Abstract
Objective:
There are some reports on hypotensive and antispasmodic effects of Teucrium polium L. (Lamiaceae) (TP).
Subjects and Methods:
The activity of different concentrations of TP extract (1, 2, 4 and 8 mg/ml) was evaluated on contractile responses of isolated aorta to potassium chloride (KCl) and phenylephrine (PE).
Results:
The cumulative concentrations of the extract induced a concentration-dependent relaxation in the aorta precontracted by PE and KCl. Extract-induced vasorelaxations in denuded aortic rings precontracted by PE and KCl at lower concentrations were considerably less than intact aortic rings, but this effect was significantly more at concentrations of 4 mg/ml for PE-, 4 and 8 mg/ml for KCl-induced contractions. All the extract concentrations (except 1 mg/ml) significantly relaxed PE-induced contraction in the presence of NG-nitro-L-arginine methyl ester. Indomethacin reduced effectively extract-induced vasorelaxation at 1 and 2 mg/ml. The extract reduced PE- and KCl-induced contractions in the presence of cumulative calcium concentrations and after incubation with diltiazem; this vasorelaxant effect of TP was decreased. TP-induced relaxation was inhibited by heparin, ruthenium red, glibenclamide, and tetraethylammonium, but 4-aminopyridine had no effect on TP-induced relaxation.
Conclusion:
TP extract has vasorelaxant effect on isolated rat thoracic aorta which mediated by endothelium-dependent and endothelium-independent mechanisms. The relaxation mainly was mediated by inhibition of calcium influx in vascular smooth muscle cells. It seems that the vasorelaxant effect of extract at lower concentrations was mediated by nitric oxide and prostacyclin.
SUMMARY
The vasodilatory effect of Teucrium polium L. was mediated by several mechanisms. First: Teucrium polium L. inhibited receptor operated ROCC and VDCC. Second: Teucrium polium L. also inhibited KATP and KCa channels. Third: Teucrium polium L. blocked IP3 receptor and reduced the release of calcium from intracellular source. Forth: Teucrium polium L. increased the release on NO and PGI2 from endothelial cells.
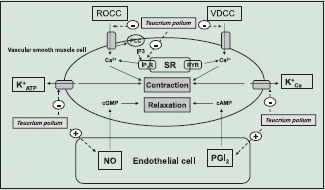
Abbreviations Used: ROCC: Receptor operated calcium channels, VDCC: Voltage dependent calcium channels, PLC: Phospholipase C, IP3: 1,4,5 triphosphate inositol, IP3R: IP3 receptors, SR: sarcoplasmic reticulum, RYR: ryanodine receptors, K+ATP: ATP-sensitive potassium channel, K+Ca: Calcium-activated potassium channel, cAMP: Cyclic adenosine monophosphate, cGMP: Cyclic guanosine monophosphate, PGI2: Prostaglandin I2, NO: Nitric oxide
Key words: Calcium channels, isolated aorta, potassium channels, Teucrium polium, vasorelaxation
INTRODUCTION
Lamiaceae or Labiatae, which also known as the mint family, comprises about 210 genera and 7000 species. One of the most popular species of this family native to the Mediterranean region and the Middle East is Teucrium polium L. (Lamiaceae) (TP) that has been used for over 2000 years in traditional medicine mainly for its antidiabetic, antipyretic, anti-inflammatory, alleviating of heart pain, and antispasmodic properties so that in some parts of Iran, it is traditionally used for the treatment of heart failure.[1] The phytochemistry and medicinal properties of TP are reviewed by Bahramikia and Yazdanparast.[2] Previous studies have demonstrated some of the pharmacological effects of TP such as antibacterial,[3,4] anti-inflammatory,[5] antioxidant,[4,6,7,8,9] antiulcerogenic,[10,11] antinociceptive,[12,13] antidiabetic,[14,15,16,17] antispasmodic,[18,19] and improve memory impairment.[20] TP contains chemical compositions such as flavonoids (rutin, luteolin, apigenin, cirsiliol, salvigenin, and cirsiliol),[17,21,22] monoterpenes (α- and β-pinene, sabinene. and myrcene), and sesquiterpenes (germacrene D, β-caryophyllene, and spathulenol).[23,24,25,26] There is increasing evidence of cardiovascular effects of TP such as positive inotropic and chronotropic,[27,28] decreasing of blood pressure,[19,29,30] and lowering blood lipid.[31] However, the exact effect of TP extract on the vascular system has not been clarified. Therefore, the present study was carried out to examine the effects of the hydroalcholic extract of TP on the vasomotor tone of the aortic rings, and its possible mechanisms of action.
