Abstract
Background:
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries. Annonacin, a natural pure compound extracted from the seeds of Annona muricata, is a potential alternative therapeutic agent to treat EC.
Objective:
To study the antitumor activity of annonacin and its mechanism of action in EC cells (ECCs).
Materials and Methods:
Viability of ECCs treated with annonacin for 72 h was determined using methyl thiazolyl tetrazolium assay. The induction of cell cycle arrest and apoptotic cell death was evaluated using propidium iodide and annexin V-PE/7-AAD assay, respectively. DNA strand breaks were visualized using transferase dUTP nick end labeling assay, and the effects of annonacin on survival signaling were determined using western blotting.
Results:
Annonacin exhibited antiproliferative effects on EC cell lines (ECC-1 and HEC-1A) and primary cells (EC6-ept and EC14-ept) with EC50values ranging from 4.62 to 4.92 μg/ml. EC cells were shown arrested at G2/M phase after treated with 4 μg/ml of annonacin for 72 h. This led to a significant increase in apoptotic cell death (65.7%) in these cells when compared to vehicle-treated cells (P < 0.005). We further showed that annonacin-mediated apoptotic cell death was associated with an increase in caspase-3 cleavage and DNA fragmentation. Cell apoptosis was accompanied with downregulation of extracellular signal-regulated kinase survival protein expression and induction of G2/M cell cycle arrest.
Conclusion:
Annonacin may be a potential novel therapeutic agent for EC patients.
SUMMARY
We aimed to study the antitumor activity of annonacin and its mechanism of action in endometrial cancer cells. Annonacin exerted antiproliferation effects on both endometrial cancer cell lines and primary cells via induction of apoptosis and inhibition of extracellular signal-regulated kinase. Our data represented that annonacin could be an alternative therapeutic treatment to combat endometrial cancer.
Abbreviations Used: 7-AAD: 7-Amino-Actinomycin, ATP: Adenosine diphosphate, BSA: Bovine serum albumin, DNA: Deoxyribonucleic acid, EC: Endometrial cancer, ECC-1: Endometrial cancer cell-1, EC50: Half maximal effective concentration, Ept: Epithelial, FBS: Fetal bovine serum, HEC-1A: Human endometrial carcinoma-1A, MTT: Methyl thiazolyl tetrazolium, NaCl: Sodium chloride, NADH: Nicotinamide adenine dinucleotide, RPMI 1640: Roswell Park Memorial Institute Medium, SDS: Sodium dodecyl sulfate
Key words: Annona muricata, apoptosis, caspase-3, cell cycle arrest, extracellular signal-regulated kinase, uterine cancer
INTRODUCTION
Endometrial cancer (EC) represents the most common gynecologic malignancy in developed countries. In 2016, American Cancer Society estimated 60,050 new cases of EC and 10,670 deaths from this cancer.[1] Approximately, 90% EC cases are sporadic and are classified into Type 1 and Type 2, according to their etiology and clinical behavior.[2] Woman with advanced or recurrent EC may benefit from hormonal manipulation, systemic chemotherapies, or radiotherapy.[3] To date, chemotherapy remains a mainstay treatment to target rapidly diving cancer cells using mainly anthracyclines, platinum, and taxanes compounds.[4] However, the systemic adverse effects of chemotherapeutic regimens are always a major concern limiting effective treatment strategies for EC.
Bioactive compounds from naturally occurring dietary components serve as chemotherapeutic agents against various type of cancers. Abundant epidemiologic studies have shown that higher intake of herbs and natural products potentiate the antitumor effects of chemotherapy and attenuate its adverse toxicity. This could be due to high levels of fiber, antioxidants, and potentially antineoplastic compounds in these fruits and vegetables.[5] Moreover, investigation on complementary and alternative medicine may be useful to improve the efficacy of chemotherapy against EC.
Annonacin is a natural pure compound extracted from the seeds of Annona muricata (Linn.) (family, Annonaceae), also known as soursop or Guanábana in Latin. A. muricata is an indigenous plant in most of the warm tropical areas such as South and North America, and Amazon.[6] The molecular formula of annonacin is C35H64O7 and [Figure 1a] represents the 2D molecular structure. Annonacin belongs to annonaceous acetogenin group featuring a mono-tetrahydrofuran with two flanking hydroxyls.
Figure 1.
