major issues that should be addressed in future studies: (1) as only a low percentage of the injected nanoparticles can pass through the coronary arteries, the targeting capabilities of these particles to the heart tissue should be precisely defined; and (2) the effect of the protein corona on the in vivo release kinetics of the payloads should be characterized. Addressing these critical issues will help scientists design safe and efficient dosage of nanoparticles for biomolecular delivery applications.
Besides cardiac ischemic disease, nonischemic cardiomyopathies are important causes of heart failure, such as those induced in cancer patients by chemotherapy. Cardiotoxic agents can significantly affect adult CMs in a patient-specific manner63,97. Nanoparticles also demonstrate great potential for diminishing the biodistribution of drugs to cardiac tissue and thus eliminate cardiac toxicities associated with many types of active agent. Widely used drugs such as classic chemotherapeutic agents, chemoprevention agents (for example, cyclooxygenase-2 inhibitors), monoclonal antibodies intended to target tyrosine kinase receptors and small-molecule tyrosine kinase inhibitors have been shown to be cytotoxic to CMs. Personalized-medicine approaches have firmly established that a particular cytotoxic agent can cause cardiotoxicity in one patient but not another62,63. New therapeutic approaches (for example, combination therapy, which decreases the likelihood of resistance) can actually exacerbate the cardiotoxicity of these drugs in some patients98. With the use of nanocarriers for targeted delivery of toxic drugs to tumour cells, with negligible penetration of heart tissue, the cytotoxicity problem can be substantially diminished. As there are very few reports to support this hypothesis99, studies comparing the induced cardiotoxicity of conventional chemotherapy and nano-chemotherapy would be of great interest to the field.
Given the recent findings that some nanoparticles (for example, TiO2) can affect the functionality of endothelial cells’ adherens junction protein (that is, VE-cadherin) and cause cell leakiness100, nanoparticles with specific physicochemical properties that avoid inducing damage to the vessels’ endothelial cells and cardiotoxicity should be designed to benefit cancer patients.
Nanostructured scaffolding strategies for myocardial repair
As a bioartificial ECM, cardiac tissue scaffolds are engineered to interact optimally with cardiac cells during their gradual degradation and neotissue formation101. A variety of nanobiomaterials have been used to recapitulate the nanoscale features of the native ECM102. In comparison with conventional tissue-engineering scaffolds, nanostructured biomaterials (for example, nanofibre/tube and nanoporous scaffolds) offer more biomimetic structural and physiomechanical cues, enhancing protein (molecular) and cellular interactions. Such an ECM-resembling nano-niche therefore provides more efficient tissue-regenerative opportunities102. A growing body of research has explored the development of multiple cyto-compatible nano-scaffold systems, with or without cells and/or other macromolecules, as culture models to mimic the tissue complexity in vitro or for drug-delivery and tissue-engineering applications in vivo.
In cardiovascular medicine, bioengineered scaffolding systems are increasingly being investigated as alternative platforms for targeted delivery of cells/therapeutics or as cardiac ‘patches’ for myocardial tissue repair103,104. The use of cardiac tissue scaffolds, however, has been hindered by poor cardiac cell viability and function (for example, electrical conductivity and contractility), lack of appropriate cells and/or molecules in the patch to guide tissue regeneration, insufficient perfusion/permeability within the 3D construct, and inadequate ultrastructural and mechanical properties105. In dynamic culture of cellular scaffolds in bioreactors in vitro, or by connecting the scaffold to the native blood vessel network in animal models, researchers have found powerful tools to enhance mass transfer, cardiac cell viability, maturation and function. A variety of physiochemical modifications (for example, compression/consolidation106 or crosslinking of hydrogels107) and microfabrication processes108 have been used to improve tissue architecture, stiffness and cellular anisotropy in cardiac scaffolds. Incorporating nanostructured features into these bioengineered 3D cardiac matrices can further enhance their conductivity and mechanical and adhesive properties, as well as direct the organization, morphogenesis and functionality of cardiac cells109–111.
