Abstract
Background:
Fruits of Withania coagulans (Solanaceae) reported to possess several bioactive compounds as curative agents for various clinical conditions. Cisplatin is a chemotherapeutic drug to treat sarcomas, carcinomas, lymphomas, cervical cancer, germ cell tumors, etc. The major factor that limits its clinical use is its dose-dependent nephrotoxicity.
Aim:
To explore the nephroprotective effect of W. coagulans extract and its modulatory effects against cisplatin-induced nephrotoxicity and genotoxicity.
Materials and Methods:
W. coagulans fruit extract was quantitatively standardized with withaferin A using high-performance thin-layer chromatography. The subacute toxicity study was performed according to OECD guidelines in experimental rats. Nephrotoxicity in rats was induced by a single dose of cisplatin (6 mg/kg, intraperitoneal). Nephroprotective role of W. coagulans fruit extract at different doses had been evaluated. It includes quantification of serum kidney toxicity markers, renal tissue oxidative stress biomarkers and pro-inflammatory cytokines level, DNA fragmentation assay, and histopathological examination of renal tissue.
Results:
Withaferin A was found 3.56 mg/g of W. coagulans fruit extract. It significantly prevented the rise in serum urea and creatinine level and also preserve rat kidneys from oxidative stress and free radical induced DNA damage. Histopathological study showed extract treatment eliminates tubular swelling, cellular necrosis, and protein cast deposition in cisplatin treated kidney tissue. It averted the decline in glutathione content, activities of superoxide dismutase and catalase. These parameters were restored to near normal levels by extract in a dose of 400 mg/kg, per oral. Conclusion: It can be justified that W. coagulans possess dose dependent protective effect against cisplatin induced kidney damages, primarily through its free radical scavenging and anti inflammatory activity
SUMMARY
Authentication and standardization of Withania coagulans fruits
Subacute oral toxicity study
Evaluation of nephroprotective activity against cisplatin-induced nephrotoxicity
DNA fragmentation assay and histopathological examination of kidney tissue in experimental rats.
Abbreviations Used: WHO: World Health Organization, SOD: Superoxide dismutase, CAT: Catalase, HPTLC: High-performance thin layer chromatography, p.o.: Per.oral, i.p.: Intraperitoneal, TNF-α: Tumor necrosis factor-alpha, IL-1β: Interleukin 1-beta, IL-6: Interleukin-6
Key words: Cisplatin, DNA fragmentation assay, nephroprotective, withaferin A, Withania coagulans
INTRODUCTION
Withania coagulans Dunal belongs to family Solanaceae and is a well-known medicinal plant in indigenous system of medicine mainly distributed in the east of the Mediterranean region extending to South Asia. It is found in many parts of India.[1] The drug has been reported to possess anti-inflammatory,[2] cardiotonic activities,[3] hepatoprotective,[4] antifungal,[5] hypoglycemic, free radical scavenging activity,[6] hypolipidemic,[7] wound healing activity,[8] and diabetic nephropathy.[9] Pharmacognostical standardization of W. coagulans fruits has also been reported in our previous paper.[10] W. coagulans has a wide range of active phytoconstituents mainly withanolides, withaferin A, and coagulins.[11,12] Traditionally, the ripen and dried fruits of W. coagulans were used for anti-inflammatory, antioxidant, and as strangury and also reported to possess nephroprotective activity in rats against diabetes-induced nephropathy.[9,13] The Withaferin A is proven to reduce the inflammation in kidney disease using in vitro model; hence, the fruit extract of W. coagulans had been chosen for evaluating its nephroprotective activity against cisplatin-induced nephrotoxicity.
Nephrotoxicity is very common toxic effect when body is exposed to drugs or some toxins. It results in failure to filter excess urea and excess of nitrogenous compounds and creatinine that leads to uremia. There is no specific therapy for acute renal failure; only the supportive care is necessary for renal function restoration. It can only be prevented by avoidance of nephrotoxic substances and maintenance of adequate hydration and perfusion.[14]
Cisplatin (cis-diamminedichloroplatinum II) is the platinum-containing antineoplastic medication of most of the chemotherapy regimens for solid or hematologic tumors.[15] Cisplatin mainly acts by binding to DNA and form inter- and intrastrand cross-links and arrest DNA synthesis and DNA replication in rapidly proliferating cells.[16] Among gastrotoxicity, myelosuppression, ototoxicity, and allergic reaction, the nephrotoxicity is the major dose-limiting side effects.[17] Approximately 20%–30% patients under cisplatin chemotherapy suffer from severe renal dysfunction and one-third of patients experience nephrotoxicity after few days of initial treatment.[18] Patients administered with cisplatin exhibit lowered glomerular filtration.[19] The organic cation transporter 2 is the critical transporter for cisplatin uptake in proximal tubules. Cisplatin is also taken up through passive diffusion[20] and get accumulated in highest concentrations in S3 segment of proximal tubular cells of the inner cortex and outer medulla.[21] It also causes impairment in liver function and marked elevation in hepatic enzymes.[22] Consequences of cisplatin-induced nephrotoxicity are oxidative stress, inflammation, direct tubular toxicity, and vascular factors. The mechanism of cisplatin-induced nephrotoxicity is complex and involves many cellular processes including DNA damage, oxidative stress, apoptosis, and inflammation.[23,24] It has been reported that cisplatin can induce the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) by renal epithelial cells and TNF-α could also produce oxidant stress by sensitizing infiltrating leukocytes. Interleukin 1-beta (IL-1β) and IL-6 level is also increased in cisplatin-induced renal injury.[24,25,26,27] From the ancient time, medicinal plants played an important role in primary health-care system. The World Health Organization suggested that about 80% of the world's population depends on medicinal plants for prevention, treatment, and cure of diseases.[28] Although there are limited evidences that suggest adverse effects associated with herbal drugs. However, the toxicological studies of medicinal plants before giving them to animals or humans are necessary to evaluate.[29] The objective of the present study is to explore the protective effect of ethanolic extract of W. coagulans (WCE) fruits against cisplatin-induced nephrotoxicity in rats.