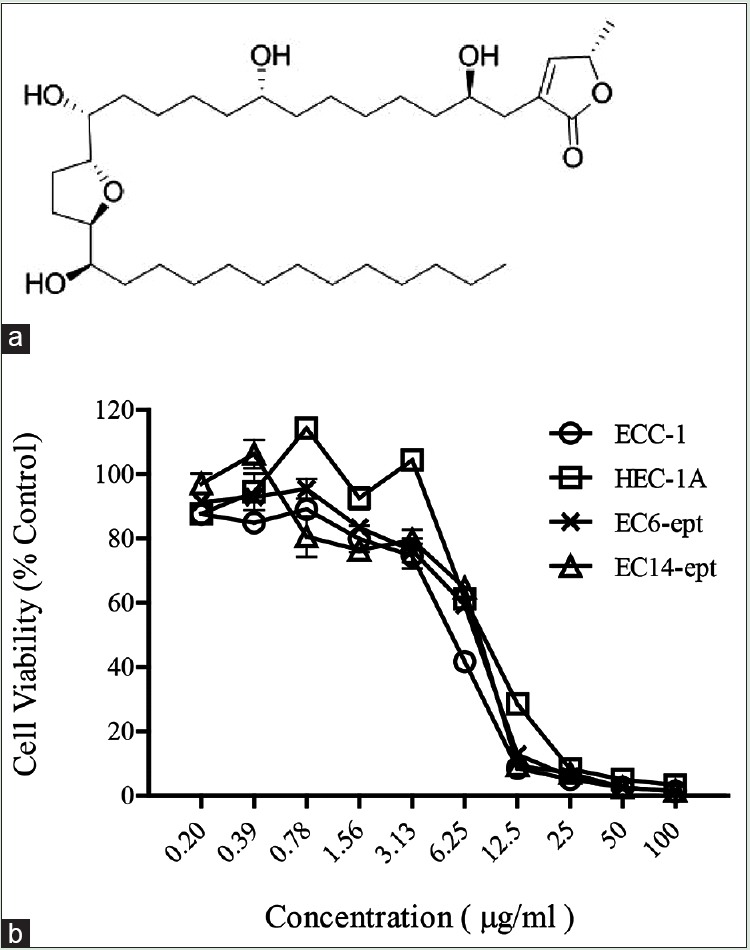
Antiproliferative effects of annonacin on endometrial cancer cells (a) molecular structure of annonacin. (b) ECC-1, HEC-1A, EC6-ept, and EC14-ept were treated with 0.2–100 μg/ml of annonacin in an increasing dose for 72 h. The cell viability was assessed by methyl thiazolyl tetrazolium assay with triplicate samples. The cell viability with annonacin treatment was normalized to control media containing 10% FBS. *P < 0.05, **P < 0.01, ***P < 0.001. ECC: Endometrial cancer cell, EC: Endometrial cancer, FBS: Fetal bovine serum
Numerous studies have demonstrated that annonacin possesses anti-inflammatory, antiulceric, wound healing, and hypoglycemic activities. In addition, preliminary studies have shown that annonacin is effective against breast, lung, and bladder cancer.[7,8,9] The accumulating evidence of the inhibitory effect of annonacin on various cancers has fuelled our interest to study its antiproliferative effects on endometrial cancer cells (ECCs). In the present study, we showed that annonacin exhibits antiproliferative and cytotoxic effects in both EC cell lines and primary ECCs. We further elucidated that the antitumor effects were mediated by apoptotic cell death, as shown by annexin V staining, caspase-3 cleavage, and DNA fragmentation. Our data indicated that annonacin acts through G2/M phase arrest and inhibition of extracellular signal-regulated kinase (ERK) signaling pathway. Together, our results demonstrated that annonacin could be a candidate for alternative therapeutic agent to treat ECs.
MATERIALS AND METHODS
Chemicals and reagents
Purified powder of annonacin (CAS#111035-65-5) extracted from seeds of A. muricata was purchased from BOC Sciences (New York, USA). The purity of annonacin is ≥96%. The annonacin content used in this study was indicated in μg/ml.
Ethics statement
Fresh EC tissues were obtained for establishment of primary culture from patients undergoing surgery at University of Malaya Medical Center. The study was approved by the Ethical Committee of University Malaya Medical Centre (Ref No. 865.19).