SUBJECTS AND METHODS
Chemicals and drugs
All chemicals were of analytical grade (Merck). Phenylephrine (PE) hydrochloride, acetylcholine (ACh), NG-nitro-L-arginine methyl ester (L-NAME), indomethacin, ruthenium red (RR), heparin (HP), tetraethylammonium chloride (TEA), 4-aminopyridine (4-AP), glibenclamide, and diltiazem were obtained from Sigma (Germany).
Plant material and preparation of the extract
The aerial part of TP was collected and identified (voucher No. 152-2016-4) and then dried at room temperature. Thirty hundred grams of aerial parts of the plant were soaked in ethanol (50%) for 48 h and paper filter was used to filter the solute after mixing. The solution was then dried using a 40°C oven for 72 h. The dried extract was dissolved in the Krebs solution to make 1, 2, 4, and 8 mg/ml concentrations.
Experimental animals
The experiment was conducted on male Wistar rats (weighing 200–250 g). The animals were kept in a 22°C ± 1°C temperature with a 12 h light/dark cycle and fed with a standard diet and drinking tap water. All experiments were conducted in accordance with the Animal Experimentation Ethics Committee (Approval No. 89145).
Preparation of rat aortas
Vascular isometric tension was determined by organ bath technique as described previously.[32] In brief, after anesthesia with ketamin (50 mg/kg, i.p), animals were decapitated by guillotine; descending thoracic aorta was rapidly dissected out and immersed in chilled Krebs solution composed of the following (in mM): NaCl 118.5, potassium chloride (KCl) 4.74, MgSO41.18, NaHCO324.9, CaCl22.5, glucose 10, and bubbled with carbogenic mix (95% O2, 5% CO2, pH = 7.4). The aorta was removed free of the perivascular tissue and cut into ring segments 5 mm in length and care was taken to avoid any damage to the endothelium. Aortic rings were suspended in organ chambers containing 20 ml of Krebs solution at 37°C and continuously aerated with carbogen. The vessel segments placed under a resting tension of 2 g allowed to stabilize for 60 min. Changes in tension were recorded by isometric transducers connected to a data acquisition system (AD Instrument, Australia). When required, endothelium was removed by gently rubbing the intimal space with a thin metal rod. The absence of functional endothelium was verified by the inability of ACh (10−5 M) to induce relaxation of rings precontracted with PE (10−6 M).
Experimental procedure
Effect of Teucrium polium extract on aortic contraction induced by phenylephrine and potassium chloride
To evaluate vasorelaxant effect of TP extract, PE (10−6 M) or KCl (6 × 10−2 M) was used to induce a steady contraction in rings with the endothelium intact or denuded, and TP was added cumulatively (1, 2, 4, and 8 mg/ml). The TP extract-induced relaxation in aortic rings was calculated as a percentage of the relaxation in response to PE and KCl.
Effect of Teucrium polium extract on aortic contraction induced by phenylephrine in the presence of NG-nitro-L-arginine methyl ester and indomethacin
To test for involvement of endothelium-dependent mechanisms in the vasorelaxant response, the intact aortic rings were exposed to L-NAME (10 μM), a nitric oxide (NO) synthase inhibitor, or indomethacin (10 μM), a cyclooxygenase (COX) inhibitor, for 30 min before the application of PE (10−6 M) to induce a steady contraction, and finally, the effects of cumulative concentrations of TP extract were evaluated.
Effect of Teucrium polium extract on extracellular influx of Ca2+ and Ca2+ channels
In the first set of these experiments, to investigate the involvement of Ca2+ influx in the vasodilator response of TP extract, the endothelium denuded aortic rings were washed four to five times with Ca2+-free Krebs solution (containing 5 × 10−5 M EGTA) before PE (10−6 M) or KCl (6 × 10−2 M) was applied to produce a steady contraction, and then, Ca2+ was added cumulatively to obtain a concentration–response curve (10−5–10−2 M) in the presence of 8 mg/ml TP extract. In the second set of experiments, to evaluate the roles of voltage-dependent calcium channels in extract-induced relaxation, endothelium-denuded aortic rings were exposed to diltiazem (10−5 M), an L-type Ca2+ channel inhibitor, for 30 min before the application of PE (10−6 M) or KCl (6 × 10−2 M) to induce a steady contraction, and then, vascular relaxation was carried out by cumulative addition of TP extract (1, 2, 4, and 8 mg/ml).