MATERIALS AND METHODS
Plant identification and extraction procedure
W. coagulans fruits were purchased from the local herbal market of Varanasi, Uttar Pradesh and authenticated by Prof. V. K. Joshi, Department of Ayurveda (Banaras Hindu University), Varanasi. The voucher specimen (Cog/wc/14-15) has been deposited in the Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi (Uttar Pradesh), for future references. Coarsely powdered drug (500 g) extracted in ethanol by maceration process for 7 days. The crude extract was filtered and dried in rotary vacuum evaporator (IKA RV 10, China) to obtain dried WCE.
Standardization of extract using high-performance thin-layer chromatography
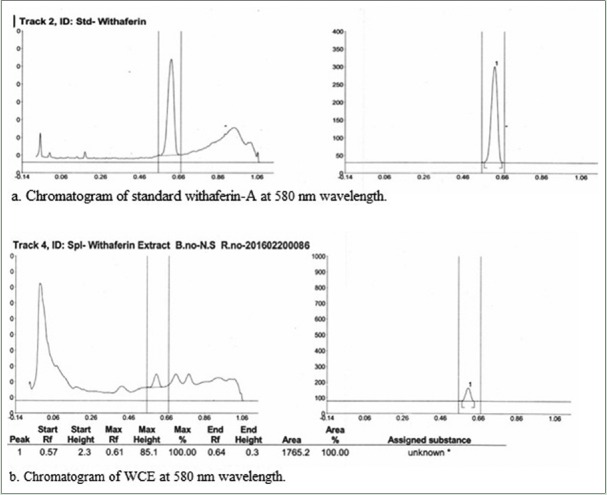
The obtained dried extract, WCE, was quantitatively standardized for the presence of withaferin A using a CAMAG Linomat 5 automatic thin-layer chromatography (TLC) applicator and a CAMAG TLC Scanner with solvent system toluene:ethyl acetate:formic acid in the ratio of (5:5:1). Merck 60F254(E. Merck) silica plates of uniform thickness of 0.2 mm were used for plate development. Visualization was done at 580 nm.
Animals
The certified pathogen-free Healthy Charles Foster albino (150–200 g) male rats were procured from the Central Animal House (Reg. No. 542/02/ab/CPCSEA), Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. Animals were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycles at an ambient temperature of 25°C and 45%–55% RH). Rats were permitted free access to standard feed and water. The animals were allowed to acclimatize to the environment for 7 days before the commencement of experiments. All experimental protocols were approved from the Central Animal Ethical Committee of Banaras Hindu University (No. Dean/2015/CAEC/1132) and were conducted in accordance with accepted standard guidelines of National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication no. 85-23, revised 1985).
Subacute oral toxicity study
Subacute oral toxicity study had been performed as per OECD guideline, repeated dose 28-day oral toxicity study in rodents (TG-407). Nulliparous and nonpregnant female and healthy male rats were used for this study. The first group was served as control and given with 0.5 ml normal saline and second, third, and fourth group were administered with (1000, 2000, and 4000 mg/kg) per oral to overnight fasted rats. Animals were then observed individually for 48 h for any behavioral and neurological changes such as tremors, convulsions, salivation, diarrhea, sleep, lacrimation, and feeding behavior as a sign of acute toxicity for 48 h. Health condition, morbidity, and mortality were observed daily for 28 days. Reversibility, persistence, or delayed occurrences of signs of toxicity were noticed, for at least 14 days posttreatment. Preclinical signs, body weight, and organ weight of different groups were compared with control. Further, blood was collected from the rats; biochemical parameters were estimated using Span Diagnostic Ltd., and hematological parameters were determined by Mythic 18 hematology analyzer.
Experimental induction of cisplatin-induced nephrotoxicity
To standardize the dose of cisplatin to induce nephrotoxicity, rats were divided into three groups with six animals each. Cisplatin, in normal saline (Cytoplatin-10, Cipla) at a dose of 4 mg/kg, 6 mg/kg, and 8 mg/kg, was administered intraperitoneal to rats in each group. After 72 h of cisplatin administration, blood was collected through retro-orbital venous plexus under light anesthesia. Serum urea and creatinine level were determined using standard kits (Erba Diagnostics Mannheim, Germany).
Drug treatment protocol
To evaluate the nephroprotective effect of WCE on cisplatin-induced nephrotoxicity, rats were randomly divided into five groups with six animals each. The WCE extract was suspended in 0.5% carboxymethylcellulose (CMC) and given by oral administration (per-oral [p.o.]) to rats. Cisplatin was freshly prepared in normal saline (0.9% NaCl) and administered by intraperitoneal (i.p.) injection. The doses of WCE were selected as one-tenth of the safe dose from subacute toxicity study. Different doses of WCE were given for total 7 days to respective groups, and cisplatin (6 mg/kg, i.p.) was administered on 4th day of the treatment. Finally, on 7th day, rats were sacrificed to collect blood and harvest kidneys for measuring various parameters (renal function test, liver function test, antioxidant parameters, and estimation of pro-inflammatory cytokines level), DNA fragmentation assay, and histological studies. Schematic presentation of treatment is shown in Figure 1.
