Abstract
Background:
Our previous study showed antidiabetic effect of aqueous extract of Solanum nigrum Linn fruit (SNE).
Objective:
This study was designed to explore the antidiabetic and nephroprotective effects of SNE in diabetic rats.
Materials and Methods:
Animals were divided into nine groups to undergo two experiment protocols: Two groups served as nondiabetic controls (NDCs), while the other groups had diabetes induced with a single injection of streptozotocin. SNE-treated diabetic (D-SNE) and SNE-treated controls (NDC-SNE) received 1 g/L of SNE added to the drinking water and insulin-treated diabetic (D-I) for 8 weeks. Furthermore, there were four groups (D-SNE, NDC-SNE, D-I, D) in the second protocol to examine diabetic nephropathy (DN) for 16 weeks. Blood urea nitrogen (BUN), creatinine (Cr) magnesium, nitric oxide (NO), and malondialdehyde (MDA) levels were measured. Both kidneys were isolated to measure MDA, NO levels, and renal damage.
Results:
SNE could decrease blood glucose level in diabetic rats. In addition, SNE was more effective than insulin in controlling blood glucose. SNE could decrease BUN, Cr levels, and kidney weight and damage after 8 and 16 weeks of administration. Plasma and kidney levels of NO and MDA also decreased.
Conclusion:
Our results support the hypothesis that SNE could play a role in the management of diabetes and the prevention of DN.
SUMMARY
The aqueous extract of Solanum nigrum Linn fruit (SNE) (1 g/L via drinking water) was studied on streptozotocin-induced diabetic rats to prevent diabetic nephropathy (DN). The results suggest that SNE in addition to the management of diabetes could have a beneficial effect on the prevention of DN.
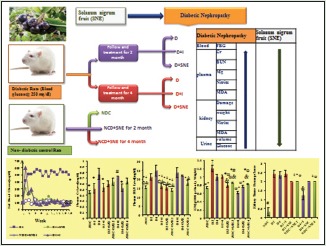
Abbreviations Used: SNE: Extract of Solanum nigrum Linn fruit, NDCs: Nondiabetic controls, STZ: Streptozotocin, D-SNE: SNE-treated diabetic, NDC-SNE: SNE-treated controls, D-I: Insulin-treated diabetic, BUN: Blood urea nitrogen, Cr: Creatinine, Mg: Magnesium, NO: Nitric oxide, MDA: Malondialdehyde, DN: Diabetic nephropathy, BW: Body weight, FBG: Fed blood glucose, KW: Kidney weight, TBA: Thiobarbituric acid, IPGTT: Intraperitoneal glucose tolerance test, AUC: Aria under the curve, GFR: Glomerular filtration rate.
Key words: Blood glucose, blood urea nitrogen, creatinine, diabetic nephropathy, malondialdehyde, Solanum nigrum fruit
INTRODUCTION
Diabetic nephropathy (DN) is described by a progressive decline in renal function, proteinuria, and hypertension. The main agent of declining renal function in DN may be mesangial cell changes[1] and thickening of the basement membrane.[2,3,4,5] In addition, tubulointerstitial fibrosis may occur in DN.[3,6] Usage of medicinal plants is recommended for prevention and treatment of diabetes because they have fewer side effects.[7,8] Solanum nigrum Linn (henceforth called S. nigrum from family Solanaceae) is a common herb that grows wildly in open fields. The morphological properties of S. nigrum have been reported in various articles.[9,10,11,12,13] It is commonly called Makoi or black nightshade.[13] The plant is easily found in most parts of Iran, India, and Southern Europe.[10,11]
There are abundant nutrients in the plant such as minerals, vitamins, proteins, and certain hormone precursors.[14] The plant possesses a wide spectrum of pharmacological properties and acts as antioxidant, anticancer, hepatoprotective, neuroprotective, antiulcerogenic,[15] sedative, diaphoretic, diuretic,[16] and tuberculosis.[17] Furthermore, it has been traditionally used in treating various ailments such as pain, inflammation, fever, cough, cold, asthma, skin diseases, liver and heart problems,[9,11] and dysentery.[16] The leaves are detected to possess hypotensive effect.[16] Furthermore, the researchers showed that administration of S. nigrum leaves to diabetic rats caused antihyperglycemic and hypolipidemic effects.[18] Recent studies have shown that administration of S. nigrum leaves in normoglycemic and diabetic rats could decrease blood glucose level and leads to an increase in insulin secretion while improving the antioxidant defense in diabetic rats.[19] Alkaloids and solanine of aqueous extract of S. nigra may be responsible for antidiabetic effects of the plant.[20]
The researchers have reported antioxidant properties of methanolic extract of S. nigrum (SNE) berries on aspirin-induced gastric mucosal damage.[16] Furthermore, antioxidant, antihyperlipidemic, and nephroprotective effects of aqueous SNE against ethanol-induced toxicity have been reported.[11,15] Moreover, the researchers recommended that the water extract has better hepatoprotective effects than the methanolic one.[21] Singh et al. have shown a successive increase in the content protein of liver and kidney after daily administration of S. nigrum.[22] In addition, different concentrations of S. nigrum significantly protected gentamicin-induced kidney cell damage.[23] Antidiabetic SNE was reported in our previous studies,[10,24] but as far as the researchers are aware, there is limited, if any, documented information about its nephroprotective effects against DN. Thus, the current study was designed to explore the antidiabetic and nephroprotective effects of aqueous SNE in streptozotocin (STZ)-induced diabetic rats.
