Abstract
Rationale
Although the synthetic cathinone 4-methylmethcathinone (4-MMC, mephedrone) has been a subject of intensive research investigation, the pharmacological mechanisms involved in its interoceptive stimulus effects have yet to be fully characterized.
Objective
The present study employed drug discrimination methods in rats to compare the interoceptive stimulus properties of two different training doses of 4-MMC to other substances with similar pharmacological actions.
Methods
Sixteen male Sprague-Dawley rats were trained to discriminate either 1.0 mg/kg (N=8) or 3.0 mg/kg (N=8) 4-MMC from saline. Substitution tests were conducted with drugs that increase extracellular monoamine levels (d-amphetamine, (+)-methamphetamine, 4-MMC, MDMA, MDPV, and (−)-cocaine), a serotonin releaser (+)-fenfluramine, and a serotonergic (5-HT2A) hallucinogen (+)-LSD.
Results
Stimulus control was established in fewer sessions in the subjects trained with 3.0 mg/kg compared to those trained with 1.0 mg/kg 4-MMC. Cocaine, MDMA, and d-amphetamine produced full substitution in the 1.0 mg/kg 4-MMC-trained rats at doses that did not decrease response rate. However, doses of test drugs that engendered >80% 4-MMC-lever selection concurrently produced rate-decreasing effects in rats trained to discriminate 3.0 mg/kg 4-MMC.
Conclusions
These findings further characterize the interoceptive stimulus effects of 4-MMC and indicate that these effects vary little with training dose, however qualitative differences in substitutability of test drugs were observed between training groups. This study expands existing knowledge regarding the psychopharmacology of 4-MMC and the potential neurochemical substrates contributing to its subjective effects.
Keywords: drug discrimination, bath salts, synthetic cathinone, mephedrone, 4-methylmethcathinone, 4-MMC
Introduction
Synthetic cathinones appeared on the recreational drug market in the early 2000s. These substances are structurally similar to the parent compound cathinone, a monoamine alkaloid contained in the leaves of Catha edulis (Khat), an indigenous plant of the Arabian Peninsula and Horn of Africa with known psychostimulant effects. Synthetic cathinones were deliberately sold with deceptive labels such as “Bath Salts”, “Plant Food”, and “Research Chemicals” to circumvent legal restrictions. Prior to 2012, their uncontrolled status provided users with a cheap and quasi-legal alternative to other popular drugs of abuse, such as cocaine, amphetamines, and 3,4-methylenedioxymethamphetamine (MDMA). Given their popularity among recreational users, scientific research on these substances has grown considerably since 2010 (for review of synthetic cathinones, Valente et al. 2014; German et al. 2014).
4-methylmethcathinone (4-MMC, mephedrone) is among the more well-studied synthetic cathinones, likely because it was a common constituent found in recreational “bath salts” mixtures in the early 2000s (e.g., Drug Enforcement Agency, 2011). 4-MMC contains chemical moieties that are similar to prototypical phenylethylamine derivatives, such as MDMA and methamphetamine, and it may thus serve as a useful model from which to predict the effects of emerging, second generation synthetic cathinones with similar chemical structures (e.g., 4-methyl-N-ethylcathinone (4-MEC) and 4'-methyl-α-pyrrolidinopropiophenone (4-MePPP)). Microdialysis experiments in rats have demonstrated that 4-MMC, administered at intravenous (IV) doses ranging from 0.3 to 1.0 mg/kg, produces acute increases in extracellular serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in the nucleus accumbens with a greater effect on 5-HT release than DA (Baumann et al. 2012). Consistent with these findings, subcutaneous (SC) administration of 3.0 mg/kg 4-MMC increased extracellular concentrations of DA and 5-HT in the rat nucleus accumbens to approximately 496% and 941% above baseline levels, respectively (Kehr et al. 2011). Baumann et al. (2012) confirmed that 4-MMC acts as a nonselective substrate for release at serotonin transporters (SERT), dopamine transporters (DAT), and norepinephrine transporters (NET) similar to the pharmacological actions of MDMA. In addition to serving as a transporter releaser, 4-MMC acts as a 5-HT and DA reuptake inhibitor with a greater affinity towards DA transporters (Martinez-Clemente et al. 2012; Hadlock et al. 2011). These findings along with separate observations that 4-MMC induces conditioned place preference (CPP) in both rats and mice (Karlsson et al. 2014; Lisek et al. 2012) and supports intravenous self-administration (Motbey et al. 2013; Aarde et al. 2013) indicate potential abuse liability.
