Abstract
Background:
Caralluma europaea (CE) has been studied for its chemical constituents, and no information is available on its toxicity or its pharmacological activities.
Objective:
To determine the toxicity of an aqueous extract of CE stems in vitro and in vivo after acute and subchronic oral gavages in Swiss albino's mice and its immunomodulatory and inflammatory activities.
Materials and Methods:
The extract was administrated in single oral dose at 5 g/kg body weight for the acute toxicity test and by gavages daily at doses of 1, 2.5, or 5 g/kg for 30 consecutive days for the subchronic toxicity test. The immunomodulatory activities and inflammatory activities were tested by the evaluation of hemagglutination antibodies (HAs) titers and delayed-type hypersensitivity (DTH) response.
Results:
For the dose of 1 g/kg, no visible toxic effects were observed. However, for the higher doses, clinical observations of toxicity were noted after 1 week of treatment. This was confirmed by the biochemical parameters values and the histology analyses of the spleen, liver, and kidney tissues. The high cellular mortality rate in vitro when treated with CE extract confirmed their toxicity potential. There was also increase of “HA titer” and “DTH” response in mice treated with nontoxic dose of CE (1 g/kg) compared to control group. This immune activity was confirmed by the high number of lymphocytes infiltrates noted in the different organs.
Conclusion:
We conclude that CE at the dose up of 1 g/kg produced toxic effect in mice that induced an immune inflammatory reaction.
SUMMARY
Caralluma europaea (CE) has been studied for its chemical constituents, and no information is available on its toxicity or its pharmacological activities. The objective is to determine the toxicity of an aqueous extract of CE stems in vitro and in vivo after acute and subchronic oral gavages in Swiss albino's mice and its immunomodulatory and inflammatory activities. For the dose of 1 g/kg, no visible toxic effects were observed. However, for the higher doses, clinical observations of toxicity were noted after 1 week of treatment. This was confirmed by the biochemical parameters values and the histology analyses of the spleen, liver, and kidney tissues. The high cellular mortality rate in vitro confirmed their toxicity potential. There was also increase of “hemagglutination antibody titer” and “delayed-type hypersensitivity” response in mice treated with nontoxic dose of CE (1 g/kg) compared to control group. This immune activity was confirmed by the high number of lymphocytes infiltrates noted in the different organs. We conclude that CE at the dose up of 1 g/kg produced toxic effect in mice that induced an immune inflammatory reaction.
Abbreviations Used: CE: Caralluma europaea, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, RRBCs: Rat red blood cells, DTH: Delayed-type hypersensitivity response, PBS: Phosphate buffer solution.
Key words: Caralluma europaea, immunomodulatory reaction, inflammatory reaction, toxicity in vivo and in vitro
INTRODUCTION
Caralluma europaea (CE) (or Apteranthes europaea Guss.) a member of Apocynaceae family[1] is distributed in Egypt, Spain, Italy, Libya, Tunisia, Algeria, and Morocco.[2] Several members of genus Caralluma have found various medicinal uses: antidiabetic, antihyperglycemic, antiparasitic, antitrypanosomal, antiulcer, neuroprotective, antipyretic, anti-inflammatory, antinocicepetive, antioxidant, antiobesogenic, and antiartherosclerotic properties.[3,4,5,6,7,8,9,10,11,12] This genus has been extensively explored for a variety of pharmacological activities as compared with other species, but not all species were tested for their biological activity. Locally known as “Daghmous,” “Zakkum,” or “Tikiwt”, CE is a plant species communally used in Moroccan traditional medicine for their presumed anticancer activity.[13,14] The only few studies on CE cited in the literature were on their chemical constituents (monoterpenoids, terpinolene [23.3%], α-terpinene [19.1%] and linalool [18.4%], flavonoids) and their possible role in the biology of pollination.[15,16] Zito et al. have reported that aromatic compounds found in stems and fruits of CE are semi-chemicals for many insects. Some constituents show antimicrobial activity against Candida albicans, Clostridium welchii, and Staphylococcus aureus[17] or antifungal activities on the plant pathogenic fungi such as Rhizoctonia solani, Pythium ultimum, Pyrenophora avenae, and Crinipellis perniciosa.[18] However, no sufficient information is available on the toxicity of CE or its derived effects in vivo.