One major drawback of porous cellular constructs as engineered myocardial scaffolds is the limited intercellular connection and electrical signal propagation due to the isolating pore walls112. Such conduction disturbances can induce cardiac arrhythmias following implantation of the scaffold onto the myocardial tissue. Inorganic nanostructures, such as gold nanowires with negligible cytotoxicity, can interact with CMs to create electronic interfaces and enhance cellular excitability113,114. Incorporating gold nanowires in 3D biopolymeric (alginate) scaffolds has been recently demonstrated to bridge the non-conducting pore walls, allowing nanocomposite cardiac matrices to be engineered with remarkably improved electrical communication, cellular alignment, tissue integration and synchronous contractile function102. These cellular nano-constructs could be further used as cardiac patches in vivo to treat ischemic heart injuries at much lower risk of patch-induced cardiac arrhythmias115,116.
Existing cardiac patch systems lack the capability to sense or stimulate resident cardiac cells. A new generation of 3D nanocomposite cardiac scaffolds, engineered by incorporating micro-electrodes into the nanocomposite fibres of polycaprolactone–gelatin matrices, now allows remote and non-destructive recording of the contractile activity of encapsulated cardiac cells60. These nano-patches also enable on-demand electrical stimulation of synchronous contraction of cardiac cells, as well as the release of therapeutics. This technology could be used as a ‘self-regulating’ nanobioengineering tool to remotely monitor the cardiac tissue’s condition and regeneration progress in situ, and to selectively trigger or stop various cellular or molecular processes underlying cardiac repair.
Although cell-based approaches to cardiac tissue engineering have made significant advances, a number of major challenges and limitations remain, including problems involving transplanted cell sources, viability and phenotype stability, as well as regulatory challenges117. Acellular biomaterials are evolving as alternatives to repair ischemic heart injury via delivery of therapeutics and/or stimulation of the native (endogenous) regenerative mechanisms68, along with basic physiomechanical support118. The use of a nano-fibrillar collagen type I-based cardiac patch as an epicardial delivery device was recently reported to regenerate adult mammalian heart tissue following MI (Fig. 4)68. Mimicking the stiffness of embryonic epicardium6, the engineered acellular patch was used for targeted delivery of cardiogenic follistatin-like 1 (FSTL1) protein into the ischemic tissue. The application of a human FSTL1-laden nanostructured patch stimulated cell cycle re-entry and proliferation of native CMs, restoring the structure and function of damaged myocardial tissue in mouse and swine models of MI (Fig. 4).
Figure 4. Application of nanostructured cardiac patch device in repair/regeneration of MI.
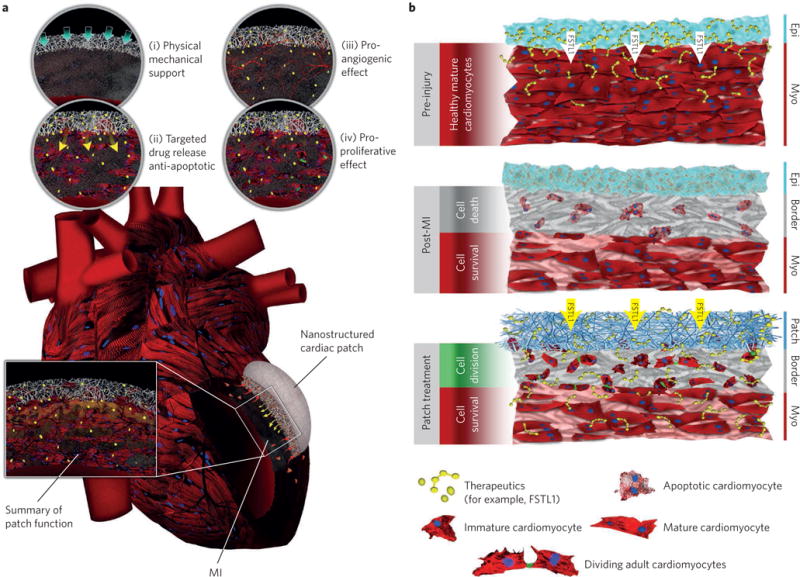
a, Different scenarios by which the engineered nanostructured scaffold can contribute to myocardial tissue repair following MI: (i) inhibition of adverse cardiac remodelling processes post-MI via physical mechanical support; (ii) targeted delivery and sustained release of anti-apoptotic factors; (iii) promoting angiogenesis (via delivery of angiogenic factors); and (iv) promoting proliferation of pre-existing native CMs (for example, through epicardial delivery of human FSTL1 peptide). b, Schematic demonstration of cellular and molecular processes in healthy (top), post-MI (middle) and patch-treated (bottom) heart tissue. The bottom panel delineates the role of a nanostructured cardiac patch in regenerating the damaged myocardium by inducing cell cycle re-entry among pre-existing CMs.