Human tissues and endometrial cancer cell lines
Human EC cell lines, ECC-1 (CRL-2923), and HEC-1A (HTB 112) were purchased from ATCC (Virginia, USA). Primary ECCs, EC6, and EC14 epithelial cells, previously established in the laboratory were used.[10] ECC-1, EC6, and EC14 epithelial cells were cultured in RPMI 1640 complete growth media (Life Technologies, New York, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Life Technologies, New York, USA) whereas HEC-1A was cultured in McCoy's complete growth media (Life Technologies, New York, USA) supplemented with 10% FBS and 1% penicillin-streptomycin (Life Technologies, New York, USA).
Methyl thiazolyl tetrazolium assay
ECC viability after treated with annonacin was measured using methyl thiazolyl tetrazolium (MTT) assay (Sigma, Missouri, USA). Briefly, cells were seeded at 5 × 103 cells/well in 96-well plates. At 24 h postseeding, the cells were treated with varying concentrations of annonacin through serial dilutions starting from 100 to 0.2 μg/ml. At the end of 72 h treatment, 20 μl of MTT solution (5 mg/ml) was added to each well. Following 4 h of incubation at 37°C, 100 μl of 10% sodium dodecyl sulfate (Life Technologies, New York, USA) was added to dissolve the formazan crystals with additional 4 h incubation at 37°C. Absorbance was measured at 575 nm with a reference of 650 nm using SpectraMax M3 (Molecular Devices, California, USA). Dose response curve was plotted using GraphPad Prism version 5.02 to calculate the EC50.
Total protein extraction and western blotting
ECCs were seeded at 1 × 104 cells/well in 6-well plates in complete media overnight before treated with either control media (containing 10% FBS), vehicle media or 1–4 μg/ml of annonacin for 72 h. Protein lysates were collected by scraping the cells in cold lysis buffer which containing a final concentration of 0.1% Triton-X, 0.1% SDS, 50mM Tris, 150mM NaCl, 1X phosphatase, and 1X protease inhibitors. Protein concentration was quantified using Bradford assay (Biorad, California, USA). Approximately, 30 μg of protein were resolved in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis before being transferred onto polyvinylidene difluoride membrane. Antibodies used were rabbit antihuman ERK, phospho-ERK, caspase-3, and β-actin (Cell Signaling Technology, Massachusetts, USA). Blots were visualized using ECL prime Western blotting detection reagent (GE Healthcare Lifesciences, Pennsylvania, USA) using BioSpectrum 410 gel documentation system (UVP, California, USA).
Cell cycle analysis
ECCs were treated with 4 μg/ml of annonacin for 24, 48, and 72 h. After trypsinization, cells were collected, washed, and suspended in 0.5 ml phosphate-buffered saline (PBS) (1 × 106 cells/ml). The cell suspension was added with 4.5 ml of 70% ethanol and stored at 4°C for 30 min. After centrifugation, cells were washed with PBS and resuspended in 1 ml PI/Triton X-100 staining solution with DNase-free RNase A at 37°C for 30 min, before acquisition using FACS Canto II flow cytometer (BD Bioscience, New Jersey, USA). The experiments were conducted in duplicate.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Cells were treated with annonacin at 1 and 4 μg/ml for 72 h before fixed with 4% paraformaldehyde in PBS and then permeabilized with 0.1% Triton X-100 in PBS. TdT reaction buffer (100 μl) was added followed by another 100 μl of the TdT reaction cocktail. The cells were washed with 3% BSA in PBS. Click-iT® Reaction cocktail (100 μl) (Life Technologies, New York, USA) were added. The cells were counterstained using 1X Hoechst 33342 solution. The cells were further assessed by Nikon Eclipse Ti fluorescence microscopy (Tokyo, Japan).
Annexin V-PE/7-AAD staining assay
Cells were treated with annonacin at 4 μg/ml for 24, 48, and 72 h. The cells were washed twice with cold PBS then resuspended with 1X Binding buffer at a concentration of 1×106 cells/ml; followed by transferring 100 μl of the solution (1 × 105 cells) to a 5 ml culture tube. Annexin V-PE and 5 μl of 7-AAD, 5 μl each (BD Bioscience, New Jersey, USA) were added to each tube. The cells were vortexed gently and incubated for 15 min at room temperature in dark. 400 μl of 1X Binding buffer were added to each tube. Analysis by FACS Canto II flow cytometer (BD Bioscience, New Jersey, USA) was carried out within 1 h of sample preparation.
Statistical analysis
The data from the MTT assay were normalized to control media and presented as mean ± standard error mean. We carried out Student's t-test using Graph Pad Prism version 5.02 to analyze the difference between the means of the treatment and control groups. The difference with P < 0.05 was considered to be statistically significant.