Figure 1.
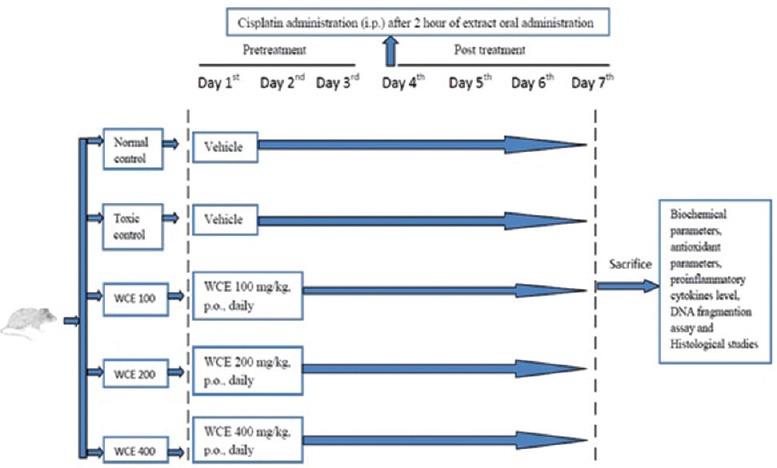
Schematic diagram represents treatment protocol
Group I (normal control): Vehicle (aqueous solution of 0.5% CMC) for 7 consecutive days and 0.9% NaCl on the 4th day
Group II (Toxic control): Vehicle (aqueous solution of 0.5% CMC) for 7 consecutive days and cisplatin (6 mg/kg, p.o.) on the 4th day
-
Group III (WCE-treated groups)
- Group IIIA: WCE (100 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
- Group IIIB: WCE (200 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
- Group IIIC: WCE (400 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day.
Determination of biochemical parameters in serum
Serum urea[30] and serum creatinine[31] had been measured by autoanalyzer using blood urea nitrogen method and modified rate Jaffe's kinetic method. Serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT),[32] total protein,[33] total bilirubin (TB) and direct bilirubin (DB),[34] and alkaline phosphate (ALP)[35] had been also analyzed according to standard methods using autoanalyzer. Enzyme assay kits were purchased from Span Diagnostic Ltd.
Determination of antioxidant parameters in renal tissue homogenate
Minced kidneys were homogenized in 0.1 M potassium phosphate buffer (pH-7) with protease inhibitor. Obtained homogenate (10% w/v) was centrifuged at 10,000 ×g for 20 min at 4°C. The supernatant was utilized for measuring antioxidant level. Lipid peroxidation (LPO) levels had been determined in terms of thiobarbituric acid reactive substance and expressed as equivalent to malondialdehyde (MDA) using 1'1'3'3'-tetramethoxypropane as standard MDA.[36] Superoxide dismutase (SOD) had been estimated in terms of its capacity to inhibit the reduction of nitroblue tetrazolium by superoxide, generated in the presence of riboflavin in reaction system through a photosensitive reaction.[37] Catalase (CAT) activity had been expressed from the rate of decomposition of H2O2 at 240 nm following the addition of tissue homogenate.[38] Reduced glutathione (GSH) level had been calculated as protein-free sulfhydryl content using 5,5-dithiobis-2-nitrobenzoic acid.[39]
Determination of pro-inflammatory cytokines in renal tissue homogenate
The pro-inflammatory cytokines, generated in kidneys due to toxicity induced by cisplatin, had been investigated by measuring levels of TNF-α, IL-6, and IL-1β on homogenized renal tissues using ELISA kit (Koma Biotech, Korea). Standard and detection antibodies provided were reconstituted in sterile water. Serial dilutions of standard and samples were prepared. Selected wells in microplate were washed with washing solution. One hundred milliliters of samples and standard were added to wells followed by addition of diluted detection antibody after incubation. Color was developed with addition of color development enzyme and color development solution. Finally, absorbance had been measured using microplate reader (BioTek Instruments Inc., USA).[40]
Qualitative DNA fragmentation assay by agarose gel electrophoresis
Harvested kidneys were washed with sterile water and homogenized with phosphate-buffered saline (PBS) buffer and centrifuged at 3000 rpm for 10 min at 4°C, and the supernatant was discarded for 3 times to remove cell debris and red blood cells. The cells were then washed twice with PBS and lysed in a buffer containing 50 mmol/l Tris–HCl, pH 8.0, and 0.5% sodium dodecyl sulfate and incubated for 30 min at 37°C. The cell pellet was stirred with a wide-bore pipette tip to ensure uniform mixing. The pellet was incubated with 1 μl of DNase-free RNase (10 mg/ml) for 1 h at 37°C. Samples were further added with Proteinase K (50 μg/ml) solution and incubated for 90 min at 50°C. The precipitated DNA was dissolved in a 5 μl of Tris-ethylenediaminetetraacetic acid buffer and quantified spectrophotometrically as described previously. An equal concentration of DNA (10 μg) had been resolved on a 1% agarose gel at 50 V for 4 h, viewed under ultraviolet light, and documented using the Alpha Innotech system (San Leandro, California, USA).