MATERIALS AND METHODS
Drugs
The drugs used in this study were STZ, purchased from Sigma-Aldrich (St. Louis, MO, USA) which was dissolved in normal saline immediately before use as well as insulin (regular) and insulin isophane (NPH), 100 units/ml, which were provided from Exir Pharmaceutical Co.
Experimental protocol
Male Wistar rats (weighting 180–220 g) were kept at a constant temperature of 23°C–25°C and 12 h light/12 h dark cycle and free access to water and rat chow. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the European Commission Directive 86/609/EEC for animal experiments. The animals were assigned into nine experimental groups (10 rats in each group) to undergo two experiment protocols. Initially, five groups were probated based on the first protocol which was investigating DN for 8 weeks after diabetes induction, in the exact order listed below:
Group 1: D2: diabetic rats, Group 2: D2+I: insulin-treated diabetic rats, Group 3: nondiabetic control (NDC)+SNE2: nondiabetic control rats treated with SNE, Group 4: D2+SNE: diabetic rats treated with SNE, Group 5: NDC: nondiabetic control rats. Furthermore, there were four groups in the second protocol to examine DN for 16 weeks after diabetes induction which are Group 6: D4: diabetic rats, Group 7: D4+SNE: diabetic rats treated with SNE, Group 8: NDC+SNE4: NDC rats treated with SNE, Group 9: D4+I: insulin-treated diabetic rats.
At first, body weight (BW) and fed blood glucose (FBG) of all animals were measured, and then, diabetes was induced with a single (IP) injection of 60 mg/kg STZ, in both protocols. Ten days after STZ administration, FBG level was determined through a tail vein using glucometer (Ascensia Elite XL) and blood glucose test strips (AscensiaElit). Afterward, the rats with FBG above 250 mg/dl were considered diabetic and were selected for the next steps of the experiment. FBG concentration and BW of all rats were recorded throughout the period on a weekly basis. Furthermore, nondiabetic and diabetic rats treated with SNE received 1 g/L of SNE added into the drinking water from 10th day onward.[10,24] As regards insulin-treated diabetic rats, they were injected with 2.5 U/kg insulin (a mixture solution from 1/2 ml regular and 1/2 ml NPH) in a determined time of morning, and then, the other injection was done using the same dosage twice a day, starting in the morning with a 12-h interval.[10,24]
Finally, in the end of the study period (late - 16th and 8th week), all animals were decapitated following anesthesia with the mixture of ketamine HCL (50 mg/kg i.p) and xylazine (10 mg/kg, i.p), and then, a blood sample was taken from the neck vascular trunk. Both kidneys were removed and weighted. The kidney weight (KW) was normalized to the BW and reported as tissue weight (g)/100 g of BW. The left kidney was used for biochemical measurements. Besides, the right kidney was fixed in 10% formalin solution for pathological assessment. Furthermore, urine sample which remained in the bladder was collected to measure volume, glucose, and protein (Combi-screen 10SL urine test).
Biochemical assay
Blood urea nitrogen (BUN), plasma creatinine (Cr), and magnesium (Mg) levels were determined using quantitative kits (Pars Azmoon, Iran). Plasma and kidney nitrite levels (stable, with nitric oxide [NO] metabolite) were measured using a colorimetric assay kit (Promega Corporation, USA) and Microplate Absorbance Reader (anthos 2020). Malondialdehyde (MDA) levels of the plasma and homogenized kidney supernatant were quantified according to the thiobarbituric acid (TBA) method.[25]
Intraperitoneal glucose tolerance test
For doing intraperitoneal glucose tolerance test, animals in the NDC, D4, D4+I, D4+SNE, and NDC+SNE4 groups were fasted overnight for15 h, and then, they were given 1.5 g/kg bodyweight glucose, through IP injection. Blood was drawn from the tail vein at 0, 10, 20, 30, 60, 90, and 120 min after glucose administration.[10,26]
Histopathological procedures
Paraffin-embedded tissues were used for histopathological staining. The hematoxylin and eosin stain were applied to examine the tissue injury. To consider the kidney damage, the presence of tubular atrophy, fibrosis, connective tissue changes, inflammation, degeneration of tubular epithelial, congestion, and glomerular damage was evaluated. Based on the damage intensity, the samples were scored from 1 to 4 while score zero was assigned to normal tissue.