Of interest to the current study are the interoceptive stimulus effects produced by 4-MMC. Drug discrimination is employed as an in vivo drug detection assay to evaluate emerging drugs of abuse in comparison to drugs with known interoceptive effects. To date, only a few published studies have examined 4-MMC using drug discrimination methods (Varner et al. 2013; Gatch et al. 2013; Harvey and Baker, 2016; Milewski et al. 2017; DeLarge et al. 2017), and three of these studies used 4-MMC as the training drug (Varner et al. 2013; Milewski et al. 2017; DeLarge et al. 2017). The results of these initial studies indicate 4-MMC produces interoceptive stimulus effects similar to those of MDMA, cocaine, methamphetamine, and fenfluramine, and the extent of stimulus substitution depends on the drug used as the training stimulus.
In addition to training drug serving as an important determinant in the substitution profiles of centrally-acting drugs, training dose can also influence qualitative and quantitative aspects of stimulus substitution (for review, Stolerman et al. 2011). To further characterize the interoceptive cues contributing to 4-MMC discrimination, the present study assessed the substitutability of centrally-acting drugs with variable pharmacological actions in rats trained to discriminate either 1.0 mg/kg or 3.0 mg/kg 4-MMC from saline. Substitution tests comprised substances with similar chemical structures or pharmacological effects (e.g., produce increases in extracellular monoamines) based on the foregoing drug discrimination studies that included 4-MMC. Drugs with similar pharmacological effects (e.g., d-amphetamine and (+)-methamphetamine, or (−)-cocaine and MDPV) were included to evaluate any differences in potency in substitution. Structurally-related, phenylethylamine derivatives (MDMA, d-amphetamine, and (+)-methamphetamine) and structurally-unrelated plasmalemmal transporter blockers (MDPV, (−)-cocaine) were expected to produce high percent substitution values in both training groups based on the aforementioned drug discrimination studies using these drugs. Based on Varner et al. (2013), the serotonin releaser (+)-fenfluramine was expected to produce only partial substitution in the training groups. Last, the serotonergic hallucinogen (+)-LSD was expected to produce partial substitution given that 4-MMC displays pharmacologically-relevant binding affinity to 5-HT2A receptors (López-Arnau et al. 2011). Results indicate that the profile of stimulus substitution with a range of test drugs varies with 4-MMC training dose and effects on response rate.
Methods
Subjects
Sixteen experimentally naïve male Sprague-Dawley rats weighing an average of 350 g (Charles River Laboratories, Wilmington, MA, USA) were singly housed in polycarbonate cages with corncob bedding (Harlan Laboratories, Haslett, MI, USA) in a room maintained at 20°C (± 2°C) and 50% humidity (±5%) and on a 12 h:12 h light/dark schedule with lights on between 0700 and 1900 hours. Rats had unlimited access to water in home cages and were fed a commercial rodent diet (Purina®, Richmond, IN, USA) in daily rations to maintain body weight at approximately 85–90% of free-feeding weights. Rats received progressive increases in daily rations to accommodate normal growth rates that occur over their developmental life spans. Experimental procedures were performed during the light cycle. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (2013) and were approved by the Institutional Animal Care and Use Committee at Western Michigan University.
Drugs
(±)-Mephedrone-hydrochloride (4-MMC), (−)-cocaine-hydrochloride (COC), (+)-fenfluramine 3,4-methylenedioxypyrovalerone-hydrochloride (MDPV), 3,4-methylenedioxymethamphetamine-hydrochloride (MDMA), (+)-lysergic acid diethylamide-tartrate (LSD), and (+)-methamphetamine-hydrochloride (METH) were provided by the National Institute on Drug Abuse Drug Control Supply Program (Bethesda, MD, USA). d-Amphetamine-hemisulfate was purchased from Sigma Chemical Company, Inc. (St. Louis, MO, USA). Drug solutions were prepared by dissolving the salt in 0.9% (wt/vol) bacteriostatic sodium chloride. All drugs were administered by intraperitoneal (IP) injections using 1 mL syringes with a pre-session injection interval of 15 minutes. All doses were expressed as weight of the salt.