Effect of Teucrium polium extract on intracellular Ca2+ release
To clarify whether the relaxation induced by TP was related to the inhibition of intracellular Ca2+ release, endothelium-denuded aortic rings were exposed to diltiazem (10−5 M) and RR (10−5 M), a ryanodine receptor (RYR) inhibitor,[33] or HP (50 mg/l), an IP3 receptor inhibitor,[34] 30 min before the application of PE (10−6 M) to induce a steady contraction; subsequently, the cumulative concentrations of TP extract (1, 2, 4 and 8 mg/ml) were added to evoke a relaxation in a separate experimental group.
Determining the role of K+ channels in Teucrium polium induced relaxation
To determine the role of K+ channels in the extract-induced relaxation, the endothelium-intact aortic rings were exposed to TEA (5 mM; a nonselective K+ channel blocker), 4-AP (1 mM; a selective voltage-dependent K+ channel blocker), or glibenclamide (10−5 M; an inhibitor of the ATP-dependent K+ channels) for 30 min before the application of PE (10−6 M) to induce a steady contraction, and finally, the effects of cumulative concentrations of TP extract were evaluated.
Data analysis
The results are expressed as mean ± standard error of mean of eight experiments. The EC50 was defined as the concentration of TP that induced 50% of the maximum relaxation from the contraction elicited by PE (10−6 M) or KCl (6 × 10−2 M) and was calculated from the concentration–response curve. Statistical comparisons were made using Student's t-test and one-way ANOVA followed by post hoc Tukey's test. A P < 0.05 was considered to be statistically significant.
RESULTS
Effect of Teucrium polium on vascular tension of aortic rings
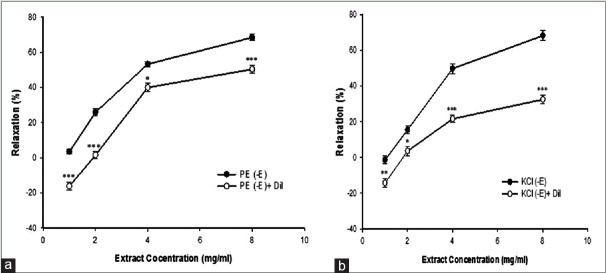
The cumulative concentrations of TP extract (1, 2, 4, and 8 mg/ml) induced concentration-dependent relaxation in aortic rings precontracted by PE and KCl with a maximum relaxation of 56.7% ± 1.37% (EC50=2.4 ± 0.4 mg/ml) and 60.2% ± 1.2% (EC50=2.15 ± 0.36 mg/ml), respectively [Figure 1a and b]. The vasorelaxant effect of TP at concentrations of 1 and 2 mg/ml in denuded aortic rings precontracted by KCl was considerably less than intact aortic rings, but this effect was significantly more at 8 mg/ml. Extract-induced vasorelaxation at 1 mg/ml in denuded aortic rings precontracted by PE was significantly less than intact aortic rings, but this effect was more at 4 and 8 mg/ml.
Figure 1.
Effect of cumulative concentrations of Teucrium polim extract (1, 2, 4 and 8 mg/ml) on PE (10−6 M) (a) and KCl (6 × 10−2 M) (b) precontracted rat aortic rings with (+E) or without (−E) endothelium. Data are expressed as mean ± SEM (n = 8). ***P < 0.001, compare to base + En; *P < 0.05, ***P < 0.001, compared to base-En. PE: Phenylephrine; KCl: Potassium chloride; SEM: Standard error of mean
Effect of NG-nitro-L-arginine methyl ester and indomethacin on Teucrium polium induced vasorelaxantion
As shown in Figure 2, pretreatment of endothelium-intact aortic rings with L-NAME significantly attenuated the TP-induced vasorelaxation at 1 mg/ml. Indomethacin also reduced effectively TP-induced vasorelaxation except at 8 mg/ml [Figure 2].
Figure 2.