Histopathological study
Isolated kidneys washed with isotonic saline were fixed in 10% neutral buffered formalin for 48 h and embedded in paraffin wax. Sections (5–6 μm thickness) had been made from paraffin blocks by microtome and stained with hematoxylin and eosin and subjected to microscopic and imaging system (Nikon, Japan).[41]
Statistical analysis
The experimental data had been expressed as mean ± standard error of the mean, with six animals in each group. Analysis of variance (ANOVA) had done by one-way ANOVA followed by Newman–Keuls multiple comparison test for determining the statistical significance between different groups. A difference in the mean values of P < 0.05 had considered to be statistically significant. GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA) had been used for all statistical analyses.
RESULTS
Standardization of extract
Chromatogram of withaferin A standard showed with maximum peak height at 270.7. Extract showed maximum peak height of withaferin A at 85.1 at Rf= 0.57. The calculated concentration of withaferin A had been found to be 3.56 mg/g of sample [Figure 2].
Figure 2.
High-performance thin layer chromatography chromatogram of standard withaferin A and Withania coagulans fruit extract showing peak of withaferin-A
Subacute oral toxicity study
Preclinical signs
Throughout the period of 28 days of the study, there were no abnormal preclinical signs found such as loss of consciousness, tremors, convulsions, irregular breathing, lethargy, aggression, diaphragmatic breathing, gait, piloerection, and licking were observed. It had been found that rats in each group were survived with a normal weight gain pattern until the termination of experiment. WCE (1000, 2000, and 4000 mg/kg, p.o. per day) administration for 28 days causes no significant difference vital organ weight, hematological and biochemical parameters when compared with the control group [Table 1]. Study established that the WCE up to 4000 mg/kg, p.o. was found to be safe.
Table 1.
Various parameters studied in subacute oral toxicity study of Withania coagulans extract

Experimental induction of cisplatin-induced nephrotoxicity
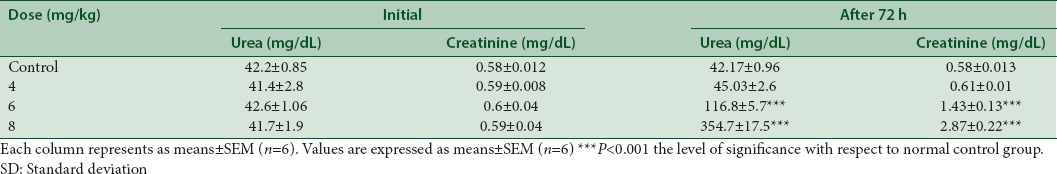
Cisplatin at a dose of 4 mg/kg, i.p. showed no significant elevation (P > 0.05) in urea and creatinine level after 72 h of cisplatin administration. Cisplatin at the dose of 6 mg/kg, i.p. and 8 mg/kg, i.p caused significant elevation (P < 0.05) in urea and creatinine concentration. Administration of cisplatin at the dose of 8 mg/kg, i.p., causes mortality (one-half) in a group of test rats. Therefore, nephroprotective activity of WCE had been evaluated in rats treated with 6 mg/kg, i.p. of cisplatin [Table 2].
Table 2.
Effect of different doses of cisplatin on blood urea and creatinine concentration
Effect of ethanolic extract of W. coagulans on cisplatin-induced nephrotoxicity
Cisplatin-induced renal toxicity was followed with WCE treatment at different doses. WCE 400 mg/kg, p.o. for 7 days significantly decreased the elevated levels of serum urea and serum creatinine level. The decreased total serum protein level in cisplatin (6 mg/kg, i.p.)-treated group was also elevated to normal level after administration of WCE (400 mg/kg, p.o.). It also significantly lowers the increased levels of other biochemical parameters such as SGOT, SGPT, TB, DB, and ALP to normal as compared to control group (P < 0.05). WCE (100 and 200 mg/kg, p.o.) showed no significant protection [Table 3].
Table 3.
Effect of different doses of Withania coagulans on biochemical parameters in rats treated with cisplatin
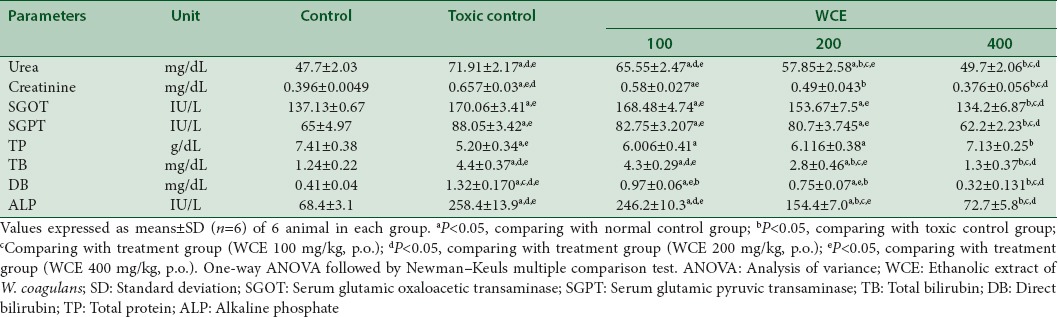
Antioxidant studies in renal tissues of rats
The activity of SOD, CAT, and GSH content had decreased while MDA level had elevated in cisplatin (6 mg/kg, i.p.)-treated rats. WCE (400 mg/kg, p.o.) treatment significantly inverted the changes in antioxidant enzymes in dose-dependent manner (P < 0.05) [Table 4].
Table 4.