The toxic effect of plant in vivo or in vitro and its immunomodulatory and anti-inflammatory potential have never been investigated before. The present study was carried out to determine the toxicity of an aqueous extract of CE after acute and subchronic oral gavages in mice at different doses and its immunomodulatory and inflammatory activities.
MATERIALS AND METHODS
Plant material
The aerial part of CE was collected in Beni-Mellal city, Morocco, and was authenticated by Professor Najat Khiyati, a Plant Taxonomist, at the Department of Biology, Faculty of Sciences, University Hassan II of Casablanca. A voucher specimen of the plant sample was deposited in the National Scientific Institute, Rabat, for future reference.
Animals
Young adult male mice Swiss (20–30 g) were purchased from the animal house of the Department of Biology, Faculty of Sciences, Mohammed V University, Rabat, Morocco. The animals were kept in plastic cages in environmental conditions (22°C–24°C, 12-h:12-h dark/light cycle) allowed to drink water ad-libitum and standard pellet diet. Mice were deprived of food but with access to water 16–18 h prior the experiments. An adaptation period of 2 weeks was allowed before each experiment.
Preparation of the aqueous extract of Caralluma europaea
The aerial part of CE has been air-dried and then pulverized. The aqueous extract was prepared by adding 1.5 L of distilled water to 150 g of CE powder, and the mixture has been heated under reflux at 60°C for 1 h in a round-bottom flask; then, the boiled decoction was centrifuged, filtered, and then concentrated in a rotary vacuum evaporator at 40°C. The extracted material was stored at −20°C until used. For oral administration (gavages), the crude extract was dissolved in water at a desired concentration which was prepared on the day of the experimental studies.
Toxic activity of aqueous extract of Caralluma europaea in vivo
Acute toxicity test
The assessment of acute toxicity was performed according to the World Health Organization (WHO) guideline (WHO 2000) and the Organisation for Economic Cooperation and Development (OECD) guideline for testing of chemicals 420 (OECD 2001).[19] To conduct these studies, we receive the ethics committee approval of the university.
The aqueous extract was administrated in single oral dose at 5 g/kg body weight in 200 μL while the control group received distilled water (5 mice/group). Signs of toxicity and mortality have been recorded at the 1st, 2nd, 4th, and 6th h after oral administration and then once daily for 14 days.[20,21]
Subacute toxicity test
Mice were divided into four groups containing five animals per group and housed in separate cages during the study. The aqueous extract was given orally by gavages to different groups daily at a dose of 1, 2.5, or 5 g/kg body weight for 30 consecutive days, while the control group received the vehicle only. The chosen doses were based on the dose of no-observed-adverse-effect-level (NOAEL) that was obtained from the acute toxicity study, 1 g/kg. The animals were closely monitored daily for general behavior and toxicity signs throughout the experimental period.[22] At the end of the treatment period (30 days), the mice were sacrificed and the blood samples and organs have been collected. Blood was collected in tubes with the anticoagulant, ethylenediaminetetraacetate. The blood was allowed to clot before centrifugation (3000 rpm at 4°C for 10 min) to obtain serum, which was analyzed for creatinine, urea, and the activity of liver enzymes: alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Freshly dissected mice's liver, kidneys, and spleen were cut carefully in small slices and fixed in buffered formaldehyde solution (10%), dehydrated in ascending series of ethanol solutions, and embedded in paraffin. Then, 4–5-μm thick sections of each tissue was prepared and stained with hematoxylin-eosin and examined under a light microscope; photomicrographs of the samples were recorded and interpreted by a pathologist.
Toxic activity of aqueous extract of Caralluma europaea in vitro
Spleen cells from mice were cultured in RPMI (Invitrogen, Merelbeke, Belgium), media supplemented with 10% heat inactivated fetal calf serum, 4 mM glutamine, 100 μg/mL gentamicin, and penicillin–streptomycin (200 U/mL and 200 μg/mL) (Invitrogen) and 100 μl of the plant extract at 0.5 or 2 mg/ml. The cells were maintained in continuous culture in a humid atmosphere at 37°C for 24 h.In vitro cytotoxicity assay was carried every 2 h, using Trypan blue (dye exclusion) method for mortality rate determination.[23]
Immunomodulatory activities of assay
Antigen
Fresh rat blood was obtained by cardiac puncture. The rat red blood cells (RRBCs) were washed three times in a large volume of phosphate buffer solution (PBS) and centrifuged at 3000 × g for 10 min before use.