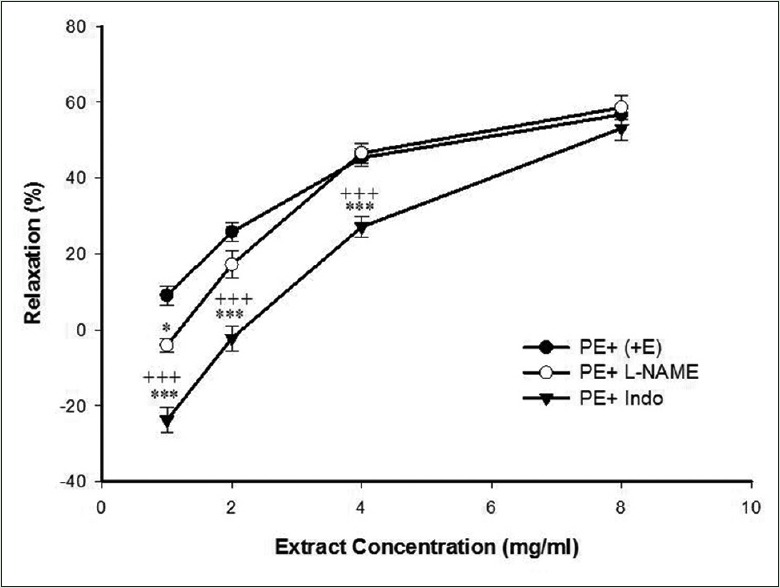
Effect of cumulative concentrations of Teucrium polim extract (1, 2, 4 and 8 mg/ml) on precontracted rat aortic rings with endothelium (PE + E) and after pretreatment with L-NAME (10 μM) (PE + L-NAME) or indomethacin (10 μM) (PE + Indo). Data are expressed as mean ± SEM (n = 8). *P < 0.05, ***P < 0.001 compared to PE + E,+++P < 0.001 compared to PE + L-NAME. PE: Phenylephrine; SEM: Standard error of mean; L-NAME: NG-nitro-L-arginine methyl ester
Effect of Teucrium polium on extracellular Ca2+-induced contraction and Ca2+ channels
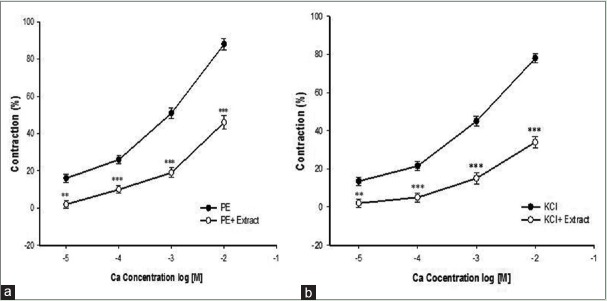
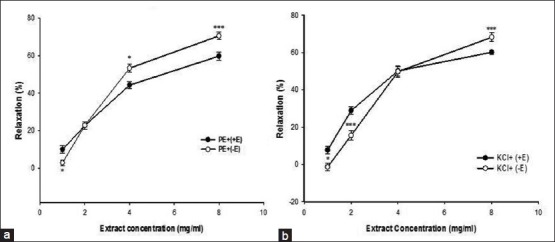
Cumulative addition of Ca2+ in a Ca2+-free medium containing PE or KCl induced a concentration-dependent contraction of aortic rings. Preincubation of the rings with 8 mg/ml of TP significantly inhibited Ca2+-induced contraction in both PE [Figure 3a] and KCl [Figure 3b] constricted rings. In the endothelium-denuded aortic rings pretreated for 30 min with diltiazem and subsequently contracted by PE or KCl, the relaxant effect of cumulative TP concentrations (1, 2, 4 and 8 mg/ml) was significantly reduced [Figure 4a and b].
Figure 3.
Effect of Teucrium polium extract at 8 mg/ml on the Ca2+-induced (0.01–10 mM) contraction of rat aortic rings without endothelium pretreated with PE (10−6 M) (a) or KCl (6 × 10−2 M) (b). Data are expressed as mean ± SEM (n = 8). **P < 0.01, ***P < 0.001 compared to control. PE: Phenylephrine; SEM: Standard error of mean. KCl: Potassium chloride
Figure 4.
Effect of cumulative concentrations of Teucrium polim extract (1, 2, 4, and 8 mg/ml) on rat aortic rings without endothelium (−E) contracted with PE (10−6 M) (PE − E) (a) or KCl (6 × 10−2 M) (KCl − E) (b) and after diltiazem (10−5 M) pretreatment (PE + Dil, KCl + Dil). Data are expressed as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to PE − E or KCl – E. PE: Phenylephrine; KCl: Potassium chloride; SEM: Standard error of mean
Effect of Teucrium polium on intracellular sources of Ca2+
The results of 30 min preincubation of the endothelium-denuded aortic rings with HP or RR in the presence of diltiazem with subsequent contraction by PE showed that HP diminished the relaxant effect of cumulative TP concentrations (1, 2, 4, and 8 mg/ml) [Figure 5].
Figure 5.
