Abstract
The etiology for the majority of congenital heart defects (CHD) is unknown. We identified a patient with unbalanced atrioventricular septal defect (AVSD) and hypoplastic left ventricle who harbored an ~0.3Mb monoallelic deletion on chromosome 3p14.1. The deletion encompassed the first 4 exons of FOXP1, a gene critical for normal heart development that represses cardiomyocyte proliferation and expression of Nkx2.5. To determine if FOXP1 mutations are found in patients with CHD, we sequenced FOXP1 in 82 patients with AVSD or hypoplastic left heart syndrome. We discovered two patients who harbored a heterozygous c.1702C>T variant in FOXP1 that predicted a potentially deleterious substitution of a highly conserved proline (p.Pro568Ser). This variant was not found in 287 controls but is present in dbSNP at a 0.2% frequency. The orthologous murine Foxp1 p.Pro596Ser mutant protein displayed deficits in luciferase reporter assays and resulted in increased proliferation and Nkx2.5 expression in cardiomyoblasts. Our data suggest that haploinsufficiency of FOXP1 is associated with human CHD.
Keywords: congenital heart defect, FOXP1, cardiomyocyte proliferation, hypoplastic left heart syndrome, atrioventricular septal defect
Congenital heart disease (CHD) is the most common type of birth defect with a birth prevalence of 6–8 per 1,000.[Hoffman and Kaplan, 2002] CHD results in significant morbidity and mortality during childhood, and even with the recent advances in medical and surgical management it remains a leading cause of infant death worldwide. CHD occurs when the normal process of cardiac morphogenesis is disrupted. The etiology of CHD remains largely unknown and is proposed to be multifactorial with genetic and environmental factors playing critical roles.[Kathiresan and Srivastava, 2012]
Evidence supporting genetic contributors comes from reports of familial cases for nearly each subset of CHD and epidemiologic studies, which have demonstrated an increased recurrence risk.[Ferencz, et al., 1989] The earliest evidence of specific genetic factors is the link between CHD that occurs in the setting of syndromes associated with chromosomal abnormalities such as Trisomy 21 and 22q11 deletion. Genetic etiologies for non-syndromic CHD have mostly been discovered by studying large kindreds with multiple affected family members using positional cloning approaches.[McBride and Garg, 2011] The identification of CHD-causing genes has been aided by our increasing molecular knowledge of the developmental pathways that govern normal heart development.[Garg, 2006] Even with this increased knowledge, the etiology for the majority of non-syndromic CHD remains unclear.
Recent studies have demonstrated the utility of microarray-based approaches to uncover subtle chromosome abnormalities in children with multiple birth defects. [Richards and Garg, 2010; Richards, et al., 2008] Chromosomal microarray or array comparative genome hybridization is a relatively new technology that has gained clinical utility to investigate genetic etiologies for complex forms of CHD, especially when it is associated with other birth defects. [Payne, et al., 2012; Richards and Garg, 2010] Use of this technology has been important for identifying chromosomal abnormalities in children with CHD, and even for discovering new genetic syndromes associated with chromosomal abnormalities. In some instances, the identified chromosomal abnormalities are small, allowing for the identification of the CHD-causing gene.