Assessment of humoral immune function
The method of Bin Hafeez et al. was used to determine the effect of the extracts on the antibody level resulting from sensitization with RRBC.[24] Briefly, mice were immunized by intraperitoneal (i.p) injection of 200 μL of RRBCs suspension (30% v/v in PBS) on day 0. Mice of the study groups (5 mice) were treated with aqueous extract at 1 g/kg body weight administered orally 3 days before immunization and continued once daily for 7 days. The control group (5 mice) received only the vehicle. The mice were sacrificed by decapitation and blood samples were collected on day 7 for serum preparation. The blood was incubated for 1 h at 37°C, centrifuged, and supernatants pooled. The sera were incubated for 30 min at 56°C to inactivate complement and stored at −20°C until use. The primary antibody titer was determined by hemagglutination technique.[25]
Hemagglutination antibody titer
A micro technique employing 96-well microplates was used. Each well of the plate received 25 μl of serial two-fold dilutions of sera in PBS. The dilution sera were challenged with 25 μl of 1% (v/v) RRBCs in the plate. After incubating the mixtures for 2 h at room temperature, the hemagglutination capacity of the sera was read visually. Titers of sera were determined as the reciprocal of the maximal dilution presenting positive hemagglutination. Each assay in this experiment was repeated three times.
Delayed-type hypersensitivity response
The effect of the plant extract on the antigen-specific cellular immune response in experimental animals was measured by determining the degree of DTH response using the footpad swelling test.[26] For sensitization, seven animals per group (control and treated) were immunized on day 0 by i.p injection with 200 μL of a RRBC suspension (30% v/v in PBS). Seven days later (day + 7), these animals were injected subcutaneous with 50 μl of same RRBC suspension in PBS into the right hind footpad for elicitation of the DTH reaction. Then, a footpad swelling was measured 24 h after the injection. The difference between the means of right and left hind footpad thickness gave a degree of footpad swelling which was used for group comparisons. To establish the effect of the extract on this immune response, a daily dose of 700 μL of plant extract at 1 g/kg body weight in PBS was administered orally at different stages of the reaction: 2 days before the sensitization (day 0) and for 7 days after the induction. Simultaneously, another group of animals (controls) was inoculated in the same conditions with 700 μL of PBS.
Statistical analysis
All studies mentioned above were done in triplicate. All values were expressed as mean ± standard error of the mean and were analyzed by one-way analysis of variance, followed by Scheffe post-hoc test, and statistically significant findings were considered those in which P < 0.05.
RESULTS
Chronic toxicity studies of aqueous extract of Caralluma europaea in mice: Clinical observations of intoxication
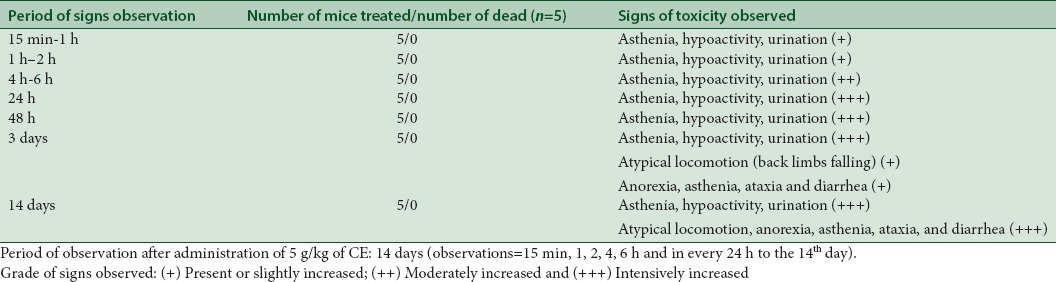
Asthenia, hypoactivity, and urination were noticed immediately after gavages (15 min) and were more pronounced and persisted until the end of experimentation. The main behavioral signs of toxicity observed from the 3rd day of daily oral administration of aqueous extract of CE at 5 g/kg were atypical locomotion, anorexia, asthenia, ataxia, diarrhea, and urination. No mortality was observed after 14 days of treatment [Table 1]. Daily clinical observations are of major importance as well as the final observations.[27,28] The doses to be evaluated in chronic toxicity must be larger than that suggested for use in humans. This dose selection is critical for the study.[28] Finalizing, the studies carried out suggest that in 1 g/kg dose, the product seems to be safe. However, in 2 and 5 g/kg doses, some adverse effects were observed despite the fact of this dose being much higher than that usually utilized in human beings (7–10 times the maximum indicated therapeutic dose in folk medicine).[13,14]
Table 1.
