Abstract
Introduction:
The harmful action of the free radicals which cause the oxidative stress can be blocked by antioxidant substances, and different plant extracts showed antioxidant activity. The aim of this study is was evaluation the antioxidant activity of total methanol extract (ME) and subfractions of Euphorbia splendida Mobayen.
Materials and Methods:
Aerial part of E. splendida was extracted by maceration with methanol and then subfractionated by liquid–liquid fractionation using petroleum ether, chloroform, ethyl acetate, and water. Antioxidant activity was assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay, reduction of ferric ions and ferrous ion chelating potential. Total phenolic contents (TPC) and total flavonoid contents (TFC) were estimated with Folin-Ciocaltue and aluminum chloride methods, respectively.
Results:
The findings revealed that E. splendida ME and subfractions showed a dose-dependent antioxidant activity. ME showed the highest antioxidant activity based on total reduction capability and ferrous ions chelating assay tests. Aqueous fraction and then ethyl acetate fraction showed the best IC50in DPPH radical scavenging test in comparison to butylated hydroxytoluene. ME showed the highest value of TPC and TFC (270.74 ± 0.005 mg/g and 208.23 ± 0.007 mg/g, respectively).
Conclusion:
This study showed that the extract and subfractions of E. splendida have antioxidant activity. The antioxidant activity of the extract and fractions might be attributed to the presence of phenolic compounds. More studies are needed to determine the active antioxidant compounds of this plant.
SUMMARY
Total extract and subfractions of Euphorbia splendida showed antioxidant activity.
Abbreviations Used: TPC: Total phenolic content, TFC: Total flavonoid content, DPPH: 2, 2’- diphenyl-1-picrylhydrazyl, BHT: Butylated hydroxytoluene, EDTA: Ethylene Diamine Tetra Acetic acid, ME: Total methanol extract, EAF: Ethyl acetate fraction, AQF: Aqueous fraction, PEF: Pertolium ether fraction, CHF: Chloroformic fraction
Key words: 1,1-Diphenyl-1-picrylhydrazyl; antioxidant; Euphorbia splendida; flavonoid; fractions; free radicals
INTRODUCTION
It has been shown that oxidative stress is one of the major causative factors in the induction of many chronic and degenerative diseases including atherosclerosis, ischemic heart disease, aging, diabetes mellitus, cancer, neurodegenerative diseases, and others.[1] The harmful action of the free radicals which cause the oxidative stress can be blocked by antioxidant substances, which scavenge the free radicals and detoxify the organism.[2] Several plant extracts and different classes of phytochemicals have been shown to have antioxidant activity.[3,4]
Genus Euphorbia (Euphorbiaceae) comprising about 2000 species and spreads in Pakistan, India, and Iran and over 82 species of Euphorbia have been found in Iran.[5] Previous studies on Euphorbia species or their active components showed different biological effects such as cytotoxic, antitumor, antioxidant, antibacterial,[6] anti-inflammatory, and antinociceptive activities.[7,8,9] Up to now, antioxidant activity of some species of Euphorbia such as Euphorbia helioscopia and Euphorbia hirta has been reported.[10,11]
Euphorbia splendida Mobayen is a plant distributed in the West of Iran with 30–50 cm height.[12] our previous studies on E. splendida showed the presence of diterpenoid, triterpenoid, and flavonoid in this plant.[13,14] According to our investigation, antioxidant activity of this plant has not been studied so far. Therefore, in this study, antioxidant activity of the methanol extract (ME) and subfractions of E. splendida has been evaluated with different methods.
MATERIALS AND METHODS
Plant material
Fresh aerial parts of E. splendida were collected in May 2014 from Arak, Markazi province, Iran. The specimen was identified by Dr. M. Noori (the Biology Department, Faculty of Science, Arak University) and was deposited under voucher number CMK10 in the herbarium of the biology department.