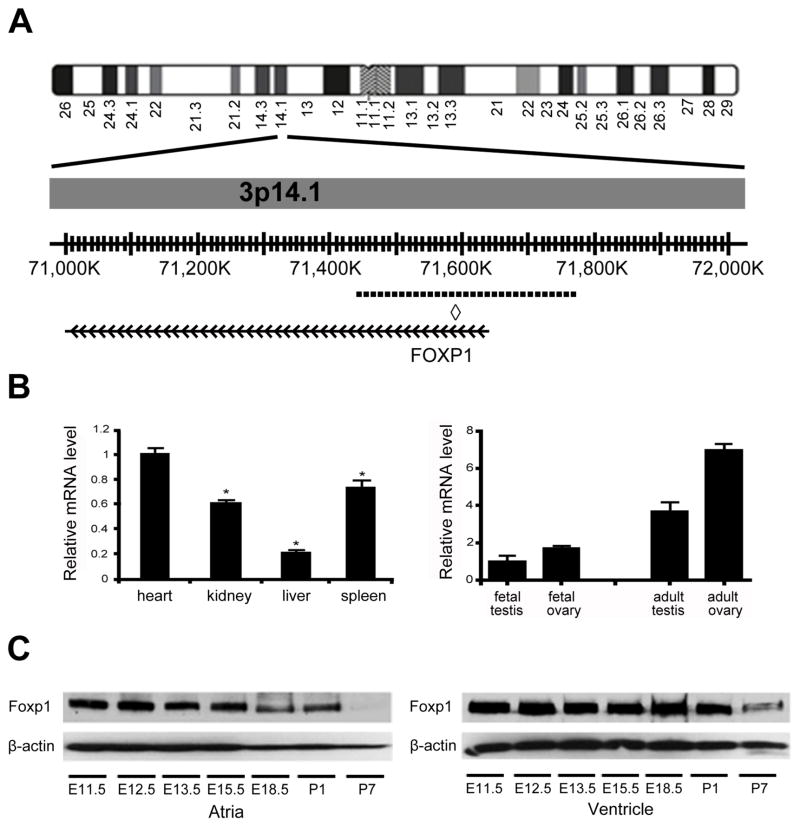
In this study, we identified a 23 day old patient with complex CHD consisting of unbalanced atrioventricular septal defect (right ventricle dominant), hypoplastic left ventricle and aortic arch, left atrioventricular valve (mitral valve) stenosis, bilateral superior vena cavae and transposed great vessels along with bilateral cryptorchidism with germ cell hypoplasia (Supp Fig. S1A). The child died at 8 months of age due to multiple complications related to a long postoperative course that followed cardiac surgery. A clinical chromosomal microarray was performed (Oligo V8.1, Baylor College of Medicine Medical Genetics Laboratories) and revealed a monoallelic microdeletion on chromosome 3p14, spanning a minimum of 0.261 Mb (chr3: 71,455,422–71,716,260) to a maximum of 0.325 Mb (chr3: 71,443,671–71,768,367). Fluorescence in situ hybridization (FISH) analysis also confirmed the deletion and revealed the same loss from the unaffected mother (Supp Fig. S1B). This deletion has been only found in this patient and his mother in the database of Baylor College of Medicine Medical Genetics Laboratories and is considered to be a very rare variant. Based upon the NCBI36/hg18 database, the deletion encompassed only the first 4 exons of the FOXP1 gene (MIM #605515) and hsa-miR-1284, a not well-studied miR that is not expressed in the developing heart (Fig. 1A and data not shown).
Figure 1. Deletion of FOXP1 on chromosome 3 and Foxp1 expression in human and mouse.
(A) Schematic of chromosome 3. Dashed line indicates location of microdeletion of FOXP1 in region of chromosome 3p14.1. ◇, miR-1284 locus. (B) FOXP1 mRNA is expressed at higher levels in the human fetal heart as compared to other fetal tissues by qRT-PCR. FOXP1 mRNA is expressed at higher levels in the human fetal and adult ovary than the fetal and adult testis by qRT-PCR. (C) Expression of Foxp1 protein in murine atria and ventricles at various embryonic and postnatal timepoints. β-actin is shown as loading control. *, p value<0.05.
Foxp1 is a member of the Forkhead box family of transcription factors and Foxp1 has a demonstrated role in murine cardiac development.[Wang, et al., 2004] To determine if FOXP1 is expressed in the developing human heart, we performed quantitative real time(qRT)-PCR using fetal cDNA. We found that FOXP1 was expressed at higher levels in the fetal human heart than in other human fetal tissues (Fig. 1B). Due to the bilateral cryptorchidism with germ cell hypoplasia, we performed qRT-PCR using fetal and adult testes and ovaries cDNA, finding that FOXP1 is expressed in the developing gonads with higher expression in the mature gonads (Fig. 1B). In the mouse embryo, a similar pattern of a high level of cardiac expression as compared to other organs was found (Supp Fig. S2). To further define the cardiac expression of Foxp1, we determined the expression of Foxp1 protein in murine atria and ventricles during different embryonic and postnatal timepoints. Western blotting was performed using wildtype mouse hearts from embryonic day (E) 11.5 to postnatal day (P) 7. Foxp1 protein expression levels were higher during embryogenesis in both the atria and ventricles as compared to postnatal day 7 (Fig. 1C).