Apparatus
Eight computer-operated standard rat operant conditioning chambers (ENV-001; MED Associates Inc., St. Albans, VT, USA) were equipped with three retractable levers, a food pellet dispenser, 28-V overhead house lamp, and a fan for ventilation. Each chamber was housed within light- and sound-attenuating cabinet. Experimental events were programmed and controlled using Version IV Med-PC software (MED Associates Inc., St. Albans, VT, USA). Reinforcers were 45 mg Dustless Precision Pellets® (F0021, BioServ, Flemington, NJ, USA).
Training Procedures
Preliminary training and discrimination training were identical to procedures described previously (Harvey and Baker, 2016). Briefly, subjects were initially trained to lever press under a fixed ratio (FR) schedule of reinforcement that was gradually increased from FR1 to FR 20. Six errorless sessions were conducted to establish schedule control on each lever separately. Subjects were administered a 0.9% bacteriostatic saline solution (vehicle, V) or the training drug of 1 or 3 mg/kg 4-MMC (D) for a total of six 20 min training sessions in the following order: V, V, D, D, V, D. The lever assigned to each stimulus condition was counterbalanced among animals in each training group.
Discrimination training proceeded in a pseudorandom order, with no more than two consecutive drug or saline sessions. Correct responses on the drug or vehicle lever were reinforced on a FR 20 resetting schedule. Criteria for stimulus control included a minimum of 8 out of 10 consecutive training sessions in which the percentage of responses during the first FR and the percentage of responses for the total session were at least 80% on the injection-appropriate lever.
Stimulus Substitution Tests
Test sessions were similar to discrimination training sessions with the exception that no food pellets were delivered and sessions ended upon completion of the first FR or 20 min, whichever occurred first. Subjects were required to complete at least one D and at least one V session with 80% or higher injection-appropriate responding between test sessions. The order of drugs tested for substitution was the same for each training group, as follows: 4-MMC (0–3.0 mg/kg), MDPV (0–3.0 mg/kg), d-amphetamine (0–3.0 mg/kg), (+)-methamphetamine (0.03–3.0 mg/kg), (−)-cocaine (0–10.0 mg/kg), MDMA (0–3.0 mg/kg), (+)-LSD (0.01–0.2 mg/kg), and (+)-fenfluramine (0.1 – 3.0 mg/kg). Hereafter, the symbol to indicate the optical isomer of particular test drugs is excluded for ease of communication (except in the figures and d-amphetamine). Individual doses of each test drug were assessed in a counterbalanced order among subjects in each training group.
Data Analysis
Acquisition of stimulus discrimination was quantified as the number of sessions required for each subject to meet the criteria necessary to begin testing procedures. An independent samples t-test was conducted to analyze the effects of training dose on this measure. Results for stimulus substitution tests are expressed as the mean (±SE) percent 4-MMC-lever selection for each compound and dose tested in each training dose group. Percent 4-MMC-lever selection was determined as the number of responses emitted on the drug-lever as a percentage of the total number of responses emitted on both levers. Full substitution was defined as satisfying the dual criteria of ≥50% of subjects displaying ≥80% drug-lever selection and a statistically significant difference between the percent drug-lever selection observed with a dose of a test compound and the percent drug-lever selection observed with vehicle. Percent drug-lever selection that was statistically no different from vehicle was considered no substitution, and percent drug-lever selection that was statistically different from vehicle, but < 80%, was considered partial substitution. Statistical significance of a test compound’s capacity to substitute for the training drug cue was examined using a one-way repeated-measures (RM) analysis of variance (ANOVA) of rats completing the FR requirement at each test dose within each training group. Dunnett’s multiple comparisons tests were used to compare all doses of test drugs to vehicle. For drugs that satisfied the foregoing criteria for full substitution, a non-linear regression analysis was performed to estimate median effective dose (ED50) values (with normalized range set at 0–100%) with a 95% confidence interval (CI). Where applicable, ED50 values were compared between training groups using an independent-samples t-test. Response rate during stimulus substitution tests are plotted as the mean (±SE) number of responses per second, calculated for each session by dividing the total number of responses emitted per second by total session time. Subjects that failed to complete the FR20 schedule within the 20 minute test session were not considered in the calculation of percent drug-lever selection, but were included in the statistical analysis of response rates. Response rates were statistically analyzed using a RMANOVA within each training group. Dunnett’s multiple comparisons tests were used to indicate statistically significant differences from vehicle control. Statistical significance was declared at p <.052-tail for all statistical analyses. All statistical analyses and creation of figures were performed using GraphPad Prism version 7 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Discrimination Acquisition
An independent-samples t-test revealed that the 1.0 mg/kg 4-MMC training group (M = 37.4; SE = 5.66; range = 20–70) required a significantly greater number of discrimination training sessions to reach training criteria than the 3.0 mg/kg 4-MMC training group (M = 17.13; SE = 2.69; range = 12–32); [t(14) = 3.24, p = .006].