Signs observed in chronic toxicity after oral administration of aqueous extract of Caralluma europaea in mice
Subchronic toxicity studies of aqueous extract of Caralluma europaea in mice
Clinical observations of intoxication
Daily oral administration of aqueous extract of CE at 1 g/kg, 2.5 g/kg, and 5 g/kg of body weight has induced an hyperactivity during the 1st week of the experimentation. At the end of the 2nd week, asthenia, hypoactivity, and urination with the loss of hair and the weight (20%) were observed with the doses of 2.5 and 5 g/kg. For the dose of 1 g/kg, no visible toxic effects were observed. The NOAEL was considered to be 1 g/kg/day. No death was observed for all doses.
Effects of aqueous extract of Caralluma europaea on biochemical parameters
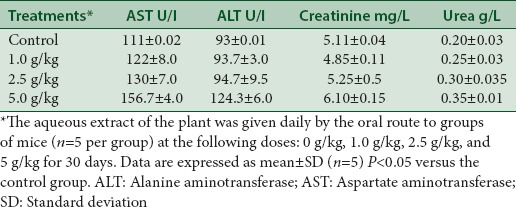
Serum levels of ALT and AST and concentration of creatinine and urea were markedly and significantly (P < 0.05) increased in mice treated by 2.5 and 5.0 g/kg of aqueous extract of the plant [Table 2]. This increase was dose dependent. In the groups that received 1 g/kg of CE, no differences were observed. The activities of AST and ALT are indicators of liver functions and the level of creatinine is an indicator for kidney activity. Therefore, CE induced serious kidney and liver injury for the higher doses 2.5 and 5 g/kg.
Table 2.
Blood chemistry values of mice in sub chronic toxicity study in control and groups treated with different doses of aqueous extract of Caralluma europaea on biochemical parameters
Effects of aqueous extract of Caralluma europaea on the spleen, kidney, and liver
Histopathological examination of the spleen, kidneys, and liver [Figure 1] at the end of the study showed that for all the doses used, there were histopathological changes. For the spleen, some hemosiderin deposits with foci of hemorrhage for the three doses tested. In the liver, lobular hepatocellular necrosis, centrilobular, macrovesicular steatosis, and centrilobular lymphocytic inflammatory infiltrations were observed for mice group treated with 1 g/kg. For the dose of 2 g/kg, splitting of nuclei and infiltration around the centrolobular veins were observed. This was more pronounced for 5 g/kg in space carries in intralobular and in centralobular cells. In the kidney, the changes were observed for the doses of 2.5 and 5 g/kg. An interstitial inflammation with nodular system composed of lymphocytes and plasmocytes indicating interstitial nephritis.
Figure 1.