RESULTS
Annonacin is a potent inhibitor of endometrial cancer cell proliferation
Antiproliferative effect of annonacin was tested on two immortalized human EC cell lines (ECC-1 and HEC-1A) and two established human primary epithelial ECCs (EC6-ept and EC14-ept), in a range of 0.2–100 μg/ml of annonacin for 72 h. Both EC cell lines showed decreasing viability in a concentration-dependent manner over 72 h (P < 0.005 when compared to control media) [Figure 1]. The EC50 of annonacin in ECC-1 and HEC-1A were within the range of 4.62–4.75 μg/ml. The antiproliferative effects of annonacin were slightly lower in primary ECCs (EC6-ept and EC14-ept), with EC50 ranging from 4.92 to 4.81 μg/ml [Figure 1 and Table 1]. These data show that annonacin exerted significant antiproliferative activity against both EC cell lines and primary cells.
Table 1.
Values of EC50 of annonacin on endometrial cancer cell lines and primary cells were determined from the dose-response curve

Annonacin induces morphological changes in endometrial cancer cells
We further examined morphological changes of ECCs on treatment with annonacin over 7 days. Both vehicle-treated ECC-1 and EC6-ept cells exhibited expected rose petal-shaped epithelial cell morphology. In contrast, annonacin-treated cells rounded off and detached after 24 h [Figure 2]. Treatment with annonacin for 7 days led to extensive cell shrinkage and membrane blebbing while vehicle-treated cells remained in monolayer cell sheet [Figure 2]. Similar observation was seen with HEC-1A and EC14-ept after treatment with 4 μg/ml of annonacin. Our data suggest that 4 μg/ml of annonacin induced cytotoxicity as early as 24 h posttreatment, as shown by cell shrinkage and detachment. Thus, the subsequent experiments were conducted using 4 μg/ml of annonacin.
Figure 2.
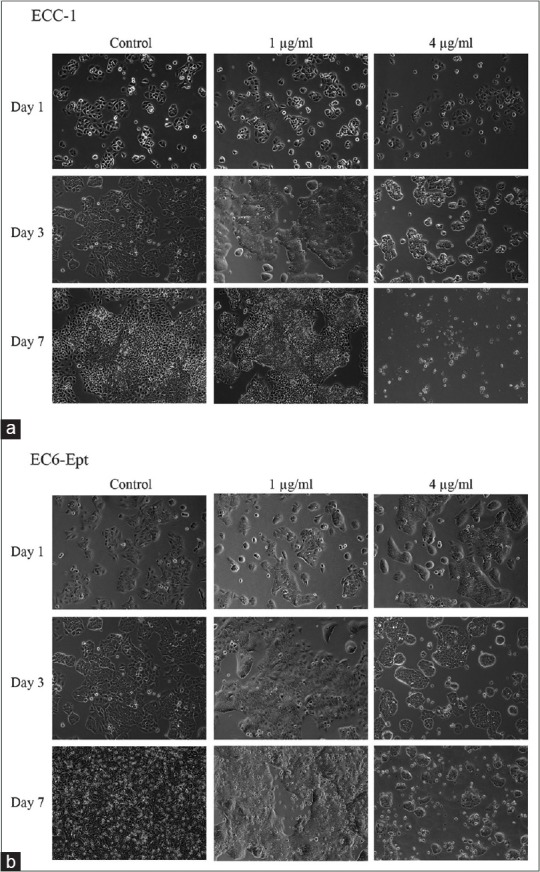
Morphology changes of endometrial cancer cells postannonacin treatment. (a and b) ECC-1 and EC6-ept were treated by control media, 1 and 4 μg/ml of annonacin. Morphology changes were observed using phase contrast microscopy at constant time points (×100). ECC: Endometrial cancer cell, EC: Endometrial cancer
Annonacin induces apoptotic cell death of endometrial cancer cells
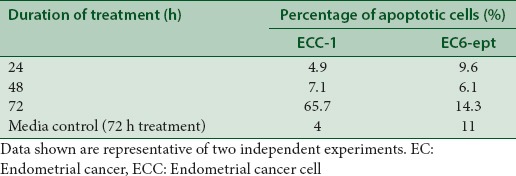
We next determined whether induction of apoptotic cell death can explain the changes in morphology observed in annonacin-treated cells. One of the earliest features of apoptosis is the translocation of phosphatidylserine to the external membrane leaflet. Thus, we performed annexin V-PE/7-AAD double staining assay to identify early apoptotic (intact membrane) and late apoptotic cells (leaky membrane) on ECCs treated with 4 μg/ml of annonacin. As early as 24 h posttreatment, annonacin induced significant apoptosis with 16% increase in apoptotic cell population, when compared to vehicle-treated cells (P < 0.005) [Figure 3 and Table 2]. Strikingly, the number of apoptotic cell population increased dramatically at 72 h, in which almost 76% of the cells were either at early or late apoptosis phase (P < 0.005). Despite having similar EC50, EC6-ept did not demonstrate the similar extent of cell apoptosis after treated with annonacin; there were about 20% apoptotic cells after 72 h treatment [Figure 3 and Table 2]. Our result suggests that annonacin induced apoptosis as part of the mechanism of its antitumor effects in EC and these effects were greater in EC cell lines than in primary cells.
