Abstract
Background:
Excoecaria lucida Sw. (Euphorbiaceae) is a plant conventionally used throughout the Caribbean in the treatment of infectious diseases.
Objective:
To evaluate, using bioassay-guided fractionation, the in vitro cytotoxicity and antimicrobial activity of E. lucida leaves.
Materials and Methods:
A 95% ethanol crude extract was dried and fractionated by solid-liquid separation in four phases (hexane, dichloromethane, ethyl acetate, and butanol). Antimicrobial activity (3 bacteria, 6 yeasts, and 2 fungi) was evaluated by the dilution method with resazurin (2048, 512, 128, 32, and 8 μg/mL). The cytotoxicity assays were evaluated in two cell lines: MRC-5 and RAW 264.7; calculating the selectivity index. Assays were performed for the total extract, the isolated compound with the highest yield, and the ethyl acetate and butanol phases. Isolated compounds were characterized by nuclear magnetic resonance and mass spectrometry techniques.
Results:
Fractionation process led to the isolation of ellagic acid (784.29 mg), 3,3',4'-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid (6.1 mg), and corilagin (6.91 mg). The most active were ethyl acetate phase and ellagic acid with IC50= 128 μg/mL against seven and five different species of microorganisms, respectively. The total extract (IC50=512 μg/mL) and the ethyl acetate phase (IC50=128 μg/mL) were cytotoxic in both cell lines, while butanol phase and ellagic acid both with IC50>2048 μg/mL seemed to be safer.
Conclusions:
The results obtained indicate that the Excoecaria leaves can be conventionally used as antimicrobial, but it should be present that some cytotoxicity could appear. In addition, the three identified compounds were reported for the first time in the species.
SUMMARY
Excoecaria lucida leaves (Euphorbiaceae) are used by the Cuban population due to their antimicrobial activity. This ethnopharmacological knowledge is confirmed by the integrated antibacterial and antifungal in vitro screening developed, using the bioassay-guided fractionation method.
Abbreviations Used: MRC-5-SV2: Diploid human lung fibroblasts cells, RAW 264.7: Murine macrophages cells, IC50: Inhibitory Concentration 50%, ATCC: American Type Culture Collection, CCEBI: Culture Collection of Industrial Biotechnology Center, CECT: Spanish Culture Collection Type, CFU: Colony forming units, CC50: 50% cytotoxic concentration, CO2: Carbon dioxide, SI: Selectivity index, IR: Infrared spectroscopy, 1H NMR: Nuclear Magnetic Resonance of hydrogen, 13C NMR: Nuclear Magnetic Resonance of carbon, HMQC: Heteronuclear Multiple-Quantum Correlation, HMBC: Heteronuclear Multiple Bond Correlation, COSY: Correlation Spectroscopy, NOESY: Nuclear Overhauser Effect Spectroscopy, KBr: Potassium bromide, DMSO-D6: Deuterated dimethyl sulfoxide, LC.MS: Liquid Chromatography-Mass Spectrometry, [α]D: Optical rotation, EL1: ellagic acid, EL2: 3,3’,4’-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid, EL3: corilagin, Active (+), inactive (-).
Key words: Corilagin, ellagic acid, ethnopharmacological use, selectivity index, tanninsSUMMARY
INTRODUCTION
The evaluation of new antimicrobial agents from natural origin is of the highest importance in the new therapeutic strategies that are alternative or complementary to antibiotic therapies.[1,2] The antimicrobial activity of some plant extracts may be related in some cases to the presence of phenolic compounds such as tannins.[3,4] In Cuba, there are reports of Excoecaria lucida (Euphorbiaceae)[5,6] used as an anti-asthmatic and antimicrobial.[5,7] The aqueous and methanolic extracts of the bark showed activity against various bacteria.[8] The lack of scientific data on E. lucida urged us to study its chemical and pharmacological properties, with a focus on the antimicrobial activity of the leaves, as this is the most common ethnopharmacological use reported by the Cuban population.