As the field of tissue engineering evolves, more attention is being given to the development of alternative biofabrication strategies to control the nano-scaffold 3D architecture in a more reproducible and patient/tissue-specific manner119. Examples include 3D bio-printing and nanoprinting technologies that use computer-assisted layer-by-layer deposition (that is, additive manufacturing) to create 3D structures with submicrometre resolution120. 3D bioprinting of cardiac patches starts with acquiring a 3D computer-aided design of the target tissue/organ using medical imaging modalities such as MRI, computerized tomography scanning, or echocardiography. A bioink is prepared by encapsulating cardiac cells (for example, CMs, endothelial and smooth muscle cells, and stem/progenitor cells) and/or support molecules (for example, growth factors) in a biomimetic hydrogel. A bioprinter then uses the digital computer-aided design model to deposit the cardiac bioink into a predefined 3D architecture. Bioprinted constructs (patches) will be subsequently cultured in vitro for further growth and maturation of cardiac tissue, and tested in vitro (for example, for disease modelling or drug screening) or as a heart tissue graft in vivo121. The delivery of printed patches onto damaged heart tissue has been primarily tested via a left thoracotomy approach and direct transplantation of the patch onto the epicardial surface of the heart122.
Current attempts at ‘writing’ with electrospun nanofibres also offer great promise in direct assembly of 3D cardiac tissue templates with customized structural hierarchy and cellular organization123. Delivery of other paracrine/cardioprotective factors (for example, micro or small interfering RNAs) may further improve the regenerative function of nanostructured patches by regulating cellular and molecular mechanisms underlying ischemic heart injury. However, bioengineering nano-scaffold systems that allow targeted transmission of physiomechanical and biochemical cues to the damaged cardiac cells to activate/amplify the regeneration processes still remains a challenge. Designing and developing new nanobiomaterial hybrid systems with superior biomimetic composition and structure to achieve significant engraftment to the heart tissue is an essential future goal in this field124.
Challenges in designing nanoparticles for clinical applications
Despite the enormously large and rapidly growing arsenal of nanoparticle technologies developed to date, few have reached clinical development and even fewer have been approved for clinical use (reviewed in refs 94,125). This is in part attributed to the challenges associated with controllable and reproducible synthesis of nanoparticles using processes and unit operations that allow for scalable manufacturing required for clinical development and commercialization. Nanoparticles also encounter unique physiological barriers in the body as compared with small molecule drugs with regard to systemic circulation, access to tissue and intra-cellular trafficking126. The regulatory considerations for nanoparticle development may create additional challenges beyond what is encountered for most small molecule drugs. What is increasingly clear is that the physicochemical identity of nanoparticles affects the composition of the protein corona (that is, biological identity of nanoparticles) at the nanobiointerfaces, underscoring the importance of reproducible nanoparticle synthesis127. We and others have revealed several previously underappreciated factors (for example, slight temperature changes128, local temperature changes in the hyperthermic nanoparticles129, plasma concentration130 and disease type94,131) that can affect the composition of the protein corona and potentially make the clinical development of nanoparticles more complicated than previously anticipated. We believe that inter- and intra-patient variation in blood plasma composition and the resulting variability in the biological identity of nanoparticles should be carefully considered as part of the development strategy that may guide personalization of therapy. So far, almost all the current literature in the field of protein coronas has overlooked the contribution of these factors (and more specifically the effect of human diseases and their stages) to the biological identity of nanoparticles. This has led to some inconsistencies among findings, including: (1) the considerable variability in the protein corona formed on the same nanoparticle; (2) a substantial gap between in vitro and in vivo readouts; (3) differences between therapeutic and targeting efficacies of the same nanoparticles in different media; and (4) unsuccessful clinical outcomes of nanoparticles with positive in vitro and in vivo outcomes. This may be one of the main reasons why, despite numerous advances in nanomedicine, few nanoparticles have reached clinical trials, and even fewer have reached clinical practice94,132.