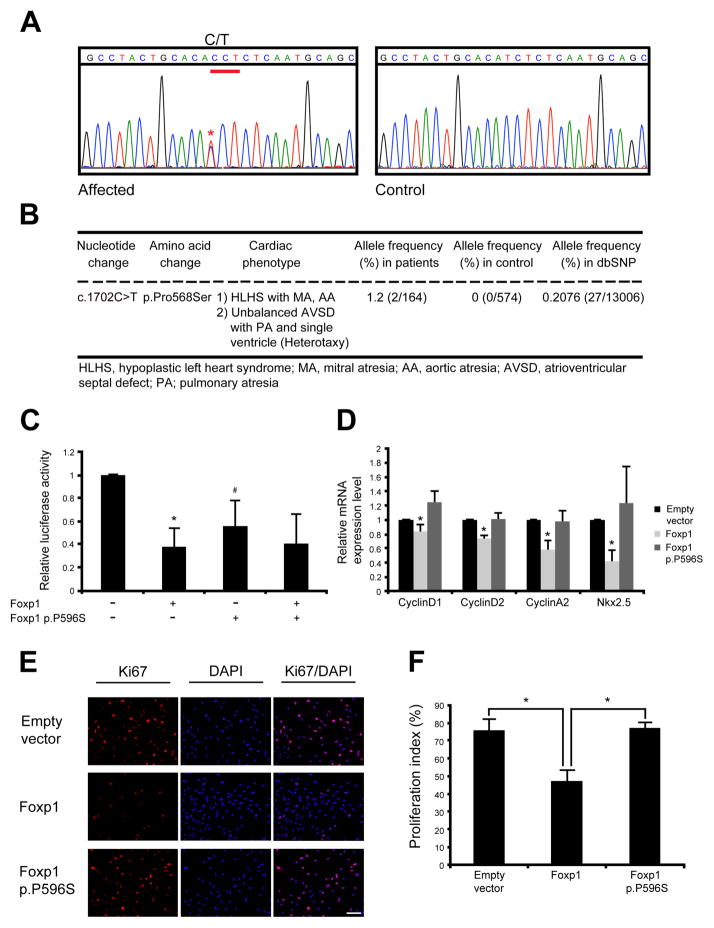
The discovery of a rare chromosomal microdeletion that harbored a cardiac transcription factor implicated in heart development in a patient with complex CHD consisting of an unbalanced atrioventricular septal defect (AVSD) and hypoplastic left ventricle suggested that FOXP1 may be contributing to the etiology of similar types of CHD. Therefore, we screened patients with AVSD or hypoplastic left heart syndrome (HLHS) for mutations in FOXP1. We sequenced the coding region of FOXP1 in DNA samples from 82 patients, 10 with AVSD and 72 with HLHS. We discovered two unrelated patients who harbored a heterozygous C to T mutation at nucleotide position 1702, c.1702C>T (with +1 correspond to the A of ATG in the GenBank accession number NM_032682.5). This variant predicts the nonsynonymous amino acid change, p.Pro568Ser and has been submitted to the NCBI Clin Var database (http://www.ncbi.nlm.nih.gov/clinvar/) (Fig. 2A). One African American patient had HLHS (with mitral valve and aortic valve atresia) while the other subject of Hispanic ethnicity had an unbalanced AVSD, pulmonary atresia and single ventricle in the setting of heterotaxy syndrome (Fig. 2B). The parental DNAs were not available to determine inheritance. The FOXP1 protein is 677 amino acids in length and contains a homologous DNA-binding forkhead domain and two nuclear localization signals (NLS) [Banham, et al., 2001] and the p.Pro568Ser variant was 22 codons from the NLS (Supp Fig. S3A). The proline residue at codon 568 is highly conserved between species and bioinformatics tools, which predict whether an amino acid substitution affects protein function, suggest deleterious effects with a score of 0.05 and a disease-causing probability value of 0.9985 based upon SIFT (Sorting Intolerant from Tolerant) and MutationTaster, respectively (Supp Fig. S3B). However, another algorithm, PolyPhen-2, predicted this sequence variant to be benign. This nucleotide change was not found in a diverse control population of 287 individuals (96 Caucasian-American, 95 Hispanic, and 96 African-American). This nucleotide variant (rs147674680) has been reported in dbSNP with a frequency of 0.6% in African Americans (27/4406) and an overall frequency of 0.2% (27/13006 alleles). It was present in 1.2% (2/164 alleles) of the affected population (Fig. 2B; Fisher’s Exact test, p value = 0.05).