Preparation of Solanum nigrum extract
The S. nigrum is abundantly found in open grasslands of Southern Iran, and in this study, the specimens were taken from an area around the city of Bandar Abbas. Following identification by a Shahid Beheshti University of Medical Sciences taxonomist, 1 kg of S. nigrum fruit, dried for 2 weeks under shade at room temperature (26°C ± 1°C), was ground into powder. Deionized water was added to the powder to get the fruit extract through boiling and continuously stirring the suspension for 15 min. Postfilteration (filter paper by Whatman No. 1) yield of the extract was 21% for the sample dried at 80°C until constant weight was achieved. Before use, the concentrated extract and fraction as stock were frozen (−20°C) in a desiccant. For every gram of S. nigrum fruit, the extract dry matter weighed 75.7 mg.
Gas chromatography/mass spectrometer analysis
A mass spectrometer (MS) connected to a gas chromatography (GC) system (GC/MSD7890A, 7000 Triple Quad series Agilent), equipped with a HP-5MS capillary column (30 mm × 0.25 mm; film thickness of 0.25 μm) was utilized to analyze the aqueous extract. The carrier gas used was helium at a flow rate of 1 ml/min. The temperature of the GC oven was set to increase from 50°C to 260°C at a rate of 10°/min and then was kept at 260°C for 26 min. Following MS at 1 scan s-1 with ionizing voltage of 70 V and ion source temperature of 280°C, the separated compounds were compared for retention times with authentic standards injected under the same chromatographic conditions. This comparison of the retention indices and mass spectra of the unknown peaks with the MS library helped identify the compounds.
Statistical analysis
Data are presented as mean ± standard error of mean; differences among groups were compared with Mann–Whitney or Kruskal–Wallis, one- and two-way ANOVA with the Turkey post hoc test. Values of P < 0.05 were considered statistically significant.
RESULTS
Identification of compounds in the extract
RT-1
RT-2
RT-3
RT-4 hydroxylamine, O-(-2-methylpropyl)-
RT-5 1, 6: 3,4-dianhydro-2-deoxy-b-d-ribo-hexopyranose
RT-6 13-docosenamide, (z)-
RT-7 1,2-benzenedicarboxylic acid, diisooctyl ester.
Changes in blood glucose level
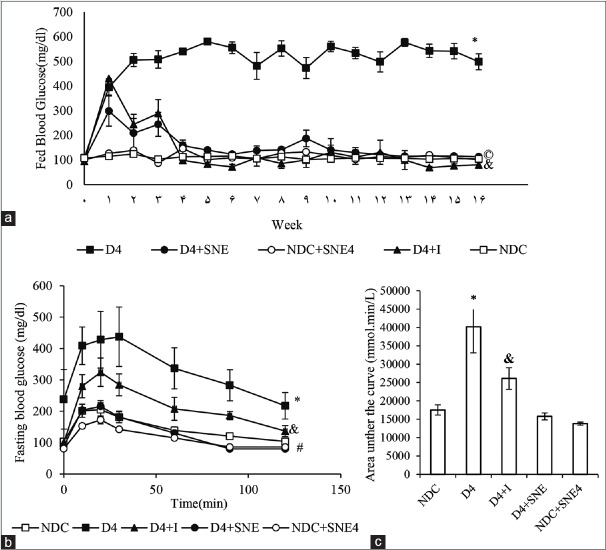
Administration of SNE and insulin for 16 weeks (from day 10 onward) significantly decreased blood glucose level in diabetic groups [Figure 1a].
Figure 1.
Comparison of weekly-fed blood glucose (a) and intraperitoneal glucose tolerance Test (IPGTT) (b and c). #P < 0.01 Significant difference between NDC group and the other groups. *P < 0.01 Significant difference between D4 group and the other groups, & P < 0.05 D4+I vs. the other groups, ©P < 0.05D4+SNE vs. the other groups.
Effect of extract of Solanum nigrum Linn fruit on intraperitoneal glucose tolerance test
Sixteen weeks after administration of SNE, insulin and STZ the D4 group displayed severe intolerance with high area under the curve (AUC), which was significantly improved in the D4+SNE and D4+I groups to a degree that was judged effective [Figure 1b and c].
Changes in plasma creatinine and blood urea nitrogen levels
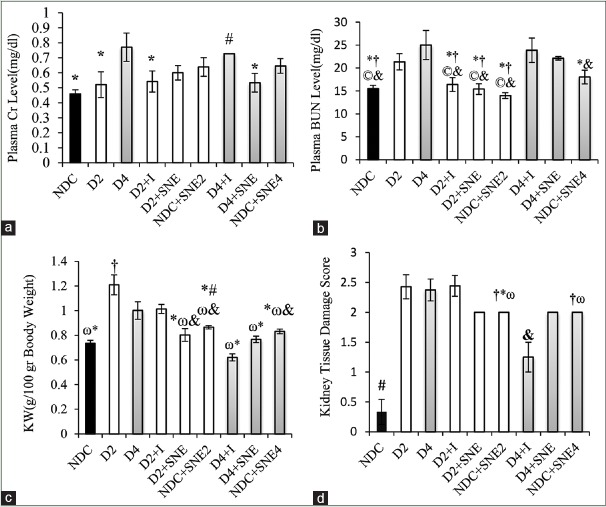
For 4 months, diabetes induction could significantly increase plasma Cr and BUN levels (P < 0.05). Plasma Cr level had no marked change within 2 months although plasma level of BUN showed a substantial rise during 2 months after diabetes induction.