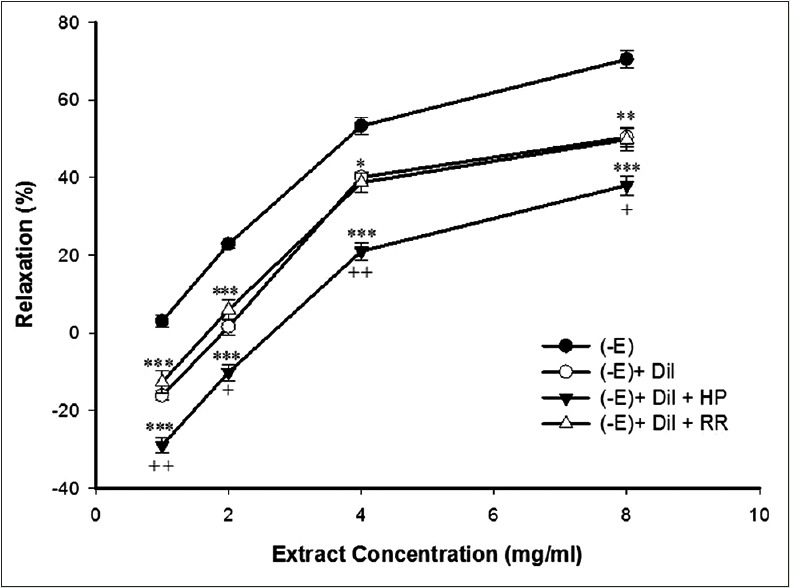
Effect of cumulative concentrations of Teucrium polim extract (1, 2, 4 and 8 mg/ml) on rat aortic rings without endothelium contracted with PE (10−6 M) in the presence of diltiazem (10−5 M) (PE + Dil), after heparin (50 mg/l) (PE + Dil + Hp) or ruthenium red (10−5 M) (PE + Dil + RR) pretreatment. Data are expressed as mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to PE + Dil. PE: Phenylephrine; SEM: Standard error of mean
Effect of Teucrium polium on K+ channels
Thirty minutes preincubation of aortic rings with glibenclamide, 4-AP, or TEA with a subsequent contraction by PE showed glibenclamide attenuated significantly TP-induced relaxation in all concentrations of extract, but TEA reduced this relaxative effect only in concentrations of 1 and 2 mg/ml. 4-AP had no effect on TP-induced relaxation [Figure 6].
Figure 6.
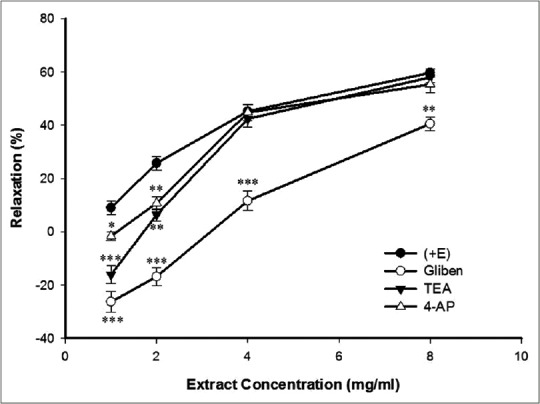
Effect of cumulative concentration of Teucrium polium extract (1, 2, 4, and 8 mg/ml) on rat aortic rings with endothelium (+E) contracted with PE (10−6 M) (PE + E), after pretreated with glibenclamide (10−5 M) (PE + Gb), tetraethylammonium chloride (5 mM) (PE + TEA) or 4-aminopyridine (PE + 4-AP). Data are expressed as means ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to PE + E. PE: Phenylephrine; SEM: Standard error of mean; TEA: Tetraethylammonium chloride
DISCUSSION
The results of this study showed that TP evoked relaxation in aortic rings precontracted by PE and KCl. Comparing the results of the TP effect in endothelium-intact and endothelium-denuded aorta precontracted by KCl or PE indicated the endothelium has a biphasic role in the inhibitory effect of the extract [Figure 1]. Endothelium is involved in contraction and relaxation responses of vascular smooth muscle cells (VSMCs). Endothelial cells synthesize and release various factors that regulate vascular tone,[35] through substances such as NO and prostacyclin inhibit contraction and by production and secretion of endothelin can cause contraction in VSMCs. NO and prostacyclin are endothelium-derived relaxing factors.[29,30] L-NAME as an inhibitor of NO production attenuated the relaxant effect of the extract at concentration of 1 mg/ml that shows the relaxant effect of the extract on this concentration is dependent to NO pathway.