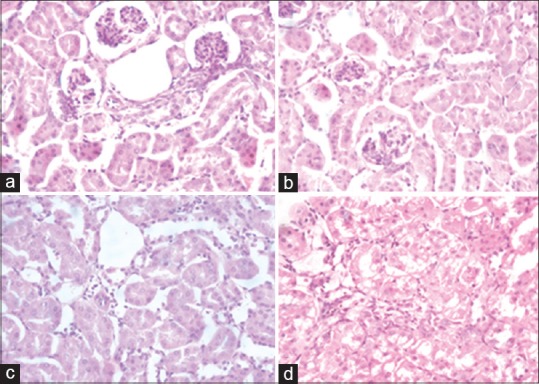
Histopathological observations of spleen, liver, and kidney of mice after subchronic treatment with aqueous extract of Caralluma europaea (H and E stain, ×400). (a) Control mice. (b) Mice treated with 1 g/kg. (c) Mice treated with 2.5 g/kg. (d) Mice treated with 5 g/kg. The aqueous extract of the plant was given daily by the oral route to groups of mice (n = 5 per group) at the following doses: 0 g/kg, 1.0 g/kg, 2.5 g/kg, and 5 g/kg for 30 days
Toxicity activity of aqueous extract of Caralluma europaea in vitro
The cytotoxicity activity of the aqueous extract of CE in vitro was tested on spleen cell suspension for 24 h with two doses: 0.5 and 2 g/ml and cell viability determined by the Trypan blue exclusion test. The results [Figure 2] have shown a mortality rate of 80% and 90% of cells after 24 h of incubation, respectively, for 0.5 and 2 g/ml doses compared at 60% for the control. The mortality rate was dose and time dependent. This confirms the toxicity effect in vivo.
Figure 2.
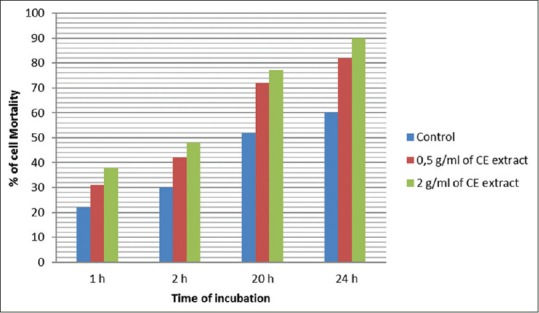
Effect of toxicity activity of aqueous extract of Caralluma europaea in vitro spleen cells from mice were cultured in RPMI supplemented with 10% heat inactivated fetal calf serum, 4 mM glutamine, 100 μg/mL gentamicin, and penicillin–streptomycin (200U/mL and 200 μg/mL) and 100 μl of the plant extract at 0.5 or 2 mg/ml. The cells were maintained in continuous culture in a humid atmosphere at 37°C for 24 h. In vitro cytotoxicity assay was carried using Trypan blue (dye exclusion) method for mortality rate determination
Effect of Caralluma europaea extract on immune functions in mice
Effect on humoral immunity
The effect of the aqueous extract treatment on the production of hemagglutination antibodies (HAs) in mice was tested. A significant (P < 0.05) increase (16 times) in primary titer values of antibodies (32,768) at limit dose of 1 g/kg was observed as compared to control (2048). The toxic effect of the plant extract on the different tissues has induced lymphocyte B-cell stimulation and antibodies production in high titers compared to controls.
Effect on the delayed-type hypersensitivity reaction
The plant extract at dose of 1 g/kg elicited a significant increase in DTH response (46%) in comparison to control animals (21%) (P < 0.05). This indicates an inflammatory reaction with attraction of inflammatory cells in the site of injection.
The CE extract has shown a significant (P < 0.05) immunostimulating effect on both humoral and cellular immune response.
DISCUSSION
There are no publications dealing with CE in its pharmacological activity or toxicity in vivo. The aqueous extract of CE was tested in vivo in mice and in vitro on spleen cell suspensions for its cytotoxic activity. Our results have shown that the dose of 1 g/kg bw was not toxic compared to control. For higher doses, when given orally, the aqueous extract of CE produced toxic effects in mice. The multiple dose study with natural products is necessary to determine the safety of drugs and plant products for human use. The doses selected for chronic and subchronic toxicity studies should be at and above the suggested human dose. In the present study, the doses used were higher than that used in folk medicine in Morocco, which is about 50–100 ml of the liquid preparation containing about 10 g of plant material per liter of water.
In the chronic toxicity study, mice administered an oral dose of 5 g/kg of the CE extract exhibited adverse effects. In the subchronic toxicity study, there was a significant decrease (20%) in bodyweight gain in mice receiving the CE extract orally at doses of 2 and 5 g/kg as compared to control group of mice. A decrease in body weight has been used as an indicator of adverse effects of drugs and chemicals. This is as results of decreased appetite. Other adverse effects have been observed; asthenia, hypoactivity, and urination with the loss of hair.