To overcome these issues, a large body of work remains to be carried out to shed light on variations in the biological identity of the exact same nanoparticles in different disease stages, and to define how these changes in biological identity may actually change their biological fates during disease progress. New mathematical modelling (such as quantitative structure–activity relationship models133,134) should be developed to help scientists apply the current literature on protein corona to predict the biological identity of nanoparticles in different diseases and disease stages. We believe that such efforts will allow scientists to better understand and control the biological identity of nanoparticles in the design of safer and more effective nanoparticles (in a disease-specific manner), and substantially accelerate clinical translation.
As nanoparticles are increasingly being used in the diagnosis and treatment of cardiac diseases, their potential cardiotoxicity should be examined in detail. Their potential toxicity for cardiac tissue and heart function is of crucial importance for the safety of such nanoparticles. Intensive studies have thoroughly probed the toxicities of a wide range of nanoparticles (organic, inorganic and polymeric) in different types of cell and organ135,136. However, the cardiotoxicity of nanoparticles has been poorly investigated, and data are still limited to a few types of nanoparticle including metal oxides, silver and carbon137. New findings on the critical role of personalized and disease-specific protein corona in the toxicity of nanoparticles add further complexity to their in vivo cardiotoxicity. Therefore, intensive research should be performed to deepen our understanding of the possible cardiotoxic effects of therapeutic nanoparticles on both the molecular (for example, cardiac genetics such as α-actinin, cardiac troponin and myosin light chain) and phenotypic (for example, beat rates and Ca2+ transient measurements) scales.
Conclusion and future challenges
This Review, from the points of view of both clinicians and nanotechnologists, offers an outline of critical issues and emerging developments in cardiac nanotechnology, which overall represent tremendous opportunities for advancing the field. We show that nanoscience and nanotechnology have enormous potential as applied to efficient predictive and therapeutic approaches for cardiovascular diseases.
Although the field is growing fast, and exciting reports of both in vitro and animal studies are steadily being published, successful clinical translation of these nano-based approaches remains elusive. Several shortcomings explain the huge gap between bench discoveries and clinical trials138. One of the main issues is the lack of reproducible large-scale synthesis of nanoparticles with similar physicochemical properties (for example, same size, shape and surface properties). Thus far, only a few promising approaches (for example, self-assembly of functionalized nanomaterials139,140, micro-fluidics enabling rapid mixing141 and particle replication in non-wetting template142 for nanoparticle fabrication) have been developed to synthesize nanoparticles with distinct physicochemical properties.
In addition to mass production of similar nanoparticles, the personalized biological identity of nanoparticles (that is, an individual’s protein corona) can significantly affect their efficacy and possible cardiotoxicity132. Recent developments in the field of nano-bio interactions have revealed that different types of disease may change the biological identity—and thus the efficacy—of the same nanoparticles131,143. As most people suffering from cardiac ischemia are likely to have a variety of other diseases, nanoparticles with predictable bio identity should be designed in a patient-specific manner for achieving maximal efficacy and safety.
Nanostructured materials are rapidly evolving towards enabling mass production of patient-specific mature and functional CMs for both therapeutic and disease-monitoring applications144. More attention should be given to developing simple culture plates to either differentiate stem cells into mature CMs or induce maturity of immature CMs obtained from chemically defined approaches. Such plates will be central to clinical applications of autologous cell transplantation and patient-specific drug monitoring/discovery.
A major factor largely overlooked in the design of in vitro approaches to evaluation of the targeting and therapeutic effects of multiscale technologies on CMs is that physiological fluids are dynamic by nature, whereas most in vitro models provide a simpler static environment145. For instance, in the human body, blood moves at different speeds ranging from a few micrometres per second (in the capillaries) up to 60 cm s−1 (in the ascending aorta)146. To achieve a precise correlation between the in vitro models and the clinical outcomes, future studies should be more focused on the use of fluidic approaches to mimic the in vivo dynamic environment in the in vitro models.