Effect of different doses of Withania coagulans on renal antioxidant enzyme levels in rats treated with cisplatin
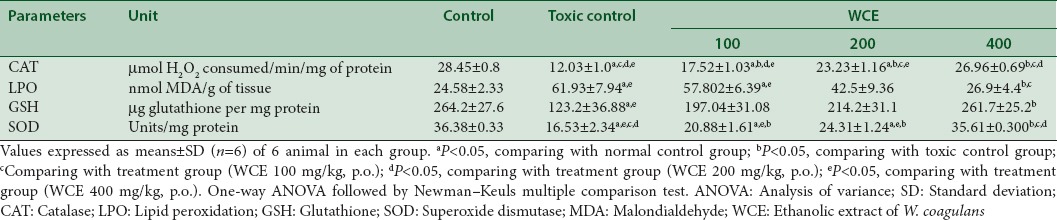
Pro-inflammatory cytokines in renal tissues of rats
The proinflammatory molecules generated after cisplatin-induced nephrotoxicity had been investigated by measuring cytokine levels of TNF-α, IL-6, and IL-1β on homogenized renal tissues using standard. The level of TNF-α, IL-6, and IL-1β in serum elevated after cisplatin injection in rats. However, WCE pretreated rats at dose of 400 mg/kg, p.o. had reduced the expression of these pro-inflammatory molecules in the kidney [Table 5].
Table 5.
Effect of Withania coagulans treatment with different doses on pro-inflammatory cytokines interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in renal tissue of rats treated with cisplatin
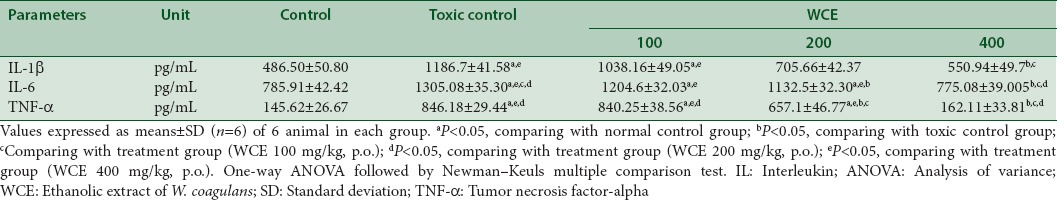
Qualitative DNA fragmentation assay by agarose gel electrophoresis
Agarose gel electrophoresis technique performed for the DNA fragmentation assay in kidneys of treated rats. As perceptible from the figure that cisplatin treatment results in a substantial increase in oligonucleosome-length degradation of DNA. However, the WCE treatment significantly inhibited this smear length. At a dose of 400 mg/kg, p.o., there was approximately no smearing, indicated complete protection of DNA damage [Figure 3].
Figure 3.
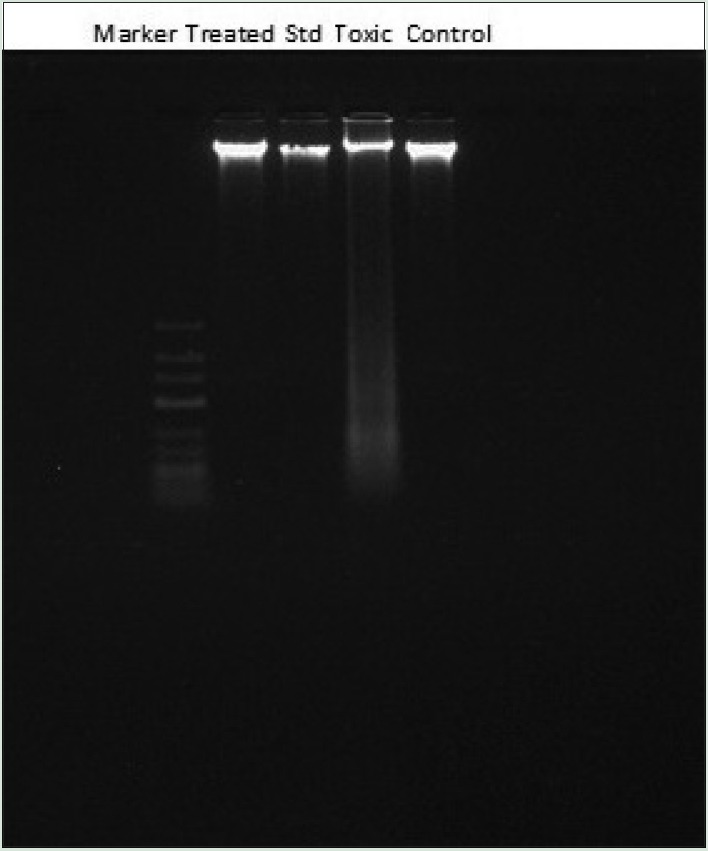
DNA fragmentation of kidney cells exposed to cisplatin, silymarin as standard, and ethanolic extract of W. coagulans (400 mg/kg, per-oral) treated rats. Each lane reflecting the presence of DNA fragments was viewed on an ethidium bromide-stained gel
Evaluation of structural changes in renal tissue of rats
Analysis of renal histology showed that control group (Group I) has normal architecture, i.e. cortex with convoluted tubules and medulla with collecting tubule and pars recta. The toxic control group showed distorted histology with atrophied glomerulus, collecting tubules showing necrosis, vacuolization, and loss of normal architecture. Rats treated with WCE (100 mg/kg, p.o.) showed no protective effect. WCE (200 mg/kg, p.o.)-treated rats manifest damaged histology with less protective effect, but WCE (400 mg/kg, p.o.) justified protective action of WCE by minimizing necrosis and inflammation and vacuolization [Figure 4].