Indomethacin as a nonselective inhibitor of COX reduced the relaxant effect of the extract except at 8 mg/ml on PE-induced contraction, so it can be concluded that the relaxant effects of the extract, especially in lower concentrations, are dependent to prostacyclin. Comparing the effects of L-NAME and indomethacin on PE-induced contraction showed this dependency to prostacyclin is more potent than NO because indomethacin attenuated the inhibitory effects of the extract more effective than L-NAME [Figure 2].
On the other hand, increasing vasorelaxant effect of the extract at higher concentrations in endothelium-denuded aortic rings precontracted by KCl or PE suggests may be TP extract activates endothelium-dependent vasoconstrictor mechanisms which are independent of NO or prostacyclin pathways because the L-NAME or indomethacin did not affect the inhibitory effect of extract at the highest concentration [Figure 2].
Ca2+ is a critical factor in the excitation-contraction coupling in smooth muscle cells.[36,37] Influx of extracellular Ca2+ through receptor-operated Ca2+ channels (ROCCs) and voltage-dependent Ca2+ channels (VDCCs) and release of Ca2+ from the sarcoplasmic reticulum by activation of 1, 4, 5 triphosphate inositol (IP3) and RYRs[38,39,40] result in increased intracellular Ca2+, which causes contraction. PE is an alpha-adrenergic agonist that induces VSMCs contractions by a Ca2+ influx through the ROCCs and by the release of intracellular Ca2+ from the sarcoplasmic reticulum after activation of IP3 receptors.[40,41] By contrast, the contraction elicited by KCl mainly results from the influx of extracellular Ca2+ induced by depolarization of the cell membrane and subsequent opening of the VDCCs.[39] To determine whether TP modified the extracellular Ca2+ influx, experiments were conducted on rings contracted with PE or KCl in a Ca2+-free Krebs solution in which Ca2+ was added subsequently. Our data reporting that TP decreased Ca2+-induced contractions after both PE- and KCl-induced contractions argue that the blockade of both ROCCs and VDCCs are a part of the vasodilating effects of TP. These results were verified by PE- or KCl-induced contractions in the presence of diltiazem, which the vasolaxant effect of TP decreased significantly [Figure 4a and b].
Relaxant effect of the extract reduced significantly in the presence of HP, as an IP3 receptor inhibitor, which shows the importance of IP3 signaling pathway in the relaxant effect of TP. These results indicate the RYRs have any role in vasorelaxant effect of TP extract.
Besides Ca2+ channels, K+ channels contribute to the regulation of the membrane potential in electrically excitable cells including VSMCs.[42] Membrane hyperpolarization due to an efflux of K+ rises of opening of the K+ channels in the VSMCs. This effect is followed by the closure of VDCCs, leading to the reduction in Ca2+ entry, and vasodilation.[39] VSMCs express both KATP and nonselective K+ channel.[43,44] Blockade of the KATP channels by glibenclamide significantly decreased the relaxant effects of the extract which confirmed the prominent role of these K+ channels in the TP-induced vasorelaxation. Reduced inhibitory effect of the extract at concentrations of 1 and 2 mg/ml by TEA showed implicating of nonselective K+ channel in the TP-induced vasorelaxation at lower concentrations of extract. On the other hand, 4-AP did not affect the vasorelaxant effect of TP extract; therefore, the voltage-dependent K+ channels are not involved in the relaxant effect of the extract.
These results support our previous study which demonstrated the hypotensive effect of TP extract.[30] Since TP extract showed inotropic and chronotropic effect,[27,30] it seems TP could be considered as a candidate for treatment of congestive heart failure as it is used traditionally in some parts of Iran.
The findings of this study demonstrate that aqueous-alcoholic extract of TP has relaxant effects on PE-and KCl-induced contractions, which are endothelium-dependent and endothelium-independent. It seems the endothelium has a biphasic role in the relaxant effect of TP. In lower concentrations of extract, prostacyclin has more important role in the relaxing properties of TP than NO; however, in higher concentrations, it shows a vasoconstrictor role. The relaxant effects of the extract are mediated through the inhibition of extracellular Ca2+ influx through ROCCs and VDCCs and also intracellular release of Ca2+ through IP3 receptors. In addition, potassium channels especially KATP channels are involved in vasorelaxant effect of TP extract.
The present work demonstrates that TP extract has vasorelaxant effect on aorta that was mainly mediates by inhibition of calcium influx in VSMCs. It seems the vasorelaxant effect of extract at lower concentrations mediate by NO and prostacyclin. In addition, potassium channels especially KATP channels and IP3 pathways are involved in the vasorelaxant effect of TP extract.