Figure 3.
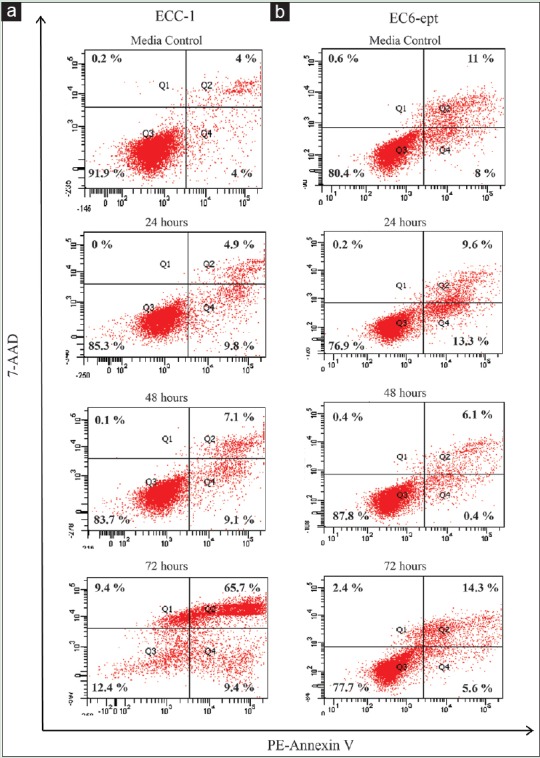
Annonacin induces apoptotic cell death in endometrial cancer cells. (a and b) Detection of apoptosis in endometrial cancer cells using Annexin V/7-AAD double staining and measured by flow cytometry posttreatment of 4 μg/ml of annonacin. Data shown are representative of two independent experiments
Table 2.
Percentage of apoptotic cells posttreatment of 4 μg/mL of annonacin at 24, 48, and 72 h were determined from annexin V/7-AAD double staining
Annonacin induces DNA fragmentation in endometrial cancer cells
We further elucidated the effect of annonacin on DNA fragmentation, one of the hallmarks of apoptosis. ECCs treated with 1 and 4 μg/ml of annonacin for 72 h were stained with transferase dUTP nick end labeling (TUNEL) solution. Total DNA was counterstained with Hoechst 33342. Both ECC-1 and EC6-ept showed negative staining of TUNEL after treatment of 1 μg/ml of annonacin. When we increased the concentration to 4 μg/ml, both cells showed significant increase in the amount of TUNEL-positive signals. Interestingly, we observed that TUNEL-positive signals were mostly formed at the perinuclear area [Figure 4]. These observations suggest that annonacin effectively triggered apoptotic cell death on ECCs which led to DNA fragmentation.
Figure 4.