Figure 4.
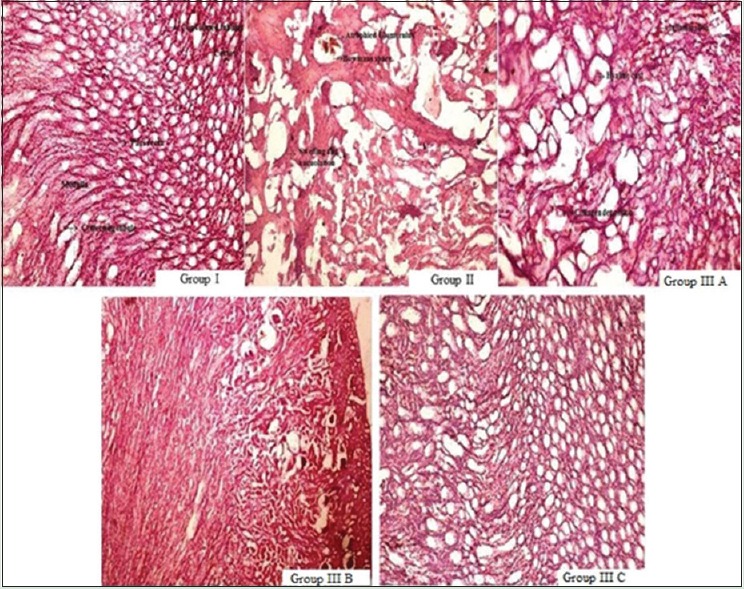
Photomicrograph of renal sections showing a protective effect of ethanolic extract of W. coagulans and cisplatin-induced tubular damage. Group I: control group, Group II: Toxic control group, Group A: Treatment group with 100 mg/kg, per-oral of extract, Group B: Treatment group with 200 mg/kg, per-oral of extract, Group C: Treatment group with 400 mg/kg, per-oral of extract
DISCUSSION
Kidneys are vital organs for survival. Their vital role is to maintain the homeostatic balance of body fluids by filtering and excreting metabolites (such as urea) and minerals from the blood. It also eliminates the nitrogenous wastes along with water from body fluid and involved in maintaining blood pressure, glucose metabolism, and erythropoiesis. Severe kidney toxicity may lead to renal failure. Cisplatin is an inorganic, divalent, water-soluble, platinum-containing anticancer drug. Its mechanism of killing tumor cells is different from tubular toxicity in kidney.[42,43] After administration of the drug, high concentration of cisplatin is found in kidney tissues as about 90% of its platinum is covalently bound to plasma proteins in blood. A small amount of cisplatin is excreted by the kidneys during first 6 h of administration, after 24 h up to 25%, and in 5 days, 43% of the administered dose is recovered in the urine. Intestinal and biliary excretion is very less. Nephrotoxicity becomes major side effect of cisplatin at higher doses.[21,44,45] Nephrotoxicity involved various pathogenic mechanisms such as reactive oxygen species (ROS)-induced cellular oxidative stress and inflammation which causes production of ROS and TNF-α and other pro-inflammatory cytokines. Relationship between nephrotoxicity and free radical oxidative stress has been reported.[27,46,47,48] Cisplatin decreases antioxidant enzymes such as GSH, CAT, and SOD due to excessive accumulation of ROS by cisplatin. The peroxidation of membrane lipids may attribute its nephrotoxicity. The cisplatin-induced nephrotoxicity is believed to be due to peroxidation of membrane lipids. Hence, the LPO assay may be the convenient method to measure oxidative damage to tissues. LPO is an autocatalytic process, MDA is one of the end products in the LPO process and releases during oxidative degeneration as a product of free oxygen radicals, which can be calculated as an indicator of LPO. The inflammation is a major issue due to its prominent role in various pathological conditions. The role of inflammation in nephrotoxicity is highly acknowledged with the participation of pro-inflammatory cytokines, chemokines, leukocytes, and adhesion molecules. TNF is an intercellular chemical messenger and is involved in the inflammatory process released by T lymphocytes, white blood cells, macrophages, and monocytes. In infection, sepsis, and ischemia, IL-1β has been reported to be a proximal mediator of the inflammatory events. IL-6 (polypeptide) secreted from activated macrophages, monocytes, adipocytes, endothelial cells, and fibroblasts in response to various stimuli such as TNF-α, IL-1β, bacterial endotoxins, physical exercise, and oxidative stress.[49,50,51,52]
W. coagulans contains wide range of withanolides (steroidal lactones), withaferin A, and coagulin.[53,54,55] withaferin-A (steroidal lactone) prevents inflammatory and oxidative stress-induced changes of cellular macromolecules such as DNA, RNA, and proteins, and subsequent dysfunction of cellular biochemical pathways. Withanolides are naturally occurring polyhydroxy C28 steroidal lactones, they are found to possess marked anti-inflammatory and antioxidant properties.[3,4,56,57,58] Furthermore, withaferin A and withanolide D have been demonstrated to inhibit inflammation in chronic kidney disease using proximal tubular epithelial cell line, NRK-52E.[59] Taking the lead from previous studies, the extract was standardized with withaferin A. The subacute oral toxicity study ensures that oral administration of WCE was considered to be safe and nontoxic in rats below the dose of 4000 mg/kg. There were no significant toxic symptoms and changes in body weight, organs weight, or biochemical parameters were found with increasing dose of WCE (1000, 2000, and 4000 mg/kg, p.o.) for 28 days. The dose of cisplatin which can cause tubular toxicity was standardized. It was found that cisplatin (6 mg/kg, i.p.) at single dose produces toxicity as evidenced from elevated level of serum urea, creatinine, and other biochemical parameters. The nephrotoxicity involved the oxidative stress and DNA damage in renal tissues also histopathological changes. Since the oxidative stress or free radical stress also affect cell components including DNA, it leads to DNA damage.