MATERIALS AND METHODS
Plant Material
Leaves from E. lucida were collected in September 2014 at the Ecological Reserve of Siboney-Juticí (Santiago de Cuba Province, Cuba). Botanical specimens were identified by Professor Félix Acosta Cantillo, and a voucher specimen (154 HAC-SC No. 7384) was deposited in the Herbarium of the Eastern Center of Ecosystems and Biodiversity.
Total extract
The dried powder (850 g) from E. lucida leaves was macerated with 95% ethanol for 72 h at room temperature, repeating this process four times. The leaf extract was concentrated and the solvent was removed by evaporation under reduced pressure at 40°C on a rotary evaporator (IKA-Werke, Germany). The extract was stored in amber glass at 5°C for further assays.
Antimicrobial assays
Antimicrobial testing was performed by two methods, a dilution method with resazurin as redox indicator of bacterial viability[9,10] and the disk diffusion test[11] as complementary test. Bacterial viability was quantified using resazurin while the choice of test organisms was Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Candida albicans (azole-resistant strain, B59630), Candida parapsilosis (ATCC J941058), Candida tropicalis (CDC49), Candida glabrata (B63155), Candida kefyr (B46120), Candida krusei (ATCC B68404), Trichophyton rubrum (B68183), and Aspergillus fumigatus (B42928). All strains were secured by the Laboratory of Microbiology, Parasitology, and Hygiene; Faculty of Pharmaceutical, Biomedical, and Veterinary Sciences; University of Antwerp; Belgium. Assays were performed in sterile 96-well microtiter plates, each well containing 10 μL of the sample together with 190 μL of bacterial inoculum (5 × 105 CFU/mL). Samples were prepared by fourfold dilutions of the extract in sterile water (2048, 512, 128, 32, and 8 μg/mL). Bacterial growth was compared to untreated-control wells (100% cell growth) and medium-control wells (0% cell growth). Seventeen hours of incubation was followed by the addition of 20 μL of resazurin to all wells. Bacterial viability was assessed fluorometrically after 30 min of incubation at 37°C. Fluorescence (λex550 nm, λem 590 nm) was measured on a GENios microplate reader (Tecan, Mechelen, Belgium). For fungi and yeasts, the same procedure was employed, except with A. fumigatus that was incubated at 27 °C and the amount of rezasurin added (10 μL). Incubation periods of 24, 48, and 168 h were used with Candida sp., A. fumigatus, and T. rubrum, respectively. After resazurin addition, incubations of 4, 17, and 24 h were used with the same strains in the order already mentioned. Miconazole and terbinafine were the reference drugs used for fungi, while doxycycline was employed as positive control for bacteria. Results were expressed in terms of percentage of microorganisms growth/viability in relation to control wells; a 50% inhibitory concentration (IC50) was also calculated.
In the disk diffusion test used as alternative method, the antimicrobial activity of the extract was evaluated using a panel of test organisms which included strains obtained from the Studies Center Industrial Biotechnology Culture Collection in the University of Oriente, Cuba. S. aureus (ATCC 25923), E. coli (CCEBI 1069), Enterococcus faecalis (ATCC 29212), P. aeruginosa (ATCC 27853), Bacillus cereus (ATCC 11778), Bacillus megaterium (ATCC 25646), C. albicans (ATCC 10231), Aspergillus niger (ATCC 9642), Aspergillus versicolor (CCEBI 3095), Alternaria alternata (CECT 2662), and Fusarium solani (CCEBI 3093) were the species used. Filter paper discs (Whatman, 6 mm diameter) were soaked with 10 μL of the extract dissolved in dimethyl sulfoxide (100 mg/mL) and then dried. Filter paper discs dampened with pure dimethyl sulfoxide were employed as negative controls while discs with 5 μg of ciprofloxacin and ketoconazole were used as positive controls of the antibacterial and antifungal action, respectively. When the inhibition diameter was >6 mm, samples were considered active.