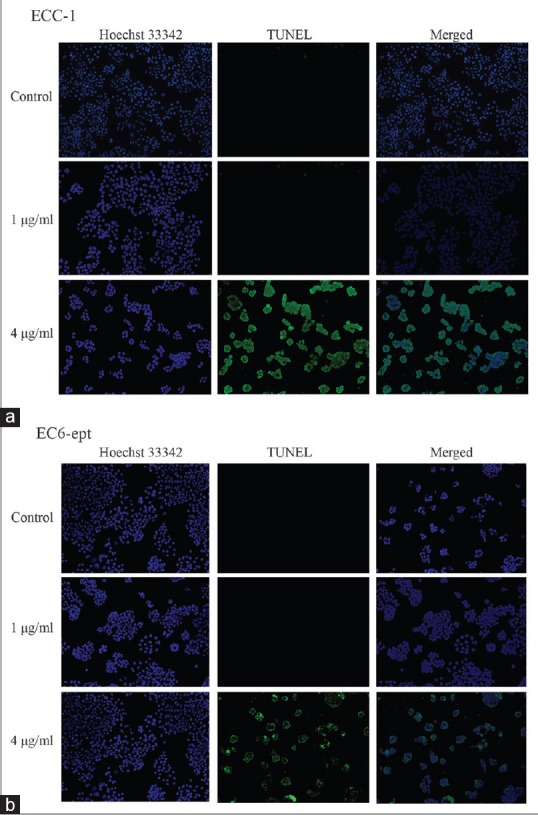
Annonacin induces DNA fragmentation in endometrial cancer cells. (a and b) DNA fragmentation examination of EC cells with 1 and 4 μg/ml of annonacin at 72 h. Total DNA of ECCs was counterstained with Hoechst 33,342. Data shown are representative of two independent experiments (×100). ECC: Endometrial cancer cell, EC: Endometrial cancer
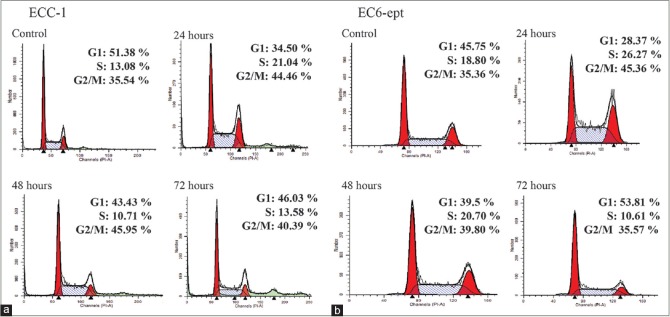
Annonacin arrests endometrial cancer cells at G2/M
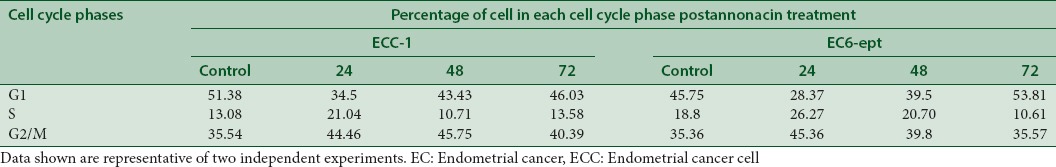
We next investigated whether annonacin could affect ECC cycle progression by performing propidium iodide staining. ECC-1 cells treated with annonacin at 4 μg/ml were shown arrested at G2/M (46%) at the peak of 48 h when compared to control (35%) (P < 0.005) [Figure 5 and Table 3]. The G2/M-phase arrest was associated with a gradual decrease in the percentage of cells in the G1 phase. In EC6-ept cells, however, a marked increase in G2/M phase was observed at 24 h posttreatment, with a similar decrease in the percentage of G1 phase cells [Figure 5 and Table 3]. These results indicate that annonacin may exert an antiproliferative effect by inducing G2/M cell cycle arrest.
Figure 5.
Annonacin induces cell cycle arrest at G2/M phase at 24 h treatment. (a and b) Cells were treated with annonacin for 24, 48, and 72 h before staining of propidium iodide to measure DNA content using flow cytometry. Data shown are representative of two independent experiments
Table 3.
Cell cycle distribution post-24, 48, and 72 h annonacin treatment were determined from propidium iodide staining
Annonacin promotes caspase-3 cleavage and inhibits extracellular signal-regulated kinase signaling pathway to induce cell death
To further investigate the mechanism involved in annonacin-induced apoptosis in ECCs, we examined the expression and cleavage of caspase-3 by immunoblotting. Caspase-3 is an effector caspase that enters the nucleus and directly interacts with its substrate, thereby promoting cell apoptosis. The cells were treated with 4 μg/ml of annonacin for 72 h before analyzed for caspase-3 cleavage. In all ECCs tested, the expression of the procaspase-3 decreased, whereas caspase-3 cleaved fragments were detected, indicating that annonacin induced a caspase-mediated apoptosis [Figure 6b]. Phosphorylation of ERK protein was significantly diminished in HEC-1A, EC6-ept, and EC14-ept, in a concentration-dependent manner, after treatment with annonacin. However, in ECC-1, the phosphorylation of ERK protein significantly reduced under treatment with annonacin at 4 μg/ml. Total ERK and housekeeping gene, β-actin protein expression were not affected by the treatment [Figure 6a]. These data suggest that annonacin may inhibit ERK survival signaling to enhance its cytotoxic action in inducing caspase-mediated apoptosis.