The recent fabrication of decellularized bioscaffolds with the molecular and cellular patterns of heart tissue has shown very promising results in creating an entirely new, functional heart147. Therefore, more attention should be given to tissue-engineering approaches, in combination with recently evolved 3D bioprinting technologies, for fabricating heart muscle or even whole hearts148. One key challenge in bioprinting cardiac tissue grafts is the incorporation of a functional, mechanically integrated vascular network into the bioengineering myocardium that can be perfused in vivo149. Access to physiologically relevant cell types and densities, clinically relevant cardiac patch size and architecture, and more bioactive inks to maintain the phenotype, function and maturation of cardiac cells are other confounding factors150.
Last but not least, to accelerate additional breakthrough discoveries in the field, funding for cardiac nanotechnology should be substantially increased. Compared with other biomedical applications of nanotechnology, such as cancer nanotechnology, cardiac nanotechnology has lagged in achieving ‘traction’, and its slower progress also mirrors (at least in part) less investment both from governments/foundations and financial and strategic investors. During the past few years, however, a growing number of funding opportunities have been created in the field of cardiac nanotechnology, and this has translated into the progress we outline above. We believe that nanomedicines will shift the paradigm of both predictive and therapeutic approaches in cardiac disease in the foreseeable future.
Acknowledgments
This work was supported by the US National Institutes of Health grants HL127464-01A1 (O.C.F.), EB015419 (O.C.F.) and HL133272 (J.C.W.), and Department of Defense grant PC140318 (O.C.F.).
Footnotes
Competing financial interests
R.L. and O.C.F. declare financial interests in Selecta Biosciences, Tarveda Therapeutics and Placon Therapeutics. R.L. declares financial interests in Moderna.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson NB, et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. MMWR Surveill Summ. 2014;63:3–27. [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Cassar A, Holmes DR, Jr, Rihal CS, Gersh BJ. Chronic coronary artery disease: diagnosis and management. Mayo Clin Proc. 2009;84:1130–1146. doi: 10.4065/mcp.2009.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 6.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11:404–415. doi: 10.1007/s11897-014-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijns W, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 11.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 12.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 13.Begum M, Sharma H. Scope of nanomedicine against coronary artery disease: a review. Eur J Pharm Med Res. 2016;3:635–641. [Google Scholar]
- 14.Mahmoudi M, et al. Protein−nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 15.Nahrendorf M, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoudi M, Serpooshan V, Laurent S. Engineered nanoparticles for biomolecular imaging. Nanoscale. 2011;3:3007–3026. doi: 10.1039/c1nr10326a. [DOI] [PubMed] [Google Scholar]
- 17.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 18.Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotech. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korin N, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 21.Fredman G, et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra220. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamaly N, et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamaly N, et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci USA. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamaly N, He JC, Ausiello DA, Farokhzad OC. Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol. 2016;12:738–753. doi: 10.1038/nrneph.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duivenvoorden R, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3531. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JM, et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci USA. 2010;107:2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chnari E, Nikitczuk JS, Wang J, Uhrich KE, Moghe PV. Engineered polymeric nanoparticles for receptor-targeted blockage of oxidized low density lipoprotein uptake and atherogenesis in macrophages. Biomacromolecules. 2006;7:1796–1805. doi: 10.1021/bm0600872. [DOI] [PubMed] [Google Scholar]
- 28.Lewis DR, et al. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc Natl Acad Sci USA. 2015;112:2693–2698. doi: 10.1073/pnas.1424594112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasini MD, Zablocki K, Petersen LK, Moghe PV, Tomassone MS. Coarse grained molecular dynamics of engineered macromolecules for the inhibition of oxidized low-density lipoprotein uptake by macrophage scavenger receptors. Biomacromolecules. 2013;14:2499–2509. doi: 10.1021/bm301764x. [DOI] [PubMed] [Google Scholar]