Stimulus Substitution
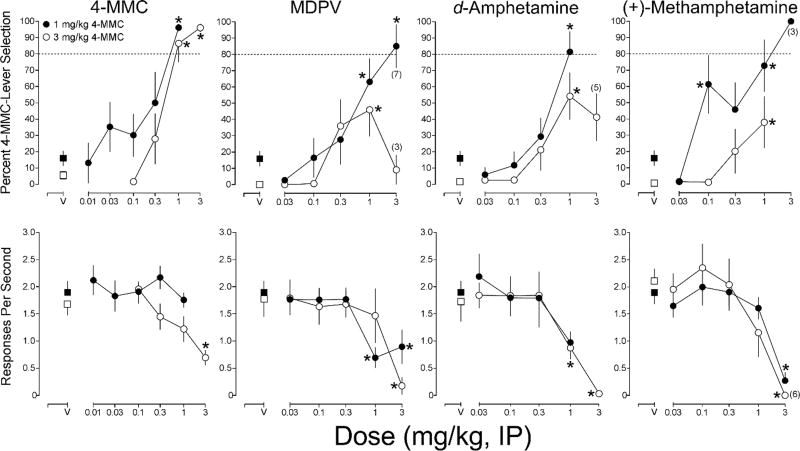
Figure 1 displays the results of drug substitution tests with 4-MMC, MDPV, d-amphetamine, and methamphetamine in each training group. The percentage of 4-MMC-lever responses is displayed in the upper graphs and response rate is depicted in the lower graphs. The statistically significant results of the Dunnett’s multiple comparisons tests on percent drug-lever selection or on response rate are also presented in Figure 1. In the 3.0 mg/kg 4-MMC training group, 4-MMC produced full substitution at 1.0 and 3.0 mg/kg [F(4, 28) = 24.05, p <.0001]. In the 1.0 mg/kg 4-MMC training group, 4-MMC produced full substitution at 1.0 mg/kg [F(5, 35) = 7.75, p < .0001].
Fig. 1.
Results of substitution tests with 4-MMC, MDPV, d-amphetamine, and (+)-methamphetamine in groups of male Sprague Dawley rats trained to discriminate 1 mg/kg (closed circles) or 3 mg/kg (open circles) 4-MMC. Upper graphs display the mean (± SE) percentage of responses on the 4-MMC lever. Lower graphs depict the mean (± SE) response rate. Numbers in parentheses indicate the number of animals included in the mean in instances when the N was less than 8. In some cases low variability was observed across subjects within a training group and the error bars are contained within a data point. Asterisks indicate statistically significant reductions in response rate compare to vehicle control for both groups combined (p < .05)
MDPV produced partial substitution for 3.0 mg/kg 4-MMC at 1.0 mg/kg [F(4, 28) = 5.50, p = .002]. In the 1.0 mg/kg 4-MMC group, MDPV produced full substitution at 3.0 mg/kg [F(6, 36) = 10.17, p <.0001]. This dose of MDPV produced behavioral disruption in five of the subjects trained to discriminate 3.0 mg/kg 4-MMC and in one subject trained to discriminate 1.0 mg/kg 4-MMC. Separate one-way ANOVAs revealed statistically significant effects of MDPV dose on response rate in the 3.0 mg/kg 4-MMC training group [F(5, 35) = 8.06, p <.0001], and in the 1.0 mg/kg 4-MMC training group [F(5, 35) = 4.89, p = .0017].