Figure 6.
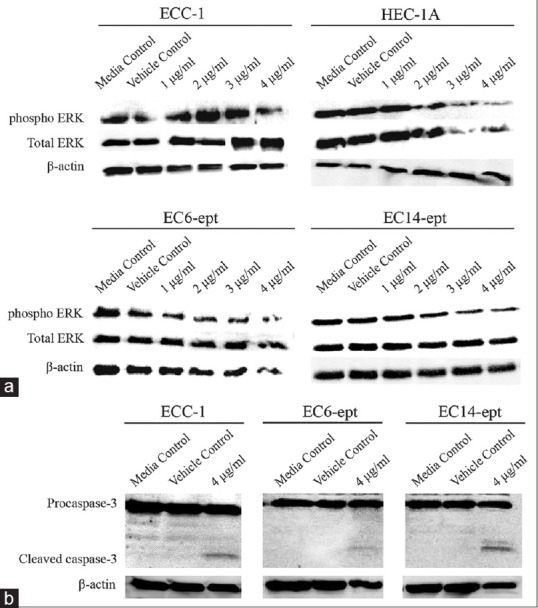
Annonacin exhibits antiproliferation effects on endometrial cancer cells through inhibition of extracellular signal-regulated kinase phosphorylation and promotion of caspase-3 cleavage. (a) Western blotting analysis of extracellular signal-regulated kinase protein expression in endometrial cancer cells after treated with control media, vehicle, or annonacin at 72 h. Data shown are representative of two independent experiments. (b) Expression of procaspase-3 and cleaved caspase-3 in endometrial cancer cells 72 h postannonacin treatment. β-actin was used as endogenous control. Data shown are representative of two independent experiments
DISCUSSION
While the antiproliferative effects of annonacin have been described in some cancer types,[11] its role and mechanism of action in EC have yet to be extensively explored. In our study, we observed that annonacin inhibited ECC proliferation in both cell lines and primary cells, ranging from 4.6 to 5 μg/ml. This compound was also shown to induce apoptotic cell death through caspase-3 cleavage and DNA fragmentation. In addition, G2/M phase in cell cycle arrest with inhibition of ERK survival signaling pathway was observed in treated cells. Hence, our data suggest that annonacin may be a potent antineoplastic compound in treating EC.
Complementary medicines have often been an alternative for diseases as the adverse effects of cytotoxic chemotherapy are often vigorous and harsh. Leaves of Annonaceae family have been used as an anti-inflammatory, antispasmodic, or antidysenteric agent for various remedies including headaches, insomnia, cystitis, liver problems, diabetes, and hypertension.[5] Annonaceous acetogenins are antineoplastic, parasiticidal, and pesticidal compounds which found only in the plant family Annonaceae.[12] A concoction from the leaves has parasiticide, antirheumatic, and antineuralgic effects when used internally, while the cooked leaves, applied topically, fight rheumatism and abscesses.[5] It has been shown that the annonacin inhibits NADH, the ubiquinone oxidoreductase (complex I) of the mitochondrial electron transport system, hence lead to a reduction of ATP production.[13]
Interestingly, according to Suresh et al., the ethanol extract of roots from Annona reticulata Linn, the acetogenins from Annonaceae family, shows an inhibitory effect against A-549, K-562, HeLa, and MDA-MB cancer cell lines with IC50 values ranging from 32 to 40 μg/ml.[14] In our study, annonacin inhibits the EC proliferation effectively at relatively low EC50(~4 μg/ml). Some pioneering studies also verified that annonacin isolated from seeds of A. reticulate is cytotoxic to T24 bladder cancer cells by arresting the cells at G1 phase. It induces apoptotic cell death of cancer cells by upregulating Bax and Bad expressions and by increasing caspase-3 activities and procaspase-3 cleavage.[11] Interestingly, our study showed that the annonacin induces apoptotic cell death in a caspase-3-related pathway and arrests cell cycle at G2/M phase. These observations suggest that annonacin may deregulate the G2/M arrest checkpoint to allow a cancer cell to enter mitosis and undergo apoptosis, which eventually reduce cell proliferation.[15]
Our findings provided a new mechanism of action for annonacin, which exhibits antiproliferation effects on ECCs by inducing G2/M arrest, inhibiting ERK survival pathway and inducing apoptosis through caspase-mediated DNA fragmentation. This is one of the few early evidences to suggest that annonacin targets ERK signaling, and hence may act as an inhibitor for ERK signaling pathway in EC. Taken together, our work provides molecular evidence that support the use of annonacin in treating cancer. Yet, comprehensive investigation is needed to better understand its mechanism of action, including its effects in vivo models. Besides, the interaction of annonacin with cytotoxic agents commonly used in therapy should be studied. This is particularly important if annonacin was to be considered as a combinatory or adjuvant treatment with current mainstay management in gynecology-related cancers. These studies will provide further justification to develop annonacin as a novel therapeutic agent in this unmet medical need for women with EC.