Extraction and isolation
The aerial parts of plant (600 g) were dried, ground, and extracted with methanol by maceration. The extraction was repeated for three times (3 days for each time) in 25°C. Different fractions of the extract were obtained by liquid–liquid fractionation using water, petroleum ether (PE), chloroform, and ethyl acetate (1500 cc of each solvent). The extract and fractions were concentrated by rotary evaporator (IKA, Model, RV 10 D) and finally dried and stored in a clean, dark container and cool place. The antioxidant activity of the extract and fractions was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, total reduction capability, and ferrous ions chelating assays.
Chemicals
DPPH, butylated hydroxytoluene (BHT), and gallic acid were purchased from Sigma-Aldrich USA. Folin–Ciocalteu was obtained from Merck (Darmstadt, Germany). Potassium ferricyanide, potassium acetate, phosphate buffer, ferrous ammonium sulfate, ascorbic acid, aluminum chloride (AlCl3), thrichloroacetic acid (TCA), ammonium molybdate, tannic acid, quercetin, acetyl acetone, and ferric chloride (FeCl3) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rutin, gallic acid, TCA, potassium ferricyanide, ferrozine, and BHT were purchased from Sigma, and iron (II) chloride was purchased from Aldrich. Methanol, chloroform, ethyl acetate, and PE were purchased from Merk (Darmstadt, Germany).
Total flavonoid content
The total flavonoid content (TFC) of E. splendida ME and subfractions was determined using AlCl3 reagent.[15] Briefly, 2.5 mL of each sample (and/or rutin as the standard), previously dissolved in 90% ethanol, was mixed with 2.5 mL of AlCl32% solution in 90% ethanol. After 40 min, the absorbance of the produced yellow solution was measured at 425 nm. The TFC of the samples was calculated on the basis of a linear calibration curve obtained using rutin.
Total phenolic content
The content of total phenolic compounds in plant extract and fractions was determined by Folin–Ciocalteu method.[16] One milliliter of plant extract (concentration of 1 mg/mL) and fractions was dissolved in methanol and mixed with 5 mL Folin–Ciocalteu reagent and 4 mL (7.5 g/100 mL) sodium carbonate. After 1 h at room temperature, the absorption of clear solutions was read at 765 nm. For the preparation of calibration curve, different concentrations of gallic acid solution were mixed with the same reagents as described above. The amount of total phenolics was expressed as gallic acid equivalent (GAE) in milligrams per gram dry plant extract.
1,1-Diphenyl-2-picrylhydrazyl free radical scavenging assay
The free radical scavenging activity of the ME and subfractions was evaluated using DPPH methods.[17] Briefly, 1 mL of the sample solution with different concentrations (ranging from 50 to 1000 μg/mL) was mixed with 3 mL of DPPH methanol solution. The reaction mixtures were incubated at room temperature and allowed to react for 30 min in the dark. After 30 min, the absorbance values were measured at 517 nm and converted into a percentage of antioxidant activity. BHT was used as a positive standard control. The percentage inhibition of DPPH (%) was calculated as follows:
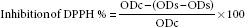
ODc = Control solution absorbance.
ODb= Blank solution absorbance.
ODS= Sample solution absorbance.
The concentration of sample required to scavenge 50% of the DPPH free radicals (IC50) was determined from the curve of percentage inhibitions plotted against the respective concentration.
Ferric reducing antioxidant power assay
The total antioxidant potential of the ME and subfractions of E. splendida was determined according to method of Oyaizu.[18] Aliquot (0.25 mL) of samples solution at different concentrations (ranging from 25 to 600 μg/mL) was mixed with 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of 1% (w/v) solution of potassium ferricyanide. Then, all the mixtures were incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of 10% (w/v) TCA solution was added and the mixture was then centrifuged at 3000 rpm for 10 min. A volume of 2.5 mL of the supernatant was combined with 2.5 mL of distilled water and 0.5 mL of a 0.1% (w/v) solution of FeCl3. The absorbance was measured at 700 nm with a spectrophotometer uv-vis (UNICO Model, UV/VIS 2100). BHT was used as positive control. All the tests were done in triplicate and results were reported as mean ± standard deviation.