Plasma Cr level improved considerably in D4+SNE group as compared to D4 group (P < 0.05). Meanwhile, there was no difference in plasma Cr level between D4+I and D4 groups, whereas it had a marked increase compared with NDC group (P < 0.05). Furthermore, plasma Cr level had no significant difference between NDC, D2, D2+I, D2+SNE, and ND+SNE2 groups. Plasma BUN level decreased similarly in D2+I and D2+SNE groups versus D2, D4, D4+I, and D4+SNE groups (P < 0.05) [Figure 2a and b].
Figure 2.
(a) Plasma levels of Cr and (b) BUN, (c) kidney tissue damage score, (d) KW (kidney weight). *P < 0.05 D4 group vs. other groups, #P < 0.05 NDC group vs. the other groups, & P < 0.05 D4+I vs. the other groups, †P<0.05 D2 group vs. the other groups, ©P < 0.05 D4+SNE vs. the other groups, ωP < 0.05 D2+I vs. the other groups.
Changes in weight and histopathology score of kidney tissue
The enlarged KW was detected in D2 group when compared with other groups (P < 0.05). Furthermore, KW of D4 and D2+I groups was markedly higher than NDC, D2+SNE, ND+SNE2, D4+I, D4+SNE, and ND+SNE4 groups (P < 0.05). In addition, in D4+I group, KW was lower than D2+SNE, ND+SNE2, and ND+SNE4 (P < 0.05) [Figure 2c].
The kidney injury of NDC and D4+I groups was significantly lower than the other groups (P < 0.05). In addition, there was no significant difference between D2, D4, D2+I, D2+SNE, and D4+SNE [Figures 2d and 3].
Figure 3.
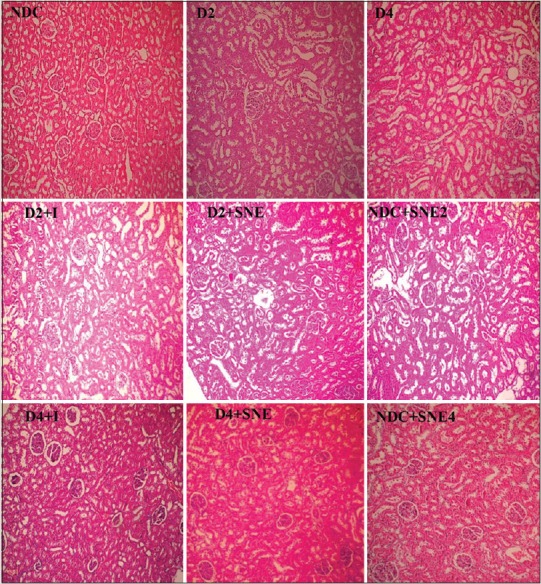
The images of kidney tissues (Magnification ×100) in all groups.
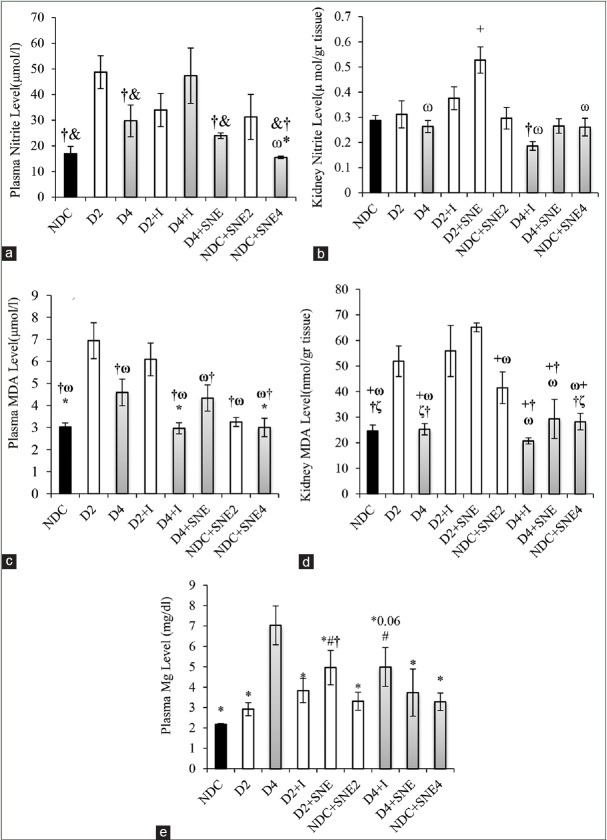
Changes in nitrite and malondialdehyde levels of plasma and kidney
The plasma nitrite levels increased significantly in the D2 group when compared with the NDC group, while the plasma nitrite level in D4 group was markedly decreased compared to D2 group. The plasma nitrite level of D4+I group dramatically rises in comparison with the other groups during the 16 weeks (P < 0.05). Administration of SNE during 16 weeks had no significant effect on the plasma nitrite level [Figure 4a]. The kidney nitrite levels in D2+SNE group were significantly higher than the other groups (P < 0.05). This factor was approximately the same in the 16-week period in all groups and the kidney nitrite levels in these groups did not change compared to NDC group [Figure 4b].