Cytotoxicity assays
Two eukaryotic cell lines were used for the purpose of assessing cytotoxicity on the in vitro assays: MRC-5-SV2 cells (diploid human lung fibroblasts) and RAW 264.7 cells (murine macrophages), both strains from the European Type Culture Collection. Cellular proliferation and viability were assessed after addition of resazurin.[10] Cytotoxicity assays were performed in duplicate and samples were prepared in a series of four aqueous solutions at concentrations of 2048, 512, 128, 32, and 8 μg/mL. In both tests, fluorescence was measured at λex550 nm, λem590 nm with a microplate spectrofluorometer (Tecan, Mechelen, Belgium) using tamoxifen as a reference drug (positive control, initial concentration of 64 μg/mL). Untreated-control wells were used as solvent control. The results were expressed as percent reduction in cell viability as compared to untreated-control wells; the 50% cytotoxic concentration (CC50) was determined.
The test with MRC-5 cells was performed in 96-well microtiter plates, each well containing 10 μl of the sample and 190 μl of cell culture (1.5 × 104 cells/mL). Cell growth was compared to untreated-control wells (100% cell growth) and medium-control wells (0% cell growth). The culture plates were kept at 37°C with 5% (v/v) CO2 for 3 days. Aftermath, a volume of 50 μL of resazurin was added to each well and the plate was incubated for 4 h at 37°C, 5% CO2 for evaluating of cellular viability at the conditions previously declared.
On the other test, RAW 264.7 cells were seeded in 96-well microtiter plates (5 × 105 cells/mL). After 24 h of incubation at 37°C and 5% (v/v) CO2, the old medium was discarded and 100 μL of fresh Dulbecco's modified eagle's medium was added to each well that contains 100 μL of the sample. The plates were incubated for 24 h at 37°C, 5% CO2. After this, a volume of 50 μL of resazurin was added to each well and the plate was incubated again for 4 h at 37°C, 5% CO2 to finally assess the cellular viability at the same conditions that in previous experiments.
Selectivity index
A calculated selectivity index (SI) allowed examining the relationship between cytotoxicity and a chosen activity. The SI was defined as the ratio between the CC50 value for cytotoxicity and the IC50 value for antimicrobial activity. Samples with an SI value of 10 or more were considered highly selective.
Bioassay-guided fractionation
The dried total extract was subjected to a solid-liquid fractioning with solvents of increasing polarity until exhaustion of its metabolites. Four phases were produced (hexane, dichloromethane, ethyl acetate, and n-butanol). The solvent was removed in the collected samples using a rotary evaporator (IKA-Werke, Germany). Dried phases were stored at 4°C and the yield was calculated.
A sample of each phase was re-dissolved in ethanol and qualitative tests for tannins (gelatin and ferric chloride) and flavonoids (Shinoda) were performed.[12,13] Phases that tested positive were further evaluated for their antimicrobial activity and cytotoxicity. Where antimicrobial activity was detected, it followed a further separation by column chromatography with the purpose of structure elucidation and identification of compounds present. Methanol was used as mobile phase and sephadex as stationary phase in the column chromatography. The eluates were pooled according to their behavior on thin layer chromatography, while ultraviolet light exposure at 254 and 365 nm (TE-540, Tecna, Brazil) and iodine vapors were employed to visualize the spots.