Among the biochemical parameters evaluated, AST and ALT are considered liver function markers. The increased values of these serum enzymes suggest changes in cell permeability in the hepatocytes, and this has been confirmed by histopathological examinations of the liver that indicate cellular lesions.
Kidney toxicity has also been reported after use of phytotherapeutic products[29,30] what makes essential its evaluation. In that case, creatinine and urea determinations are critical as these substances are markers of kidney function. In the present study, significant differences in the parameters were detected between treated mice and controls. This was also confirmed through histopathological examination of the kidney where an interstitial inflammation with nodular system composed of lymphocytes and plasmocytes, indicating interstitial nephritis.
A hyperreactivity in spleen white pulp with lymphoid follicles presenting increased germinal centers was observed for the doses 2 and 5 g/kg. This change suggests a reaction of the immune system to the effects of the plant extract.
Most common uses of this genus have been recorded as food without any reported adverse effects till date.[31,32] Caralluma genus seems to be safe for most people when 500 mg of the extract is taken twice a day for up to 60 days; however, the long-term safety is not known. Caralluma might cause some mild side effects such as stomach upset, gastric problem, and constipation. These side effects usually go away after a week of use.
Only six Caralluma species have reported toxic activity among the 2500 species of the genus.[33] Methanolic, ethanolic, and ethyl acetate extracts of Caralluma tuberculata show significant toxicity while aqueous extract was found to be nontoxic.[34] Caralluma dalzielii, Caralluma retrospiciens, Caralluma quadrangula, Caralluma negevensis, and C. tuberculata have also shown cytotoxic activity in vitro. Caralluma fimbriata has shown genotoxicity activity.[7] In an animal-conducted trail on Wistar rats, acute toxicity of the ethanolic extract of C. dalzielli was found at 2.154 mg/kg orally.[35]
We report for the first time the acute and subchronic toxicity of the aqueous extract of the CE in vivo at dose up to 1 g/kg bw. This toxicity has been confirmed by the variations of the biochemical parameters and the histopathology changes in the liver, spleen, and kidney and the high rate of cellular mortality in vitro compared to the control. The lymphocytic infiltrates were very pronounced in all tissues examined suggesting an immunostimulating effect on both humoral and cellular immune functions in mice. The plant showed a significant enhancement of antibody responsiveness to RRBC in mice as a result of both pre- and post-plant treatment which indicates the enhanced responsiveness of B-lymphocytes involved in antibody synthesis. The mechanism behind this elevated DTH response indicates a stimulatory effect of the plant extract, which has occurred on the lymphocytes and accessory cell types required for the expression of this reaction. Increase in both, antibodies titer, and DTH response indicated that CE extract potentiates humoral as well as the cellular immunity. This plant is a rich source of terpenoids and flavonoids which may act as immunomodulatory, which could justify the high number of lymphocytes infiltrates found in all tissues examined.[1]
Since the toxicity studies in experimental animals cannot always be totally extrapolated to humans, and a reasonable estimate of the self-administered dose is difficult to make, and in view of the widespread traditional use of this plant,[13,14] recommendations are necessary to protect the population from possible toxic effects of the plant, especially in patients treated for cancer who are already taking cytotoxic treatments.[36]
CONCLUSION
At the dose consumed empirically in traditional Moroccan medicine, CE appears to be relatively toxic. It can cause liver, spleen, and kidney toxicity. Considering these data, we could state that CE stems possess immunomodulatory properties and suggest their involvement of immune responses in the toxic lesions. Caralluma species may be tested against different tumor cell lines to explore further its anticancer activity or their inhibiting action on the cancer cell proliferation. Indeed, Caralluma species screened for phytochemical constituent seemed to have the potential to act as a source of useful drugs and also to improve the health status.