Financial support and sponsorship
This study was financially supported by Research Affairs of Mashhad University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Research Affairs of Mashhad University of Medical Sciences for their financial support (grant number 89145).
REFERENCES
- 1.Mir Heidar H. Encyclopedia of medicinal plant of Iran. 5th ed. Tehran: Islamic Culture Press; 2004. pp. 220–2. [Google Scholar]
- 2.Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae) Phytother Res. 2012;26:1581–93. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 3.Autore G, Capasso F, De Fusco R, Fasulo MP, Lembo M, Mascolo N, et al. Antipyretic and antibacterial actions of Teucrium polium (L.) Pharmacol Res Commun. 1984;16:21–9. doi: 10.1016/s0031-6989(84)80101-0. [DOI] [PubMed] [Google Scholar]
- 4.Samec D, Gruz J, Strnad M, Kremer D, Kosalec I, Grubesic RJ, et al. Antioxidant and antimicrobial properties of Teucrium arduini L. (Lamiaceae) flower and leaf infusions (Teucrium arduini L. antioxidant capacity) Food Chem Toxicol. 2010;48:113–9. doi: 10.1016/j.fct.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tissue React. 1989;11:185–8. [PubMed] [Google Scholar]
- 6.Ardestani A, Yazdanparast R, Jamshidi SH. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11:525–32. doi: 10.1089/jmf.2006.0230. [DOI] [PubMed] [Google Scholar]
- 7.Kadifkova Panovska T, Kulevanova S, Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–14. [PubMed] [Google Scholar]
- 8.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in vitro. Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golfakhrabadi F, Yousefbeyk F, Mirnezami T, Laghaei P, Hajimahmoodi M, Khanavi M. Antioxidant and antiacetylcholinesterase activity of Teucrium hyrcanicum. Pharmacognosy Res. 2015;7(Suppl 1):S15–9. doi: 10.4103/0974-8490.157993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–5. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 11.Galati EM, Mondello MR, D'Aquino A, Miceli N, Sanogo R, Tzakou O, et al. Effects of Teucrium divaricatum Heldr. ssp. divaricatum decoction on experimental ulcer in rats. J Ethnopharmacol. 2000;72:337–42. doi: 10.1016/s0378-8741(00)00280-4. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi M, Karimpour H, Monsef-Esfehani HR. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacol Res. 2003;48:31–5. [PubMed] [Google Scholar]
- 13.Baluchnejadmojarad T, Roghani M, Roghani-Dehkordi F. Antinociceptive effect of Teucrium polium leaf extract in the diabetic rat formalin test. J Ethnopharmacol. 2005;97:207–10. doi: 10.1016/j.jep.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Esmaeili MA, Yazdanparast R. Hypoglycaemic effect of Teucrium polium: Studies with rat pancreatic islets. J Ethnopharmacol. 2004;95:27–30. doi: 10.1016/j.jep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Esmaeili MA, Zohari F, Sadeghi H. Antioxidant and protective effects of major flavonoids from Teucrium polium on beta-cell destruction in a model of streptozotocin-induced diabetes. Planta Med. 2009;75:1418–20. doi: 10.1055/s-0029-1185704. [DOI] [PubMed] [Google Scholar]
- 16.Gharaibeh MN, Elayan HH, Salhab AS. Hypoglycemic effects of Teucrium polium. J Ethnopharmacol. 1988;24:93–9. doi: 10.1016/0378-8741(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 17.Stefkov G, Kulevanova S, Miova B, Dinevska-Kjovkarovska S, Mølgaard P, Jäger AK, et al. Effects of Teucrium polium spp. capitatum flavonoids on the lipid and carbohydrate metabolism in rats. Pharm Biol. 2011;49:885–92. doi: 10.3109/13880209.2011.552187. [DOI] [PubMed] [Google Scholar]
- 18.Parsaee H, Shafiee-Nick R. Anti-spasmodic and anti-nociceptive effects of Teucrium polium aqueous extract. Iran Biomed J. 2006;10:145–59. [Google Scholar]
- 19.Suleiman MS, Abdul-Ghani AS, Al-Khalil S, Amin R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J Ethnopharmacol. 1988;22:111–6. doi: 10.1016/0378-8741(88)90236-x. [DOI] [PubMed] [Google Scholar]
- 20.Mousavi SM, Niazmand S, Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, et al. Beneficial effects of Teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int J Alzheimers Dis. 2015;2015:493729. doi: 10.1155/2015/493729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizk AM, Hammouda FM, Rimpler H, Kamel A. Iridoids and flavonoids of Teucrium polium herb. Planta Med. 1986;52:87–8. [PubMed] [Google Scholar]