In the 3.0 mg/kg 4-MMC training group, d-amphetamine produced partial substitution at 1.0 mg/kg [F(5, 20) = 6.15, p = .0013]. In the 1.0 mg/kg 4-MMC training group, d-amphetamine produced full substitution at 1.0 mg/kg [F(4, 28) = 11.06, p <.0001]. A statistically significant effect of d-amphetamine dose on response rate was observed in the 3 mg/kg 4-MMC training group [F(5, 35) = 14.02, p <.0001].
Methamphetamine produced partial substitution for 3.0 mg/kg 4-MMC at 1.0 mg/kg [F(4, 28) = 3.31, p =.024] and 3.0 mg/kg methamphetamine produced behavioral disruption in all eight subjects in this training group. In the 1.0 mg/kg 4-MMC group, methamphetamine produced partial substitution at 0.1 and 1.0 mg/kg, but produced behavioral disruption in five subjects at 3.0 mg/kg. Separate one-way ANOVAs revealed statistically significant effects of methamphetamine dose on response rate in the 3.0 mg/kg 4-MMC training group [F(5, 25) = 13.97, p <.0001], and in the 1.0 mg/kg 4-MMC training group [F(5, 35) = 10.32, p <.0001].
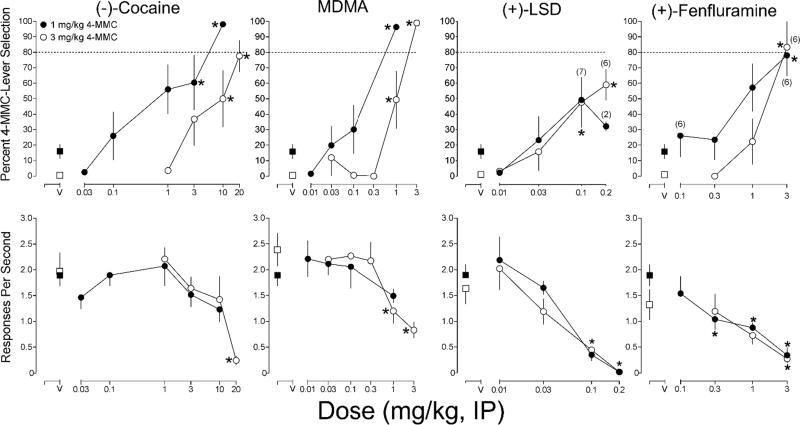
Results of stimulus substitution tests with cocaine, MDMA, LSD, and fenfluramine, and the statistically significant results of the Dunnett’s multiple comparisons tests on percent substitution and response rate are presented in Figure 2. Cocaine produced partial substitution at 10 and 20 mg/kg [F(4, 28) = 8.33, p =.0001], and 20 mg/kg produced behavioral disruption. In the 1.0 mg/kg 4-MMC group, cocaine produced full substitution at 10 mg/kg and partial substitution at 3 mg/kg [F(6, 42) = 11.23, p <.0001]. A statistically significant effect of cocaine dose on response rate was observed in the 3.0 mg/kg 4-MMC training group [F(4, 28) = 7.76, p =.0002]. MDMA produced partial substitution at 1.0 mg/kg and full substitution at 3.0 mg/kg in the 3.0 mg/kg 4-MMC group [F(5, 35) = 18.3, p <.0001]. In the 1.0 mg/kg 4-MMC group, MDMA produced full substitution at 1.0 mg/kg [F(4, 28) = 18.24, p <.0001]. A t-test comparing the ED50 values revealed that the ED50 in the 3.0 mg/kg 4-MMC group was greater than in the 1.0 mg/kg 4-MMC group [t(14) = 2.73, p = .02]. A statistically significant effect of MDMA dose on response rate was observed in the 3.0 mg/kg 4-MMC training group [F(5, 35) = 18.29, p <.0001].