- 30.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotech. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoudi M. Protein corona: The golden gate to clinical applications of nanoparticles. Int J Biochem Cell Biol. 2016;75:141–142. doi: 10.1016/j.biocel.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona. Trends Biotechnol. 2017;35:257–264. doi: 10.1016/j.tibtech.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotech. 2012;7:779–786. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 34.Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML. Protein corona significantly reduces active targeting yield. Chem Commun. 2013;49:2557–2559. doi: 10.1039/c3cc37307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behzadi S, et al. Protein corona change the drug release profile of nanocarriers: the “overlooked” factor at the nanobio interface. Colloids Surf B. 2014;123:143–149. doi: 10.1016/j.colsurfb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Moyano DF, et al. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirshafiee V, Kim R, Park S, Mahmoudi M, Kraft ML. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials. 2016;75:295–304. doi: 10.1016/j.biomaterials.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotech. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 39.Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol. 2014;11:232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen PK, Rhee JW, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1:831–841. doi: 10.1001/jamacardio.2016.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen PK, Neofytou E, Rhee JW, Wu JC. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 2016;1:953–962. doi: 10.1001/jamacardio.2016.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmoudi M, et al. Novel MRI contrast agent from magnetotactic bacteria enables in vivo tracking of iPSC-derived cardiomyocytes. Sci Rep. 2016;6:26960. doi: 10.1038/srep26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riegler J, et al. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Rep. 2016;6:176–187. doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng K, et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat Commun. 2014;5:4880. doi: 10.1038/ncomms5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiba Y, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawamura M, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 51.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mirotsou M, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han J, et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano. 2015;9:2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- 54.Vandergriff AC, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials. 2014;35:8528–8539. doi: 10.1016/j.biomaterials.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 55.Xu C, et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly (lactide-co-glycolide) microparticles. Nano Lett. 2012;12:4131–4139. doi: 10.1021/nl301658q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X. Magnetic Resonance Imaging of Stem Cell Applications. Nova Science; 2015. [Google Scholar]
- 57.Mahmoudi M, Bertrand N, Zope H, Farokhzad O. Emerging understanding of the nano-bio interface in nanomedicin. Nano Today. 2016;11:817–832. [Google Scholar]
- 58.Chen IY, et al. Comparison of optical bioluminescence reporter gene and superparamagnetic iron oxide MR contrast agent as cell markers for noninvasive imaging of cardiac cell transplantation. Mol Imaging Biol. 2009;11:178–187. doi: 10.1007/s11307-008-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terrovitis J, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahama H, et al. Liposomal amiodarone augments anti-arrhythmic effects and reduces hemodynamic adverse effects in an ischemia/reperfusion rat model. Cardiovasc Drugs Ther. 2013;27:125–132. doi: 10.1007/s10557-012-6437-6. [DOI] [PubMed] [Google Scholar]
- 62.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 63.Burridge P, et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X, Pabon L, Murry CE. Engineering adolescence maturation of human pluripotent stem cell–derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Sharma A, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017;9:eaaf2584. doi: 10.1126/scitranslmed.aaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro AJ, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Cearbhaill ED, Ng KS, Karp J. Emerging medical devices for minimally invasive cell therapy. Mayo Clin Proc. 2014;89:259–273. doi: 10.1016/j.mayocp.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang HS, et al. Electroconductive nanopatterned substrates for enhanced myogenic differentiation and maturation. Adv Healthcare Mater. 2016;5:137–145. doi: 10.1002/adhm.201500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang PY, Yu J, Lin JH, Tsai WB. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7:3285–3293. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 75.Macadangdang J, et al. Nanopatterned human iPSC-based model of a dystrophin-null cardiomyopathic phenotype. Cell Mol Bioeng. 2015;8:320–332. doi: 10.1007/s12195-015-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carson D, et al. Nanotopography-induced structural anisotropy and sarcomere development in human cardiomyocytes derived from induced pluripotent stem cells. ACS Appl Mater Interfaces. 2016;8:21923–21932. doi: 10.1021/acsami.5b11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.French A, et al. Enabling consistency in pluripotent stem cell-derived products for research and development and clinical applications through material standards. Stem Cells Transl Med. 2015;4:217–223. doi: 10.5966/sctm.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laflamme MA, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 79.Mashinchian O, et al. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine. 2015;10:829–847. doi: 10.2217/nnm.14.225. [DOI] [PubMed] [Google Scholar]