Figure 2. Identification of FOXP1 c.1702C>T variation (p.Pro568Ser) in individual with CHD and functional analysis of murine Foxp1 P596S protein.
(A) Sequence chromatogram showing heterozygous C to T transition at nucleotide position 1702 in affected subject as compared to control individual. Asterisk (*) indicates the location of c.1702C>T transition as +1 corresponds to the A of the ATG of FOXP1 (GenBank accession number NM_032682.5). (B) Summary of non-synonymous FOXP1 p.Pro568Ser variation identified in children with CHD. (C) Luciferase reporter assays using the 3kb mouse Nkx2.5 promoter/enhancer demonstrates that the murine Foxp1 p.P596S mutant protein without wildtype Foxp1 does not repress the Nkx2.5-luciferase reporter as compared to wildtype Foxp1. The addition of Foxp1 p.P596S mutant did not affect the ability of wildtype Foxp1 to repress reporter activation. *, p<0.05 comparison between empty vector and Foxp1; #, p<0.05 comparison between wildtype Foxp1 and Foxp1 p.P596S. Luciferase activity is normalized to β-gal. (D) Expression of CyclinD1, CyclinD2, CyclinA2 and Nkx2.5 mRNA was increased with transfection of Foxp1 p.P596S plasmid alone as compared to wildtype Foxp1 in H9C2 cardiomyoblasts as measured by qRT-PCR. (E) Transient transfection of Foxp1 p.P596S mutation without wildtype Foxp1 is unable to repress proliferation of H9C2 cardiomyoblasts as compared to wildtype Foxp1 as measured by immunofluorescent staining for Ki67 and quantified in (F). DAPI is shown in blue. *, p<0.05. Scale bar indicates 100μm.
Foxp1 functions as a direct transcriptional repressor of Nkx2.5, a key regulator of cardiomyocyte proliferation during cardiac development.[Tu, et al., 2009] As previously reported, Foxp1 is able to repress the activity of a luciferase reporter containing a previously identified −3kb murine Nkx2.5 promoter.[Zhang, et al., 2010] We generated the orthologous mutation in murine Foxp1, p.Pro596Ser (Supp Fig. 3B) and found that the Foxp1 p.P596S mutant protein without wildtype Foxp1 had a decreased ability to repress the Nkx2.5-luciferase reporter when transiently transfected in HEK293 cells. However, it did not show this decreased ability with wildtype Foxp1 co-transfection, demonstrating the lack of dominant negative effect of Foxp1 p.P596S mutation (Fig. 2C). Wildtype Foxp1 and Foxp1 p.P596S mutant express at comparable protein levels in HEK293 cells (Supp Fig. S3C). This in vitro assay suggested that the p.P596S mutation in Foxp1 had functional deficits.
Murine studies have demonstrated that the loss of Foxp1 in the developing myocardium results in increased cardiomyocyte proliferation, leading to increased myocardial mass and neonatal lethality.[Zhang, et al., 2010] To investigate the effects of Foxp1 p.P596S mutation on cardiomyocyte proliferation, we transfected wildtype Foxp1 and the mutant Foxp1 p.P596S in H9C2 cells, a rat cardiomyoblast cell line, and assessed cell proliferation using the proliferation marker, Ki67. Wildtype and mutant Foxp1 were expressed at comparable levels in H9C2 cells (Supp Fig. S3C), and as expected, transfection of Foxp1 decreased cell proliferation by ~40% as compared to cells transfected with empty vector. However, the Foxp1 p.P596S mutation without wildtype Foxp1 demonstrated an inability to repress cell proliferation and levels of proliferation were similar to control (Fig. 2E and 2F). Consistent with this, the mRNA levels of CyclinD1, CyclinD2 and CyclinA2 assessed by qRT-PCR were not repressed in H9C2 cells transfected with Foxp1 p.P596S mutant compared to wildtype Foxp1 (Fig. 2D). Interestingly, we also noted increased expression of Nkx2.5, a key regulator of cardiomyocyte proliferation (Fig. 2D). Similar results were seen with HL-1 cardiomyocytes, a murine atrial cardiomyocyte cell line (data not shown). These in vitro studies suggest that murine Foxp1 p.P596S mutation, which is orthologous to the human FOXP1 p.P568S mutation, has decreased ability to repress cardiomyocyte proliferation potentially via a Nkx2.5-mediated mechanism.