Fig. 2.
Results of substitution tests with (−)-cocaine, MDMA, (+)-LSD, and (+)-fenfluramine in groups of male Sprague-Dawley rats trained to discriminate 1 mg/kg (closed circles) or 3 mg/kg (open squares) 4-MMC. See Fig. 1 caption for additional details
LSD produced partial substitution for 3.0 mg/kg 4-MMC at 0.1 and 0.2 mg/kg [F(3, 15) = 12.27, p =.0003]. In the 1.0 mg/kg 4-MMC group, LSD produced a statistically significant effect on percent 4-MMC-lever selection [F(3, 18) = 3.68, p =.03], but Dunnett’s multiple comparisons tests failed to reveal significant differences between LSD doses. Separate one-way ANOVAs revealed statistically significant effects of LSD dose on response rate in the 3.0 mg/kg 4-MMC training group [F(4, 28) = 12.07, p <.0001], and in the 1.0 mg/kg 4-MMC training group [F(4, 28) = 19.67, p <.0001]. Fenfluramine fully substituted for 3.0 mg/kg 4-MMC at 3.0 mg/kg [F(3, 15) = 12.27, p =.0003]. In the 1.0 mg/kg 4-MMC group, fenfluramine produced partial substitution at 3.0 mg/kg [F(4, 16) = 3.95, p =.02]. Separate one-way ANOVAs revealed statistically significant effects of fenfluramine dose on response rate in the 3.0 mg/kg 4-MMC training group [F(3, 21) = 4.98, p = .0092], and in the 1.0 mg/kg 4-MMC training group [F(4, 28) = 9.10, p <.0001]. A summary of peak effects and ED50 values determined with all substitution test drugs are presented in Table 1.
Table 1.
ED50 values and 95% confidence intervals of stimulus substitution test drugs in groups of rats trained to discriminate 1.0 or 3.0 mg/kg 4-MMC (IP) from saline
| Test Compound | ED50 (mg/kg) (95% CI) in 1.0 mg/kg 4-MMC group |
ED50 (mg/kg) (95% CI) in 3.0 mg/kg 4-MMC group |
|---|---|---|
|
| ||
| 4-MMC | 0.18 (0.08–0.38) | 0.50 (0.27–0.96) |
| MDPV | 0.63 (0.17–1.08) | ------ |
| d-Amphetamine | 0.48 (0.16–0.79) | ------ |
| (+)-Methamphetamine | ------ | ------ |
| Cocaine | 0.92 (0.20–1.64) | ------ |
| MDMA | 0.16 (0.07–0.36) | 1.77 (1.00–3.17)* |
| (+)-LSD | ------ | ------ |
| (+)-Fenfluramine | ------ | 1.96 (0.40–3.52) |
indicates a statistically significant difference between training groups
Discussion
This study successfully established stimulus discrimination with 4-MMC using a training dose of 1.0 or 3.0 mg/kg in separate groups of rats. Not surprisingly, stimulus control was established in significantly fewer sessions with the higher training dose. In contrast to the performance of the 3.2 mg/kg 4-MMC training group used in a study reported by Varner et al. (2013), the 3.0 mg/kg 4-MMC group in the present study required considerably fewer training sessions to acquire the discrimination of 4-MMC, but this comparison should be considered with caution. The training procedures used in the present study differ greatly from those used by Varner et al. (e.g., the present experiment included an errorless training component) that likely influenced the speed of discrimination acquisition. Moreover, those authors used Long-Evans rats as experimental subjects, whereas the present study used Sprague-Dawley rats. It is also worth noting that Varner et al. (2013) initiated training with a 0.56 mg/kg 4-MMC and some animals failed to acquire the discrimination. The training dose was subsequently increased to 1.8 and then to 3.2 mg/kg to establish stimulus control with all subjects.