[60] Therefore, it was considered to examine the effect of WCE on DNA damage in renal tissue. The treatment with WCE (400 mg/kg, p.o.) lowered the elevated levels of biochemical markers mainly urea, creatinine, SGOT, SGPT, ALP, ALT, AST, and total protein. Our result explores that MDA production was significantly reduced and decreased activity of GSH, CAT, and SOD was restored by WCE administration. It indicates the ability of WCE to reduce oxidative stress. There was significant increase in IL-1β, IL-6, and TNF-α in kidneys of rats injected with cisplatin. The study demonstrated that cytokines IL-6, IL-1β, and TNF-α orchestrate the inflammatory response. It was evident from the study that the treatment with WCE significantly protects the cisplatin-induced nephrotoxicity in dose-dependent manner by attenuating free radical oxidative stress and pro-inflammatory cytokines level. WCE also prevented DNA damage in renal tissues which may be probably due to its phytoconstituents. Histology was impaired including swelling, vacuolization, proximal tubular necrosis, glomerular congestion, and inflammation by single dose of cisplatin in toxic control rats. In transverse sections of kidney, damaged tissue architecture brought to normal by WCE treatment at a dose of 400 mg/kg, p.o.
CONCLUSION
On the basis of results obtained, it can be justified that WCE fruit is nontoxic up to the dose of 4000 mg/kg, p.o. to rats. The pretreatment with WCE (400 mg/kg, p.o.) attenuated the cisplatin-mediated renal damage and upregulation of serum urea, creatinine, and other biochemical parameters. It also prevents the cisplatin-induced oxidative stress. Since the oxidative stress is also associated with DNA damage. It was therefore considered to appraise the effect of WCE on DNA damage in renal tissue. It also normalizes tissue architecture by reducing necrosis. It is concluded that the pretreatment with WCE prevents cisplatin-mediated tubular damage. Nephroprotective effect may be due to its direct free radical scavenging activity and anti-inflammatory property as it maintains antioxidant defense system and elevation in pro-inflammatory cytokines.
Financial support and sponsorship
Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi is highly acknowledged for providing financial support to Ms. Sonam Sharma as Teaching Assistantship.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kiritikar KR, Basu BD. Indian Medicinal Plants. Vol. 3. Dehradun, India: International Booksellers and Publishers; 1999. pp. 1160–1. [Google Scholar]
- 2.Budhiraja RD, Bala S, Garg KN. Pharmacological investigations on fruits of Withania coagulans, Dunal. Planta Med. 1977;32:154–7. doi: 10.1055/s-0028-1097575. [DOI] [PubMed] [Google Scholar]
- 3.Budhiraja RD, Sudhir S, Garg KN. Cardiovascular effects of a withanolide from Withania coagulans, Dunal fruits. Indian J Physiol Pharmacol. 1983;27:129–34. [PubMed] [Google Scholar]
- 4.Budhiraja RD, Garg KN, Sudhir S, Arora B. Protective effect of 3 beta hydroxy 2,3 dihydrowithanolide F against CCl4 induced hepatotoxicity. Planta Med. 1986;1:28–9. doi: 10.1055/s-2007-969059. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary MI, Shahwar DE, Parveen Z, Jabbar A, Ali I, Rahman AU. Antifungal steroidal lactones from Withania coagulance. Phytochemistry. 1995;40:1243–6. doi: 10.1016/0031-9422(95)00429-b. [DOI] [PubMed] [Google Scholar]
- 6.Hemalatha S, Wahi AK, Singh PN, Chansouria JP. Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J Ethnopharmacol. 2004;93:261–4. doi: 10.1016/j.jep.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Hemalatha S, Wahi AK, Singh PN, Chansouria JP. Hypolipidemic activity of aqueous extract of Withania coagulans Dunal in albino rats. Phytother Res. 2006;20:614–7. doi: 10.1002/ptr.1916. [DOI] [PubMed] [Google Scholar]
- 8.Hemalatha S, Mishra N, Kumar M, Singh PN, Chansouria JP, Mandal V. 12th Annual National Convention of Indian Society of Pharmacognosy at Moga. Punjab: OP 13; 2008. Wound Healing Activity of Withania coagulans Fruits. [Google Scholar]
- 9.Ojha S, Alkaabi J, Amir N, Sheikh A, Agil A, Fahim MA, et al. Withania coagulans fruit extract reduces oxidative stress and inflammation in kidneys of streptozotocin-induced diabetic rats. Oxid Med Cell Longev. 2014;2014:201436. doi: 10.1155/2014/201436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad SK, Singh PN, Wahi AK, Hemalatha S. Pharmacognostical standardization of Withania coagulans Dunal. Pharmacognosy J. 2010;2:386–94. [Google Scholar]
- 11.Valizadeh M, Bagheri A, Valizadeh J, Mirjalili MH. Phytochemical investigation of Withania coagulans (stocks) Dunal in natural habits of sistan and baluchestan province of Iran. Iran J Med Aromat Plants. 2015;31:406–17. [Google Scholar]
- 12.Hemalatha S, Kumar R, Kumar M. Withania coagulans Dunal: A review. Pharmacognosy Rev. 2008;2:351–8. [Google Scholar]
- 13.Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. New York. USA: Springer Science & Business Media; 2008. p. 719. [Google Scholar]
- 14.Hausberg M, Schaefer RM. Management of acute renal failure in intensive care patients. Med Klin (Munich) 2006;101(Suppl 1):90–4. [PubMed] [Google Scholar]
- 15.Osanto S, Bukman A, Van Hoek F, Sterk PJ, De Laat JA, Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10:574–9. doi: 10.1200/JCO.1992.10.4.574. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 17.Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol. 2005;22:441–5. doi: 10.1080/08880010590964381. [DOI] [PubMed] [Google Scholar]