Isolated compounds were characterized by infrared (IR) spectroscopy, optical rotation, mass spectrometry, and 1H and 13C nuclear magnetic resonance (NMR), either uni- or bi-dimensional (HMQC, HMBC, COSY, NOESY). IR spectra were obtained in potassium bromide (KBr) at a proportion of 0.5 mg of sample/100 mg of KBr (FTIR-8400S, Shimadzu, Japan). 1H and 13CNMR spectra, either uni- or bi-dimensional, were recorded using a spectrometer Varian NMR Systems (USA) operating at 500 MHz (1H) and 125 MHz (13C). Solvents used were deuterated pyridine (Pyridine-D5, CIL, Cambridge Isotope Laboratories, Inc.) and deuterated dimethyl sulfoxide (DMSO-D6, CIL, Cambridge Isotope Laboratories, Inc.). Mass spectra were obtained by direct injection into an LC-MS (amaZonX, Bruker, USA) in a mass range from 70 to 1000 m/z and mode of electrospray ionization. The mass spectra of samples were compared with those stored in the LC-MS database (Wiley 275), the database of the National Institute of Standards and Technology, and with data published in the literature. Optical rotation values [α]D of pure substances in methanol (1 mg/mL) were measured on a P-2000 polarimeter (Jasco, Japan) at 589 nm. Of the isolated compounds, the major component underwent the same protocol of tests (activity/toxicity) as the total extract or the phases. The only variation was on the concentrations applied in the diffusion disk method, in which 20 mg/mL was employed instead of 100 mg/mL.
RESULTS
Total extract preparation and bioassay-guided isolation
An amount of 65 g from the concentrated and totally dried total extract was dissolved in a mixture of methanol and water (8:2) to proceed to the fractioning. As a result, four phases were obtained, with a yield of 18.34 g (hexane), 10.37 g (dichloromethane), 6.01 g (ethyl acetate), and 23.48 g (butanol). Tannins and flavonoids were found only in the ethyl acetate and butanol phases, which were then chosen for the chemical and biological assays.
When the ethyl acetate phase was concentrated to dryness, 750 mg of a pure compound (pale yellow) was crystallized and designated as EL1. Furthermore, one of the fractions produced by the separation in column chromatography of the ethyl acetate phase yielded 6.1 mg of white amorphous crystals. The substance was pure and it was named EL2. By separation of the butanol phase in column chromatography, two pure substances were obtained. One was a compound of dark brown appearance (6.91 mg) which was designated as EL3 and the already mentioned EL1 (34.29 mg). Chemical structures of compounds EL1 (ellagic acid), EL2 (3,3',4'-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid), and EL3 (corilagin) were elucidated by analysis of the information provided by IR spectroscopy, mass spectroscopy, 1H and 13C NMR (one- and two-dimensional), optical rotation, and published literature data [Figure 1].