- 22.Ansari M, Sharififar F, Kazemipour M, Sarhadinejad Z, Mahdavi H. Teucrium polium L. extract adsorbed on zinc oxide nanoparticles as a fortified sunscreen. Int J Pharm Investig. 2013;3:188–93. doi: 10.4103/2230-973X.121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Çakira A, Emin Durua M, Harmandar M, Ciriminnab R, Passannantic S. Volatile constituents of Teucrium polium L. from Turkey. J Essent Oil Res. 1998;10:113–5. [Google Scholar]
- 24.De Martino L, Formisano C, Mancini E, De Feo V, Piozzi F, Rigano D, et al. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat Prod Commun. 2010;5:1969–76. [PubMed] [Google Scholar]
- 25.Talal A, Mohammad H, Vanni C. Composition of the essential oil from Jordanian germander (Teucrium polium L.) J Essent Oil Res. 2006;18:97–9. [Google Scholar]
- 26.Vokoun D, Bessiere JM. Volatile constituents of Teucrium polium. J Nat Prod. 1985;48:498–9. [Google Scholar]
- 27.Niazmand S, Erfanian Ahmadpoor M, Moosavian M, Derakhshan M. The positive inotropic and chronotropic effects of Teucrium polium L. extract on guinea pig isolated heart. Pharmacologyonline. 2008;2:588–94. [Google Scholar]
- 28.Mahmoudabady M, Shafei MN, Niazmand S, Khodaee E. The effects of hydroalchoholic extract of Teucrium polium L. on hypertension induced by angiotensin II in rats. Int J Prev Med. 2014;5:1255–60. [PMC free article] [PubMed] [Google Scholar]
- 29.Bello R, Calatayud S, Moreno L, Beltran B, Primo-Yufera E, Esplugues J. Effects on arterial blood pressure of the methanol extracts from different Teucrium species. Phytother Res. 1997;11:330–1. [Google Scholar]
- 30.Niazmand S, Esparham M, Hassannia T, Derakhshan M. Cardiovascular effects of Teucrium polium L. extract in rabbit. Pharmacogn Mag. 2011;7:260–4. doi: 10.4103/0973-1296.84244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasekh HR, Khoshnood-Mansourkhani MJ, Kamalinejad M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia. 2001;72:937–9. doi: 10.1016/s0367-326x(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 32.Niazmand S, Fereidouni E, Mahmoudabady M, Mousavi SM. Endothelium-independent vasorelaxant effects of hydroalcoholic extract from Nigella sativa seed in rat aorta: The roles of Ca2+and K+channels. Biomed Res Int. 2014;2014:247054. doi: 10.1155/2014/247054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggi CA, Patacchini R, Perretti F, Tramontana M, Manzini S, Geppetti P, et al. Sensory nerves, vascular endothelium and neurogenic relaxation of the guinea-pig isolated pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:78–84. doi: 10.1007/BF00178976. [DOI] [PubMed] [Google Scholar]
- 34.Mohanty MJ, Li X. Stretch-induced Ca(2+) release via an IP(3)-insensitive Ca(2+) channel. Am J Physiol Cell Physiol. 2002;283:C456–62. doi: 10.1152/ajpcell.00057.2002. [DOI] [PubMed] [Google Scholar]
- 35.Félétou M, Vanhoutte PM. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 36.Löhn M, Fürstenau M, Sagach V, Elger M, Schulze W, Luft FC, et al. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–9. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 37.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–29. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 38.Imtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+oscillations. Biophys J. 2006;90:1–23. doi: 10.1529/biophysj.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 40.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: Regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–42. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- 41.McCarron JG, Bradley KN, MacMillan D, Muir TC. Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+oscillations and waves in smooth muscle. Biochem Soc Trans. 2003;31(Pt 5):920–4. doi: 10.1042/bst0310920. [DOI] [PubMed] [Google Scholar]
- 42.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 43.Côrtes SF, Rezende BA, Corriu C, Medeiros IA, Teixeira MM, Lopes MJ, et al. Pharmacological evidence for the activation of potassium channels as the mechanism involved in the hypotensive and vasorelaxant effect of dioclein in rat small resistance arteries. Br J Pharmacol. 2001;133:849–58. doi: 10.1038/sj.bjp.0704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35(1 Pt 2):173–8. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]