- 18.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334:115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 19.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 20.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: A review. Br J Cancer. 1993;67:1171–6. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhlmann MK, Burkhardt G, Köhler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant. 1997;12:2478–80. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- 22.Iseri S, Ercan F, Gedik N, Yüksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–64. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther. 2008;327:300–7. doi: 10.1124/jpet.108.139162. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003;285:F610–8. doi: 10.1152/ajprenal.00101.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004;66:S56–61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- 27.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 28.Azaizeh H, Fulder S, Khalil K, Said O. Ethnobotanical knowledge of local Arab practitioners in the Middle Eastern Region. Fitoterapia. 2003;74:98–108. doi: 10.1016/s0367-326x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 29.da Silva AR, Moreira Lda R, Brum Eda S, de Freitas ML, Boligon AA, Athayde ML, et al. Biochemical and hematological effects of acute and sub-acute administration to ethyl acetate fraction from the stem bark Scutia buxifolia Reissek in mice. J Ethnopharmacol. 2014;153:908–16. doi: 10.1016/j.jep.2014.03.063. [DOI] [PubMed] [Google Scholar]
- 30.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972;41:209–17. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 32.Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002;40:725–53. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- 33.Doumas BT, Waston WA, Biggs AG. Biuret method for quantitative estimation of total protein in serum or plasma. Clin Chem Acta. 1971;31:87–91. [Google Scholar]
- 34.Garber CC. Jendrassik-Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clin Chem. 1981;27:1410–6. [PubMed] [Google Scholar]
- 35.Kind PR, King D. In vitro determination of serum alkaline phosphatase. J Clin Pathol. 1972;7:322. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 37.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 38.Aebi HE. Catalase in Methods of Enzymatic Analysis. In: Burgmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1983. p. 273. [Google Scholar]
- 39.Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–53. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- 40.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 41.Tan PV, Mezui C, Enow-Orock G, Njikam N, Dimo T, Bitolog P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J Ethnopharmacol. 2008;115:232–7. doi: 10.1016/j.jep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 43.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, et al. Cytotoxic agents. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. pp. 1677–730. [Google Scholar]
- 45.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 46.Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–26. doi: 10.1016/s0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 47.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–74. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 48.Devipriya S, Shyamaladevim CS. Protective effect of quercetin in cisplatin induced cell injury in the rat kidney. Indian J Pharmacol. 1999;31:422–6. [Google Scholar]
- 49.Sa firstein R, Miller P, Guttenplan JB. Uptake and metabolism of cisplatin by rat kidney. Kidney Int. 1984;25:753–8. doi: 10.1038/ki.1984.86. [DOI] [PubMed] [Google Scholar]
- 50.Sa firstein RL, Schrier RW. Diseases of the Kidney and Urinary Tract. Philadelphia: Lippincott Williams & Wilkins; 2007. Renal disease induced by antineoplastic agents; pp. 1068–81. [Google Scholar]
- 51.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–3. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 52.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 53.Mathur D, Agrawal RC, Shrivastava V. Phytochemical screening and determination of antioxidant potential of fruits extracts of Withania coagulans. Recent Res Sci Technol. 2011;3:26–9. [Google Scholar]
- 54.Sankara SS, Sethi PD. Withaferin A from the roots of Withania coagulans. Curr Sci. 1969;38:267–8. [Google Scholar]
- 55.Atta-ur-Rahman, Dur-e-Shahwar, Naz A, Choudhary MI. Withanolides from Withania coagulans. Phytochemistry. 2003;63:387–90. doi: 10.1016/s0031-9422(02)00727-6. [DOI] [PubMed] [Google Scholar]
- 56.Velde VV, Lavie D, Budhiraja RD, Sudhir S, Garg KN. Potential biogenetic precursors of withanolides from Withania coagulans. Phytochemistry. 1983;22:2253–7. [Google Scholar]
- 57.Budhiraja RD, Sudhir S, Garg KN. Anti-inflammatory activity of 3 beta-hydroxy-2,3-dihydro-withanolide F. Planta Med. 1984;50:134–6. doi: 10.1055/s-2007-969651. [DOI] [PubMed] [Google Scholar]
- 58.Budhiraja RD, Sudhir S. Review of biological activity of withanolides. J Sci Ind Res. 1987;46:488–91. [Google Scholar]
- 59.Grunz-Borgmann E, Mossine V, Fritsche K, Parrish AR. Ashwagandha attenuates TNF-α- and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells. BMC Complement Altern Med. 2015;15:434. doi: 10.1186/s12906-015-0958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: Current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]