Figure 1.
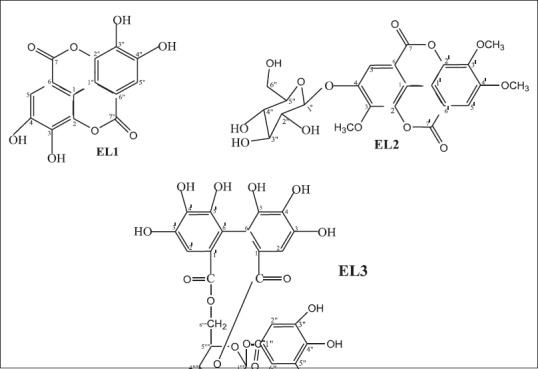
Chemical structure of compounds isolated from Excoecaria lucida Sw leaves
Ellagic acid (EL1)
Pale yellow (784.29 mg); IR (cm−1): ν 3554.81, 3157.47, 1699.29, 1616.35, 1583.56, 1508.33, 1448.54. 1H NMR (500 MHz, DMSO-d6, ppm) δH: 7.44 (2H, s, H5/H5”); 13C NMR (125 MHz; DMSO-d6, ppm) δC: 112.3 (C1/C1”), 136.4 (C2/C2”), 139.6 (C3/C3”), 148.1 (C4/C4”), 110.2 (C5/C5”), 107.6 (C6/C6”), 159.1 (C7/C7”); HMQC 1H-13C 1J (125 MHz; DMSO-d6, ppm) δH/δC: 7.44/110.2; HMBC 1H-13 Cn J (n = 2,3) (125 MHz; DMSO-d6, ppm) δH/δC: 7.44/107.6 (2 J), 7.44/112.3 (3 J), 7.44/139.6 (3 J), 7.44/148.1 (2 J) y 7.44/159.1 (3 J); EIMS: M/z 302 [M+], m/z 284 [M-18]+, m/z 256 [M-28]+, m/z 212 (M-44)+. Analysis of test results and the data described in literature allowed the identification of this substance as ellagic acid.[14,15,16,17,18]
3,3′,4′-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid (EL2)
Amorphous crystals (6.1 mg); IR (cm−1): ν 3433.29, 1743.65, 1720.50, 1620.21, 1080.14; 1H NMR: (500 MHz, C5D5N, ppm) δH: 8.44 (1H, s, H5), 7.81 (1H, s, H-5'), 4.25 (3H, s, 3-OMe), 4.12 (3H, s, 3'-OMe), 3.85 (3H, s, 4'-OMe); glucose: 5.88 (1H, d, J7.5 Hz, H-1”), 4.38 (1H, m, H-2”), 4.37 (1H, m, H-3”), 4.35 (1H, m, H-4”), 4.16 (1H, m, H-5”), 4.58 (1H, dd, J12,2.5 Hz, H-6”), 4.38 (1H, m, H-6”); 13C NMR (125 MHz, C5D5N, ppm) δC: 113.3 (C1/C1'), 141.7 (C2/C2'), 142.7 (C3), 141.8 (C3'), 153.0 (C4), 155.0 (C4'), 113.1 (C5), 108.1 (C5'), 114.1 (C6), 113.5 (C6'), 159.0 (C7), 158.8 (C7'), 61.9 (OCH3C3), 61.5 (OCH3C3'), 56.5 (OCH3C4'); glucose: 102.8 (C1”), 74.8 (C2”), 78.5 (C3”), 71.01 (C4”), 79.1 (C-5”), 62.2 (C-6”); HMQC 1H-13C 1J (125 MHz, C5D5N, ppm) δH/δC: 7.81/108.1, 8.44/113.1, 3.85/56.5, 4.12/61.5, 4.25/61.9, 5.88/102.8, 4.58/62.2 ppm; HMBC 1H-13C n J (n = 2.3) (125 MHz, C5D5N, ppm) δH/δC: 5.88/153 (2 J), 8.44/153 (2 J); COSY 1H-1H (500 MHz, C5D5N, ppm) δH/δH: 5.88/4.25. Analysis of test results and the data described in literature allowed the identification of this substance as 3,3',4'-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid.[16,19]
Corilagin: 1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-β-D-glucose (EL3)