Metal chelating activity
The chelating of ferrous ions by the ME and subfractions of E. splendida was estimated by the method of Dinis et al., 1994.[19] Briefly, 1 mL of each test sample (1 mg/mL) was taken and added to 0.5 mL of 2 mM FeCl2. The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine into the mixture, which was then left at room temperature for 10 min and then the absorbance of the mixture was read at 562 nm.
RESULTS
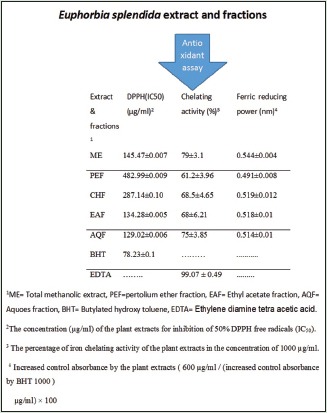
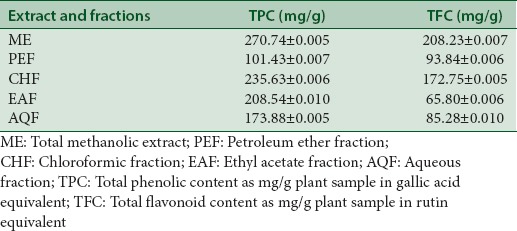
Total flavonoid content and total phenolic content of the extract and subfractions of Euphorbia splendida
The total phenolic content (TPC) of the ME and subfractions calculated from regression equation of calibration curve ([y = 5.0121x, R2 = 0.99]) and expressed in GAEs, varied between 101.43 and 270.74 mg GAE/g plant sample [Table 1]. The content of total flavonoids in plant samples (mg/g) calculated from regression equation of calibration curve (y = 5.3267x, R2 = 0.99) was expressed in rutin equivalents (REs) varied between 65.80 and 208.23 mg RE/g plant sample [Table 1]. Total methanolic extract showed the highest TFC and TPC values.
Table 1.
Total flavonoid content and total phenolic content of Euphorbia splendida extract and fractions (data are mean±standard deviation)
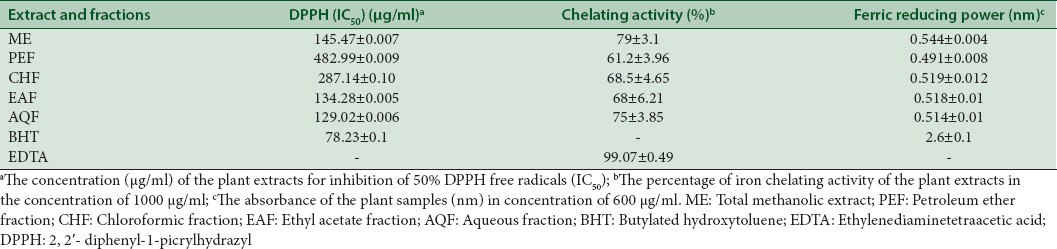
DPPH free radical scavenging assay
ME of the aerial parts of E. splendida and aqueous, ethyl acetate, chloroform, and PE subfractions were analyzed for DPPH radical scavenging activity to obtain their concentrations to scavenge 50% DPPH (IC50) as shown in Table 2. The aqueous and then ethyl acetate subfractions of E. splendida were shown the best results in inhibition of DPPH radical in comparison to standard (BHT). PE subfraction was shown the least activity with IC50= 482.99 ± 0.01 μg/mL.
Table 2.
Radical scavenging activity in the 2, 2’- diphenyl-1-picrylhydrazyl assay, metal chelating activity, and ferric reducing power activity of methanol extract and subfractions of Euphorbia splendida (mean±standard deviation)
Metal chelating activity
Metal chelating activity (%) of the plant ME, subfractions, and ethylenediaminetetraacetic acid (EDTA) in concentrations of 100, 200, 400, 600, and 1000 μg/mL are shown in Figure 1. The ME and subfractions of E. splendida showed a dose-dependent antioxidant activity in this method comparing to EDTA. In concentration of 1000 μg/mL, ME showed the strongest activity (79±3.07%) and petroleum ether fraction (PEF) showed the lowest activity (61.2±3.96%) [Table 2].