Genetic abnormalities involving genes critical for normal cardiomyocyte proliferation and gonadal differentiation are becoming increasingly recognized as etiologic contributors to human congenital anomalies.[Lourenco, et al., 2011; Richards and Garg, 2010] Here, we have identified three affected unrelated individuals with rare genetic abnormalities of FOXP1. The individuals have complex CHD spanning a spectrum of unbalanced AVSD with an associated hypoplastic ventricle to HLHS. The rare heterozygous p.Pro568Ser variant in FOXP1 was found in 2/82 affected individuals and demonstrated decreased repressive ability in transactivation assays. Consistent with this, the orthologous murine Foxp1 mutant protein (p.P596S) demonstrated the inability to repress cardiomyocyte proliferation and Nkx2.5 expression in rodent cardiomyocyte cell lines. These findings suggest that mutations in FOXP1 are associated with congenital cardiac defects.
The FOXP1 microdeletion that we identified in our index case was inherited from his mother who did not manifest complex CHD. Similarly, the FOXP1 p.Pro568Ser mutation has been reported in public databases at very low frequency. Due to the relatively higher allele frequency for the p.Pro568Ser variant in African Americans (0.6%), we cannot rule out the possibility that it represents an uncommon polymorphism in this population. While our observations with the FOXP1 microdeletion and p.Pro568Ser mutation could be accounted for by incomplete penetrance or potentially be explained by genetic modifiers, it is becoming recognized that rare deleterious variants may play a role in the etiology of birth defects, especially non-syndromic CHD. This is consistent with the rare variant hypothesis, in which disease susceptibility is due to the additive effect of genetic variants.[McBride and Ware, 2012] For example, some mutations in GATA4, a transcription factor crucial for normal cardiac and testicular development, have been linked to CHD in a highly penetrant Mendelian fashion,[Garg, et al., 2003] while other rare heterozygous point mutations in GATA4, can also show variable penetrance. [Lourenco, et al., 2011; Schluterman, et al., 2007; Tomita-Mitchell, et al., 2007] Accordingly, our literature review has revealed two additional cases of chromosome 3p deletions involving FOXP1 in patients with cardiac anomalies or cryptorchidism. One patient has speech delay, contractures, hypertonia and blepharohimosis and while the other has cardiac, genital, and cerebral malformations along with delayed neuro- and psychomotor development. [Pariani, et al., 2009; Tutulan-Cunita, et al., 2012] These and additional patients with chromosomal deletions encompassing FOXP1 have a spectrum of anomalies primarily learning deficits.[Horn, et al., 2010] The patient with the FOXP1 deletion that we describe died at 8 months of age precluding the assessment of developmental outcomes. Microcephaly with mild hydrocephalus and mild hippocampal neuronal depopulation was noted at autopsy, but this may have been related to the prolonged hospitalization.
The Forkhead box (Fox) family of transcriptional repressors is defined by a highly conserved forkhead DNA-binding domain. [Zhang, et al., 2010] FOXP1 is a member of the Forkhead box family, and has been identified as an important regulator of cardiac development, lung alveolar morphogenesis, esophageal myogenesis, and neurogenesis. Loss of Foxp1 in mice results in lethality by embryonic day (E) 14.5 with severe cardiac anomalies consisting of defects in endocardial cushion formation, ventricular septation, and myocardial maturation, while abnormalities in gonadal development have not been reported.[Wang, et al., 2004] Foxp1 is expressed in the myocardium as well as endocardium of the developing heart and has been shown to be important for endocardial cushion development and cardiomyocyte proliferation.[Zhang, et al., 2010] In the myocardium, Foxp1 has been shown to negatively regulate cardiomyocyte proliferation by repressing Nkx2.5 expression; however, in the endocardium, Foxp1 promotes cardiomyocyte proliferation through regulation of Fgf signaling.[Zhang, et al., 2010] These studies highlight the role of Foxp1 in cardiomyocyte proliferation but the link between cardiomyocyte proliferation and human CHD is not well understood.