Figure 4.
(a) Plasma levels of Nitrite, (b) kidney levels of Nitrite, (c) plasma MDA level, (d) kidney MDA level and (e) plasma Mg level. *P < 0.05 D4 group vs. other groups, #P < 0.05 NDC group vs. other groups, †P < 0.05 D2 group vs. other groups, & P < 0.05 D4+I group vs. other groups, ωP < 0.05 D2+I group vs. VS the other groups, +P < 0.05 D2+SNE vs. other groups, ζP < 0.05 NDC+SNE2 vs. other groups.
The plasma and kidney MDA levels of D2 group were markedly higher than NDC and D4 groups (P < 0.05). Furthermore, the plasma MDA level in D4 group significantly increased in comparison with NDC group, but there was not a significant change in the kidney MDA level of D4 compared to NDC group [Figure 4c and d]. The plasma MDA concentration in D2+I group did not substantially change compared with D2 group. Moreover, that was markedly higher than the plasma MDA level of NDC group (P < 0.05) [Figure 4c]. In kidney tissue, the MDA level of D2+I and D2+SNE groups had no significant difference when compared to D2 group (P < 0.05). Furthermore, the amount of MDA of D2+I, D2+SNE, and ND+SNE2 groups were significantly more than the MDA level of NDC group (P < 0.05). The MDA level of NDC, D4, D4+I, D4+SNE, and NDC+SNE4 groups was approximately the same although extract consumption of S. nigrum could significantly decrease the MDA level in NDC+SNE4 group compare to NDC+SNE2 [Figure 4d].
Changes in plasma level of magnesium
The plasma Mg concentration significantly rose in D4 group (P < 0.05), but there were no significant differences in plasma Mg level in D2, D2+I, NDC+SNE2, D4+SNE, and NDC+SNE4 groups compared with NDC group. Eight weeks after SNE treatment, plasma Mg level substantially increased in D2+SNE group as compared with NDC and D2 groups (P < 0.05). Plasma Mg level in D4+SNE and D4+I groups decreased in comparison with D4 group. In addition, Mg level in D4+I group was significantly higher than NDC group [Figure 4e].
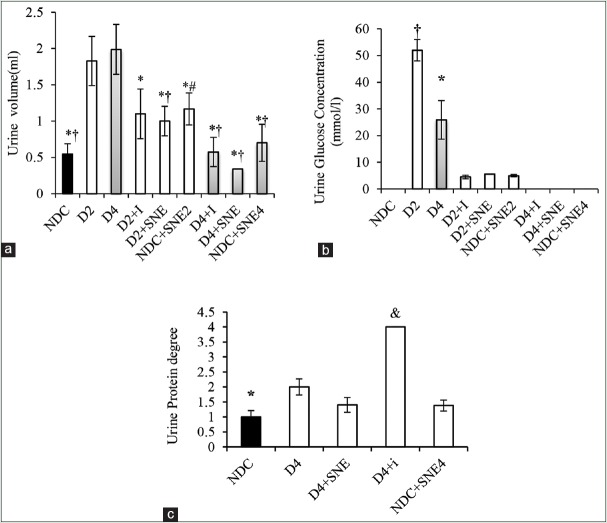
Changes in glucose, protein concentrations, and urine volume
In D2 and D4 groups, the urine volume showed a sharp rise compared with NDC group (P < 0.05). Insulin administration in D4+I and D2+I diminished the amount of urine. Furthermore, urine volume was markedly decreased by SNE in D2+SNE and D4+SNE compared to D2 and D4 groups (P < 0.05) [Figure 5a].
Figure 5.
Urine volume (a), urine glucose concentration (b) and urine protein degree (c) in NDC Group (non- diabetic control rats), D2 (Diabetic rats for 8 weeks), D2+I Group (Insulin - treated diabetic rats for 8 weeks), NDC+SNE2 (S. nigrum extract – treated non-diabetic rats for 8 weeks, D2+SNE (S. nigrum extract–treated diabetic rats for 8 weeks), D4 (diabetic rats for 16 weeks), D4+SNE (S. nigrum extract–treated diabetic rats for 16 weeks), NDC+SNE4 (S. nigrum extract–treated non-diabetic rats for 16 weeks), and D4+I (Insulin treated diabetic rats for 16 weeks). *P < 0.05 D4 group vs. other groups, #P < 0.05 NDC group vs. other groups, & P < 0.05 D4+I vs. other groups, †P < 0.05 D2 vs. other groups.
Diabetes dramatically rose the urine glucose concentration in D2 and D4 groups (P < 0.05). Urine glucose concentration in D2+I, D2+SNE, NDC+SNE2, D4+I, D4+SNE, and NDC+SNE4 groups was considerably less than D2 and D4 groups (P < 0.05). Moreover, there was no difference between these groups and NDC group [Figure 5b].
Diabetes could markedly increase urine protein degree in D4 (P < 0.05). D4+I group had the highest concentration of urine protein when compared with the other groups throughout the 16 weeks. There was no significant difference in urine protein level between NDC, D4+SNE, and NDC+SNE4 groups. Furthermore, this factor had no difference between D4 and D4+SNE groups [Figure 5c].