- 80.Mahmoudi M, et al. Cell-imprinted substrates direct the fate of stem cells. ACS Nano. 2013;7:8379–8384. doi: 10.1021/nn403844q. [DOI] [PubMed] [Google Scholar]
- 81.Mashinchian O, et al. Cell-imprinted substrates act as an artificial niche for skin regeneration. ACS Appl Mater Interfaces. 2014;6:13280–13292. doi: 10.1021/am503045b. [DOI] [PubMed] [Google Scholar]
- 82.Bonakdar S, et al. Cell-imprinted substrates modulate differentiation, redifferentiation, and transdifferentiation. ACS Appl Mater Interfaces. 2016;8:13777–13784. doi: 10.1021/acsami.6b03302. [DOI] [PubMed] [Google Scholar]
- 83.Wekerle T, Grinyó JM. Belatacept: from rational design to clinical application. Transpl Int. 2012;25:139–150. doi: 10.1111/j.1432-2277.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- 84.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 85.Rong Z, et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 87.Evans CW, Iyer KS, Hool LC. The potential for nanotechnology to improve delivery of therapy to the acute ischemic heart. Nanomedicine. 2016;11:817–832. doi: 10.2217/nnm.16.7. [DOI] [PubMed] [Google Scholar]
- 88.Heusch G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dvir T, et al. Nanoparticles targeting the infarcted heart. Nano Lett. 2011;11:4411–4414. doi: 10.1021/nl2025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 91.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 92.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Delivery Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Delivery Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardy N, et al. Nanoparticle-mediated dual delivery of an antioxidant and a peptide against the L-Type Ca2+ channel enables simultaneous reduction of cardiac ischemia-reperfusion injury. ACS Nano. 2014;9:279–289. doi: 10.1021/nn5061404. [DOI] [PubMed] [Google Scholar]
- 96.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 98.Yeh ETH, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 99.Nasr M, Nafee N, Saad H, Kazem A. Improved antitumor activity and reduced cardiotoxicity of epirubicin using hepatocyte-targeted nanoparticles combined with tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2014;88:216–225. doi: 10.1016/j.ejpb.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 100.Setyawati M, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat Commun. 2013;4:1673. doi: 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- 101.Smith I, Liu X, Smith L, Ma P. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotech. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patel DN, Bailey SR. Nanotechnology in cardiovascular medicine. Catheter Cardiovasc Interv. 2007;69:643–654. doi: 10.1002/ccd.21060. [DOI] [PubMed] [Google Scholar]
- 104.Iverson N, Plourde N, Chnari E, Nackman GB, Moghe PV. Convergence of nanotechnology and cardiovascular medicine. BioDrugs. 2008;22:1–10. doi: 10.2165/00063030-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 105.Riegler J, et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serpooshan V, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34:9048–9055. doi: 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasan A, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci. 2015;2:1500122. doi: 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engelmayr GC, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Souza GR, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotech. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dvir T, et al. Nanowired three-dimensional cardiac patches. Nat Nanotech. 2011;6:720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feiner R, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016;15:679–85. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun. 2007;361:847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Timko BP, Cohen-Karni T, Qing Q, Tian B, Lieber CM. Design and implementation of functional nanoelectronic interfaces with biomolecules, cells, and tissue using nanowire device arrays. IEEE Trans Nanotechnol. 2010;9:269–280. doi: 10.1109/TNANO.2009.2031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian B, et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miura M, et al. Effect of nonuniform muscle contraction on sustainability and frequency of triggered arrhythmias in rat cardiac muscle. Circulation. 2010;121:2711–2717. doi: 10.1161/CIRCULATIONAHA.109.907717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liau B, Zhang D, Bursac N. Functional cardiac tissue engineering. Regen Med. 2012;7:187–206. doi: 10.2217/rme.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: macro-and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Delivery Rev. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burdick JA, Mauck RL, Gorman JH, Gorman RC. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med. 2013;5:176ps4. doi: 10.1126/scitranslmed.3003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baker BM, Handorf AM, Ionescu LC, Li WJ, Mauck RL. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev Med Devices. 2009;6:515–532. doi: 10.1586/erd.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee JW. 3D nanoprinting technologies for tissue engineering applications. J Nanomater. 2015;2015:213521. [Google Scholar]