Among the many complex forms of CHD, atrioventricular septal defects (AVSDs) and hypoplastic left heart syndrome (HLHS) are both common disorders. AVSDs, which comprise 5–7% of all CHD, arise from abnormal endocardial cushion development. [Hoffman and Kaplan, 2002] While the majority of AVSD are termed “balanced”, with a common atrioventricular (AV) valve associated with equal-sized ventricles, in a small percentage of AVSD patients, the “unbalanced” location of the common AV valve will lead to the unequal size of the right and left ventricles. Hypoplastic left heart syndrome (HLHS) is the most common form of CHD associated with a hypoplastic left ventricle and is defined as when all of the structures on the left side of the heart are severely underdeveloped.[Hickey, et al., 2012] The etiology of HLHS is likely related to multiple factors including defects in endocardial and myocardial development.[Hickey, et al., 2012] As Foxp1 is expressed in the developing endocardium and myocardium and has been shown to be critical for normal development of the endocardial cushions and myocardium in mouse models, it is plausible that genetic abnormalities in FOXP1 could contribute to the development of AVSD and HLHS. Further studies are required to determine if FOXP1 mutations are found in children with other types of CHD.
We found that the murine Foxp1 p.Pro596Ser mutation demonstrated abnormal activation of the cardiac transcription factor, Nkx2.5. Mutations in NKX2.5 have been identified as a cause of human CHD, predominantly atrial septal defects and tetralogy of Fallot.[McElhinney, et al., 2003; Schott, et al., 1998] The majority of NKX2.5 mutations have been demonstrated to result in loss-of-function primarily due to reduced DNA binding affinity.[Kasahara, et al., 2000] Interestingly, a rare sequence variant in NKX2.5 that results in a missense mutation (R25C) has been linked to HLHS in multiple studies.[Elliott, et al., 2003; McElhinney, et al., 2003; Stallmeyer, et al., 2010] This R25C mutant protein was shown to increase activation ability unlike other NKX2.5 mutations.[Kasahara, et al., 2000] It is interesting to speculate that abnormal activation of NKX2.5 and its targets may contribute to HLHS.
In conclusion, our study has demonstrated that genetic abnormalities that result in loss of FOXP1 function are associated with complex forms of CHD in humans. Additional investigation is required to determine the mechanisms by which FOXP1 mutations contribute to CHD but we speculate that it may involve abnormal regulation of cardiomyocyte proliferation.
Supplementary Material
Acknowledgments
The authors wish to thank participating subjects and families. M.M. was supported by the American Pediatric Society/Society of Pediatric Research Student Research Program, and this work was supported by funding from the Children’s Heart Foundation and the Research Institute at Nationwide Children’s Hospital to V.G.
References
- Banham AH, Beasley N, Campo E, Fernandez PL, Fidler C, Gatter K, Jones M, Mason DY, Prime JE, Trougouboff P, Wood K, Cordell JL. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–8829. [PubMed] [Google Scholar]
- Elliott DA, Kirk EP, Yeoh T, Chandar S, McKenzie F, Taylor P, Grossfeld P, Fatkin D, Jones O, Hayes P. Cardiac homeobox gene NKX2-5 mutations and congenital heart disease: associations with atrial septal defect and hypoplastic left heart syndrome. J Am Coll Cardiol. 2003;41:2072–2076. doi: 10.1016/s0735-1097(03)00420-0. [DOI] [PubMed] [Google Scholar]
- Ferencz C, Boughman JA, Neill CA, Brenner JI, Perry LW. Congenital cardiovascular malformations: questions on inheritance. Baltimore-Washington Infant Study Group. J Am Coll Cardiol. 1989;14:756–763. doi: 10.1016/0735-1097(89)90122-8. [DOI] [PubMed] [Google Scholar]