While the current manuscript was under review, a study was published in abstract form indicating rats trained to discriminate 0.5 mg/kg or 3.2 mg/kg 4-MMC from saline required an average of 74 or 175 sessions to meet training criteria, respectively (Milewski et al. 2017). This finding conflicts with the current results, wherein stimulus discrimination was established in fewer sessions in rats trained to discriminate the higher dose. Other reports that used the structurally-related, ring-substituted phenethylamine, MDMA, as the training drug are partially consistent with number of sessions to criterion obtained in the present study. For example, Harvey and Baker (2016) reported that rats required an average of approximately 29 discrimination training sessions to discriminate 1.5 mg/kg from saline, an outcome that is comparable to the average of ~37 sessions observed in the 1.0 mg/kg 4-MMC group. Additional drug discrimination experiments that include experimental procedures comparable to those used herein are warranted to further evaluate discrimination acquisition of 4-MMC and related drugs.
The results of the present study revealed that stimulus substitution with test drugs varied with training dose and depended on rate-altering effects. In rats trained to discriminate 3 mg/kg 4-MMC, the dopaminergic stimulants MDPV, cocaine, methamphetamine, and d-amphetamine produced only partial substitution and disrupted response rate at the highest doses tested. The serotonergic entactogen, MDMA produced complete substitution for 3 mg/kg 4-MMC, although at a dose that also significantly decreased response rate. These results are consistent with those of Varner et al. (2013), who reported that MDMA engendered >80% drug-appropriate responding, but also decreased response rate at the highest dose tested. In addition, previous studies in which rats were trained to discriminate 3.2 mg/kg 4-MMC from saline, substitution tests with cocaine, MDPV, d-amphetamine, and methamphetamine produced only partial substitution and rate-decreasing effects (Varner et al. 2013; DeLarge et al. 2017). Contrariwise, rats trained to discriminate 1 mg/kg 4-MMC in the present study displayed complete generalization to a wider range of drug stimuli than those trained to discriminate 3 mg/kg. Full substitution was observed with 4-MMC, cocaine, d-amphetamine, and MDMA in rats trained to discriminate the lower dose, while only partial substitution and rate disruption was observed with MDPV, methamphetamine, and fenfluramine. The serotonergic hallucinogen, LSD, produced partial substitution or no substitution in the 3 mg/kg or 1 mg/kg 4-MMC training group, respectively.
Slightly greater substitutability of test drugs in the 1 mg/kg 4-MMC training dose group appear to be consistent with previous drug discrimination study findings that lower training doses often produce greater sensitivity compared to relatively higher training doses using drugs from various pharmacological classes (e.g., Stolerman, 2011). Potency differences between training dose groups were only observed with MDMA. A possible explanation for this difference is that the interoceptive cue produced by the training drug changed over time in one of the training groups. Unfortunately, the dose-response curves for 4-MMC were not re-determined throughout testing to evaluate this possibility. A second explanation is that rats trained to discriminate 1 mg/kg 4-MMC were more likely to display a broader stimulus generalization gradient compared to rats trained to discriminate 3 mg/kg 4-MMC. This interpretation is supported by a recent report indicating higher substitution values with DA agonists in rats trained to discriminate 0.5 mg/kg 4-MMC compared to rats trained to discriminate 3.2 mg/kg 4-MMC (Milewski et al. 2017). Another possible explanation for the observed differences in substitution of test drugs between training groups are differential changes in neural plasticity due to prolonged exposure to each training dose of 4-MMC. Mayerhofer et al. (2001) demonstrated that repeated exposure to the structurally- and pharmacologically-similar drug MDMA produced changes in brain monoamine levels for at least four weeks following cessation of MDMA treatment. Moreover, Khorona et al. (2004) speculated that repeated injections of MDMA, but not cocaine, may account for the asymmetrical substitution observed in groups of rats trained to discriminate 1.5 mg/kg MDMA or 8 mg/kg cocaine and subsequently tested for generalization to cocaine or MDMA, respectively. Thus, the differences in substitution between the training groups in the present study may be due to the long-term effects of 4-MMC on neurochemical transmission. Future studies investigating the effects of repeated 4-MMC exposure on brain monoamine levels would be necessary to evaluate this possibility.