DISCUSSION
The present study evaluated therapeutic effects of aqueous SNE as an antioxidant and nephroprotective substance against DN. Treatment with insulin and SNE for 8 and 16 weeks could decrease FBG in diabetic rats. In addition, SNE was more effective than insulin in controlling blood glucose, according to AUC result. These data are in agreement with our previous studies.[10,24]
In the present study, nephropathy induction is successfully confirmed in uncontrolled diabetic rats form 8th week according to literature.[27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] Kidney tissue damages such as lymphocytic infiltration, capillary congestion, degeneration of some tubular epithelial cells, slight connective tissue formation, mild fibrosis, and severe tubular atrophy were seen. Plasma Cr concentration had a significant increase 16 weeks after diabetes induction, but plasma BUN level showed a marked rise from 8th week.
Moreover, elevation of BUN can reflect decreasing in glomerular filtration rate (GFR)[29,32] from 8th week after diabetes induction. In fact, both BUN and Cr levels are hallmark of DN,[35] but BUN is a more sensitive marker for kidney injury.[34] In the present study, KW/BW ratio of uncontrolled diabetic rats, an indicator of glomerular expansion due to diabetes,[43,44] increased markedly compared to NDC group, but this figure decreased in D4 group in comparison with D2 group. Renal hypertrophy in type 1 diabetes was reported,[45] and Avram and Hurtado showed a negative correlation between kidney size and serum Cr level during uncontrolled diabetes.[46] In fact, in the initial stages of DN, renal hypertrophy occurs and it can persist for years despite glucose control. However, the kidneys become smaller with advanced renal insufficiency.[47,48,49]
In our study, treatment of DN by insulin and SNE could decrease weight of enlarged kidneys throughout the period of study, but SNE was more successful than insulin in 8 weeks. Kidney tissue damage score decreased by SNE and insulin administration in D2 and D4 groups. Insulin and SNE administration could decrease plasma BUN level after 2 months. Furthermore, SNE diminished Cr level, whereas insulin did not change it. Both SNE and insulin could improve urine volume and urine glucose concentration. In addition, urine protein became less by SNE, but insulin treatment increased it significantly. Our previous results showed that aqueous fruit extract of S. nigrum could improve plasma lipid profiles. In addition, we showed that the administration of SNE could decrease the alteration in vascular reactivity to vasoconstrictor agents and also could decrease vessel atherosclerosis[10] as cardiovascular complications of diabetes.[50] Moreover, researchers have shown antioxidant and antihyperlipidemic activity of S. nigrum fruit extract by decreasing the level of TBA, increasing Vitamin E, C, and reduced glutathione in kidney tissue, and altering the lipid profiles to near-normal including decreasing free fatty acid and improved kidney phospholipids. Furthermore, S. nigrum fruit diminished levels of urea, uric acid, and Cr in kidney against ethanol-induced toxicity.[11,15]
In various studies, the relationship between oxidative stress and DN has been demonstrated.[27,34,37,51,52,53] Furthermore, they have shown high MDA (as a marker of lipid peroxidation) and low antioxidant enzyme levels in renal homogenates of diabetic rats and in mesangial cells.[27,32,34,35,37,51,52,53] On the other hand, abnormalities of vasodilatation and generates reactive oxygen species mediated by endothelial cell is recognized in DN.[28,42] In our study, nitrite and MDA levels elevated both in serum and kidney tissues in D2 animals which are in agreement with previous studies,[27,32,34,35,37,51,52,53] but they decreased after 4 months due to increasing plasma Mg level and content limitation of L-arginine, a precursor of NO.[54] As a result, reduction of the nitrite production causes a drop in the amount of peroxynitrite. Thus, MDA concentration in the end of 4 months is lower than before. Continuation of diabetes for 4 months has increased serum Mg level in our study that can be as a result of sharp decline of GFR. Nitrite and MDA levels of kidney decreased significantly in D4+I and D4+SNE compared to D2+I and D2+SNE groups. Moreover, SNE and insulin administration in D4+SNE and D4+I groups could decrease plasma Mg level. Finally, the results indicate that aqueous fruit extract of S. nigrum was better than insulin in improving STZ-induced kidney damage and our results support the hypothesis that SNE could play a role in prevention of DN.
Financial support and sponsorship
This study was supported by Hormozgan University of Medical Science.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors of this study thank the physiology department for use of their laboratory equipment.