- Garg V. Insights into the genetic basis of congenital heart disease. Cell Mol Life Sci. 2006;63:1141–1148. doi: 10.1007/s00018-005-5532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuika R, Cohen JC, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Hickey EJ, Caldarone CA, McCrindle BW. Left ventricular hypoplasia: a spectrum of disease involving the left ventricular outflow tract, aortic valve, and aorta. J Am Coll Cardiol. 2012;59:S43–54. doi: 10.1016/j.jacc.2011.04.046. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Horn D, Kapeller J, Rivera-Brugues N, Moog U, Lorenz-Depiereux B, Eck S, Hempel M, Wagenstaller J, Gawthrope A, Monaco AP, Bonin M, Riess O, et al. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010;31:E1851–1860. doi: 10.1002/humu.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Lee B, Schott JJ, Benson DW, Seidman JG, Seidman CE, Izumo S. Loss of function and inhibitory effects of human CSX/NKX2.5 homeoprotein mutations associated with congenital heart disease. J Clin Invest. 2000;106:299–308. doi: 10.1172/JCI9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Srivastava D. Genetics of human cardiovascular disease. Cell. 2012;148:1242–1257. doi: 10.1016/j.cell.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco D, Brauner R, Rybczynska M, Nihoul-Fekete C, McElreavey K, Bashamboo A. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc Natl Acad Sci USA. 2011;108:1597–1602. doi: 10.1073/pnas.1010257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KL, Garg V. Heredity of bicuspid aortic valve: is family screening indicated? Heart. 2011;97:1193–1195. doi: 10.1136/hrt.2011.222489. [DOI] [PubMed] [Google Scholar]
- McBride KL, Ware SM. Modifying Mendel: approaches for identification of susceptibility alleles for human cardiovascular malformations. Circ Cardiovasc Genet. 2012;5:274–276. doi: 10.1161/CIRCGENETICS.112.963579. [DOI] [PubMed] [Google Scholar]
- McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol. 2003;42:1650–1655. doi: 10.1016/j.jacc.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Pariani MJ, Spencer A, Graham JM, Jr, Rimoin DL. A 785kb deletion of 3p14.1p13, including the FOXP1 gene, associated with speech delay, contractures, hypertonia and blepharophimosis. Eur J Med Genet. 2009;52:123–127. doi: 10.1016/j.ejmg.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AR, Chang SW, Koenig SN, Zinn AR, Garg V. Submicroscopic chromosomal copy number variations identified in children with hypoplastic left heart syndrome. Pediatr Cardiol. 2012;33:757–763. doi: 10.1007/s00246-012-0208-9. [DOI] [PubMed] [Google Scholar]
- Richards AA, Garg V. Genetics of congenital heart disease. Curr Cardiol Rev. 2010;6:91–97. doi: 10.2174/157340310791162703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards AA, Santos LJ, Nichols HA, Crider BP, Elder FF, Hauser NS, Zinn AR, Garg V. Cryptic chromosomal abnormalities identified in children with congenital heart disease. Pediatr Res. 2008;64:358–363. doi: 10.1203/PDR.0b013e31818095d0. [DOI] [PubMed] [Google Scholar]
- Schluterman MK, Krysiak AE, Kathiriya IS, Abate N, Chandalia M, Srivastava D, Garg V. Screening and biochemical analysis of GATA4 sequence variations identified in patients with congenital heart disease. Am J Med Genet A. 2007;143A:817–823. doi: 10.1002/ajmg.a.31652. [DOI] [PubMed] [Google Scholar]
- Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Stallmeyer B, Fenge H, Nowak-Gottl U, Schulze-Bahr E. Mutational spectrum in the cardiac transcription factor gene NKX2.5 (CSX) associated with congenital heart disease. Clin Genet. 2010;78:533–540. doi: 10.1111/j.1399-0004.2010.01422.x. [DOI] [PubMed] [Google Scholar]
- Tomita-Mitchell A, Maslen CL, Morris CD, Garg V, Goldmuntz E. GATA4 sequence variants in patients with congenital heart disease. J Med Genet. 2007;44:779–783. doi: 10.1136/jmg.2007.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CT, Yang TC, Tsai HJ. Nkx2.7 and Nkx2.5 function redundantly and are required for cardiac morphogenesis of zebrafish embryos. PLoS One. 2009;4:e4249. doi: 10.1371/journal.pone.0004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutulan-Cunita AC, Papuc SM, Arghir A, Rotzer KM, Deshpande C, Lungeanu A, Budisteanu M. 3p interstitial deletion: novel case report and review. J Child Neurol. 2012;27:1062–1066. doi: 10.1177/0883073811431016. [DOI] [PubMed] [Google Scholar]
- Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li S, Yuan L, Tian Y, Weidenfeld J, Yang J, Liu F, Chokas AL, Morrisey EE. Foxp1 coordinates cardiomyocyte proliferation through both cell-autonomous and nonautonomous mechanisms. Genes Dev. 2010;24:1746–1757. doi: 10.1101/gad.1929210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.