Peak drug-lever responses differed among the groups, as evidenced by the relatively greater number of drugs producing full substitution in the 1 mg/kg 4-MMC group compared to the 3 mg/kg 4-MMC group. However, there were reductions in response rate by several test drugs that produced full substitution when compared to saline. That is, substitution of a test compound increased as its effects on response rate decreased. Thus, it appears that rats trained to discriminate 1 mg/kg 4-MMC were less likely to display behavioral disruption following substitution tests than rats trained to discriminate 3 mg/kg 4-MMC, probably because the response rate produced by 3 mg/kg 4-MMC was already relatively low (i.e., a floor effect). Nevertheless, this study is the first to demonstrate that rats can learn to discriminate 1 mg/kg 4-MMC, and in so doing, may display stimulus generalization to a broader range of drugs because of increased resistance to the rate-decreasing effects of drugs, slightly different interoceptive cues (when compared to a higher training dose), or a combination of these factors. Future experiments should use lower training doses of 4-MMC (e.g., Milewski et al. 2017) to further broaden these findings and to determine if resistance to the rate-decreasing effects of test drugs varies with training dose.
In conclusion, efforts to learn more about drug actions on the CNS are an invaluable part of addressing the global crisis of drug abuse and addiction. As the technological capability of synthesizing novel drugs becomes more readily accessible worldwide, the need for pharmacological investigations of such substances must not be overlooked. Although the synthetic cathinones are no longer considered novel, their pharmacological effects are not yet fully understood. In order for healthcare professionals to adequately address cases involving synthetic cathinone abuse, determined scientific inquisition of these substances must continue. The current study adds to the existing literature on the interoceptive stimulus effects of 4-MMC and presents valuable data regarding the dose-dependent similarities to other commonly abused psychostimulants. Further characterization of 4-MMC’s in vivo pharmacological activities with receptor-selective antagonists can help elucidate the mechanisms underlying the subjective and abuse-related effects of this and related synthetic cathinones.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (R15DA038295). The National Institute on Drug Abuse drug control supply program provided several of the test drugs used in this study.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Aarde SM, Deepshikha A, Barlow DJ, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, Felice L, Glennon R. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 2013;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarge AF, Erwin LL, Winsauer PJ. Atypical binding at dopamine and serotonin transporters contribute to the discriminative stimulus effects of mephedrone. Neuropharmacology. 2017;119:62–75. doi: 10.1016/j.neuropharm.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Agency (DEA) Federal Register. 204. Vol. 76. Washington, DC: U.S. Government Printing Office; 2011. Oct 21, Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cathinones Into Schedule I; pp. 65371–65375. [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1016/j.drugalcdep.2014.02.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, Mcfadden LM, et al. 4-Methylmethcathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Baker LE. Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine + MDMA mixture. Psychopharmacology (Berl) 2016;233:673–680. doi: 10.1007/s00213-015-4142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana N, Pullagurla MR, Young R, Glennon RA. Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: asymmetric generalization. Drug Alcohol Depend. 2004;74:281–287. doi: 10.1016/j.drugalcdep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, et al. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: Butylone, mephedrone, and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Kovar KA, Schmidt WJ. Changes in serotonin, dopamine and noradrenaline levels in striatum and nucleus accumbens after repeated administration of the abused drug MDMA in rats. Neurosci Lett. 2001;308:99–102. doi: 10.1016/S0304-3940(01)01992-9. [DOI] [PubMed] [Google Scholar]
- Milewski AJ, Rawls S, Walker EA, Saber I. Exploring the pharmacologic and discriminative effects of abused psychostimulants and the bath salt component mephedrone in rats. FASEB J. 2017;31:987.10. [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, et al. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: Neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8. Washington D.C.: The National Academies Press; 2011. [Google Scholar]
- Stolerman IP, Childs E, Ford MM, Grant KA. Role of training dose in drug discrimination: A review. Behav Pharmacol. 2011;22:415–429. doi: 10.1097/FBP.0b013e328349ab37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente MJ, Guedes de Pinho P, de Lourdes Bastos M, Carvalho F, Carvalho M. Khat and synthetic cathinones: A review. Arch Toxicol. 2014;88:15–45. doi: 10.1007/s00204-013-1163-9. [DOI] [PubMed] [Google Scholar]
- Varner K, Daigle K, Weed P, Lewis P, Mahne S, Sankaranarayanan A, Winsauer P. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl) 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]