Brown solid (6.91 mg); IR (cm−1): 3379.29, 1720.50, 1616.35, 1446.51, 1026.13, 763.81; 1H NMR (500 MHz, DMSO-d6, ppm) δH: galloyl: 7.00 (s, H2”/H6”); hexahydroxydiphenoyl: 6.48 (s, H2), 6.55 (s, H2'); glucose: 6.19 (d, J = 7 Hz, H1””), 3.87 (d, J = 7.0 Hz, H2””), 4.59 (m, H3”'), 4.21 (m, H4””), 4.34 (t; J = 8 Hz, H5””), 3.95 and 4.24 (dd, J = 9 and 11 Hz; m, H6””); 13C NMR (125 MHz, DMSO-d6, ppm) δC: galloyl: 118.7 (C1”), 109.02 (C2”/C6”); 145.6 (C3”/C5”), 139.03 (C4”), 164.8(C = O); hexahydroxydiphenoyl: 123.1 (C1), 106.07 (C2), 143.9 (C3), 135.4 (C4), 144.8 (C5), 115.5 (C6), 166.7/167.1 (C = O), 123.9 (C1'), 106.9 (C2'), 144.3 (C3'), 135.5 (C4'), 144.9 (C5'), 115.8 (C-6'); glucose: 92.2 (C1””), 71.6 (C2””), 77.6 (C3””), 62.2 (C4””), 76.4 (C5””), 64.01 (C6””); [α]D: −8.5° (25°C, MeOH, c 1 mg/mL). Analysis of test results and the data described in literature allowed the identification of this substance as corilagin.[20,21,22,23,24]
Antimicrobial assays
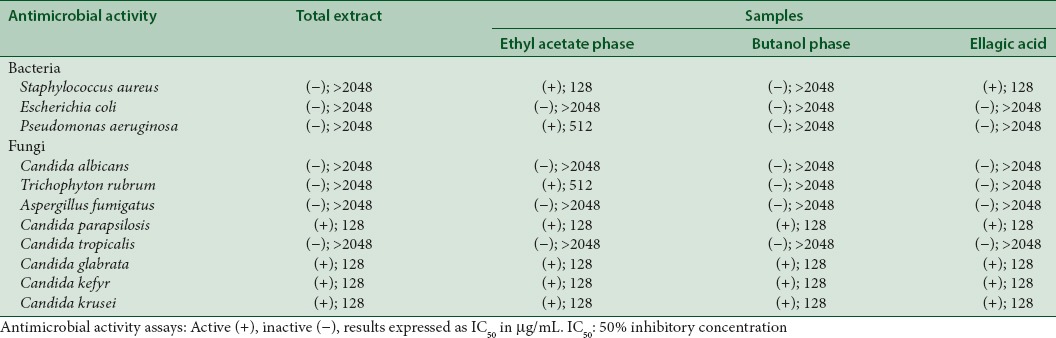
When testing antibacterial activity with the broth dilution assay and resazurin as indicator of cell growth, activity appeared in the ethyl acetate phase and in ellagic acid. Results of this antimicrobial activity tests are shown in Table 1. No activity was found for the butanol phase or for the total extract. Ellagic acid and the ethyl acetate phase were active against S. aureus, while the ethyl acetate phase was also active against P. aeruginosa. When antifungal activity is considered, moderate activities were found in the four samples. They were active against C. parapsilosis, C. glabrata, C. kefyr, and C. krusei. The ethyl acetate phase also showed activity against T. rubrum, being the sample with the broadest antifungal spectrum.
Table 1.
Results of antimicrobial activity tests in the broth dilution assay with resazurin as indicator of cell growth (50% inhibitory concentration μg/mL)
When employing the disk diffusion method (Kirby-Bauer), antibacterial activity was detected on the total extract, but not antifungal activity. The same behavior was observed for the ethyl acetate phase and the butanol phase at tested concentrations. The total extract and the ethyl acetate phase showed the broadest antibacterial spectrum as they were moderate active against four of the six bacterial strains employed. The butanol phase was only active against Gram-positive strains. The ethyl acetate phase produced the biggest inhibition zones for S. aureus, B. cereus, and B. megaterium. In E. faecalis, the inhibition zone was the largest for the total extract, suggesting that nonpolar compounds might be responsible for the antimicrobial activity against this strain.
Cytotoxicity assay and selectivity index
Results from in vitro cytotoxicity evaluations are shown in Table 2. The total extract and the ethyl acetate phase were toxic against the two cellular strains tested. In the ethyl acetate phase, cytotoxicity appeared at a lower concentration that in the total extract. The butanol phase and ellagic acid did not show cytotoxicity even at the highest concentration tested (2048 μg/mL).
Table 2.