Figure 1.
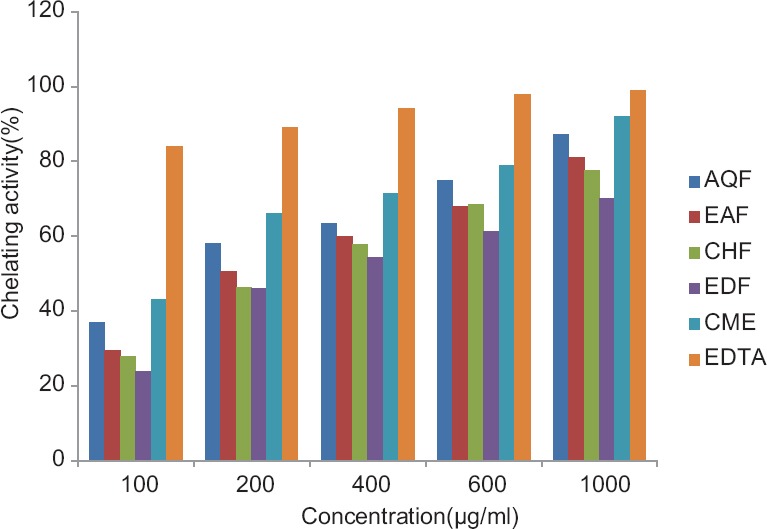
Iron chelating activity of the extract and subfractions of Euphorbia splendida. EDTA: Ethylenediaminetetraacetic acid; CME: Methanol extract; EDF: Petroleum ether fraction; CHF: Chloroform fraction; EAF: Ethyl acetate fraction; AQF: Aqueous fraction
Ferric reducing antioxidant power assay
In ferric reducing power assay, all the samples increased the absorbance of the control solution (0.210 ± 0.098) in 700 nm. As shown in Figure 2, all the concentrations had lower absorbance and consequently lower reduction capability toward BHT.
Figure 2.
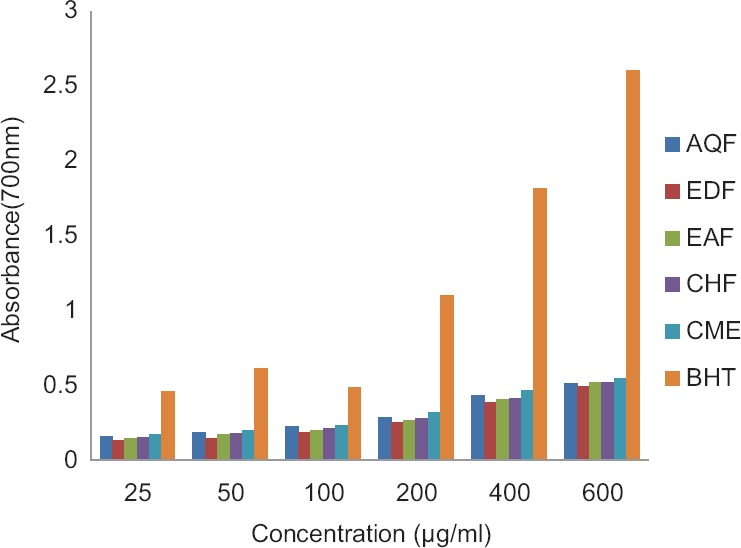
Ferric reducing power activity of the extract and subfractions of Euphorbia splendida. Standard; butylated hydroxytoluene. All the samples showed activity lower than butylated hydroxytoluene. Control absorbance: 0.210 ± 0.098. CME: Methanol extract; EDF: Petroleum ether fraction; CHF: Chloroform fraction; EAF: Ethyl acetate fraction; AQF: Aqueous fraction
DISCUSSION
Several plant extracts and different classes of phytochemicals have been shown to have antioxidant activity. Different species in genus Euphorbia showed antioxidant and free radical scavenging activity.[10,11] E. splendida is a plant which contains different chemical compounds,[13,14] and until now, it is not studied for antioxidant activity. Therefore, in the present study, antioxidant activity of the ME and subfractions of this plant was studied using DPPH free radical scavenging assay, metal chelating activity, and ferric reducing antioxidant power assay.