CONCLUSION
Annonacin exhibits an antiproliferative effect on ECCs, partly by caspase-3 cleavage and DNA fragmentation-mediated apoptosis, downregulation of ERK signaling, and G2/M cell cycle arrest.
Financial support and sponsorship
This study was funded by University of Malaya Research Grant (RP019b).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Tissue collection was undertaken by the University of Malaya's Faculty of Medicine Biobank Unit and laboratory works were performed at the Translational Core Laboratory, Faculty of Medicine, University of Malaya.
REFERENCES
- 1.Cancer.org. Endometrial Cancer. [Last updated on 2017 Jun 12; Last cited on 2017 Jun 13]. Available from: http://www.cancer.org/endometrial-cancer.html .
- 2.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 3.Kidera Y, Tsubaki M, Yamazoe Y, Shoji K, Nakamura H, Ogaki M, et al. Reduction of lung metastasis, cell invasion, and adhesion in mouse melanoma by statin-induced blockade of the Rho/Rho-associated coiled-coil-containing protein kinase pathway. J Exp Clin Cancer Res. 2010;29:127. doi: 10.1186/1756-9966-29-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Hogan S, Schmelz EM, Ju YH, Canning C, Zhou K. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr Cancer. 2011;63:795–801. doi: 10.1080/01635581.2011.563027. [DOI] [PubMed] [Google Scholar]
- 6.Adewole SO, Caxton-Martins EA. Morphological changes and hypoglycemic effects of Annona muricata linn. (Annonaceae) leaf aqueous extract on pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Biomed Res. 2006;9:173–87. [Google Scholar]
- 7.Lu W, Xia YH, Qu JJ, He YY, Li BL, Lu C, et al. p21-activated kinase 4 regulation of endometrial cancer cell migration and invasion involves the ERK1/2 pathway mediated MMP-2 secretion. Neoplasma. 2013;60:493–503. doi: 10.4149/neo_2013_064. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–8. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 9.Naik MU, Naik TU, Suckow AT, Duncan MK, Naik UP. Attenuation of junctional adhesion molecule-A is a contributing factor for breast cancer cell invasion. Cancer Res. 2008;68:2194–203. doi: 10.1158/0008-5472.CAN-07-3057. [DOI] [PubMed] [Google Scholar]
- 10.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: Molecular and cellular perspective. Landes Biosciences. 2000 [Google Scholar]
- 11.Yuan SS, Chang HL, Chen HW, Yeh YT, Kao YH, Lin KH, et al. Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Bax- and caspase-3-related pathway. Life Sci. 2003;72:2853–61. doi: 10.1016/s0024-3205(03)00190-5. [DOI] [PubMed] [Google Scholar]
- 12.Alali FQ, Liu XX, McLaughlin JL. Annonaceous acetogenins: Recent progress. J Nat Prod. 1999;62:504–40. doi: 10.1021/np980406d. [DOI] [PubMed] [Google Scholar]
- 13.Tormo JR, Gallardo T, Aragón R, Cortes D, Estornell E. Specific interactions of monotetrahydrofuranic annonaceous acetogenins as inhibitors of mitochondrial complex I. Chem Biol Interact. 1999;122:171–83. doi: 10.1016/s0009-2797(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 14.Suresh HM, Shivakumar B, Hemalatha K, Heroor SS, Hugar DS, Rao KR. In vitro antiproliferativeactivity of Annona reticulata roots on human cancer cell lines. Pharmacognosy Res. 2011;3:9–12. doi: 10.4103/0974-8490.79109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPaola RS. To arrest or not to G (2)-M Cell-cycle arrest: Commentary re: A. K. Tyagi et al. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G (2)-M arrest, and apoptosis. Clin. cancer res., 8: 3512-3519, 2002. Clin Cancer Res. 2002;8:3311–4. [PubMed] [Google Scholar]