Financial support and sponsorship
Hassan II University of Casablanca, Morocco.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Meve U, Liede S. Subtribal division of Ceropegieae (Apocynaceae-Asclepiadoideae) Taxon. 2004;53:61–72. [Google Scholar]
- 2.Meve U, Heneidak S. A morphological, karyological and chemical study of the Apteranthes (Caralluma) Europaea complex. Biol J Linn Soc. 2005;149:419–32. [Google Scholar]
- 3.Zakaria MN, Islam MW, Radhakrishnan R, Chen HB, Kamil M, Al-Gifri AN, et al. Anti-nociceptive and anti-inflammatory properties of Caralluma arabica. J Ethnopharmacol. 2001;76:155–8. doi: 10.1016/s0378-8741(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh S, Reddy GD, Reddy BM, Ramesh M, Rao AV. Antihyperglycemic activity of Caralluma attenuata. Fitoterapia. 2003;74:274–9. doi: 10.1016/s0367-326x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 5.Habibuddin M, Daghriri HA, Humaira T, Al Qahtani MS, Hefzi AA. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on streptozotocin-induced diabetic rabbits. J Ethnopharmacol. 2008;117:215–20. doi: 10.1016/j.jep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Sattar E, Harraz FM, Al-ansari SM, El-Mekkawy S, Ichino C, Kiyohara H, et al. Acylated pregnane glycosides from Caralluma tuberculata and their antiparasitic activity. Phytochemistry. 2008;69:2180–6. doi: 10.1016/j.phytochem.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Kamalakkannan S, Rajendran R, Venkatesh RV, Clayton P, Akbarsha MA. Antiobesogenic and antiatherosclerotic properties of Caralluma fimbriata extract. J Nutr Metab. 2010;2010:285301. doi: 10.1155/2010/285301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adnan M, Jan S, Mussarat S, Tariq A, Begum S, Afroz A, et al. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J Pharm Pharmacol. 2014;66:1351–68. doi: 10.1111/jphp.12265. [DOI] [PubMed] [Google Scholar]
- 9.Gujjala S, Putakala M, Gangarapu V, Nukala S, Bellamkonda R, Ramaswamy R, et al. Protective effect of Caralluma fimbriata against high-fat diet induced testicular oxidative stress in rats. Biomed Pharmacother. 2016;83:167–76. doi: 10.1016/j.biopha.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Khan MZ, Atlas N, Nawaz W. Neuroprotective effects of Caralluma tuberculata on ameliorating cognitive impairment in a d-galactose-induced mouse model. Biomed Pharmacother. 2016;84:387–94. doi: 10.1016/j.biopha.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 11.Garg S, Srivastava S, Singh K, Sharma A, Garg K. Ulcer healing potential of ethanolic extract of Caralluma attenuata on experimental diabetic rats. Anc Sci Life. 2016;35:222–6. doi: 10.4103/0257-7941.188182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen S, Chen Y, Lu Y, Wang Y, Ding L, Jiang M, et al. Cardenolides from the apocynaceae family and their anticancer activity. Fitoterapia. 2016;112:74–84. doi: 10.1016/j.fitote.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. J Ethnopharmacol. 1991;35:123–43. doi: 10.1016/0378-8741(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 14.Bellakhdar J. The Traditional Moroccan Pharmacopoeia. Paris: Ibis Press; 1997. p. 764. [Google Scholar]
- 15.Formisano C, Senatore F, Della Porta G, Scognamiglio M, Bruno M, Maggio A, et al. Headspace volatile composition of the flowers of Caralluma europaea N.E. Br. (Apocynaceae) Molecules. 2009;14:4597–613. doi: 10.3390/molecules14114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zito P, Sajeva M, Bruno M, Maggio A, Rosselli S, Formisano C, et al. Essential oil composition of stems and fruits of Caralluma europaea N.E. Br. (Apocynaceae) Molecules. 2010;15:627–38. doi: 10.3390/molecules15020627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodoprost J, Rosemeyer H. Analysis of phenacyl ester derivatives of fatty acids from human skin surface sebum by reversed-phase HPLC: Chromatographic mobility as a function of physico chemical properties. Int J Mol Sci. 2007;8:1111–24. [Google Scholar]