Results of the cytotoxicity assay (50% cytotoxic concentration μg/mL) and calculated selectivity indexes
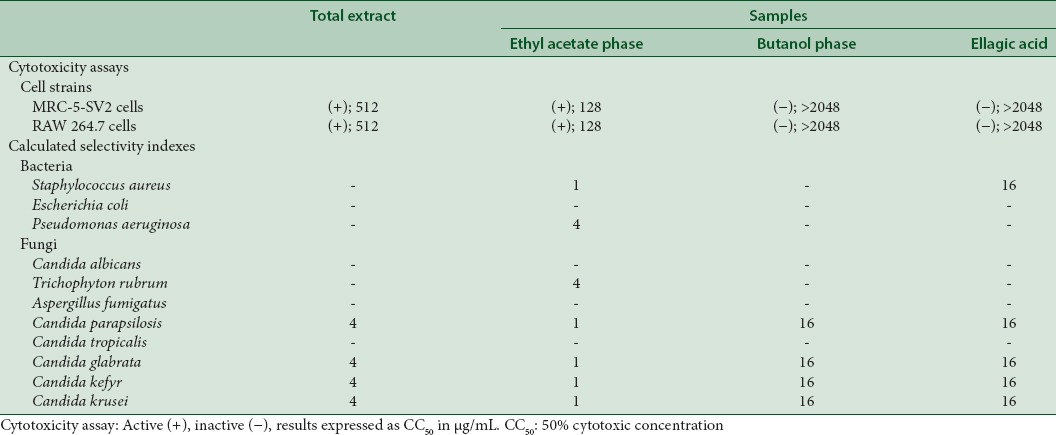
Calculated SIs are also shown in Table 2. The highest values appear in the butanol phase and in ellagic acid, with indices up to 16. The ethyl acetate phase showed an SI of 1, hampering its potential use as an antimicrobial.
DISCUSSION
The bioassay-guided isolation allowed the identification of bioactive components in the leaves of E. lucida by in vitro tests of cytotoxicity and antimicrobial activity. The total extract, its butanol, and ethyl acetate phase and an isolated compound were tested. The ethyl acetate phase showed the highest antimicrobial activity, but it was also the most cytotoxic phase. Ellagic acid was the major component in the leaves of E. lucida. It was isolated in the butanol and the ethyl acetate phase. Ellagic acid showed antimicrobial activity against five of the eleven test strains. For four of those microorganisms, antimicrobial activity was coincident with that of the butanol and the ethyl acetate phases and with that of the total extract. Our results suggest that ellagic acid might be responsible for the exhibited antifungal activity. Moreover, ellagic acid and the ethyl acetate phase were moderately active (IC50=128 μg/mL) against S. aureus while the butanol phase and the total extract were not.
Activity of a plant extract is not only governed by its main components but also governed by the activity of other substances as some of them might even have a synergic effect. In the ethyl acetate phase, the compound 3,3',4'-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid was isolated besides ellagic acid. For this compound (EL2), no published reports of antimicrobial action have been found although its unglycosylated structure has been reported as an efficient antimicrobial when tested against S. aureus, B. cereus, B. megaterium, and P. aeruginosa.[25,26] In the case of the butanol phase, corilagin was isolated in addition to ellagic acid. There are some reports of corilagin antibacterial activity against several wild strains of S. aureus,[27] C. albicans, and E. coli.[28] Despite the chemical composition of the butanol phase and the reported activity of corilagin, activity differences appeared in this sample when compared to others. These differences could be related with the low concentration at which corilagin was found.
Cytotoxicity was the highest in the ethyl acetate phase and it was followed in cytotoxicity by the total extract although it was absent in the butanol phase. The antimicrobial activity of the butanol phase and ellagic acid was specific since their SIs were higher than 10.
CONCLUSIONS
Tests on the antimicrobial effects of the total extract from E. lucida leaves confirmed the antimicrobial activity cited in its traditional use. Nevertheless, observed cytotoxicity in the extract does not suggest a safe use of it. On the other hand, the relevance of the bioassay-guided isolation was made clear when the total extract yielded the butanol phase and ellagic acid, both with high SIs. In addition, ellagic acid, 3,3',4'-tri-O-methyl ellagic 4-O-β-D-glucopyranoside acid, and corilagin are reported for the first time in E. lucida Sw.
Financial support and sponsorship
This study was supported by CAPES-MES 144-11 project: “Phytochemical Investigation of the Cuban Vegetal Species for Pharmaceutical Application” and the Belgian Development Cooperation through VLIR-UOS project (Flemish Interuniversity Council-University Cooperation for Development) in the context of the Institutional University Cooperation Program with Universidad de Oriente, especially by means of the P-3 project “Biopharmaceutical Products from Natural Sources in the Development of Biotechnology.”