- 121.Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM. Bioengineering cardiac constructs using 3D printing. J 3D Printing Med. 2017;1:123–139. [Google Scholar]
- 122.Giannopoulos AA, et al. Applications of 3D printing in cardiovascular diseases. Nat Rev Cardiol. 2016;13:701–718. doi: 10.1038/nrcardio.2016.170. [DOI] [PubMed] [Google Scholar]
- 123.Dalton PD, Joergensen NT, Groll J, Moeller M. Patterned melt electrospun substrates for tissue engineering. Biomed Mater. 2008;3:034109. doi: 10.1088/1748-6041/3/3/034109. [DOI] [PubMed] [Google Scholar]
- 124.Chaudhury K, Kumar V, Kandasamy J, RoyChoudhury S. Regenerative nanomedicine: current perspectives and future directions. Int J Nanomed. 2014;9:4153–4167. doi: 10.2147/IJN.S45332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:4153–4167. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Behzadi S, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46:4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertrand N, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. doi: 10.1038/s41467-017-00600-w. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mahmoudi M, et al. Temperature: the “ignored” factor at the nanobio interface. ACS Nano. 2013;7:6555–6562. doi: 10.1021/nn305337c. [DOI] [PubMed] [Google Scholar]
- 129.Mahmoudi M, et al. Variation of protein corona composition of gold nanoparticles following plasmonic heating. Nano Lett. 2014;14:6–12. doi: 10.1021/nl403419e. [DOI] [PubMed] [Google Scholar]
- 130.Monopoli MP, et al. Physical−chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 131.Hajipour MJ, Laurent S, Aghaie A, Rezaee F, Mahmoudi M. Personalized protein coronas: a “key” factor at the nanobiointerface. Biomater Sci. 2014;2:1210–1221. doi: 10.1039/c4bm00131a. [DOI] [PubMed] [Google Scholar]
- 132.Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M. Personalized protein corona on nanoparticles and its clinical implications. Biomater Sci. 2017;5:378–387. doi: 10.1039/c6bm00921b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walkey CD, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 134.Bigdeli A, et al. Exploring cellular interactions of liposomes using protein corona fingerprints and physicochemical properties. ACS Nano. 2016;10:3723–3737. doi: 10.1021/acsnano.6b00261. [DOI] [PubMed] [Google Scholar]
- 135.Sharifi S, et al. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 137.Bostan HB, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Life Sci. 2016;165:91–99. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 138.Fleischer S, Feiner R, Dvir T. Cutting-edge platforms in cardiac tissue engineering. Curr Opin Biotechnol. 2017;47:23–29. doi: 10.1016/j.copbio.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 139.Hrkach J, et al. Cardiotoxicity of nanoparticles. Sci Transl Med. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 140.Gu F, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci USA. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim Y, et al. Mass production and size control of lipid–polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012;12:3587–3591. doi: 10.1021/nl301253v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu J, et al. Future of the particle replication in nonwetting templates (PRINT) technology. Angew Chem Int Ed. 2013;52:6580–6589. doi: 10.1002/anie.201209145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hajipour MJ, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7:8978–8994. doi: 10.1039/c5nr00520e. [DOI] [PubMed] [Google Scholar]
- 144.Smith AST, Macadangdang J, Leung W, Laflamme MA, Kim DH. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol Adv. 2017;35:77–94. doi: 10.1016/j.biotechadv.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tenzer S, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotech. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 146.Pozzi D, et al. The biomolecular corona of nanoparticles in circulating biological media. Nanoscale. 2015;7:13958–13966. doi: 10.1039/c5nr03701h. [DOI] [PubMed] [Google Scholar]
- 147.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 148.Tian B, et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sooppan R, et al. In vivo anastomosis and perfusion of a three-dimensionally-printed construct containing microchannel networks. Tissue Eng Part C Methods. 2015;22:1–7. doi: 10.1089/ten.tec.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]