In DPPH method which is a good method to evaluate radical scavenging activity of the plants, the potency of E. splendida ME and subfractions was as below:
Aqueous fraction (AQF) > ethyl acetate fraction (EAF) > Total methanol extract (ME) > chloroformic fraction (CHF) > petroleum ether fraction (PEF).
The free radical scavenging activity was expressed as the effective concentration required for 50% of DPPH radical (DPPH) reduction (IC50) obtained from a plot of graph of scavenging activity against the concentration of the extract and its fractions. The AQF showed the highest activity (IC50=129.02 ± 0.01 μg/mL) compared to other extract and fractions which was lower than IC50 of BHT (78.23 ± 0.1 μg/mL).
For the other tests, the order of potency of ME and subfractions was as below:
Metal chelating activity: ME > AQF > CHF > EAF > PEF
Total reduction capability: ME > CHF > EAF > AQF > PEF
Phenolic contents: ME > CHF > EAF > AQF > PEF
Flavonoid contents: ME > CHF > PEF > AQF > EAF.
The obtained results for total reduction capability are in agreement with the TPCs determined for extract and subfractions. There are acceptable correlation coefficients (R) between phenolic content and the data of DPPH scavenging activity, metal chelating activity, and total reduction capability of the plant extract and subfractions (R = −0.68, 0.73, and 0.95, respectively); such correlation with flavonoid content was R = −0.06, 0.51, and 0.69, respectively, which was not as significant as phenolic contents.
Different species of Euphorbia showed antioxidant activity in different antioxidant assays.[10,20] Comparing to E. splendida, some of Euphorbia species such as E. hirta showed lower IC50 value in DPPH scavenging assay which was comparable to standards such as BHT.[21] Hence, although E. splendida showed significant antioxidant activity in comparison to standards, especially in DPPH and metal chelating activity, this antioxidant activity may be lower than antioxidant activity of some Euphorbia species such as E. hirta. The extract and subfractions of this plant are found to have different levels of antioxidant activity and phenolic and flavonoid contents. There are some flavonoids such as quercetin and rutin which have been reported from E. splendida.[14] These flavonoids showed significant antioxidant activity in different assays.[22,23,24] Plant polyphenols act as reducing agents and antioxidants by the hydrogen-donating property of their hydroxyl groups,[25] so these polyphenols may be responsible for the observed antioxidant activity. More studies are recommended to determine the active antioxidant compounds of this plant.