REFERENCES
- 1.Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–81. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–8. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziyadeh FN. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993;22:736–44. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchida K, Makita Z, Yamagishi S, Atsumi T, Miyoshi H, Obara S, et al. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999;42:579–88. doi: 10.1007/s001250051198. [DOI] [PubMed] [Google Scholar]
- 5.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–55. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Kleppel MM, Butkowski R, Mauer SM, Wieslander J, Michael AF. Differential expression of basement membrane collagen chains in diabetic nephropathy. Am J Pathol. 1991;138:413–20. [PMC free article] [PubMed] [Google Scholar]
- 7.Peesa JP. Nephroprotective potential of herbal medicines: A review. Asian J Pharm Technol. 2013;3:115–8. [Google Scholar]
- 8.Sila A, Ghlissi Z, Kamoun Z, Makni M, Nasri M, Bougatef A, et al. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur J Nutr. 2015;54:301–7. doi: 10.1007/s00394-014-0711-2. [DOI] [PubMed] [Google Scholar]
- 9.Aali NS, Singh K, Khan ML, Rani S. Protective effect of ethanolic extract of Solanum nigrum on the blood sugar of albino rats. Int J Pharm Sci Res. 2010;1:97–9. [Google Scholar]
- 10.Sohrabipour S, Kharazmi F, Soltani N, Kamalinejad M. Effect of the administration of Solanum nigrum fruit on blood glucose, lipid profiles, and sensitivity of the vascular mesenteric bed to phenylephrine in streptozotocin-induced diabetic rats. Med Sci Monit Basic Res. 2013;19:133–40. doi: 10.12659/MSMBR.883892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankaran M. Protective effect of Solanum nigrum fruit extract on the functional status of liver and kidney against ethanol induced toxicity. J Biochem Technol. 2012;3:339–43. [Google Scholar]
- 12.Atanu FO, Ebiloma UG, Ajayi EI. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol Mol Biol Rev. 2011;6:1–7. [Google Scholar]
- 13.Potawale SE, Sinha SD, Shroff KK, Dhalawat HJ, Boraste SS, Gandhi SP, et al. Solanum nigrum Linn: A phytopharmacological review. Pharmacologyonline. 2008;3:140–63. [Google Scholar]
- 14.Dhellot JR, Matouba E, Maloumbi MG, Nzikou JM, Dzondo MG, Linder M, et al. Extraction and nutritional properties of Solanum nigrum L. seed oil. Afr J Biotechnol. 2006;5:987–91. [Google Scholar]
- 15.Arulmozhi V, Krishnaveni M, Karthishwaran K, Dhamodharan G, Mirunalini S. Antioxidant and antihyperlipidemic effect of Solanum nigrum fruit extract on the experimental model against chronic ethanol toxicity. Pharmacogn Mag. 2010;6:42–50. doi: 10.4103/0973-1296.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jainu M, Devi CS. Antioxidant effect of methanolic extract of Solanum nigrum berries on aspirin induced gastric mucosal injury. Indian J Clin Biochem. 2004;19:57–61. doi: 10.1007/BF02872391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravi V, Saleem TS, Maiti PP, Gauthaman K, Ramamurthy J. Phytochemical and pharmacological evaluation of Solanum nigrum Linn. Afr J Pharm Pharmacol. 2009;3:454–7. [Google Scholar]
- 18.Poongothai K, Ahmed K, Ponmurugan P, Jayanthi M. Assessment of antidiabetic and antihyperlipidemic potential of Solanum nigrum and Musa paradisiaca in alloxan induced diabetic rats. J Pharm Res. 2010;3:2203–5. [Google Scholar]
- 19.Maharana L, Pattnaik S, Kar DM, Sahu PK, Si SC. Assessment of antihyperglycaemic and antioxidant and potential of leaves of Solanum nigrum Linn. in alloxan induced diabetic rats. Pharmacologyonline. 2011;1:942–63. [Google Scholar]
- 20.Kar A, Choudhary BK, Bandyopadhyay NG. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84:105–8. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 21.Elhag RA, El Badwi SM, Bakhiet AO, Galal M. Hepatoprotective activity of Solanum nigrum extracts on chemically induced liver damage in rats. J Vet Med Anim Health. 2011;3:45–50. [Google Scholar]
- 22.Singh K, Aali NS, Khan MI, Ahirwar V. Effect of Solanum nigrum on protein content of liver and kidney of albino rats. Pharm Globale (IJCP) 2011;4:1–3. [Google Scholar]
- 23.Prashanth Kumar V, Shashidhara S, Kumar MM, Sridhara BY. Cytoprotective role of Solanum nigrum against gentamicin-induced kidney cell (Vero cells) damage in vitro. Fitoterapia. 2001;72:481–6. doi: 10.1016/s0367-326x(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 24.Sohrabipour S, Kharazmi F, Soltani N, Kamalinejad M. Biphasic effect of Solanum nigrum fruit aqueous extract on vascular mesenteric beds in non-diabetic and streptozotocin-induced diabetic rats. Pharmacognosy Res. 2014;6:148–52. doi: 10.4103/0974-8490.129036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 26.Bahrani AH, Zaheri H, Soltani N, Kharazmi F, Keshavarz M, Kamalinajad M. Effect of the administration of Psidium guava leaves on blood glucose, lipid profiles and sensitivity of the vascular mesenteric bed to phenylephrine in streptozotocin-induced diabetic rats. J Diabetes Mellit. 2012;2:138–45. [Google Scholar]