- 18.Walters D, Raynor L, Mitchell A, Walker R, Walker K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia. 2004;157:87–90. doi: 10.1023/b:myco.0000012222.68156.2c. [DOI] [PubMed] [Google Scholar]
- 19.Organization of Economic Co-Operation and Development (OECD) Guideline for Testing of Chemicals, TG420 (OECD, The OECD Guideline for Testing of Chemical: 407 Repeated Dose Oral Toxicity Rodent: 28-day or 14-Day Study. Paris, France: OECD; 2001. [Google Scholar]
- 20.Ha H, Lee JK, Lee HY, Seo CS, Kim JH, Lee MY, et al. Evaluation of safety of the herbal formula Ojeok-san: Acute and sub-chronic toxicity studies in rats. J Ethnopharmacol. 2010;131:410–6. doi: 10.1016/j.jep.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 21.United States Environmental Protection Agency; 2002. Health Effects Test Guidelines: Acute oral toxicity OPPTS 870.1100. 2002 [Google Scholar]
- 22.United States Environmental Protection Agency; 2000. Health Effects Test Guidelines: Repeated dose 28 day oral toxicity study in rodents. OPPTS 870.3050. 2000 [Google Scholar]
- 23.Forabosco A, Zaffe D, Tosato L. On the dye exclusion of test cell vitality. I. Evaluation of the optimal concentration. Boll Soc Ital Biol Sper. 1972;48:33–6. [PubMed] [Google Scholar]
- 24.Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. On cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–8. doi: 10.1016/s0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 25.Sallander S, Shanwell A, Aqvist M. Evaluation of a solid-phase test for erythrocyte antibody screening of pregnant women, patients and blood donors. Vox Sang. 1996;71:221–5. doi: 10.1046/j.1423-0410.1996.7140221.x. [DOI] [PubMed] [Google Scholar]
- 26.Benencia F, Courrèges MC, Coulombié FC. In vivo and in vitro immunomodulatory activities of Trichilia glabra aqueous leaf extracts. J Ethnopharmacol. 2000;69:199–205. doi: 10.1016/s0378-8741(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 27.Stevens KR, Mylecraine L. Issues in chronic toxicology. In: Hayes AW, editor. Principles and Methods of Toxicology. 3rd ed. New York: Raven Press; 1994. p. 673. [Google Scholar]
- 28.Eaton DL, Klaassen CD. Principles of toxicology. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 5th ed. New York: McGraw-Hill; 1996. p. 13. [Google Scholar]
- 29.Corns CM. Herbal remedies and clinical biochemistry. Ann Clin Biochem. 2003;40(Pt 5):489–507. doi: 10.1258/000456303322326407. [DOI] [PubMed] [Google Scholar]
- 30.Isnard Bagnis C, Deray G, Baumelou A, Le Quintrec M, Vanherweghem JL. Herbs and the kidney. Am J Kidney Dis. 2004;44:1–11. doi: 10.1053/j.ajkd.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Naik RM, Venugopalan V, Kumaravelayutham P, Krishnamurthy YL. Nutritive value and mineral composition of edible Caralluma and Boucerosia species from the arid areas of Karnataka. Int J Agric Environ Biotechnol. 2012;5:117–25. [Google Scholar]
- 32.Gilbert MG. A review of Caralluma R. Br. and its segregates. Bradleya. 1990;8:1–32. [Google Scholar]
- 33.Khan MZ, Khan RA, Ahmed M, Muhammad N, Khan MR, Khan HU, et al. Biological screening of methanolic crude extracts of Caralluma tuberculata. Int J Indig Med Plants. 2013;46:2051–4263. [Google Scholar]
- 34.Rizwani GH. Phytochemical and Biological Studies on Medicinal Herbs, C. Tuberculata and C. Edulis. A Thesis Submitted to the University of Karachi for the Degree of Doctor of Philosophy, Department of Pharmacognosy, Faculty of Pharmacy, and University of Karachi. 1991 [Google Scholar]
- 35.Tanko Y, Sada NH, Mohammed K, Jimoh A, Yerima M. Effect of ethanolic extract of Caralluma diazielli on serum lipid profiles on fructose induced diabetes in Wistar rats. Ann Biol Res. 2013;4:157–61. [Google Scholar]
- 36.Chebat A, Skalli S, Errihani H, Boulaâmane L, Mokrim M, Mahfoud T, et al. Prevalence study of undesirable effects related to the use of medicinal plants by patients of National Institute of Oncology. Rabat. 2014;12:25–32. [Google Scholar]