CONCLUSION
This study showed that the extract and subfractions of the aerial parts of E. splendida have antioxidant activity. The antioxidant activity of the extract and fractions might be attributed to the presence of phenolic compounds. More studies are needed to determine the active antioxidant compounds of this plant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Souri E, Amin G, Farsam H, Jalalizadeh H, Barezi S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran J Pharm Res. 2008;7:149–54. [Google Scholar]
- 2.Gerber M, Boutron-Ruault MC, Hercberg S, Riboli E, Scalbert A, Siess MH. Food and cancer: State of the art about the protective effect of fruits and vegetables. Bull Cancer. 2002;89:293–312. [PubMed] [Google Scholar]
- 3.Cao GH, Sofic E, Prior RL. Antioxidant capacity of tea and vegetables. J Agric Food Chem. 1996;44:3426–31. [Google Scholar]
- 4.Bergman M, Varshavsky L, Gottlieb HE, Grossman S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry. 2001;58:143–52. doi: 10.1016/s0031-9422(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 5.Jassbi AR. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006;67:1977–84. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Singla A, Pathak k. Phytoconstituents of Euphorbia species. Fitoterapia. 1990;41:483–516. [Google Scholar]
- 7.Lanhers MC, Fleurentin J, Dorfman P, Mortier F, Pelt JM. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991;57:225–31. doi: 10.1055/s-2006-960079. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad VU, Hussain H, Bukhari IA, Hussain J, Jassbi AR, Dar A. Antinociceptive diterpene from Euphorbia decipiens. Fitoterapia. 2005;76:230–2. doi: 10.1016/j.fitote.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Duarte N, Gyémánt N, Abreu PM, Molnár J, Ferreira MJ. New macrocyclic lathyrane diterpenes, from Euphorbia lagascae, as inhibitors of multidrug resistance of tumour cells. Planta Med. 2006;72:162–8. doi: 10.1055/s-2005-873196. [DOI] [PubMed] [Google Scholar]
- 10.Basma AA, Zakaria Z, Latha LY, Sasidharan S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac J Trop Med. 2011;4:386–90. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 11.Maoulainine L, Jellasi A, Hassen I, Ould Boukhari O. Antioxidant proprieties of methanolic and ethanolic extracts of Euphorbia helioscopia, (L.) aerial parts. Int Food Res J. 2012;19:1125–30. [Google Scholar]
- 12.Ghahraman A. Color Flor of Iran. A Joint Project by the Research Institute of Forests and Rangelands Tehran: Ministry of Reconstruction, Jahad Research Institute of Forests and Rangeland, Tehran, Iran. 2005;7:797. [Google Scholar]
- 13.Ayatollahi S, Shojaii A, Kobarfard F, Nori M, Fathi M, Choudhari MI. Terpens from aerial parts of Euphorbia splendida. J Med Plants Res. 2009;3:660–5. [Google Scholar]
- 14.Noori M, Chehreghanib A, Kaveh M. Flavonoids of 17 species of Euphorbia (Euphorbiaceae) in Iran. Toxicol Environ Chem. 2009;91:631–41. [Google Scholar]
- 15.Nickavar B, Kamalinejad M, Haj-Yahya M, Shafaghi B. Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm Biol. 2006;44:208–12. [Google Scholar]
- 16.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999;299:152–78. [Google Scholar]
- 17.Kaji H, Inukai Y, Maiguma T, Ono H, Teshima D, Hiramoto K, et al. Radical scavenging activity of bisbenzylisoquinoline alkaloids and traditional prophylactics against chemotherapy-induced oral mucositis. J Clin Pharm Ther. 2009;34:197–205. doi: 10.1111/j.1365-2710.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 18.Oyaizu M. Studies on products of browning reactions: Antioxidative-17 activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44:307–15. [Google Scholar]
- 19.Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 20.Ul-Haq I, Ullah N, Bibi G, Kanwal S, Sheeraz Ahmad M, Mirza B. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran J Pharm Res. 2012;11:241–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Kandalkar A, Patel A, Darade S, Baviskar D. Free radical scavenging activity of Euphorbia hirta Linn. leaves and isolation of active flavonoid myricitrin. Asian J Pharm Clin Res. 2010;3:234–7. [Google Scholar]
- 22.Zhang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, et al. Antioxidant properties of quercetin. Adv Exp Med Biol. 2011;701:283–9. doi: 10.1007/978-1-4419-7756-4_38. [DOI] [PubMed] [Google Scholar]
- 23.Das M, Ray PK. Lipid antioxidant properties of quercetin in vitro. Biochem Int. 1988;17:203–9. [PubMed] [Google Scholar]
- 24.Yang JX, Guo J, Yuan JF. In vitro antioxidant properties of rutin. Lwt Food Sci Technol. 2008;41:1060–6. [Google Scholar]
- 25.Aberoumand A, Deokule S. Comparison of phenolic compounds of some edible plants of Iran and India. Pak J Nutr. 2008;7:582–5. [Google Scholar]