- 27.Makni M, Sefi M, Fetoui H, Garoui el M, Gargouri NK, Boudawara T, et al. Flax and Pumpkin seeds mixture ameliorates diabetic nephropathy in rats. Food Chem Toxicol. 2010;48:2407–12. doi: 10.1016/j.fct.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 28.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: Effects of ACEI and ARB. Kidney Int. 2002;61:186–94. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 29.Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat. Effects of insulin treatment. Diabetes. 1987;36:1014–8. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 30.Simán CM, Eriksson UJ. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes. 1997;46:1054–61. doi: 10.2337/diab.46.6.1054. [DOI] [PubMed] [Google Scholar]
- 31.Maiti R, Das UK, Ghosh D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol Pharm Bull. 2005;28:1172–6. doi: 10.1248/bpb.28.1172. [DOI] [PubMed] [Google Scholar]
- 32.Parvizi MR, Parviz M, Tavangar SM, Soltani N, Kadkhodaee M, Seifi B, et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diabetes Metab Disord. 2014;13:84. doi: 10.1186/s40200-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taskiran E, Erbas O, Yigittürk G, Meral A, Akar H, Taskiran D. Exogenously administered adenosine attenuates renal damage in streptozotocin-induced diabetic rats. Ren Fail. 2016;38:1276–82. doi: 10.1080/0886022X.2016.1207054. [DOI] [PubMed] [Google Scholar]
- 34.Kim MJ, Lim Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediators Inflamm. 2013;2013:510212. doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed S, Mundhe N, Borgohain M, Chowdhury L, Kwatra M, Bolshette N, et al. Diosmin modulates the NF-kB signal transduction pathways and downregulation of various oxidative stress markers in alloxan-induced diabetic nephropathy. Inflammation. 2016;39:1783–97. doi: 10.1007/s10753-016-0413-4. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Zhang X, Zheng W, Zhou H, Zhang BY, Zhao D. Minocycline attenuates kidney injury in a rat model of streptozotocin-induced diabetic nephropathy. Biol Pharm Bull. 2016;39:1231–7. doi: 10.1248/bpb.b15-00594. [DOI] [PubMed] [Google Scholar]
- 37.Sharavana G, Joseph GS, Baskaran V. Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. Eur J Nutr. 2016;3:1–11. doi: 10.1007/s00394-016-1283-0. [DOI] [PubMed] [Google Scholar]
- 38.Yan N, Wen L, Peng R, Li H, Liu H, Peng H, et al. Naringenin ameliorated kidney injury through let-7a/TGFBR1 signaling in diabetic nephropathy. J Diabetes Res. 2016;2016:8738760. doi: 10.1155/2016/8738760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet. 1998;352:213–9. doi: 10.1016/S0140-6736(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 40.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 41.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 42.Arora S. Renal function in diabetic nephropathy. World J Diabetes. 2010;1:48–56. doi: 10.4239/wjd.v1.i2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malatiali S, Francis I, Barac-Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res. 2008;2008:305403. doi: 10.1155/2008/305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren XJ, Guan GJ, Liu G, Zhang T, Liu GH. Effect of activin A on tubulointerstitial fibrosis in diabetic nephropathy. Nephrology (Carlton) 2009;14:311–20. doi: 10.1111/j.1440-1797.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 45.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44:1139–46. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 46.Avram MM, Hurtado H. Renal size and function in diabetic nephropathy. Nephron. 1989;52:259–61. doi: 10.1159/000185653. [DOI] [PubMed] [Google Scholar]
- 47.Rigalleau V, Garcia M, Lasseur C, Laurent F, Montaudon M, Raffaitin C, et al. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol. 2010;11:3. doi: 10.1186/1471-2369-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerbini G, Bonfanti R, Meschi F, Bognetti E, Paesano PL, Gianolli L, et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes. 2006;55:2620–5. doi: 10.2337/db06-0592. [DOI] [PubMed] [Google Scholar]
- 49.Christiansen JS, Gammelgaard J, Tronier B, Svendsen PA, Parving HH. Kidney function and size in diabetics before and during initial insulin treatment. Kidney Int. 1982;21:683–8. doi: 10.1038/ki.1982.81. [DOI] [PubMed] [Google Scholar]
- 50.Farsi L, Keshavarz M, Soltani N. Relaxatory effect of gamma-aminobutyric acid (GABA) is mediated by same pathway in diabetic and normal rat mesenteric bed vessel. Iran J Basic Med Sci. 2011;14:94–8. [Google Scholar]
- 51.Cam M, Yavuz O, Guven A, Ercan F, Bukan N, Ustündag N. Protective effects of chronic melatonin treatment against renal injury in streptozotocin-induced diabetic rats. J Pineal Res. 2003;35:212–20. doi: 10.1034/j.1600-079x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 52.Badalzadeh R, Layeghzadeh N, Alihemmati A, Mohammadi M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: Histopathological alterations and antioxidation mechanism. Int J Endocrinol Metab. 2015;13:e25969. doi: 10.5812/ijem.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aydin M, Celik S. Effects of lycopene on plasma glucose, insulin levels, oxidative stress, and body weights of streptozotocin-induced diabetic rats. Turk J Med Sci. 2012;42(Suppl 2):1406–13. [Google Scholar]
- 54.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]