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Iwu MW, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on New Crops and New Uses. Alexandria, VA: ASHS Press; 1999. [Google Scholar]
- 2.Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13:161–71. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Jones GA, McAllister TA, Muir AD, Cheng KJ. Effects of Sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl Environ Microbiol. 1994;60:1374–8. doi: 10.1128/aem.60.4.1374-1378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roig JT. Medicinal, Aromatic and Poisonous Plants of Cuba. Havana, Cuba: Science and Technology Editorial; 1974. [Google Scholar]
- 6.Borroto PR, Labrada PM, Mancina CA, Oviedo R. Rapid assessment of the biodiversity in keys Southeast Cienaga de Zapata (Cuba) ORSIS; 2007;22:9–33. [Google Scholar]
- 7.Hernández CJ, Temó VD, Acosta CF. Use of plants: Biological Diversity Project of the Sierra Maestra Mountain Range. Santiago de Cuba, Cuba: BIOECO Editorial; 2004. [Google Scholar]
- 8.Pérez GS, Zavala SM, Arias GL, Pérez GC, Pérez GR. Antimicrobial study of bark from five tree species. Phytother Res. 2001;15:356–9. doi: 10.1002/ptr.726. [DOI] [PubMed] [Google Scholar]
- 9.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–3. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 10.Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro 'proof-of-concept'. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.XII Informational Supplement. M100-S12. Pennsylvania, Wayne: National Committee for Clinical Laboratory Standards; 2002. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 12.Matos FJ. Introduction to the Experimental Phytochemistry. São Paulo, Brazil: UFC Editorial; 1997. [Google Scholar]
- 13.Sarker SD, Latif Z, Gray AI. Methods in Biotechnology, Natural Products Isolation. New Jersey, USA: Humana Press Inc.; 2006. [Google Scholar]
- 14.Su JD, Osawa T, Kawakishi S, Namiki M. Tannin antioxidants from Osbeckia chinensis. Phytochemistry. 1988;27:1315–9. [Google Scholar]
- 15.Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T, et al. Evaluation of the antioxidant activity of environmental plants: Activity of the leaf extracts from seashore plants. J Agric Food Chem. 1999;47:1749–54. doi: 10.1021/jf980864s. [DOI] [PubMed] [Google Scholar]
- 16.Li XC, Elsohly HN, Hufford CD, Clark AM. NMR assignments of ellagic acid derivatives. Magnetic Resonance in Chemistry. 1999;37:856–9. [Google Scholar]
- 17.Shin MS, Kang EH, Lee YI. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antiviral Res. 2005;67:163–8. doi: 10.1016/j.antiviral.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Rocha H, Silva H, Silva CC, Cala LB, Dantas JA, Graças AM, et al. Chemical constituents from bark of Cenostigma macrophyllum: Cholesterol occurrence. Quim Nova. 2007;30:1877–81. [Google Scholar]
- 19.Yan XH, Guo YW. Two new ellagic acid glycosides from leaves of Diplopanax stachyanthus. J Asian Nat Prod Res. 2004;6:271–6. doi: 10.1080/10286020310001595944. [DOI] [PubMed] [Google Scholar]
- 20.Nawwar MA, Hussein SA, Merfort I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry. 1994;36:793–8. [Google Scholar]
- 21.Souza CL, Massae IR, Samara NA, Sarragiotto MH, Prado DF, Vataru NC, et al. Chemical contituents of Alchornea glandulosa (Euphorbiaceae) Quim Nova. 2003;26:825–7. [Google Scholar]
- 22.Thiem B, Goslinska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75:93–5. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Markom M, Hasan M, Daud WR, Singh H, Jahim JM. Extraction of hydrolysable tannins from Phyllanthus niruri Linn: Effects of solvents and extraction methods. Sep Purif Technol. 2007;52:487–96. [Google Scholar]
- 24.Rocha-Martins LR. Thesis, Chemistry Department, University Federal of São Carlos, Sao Paulo, Brazil. 2008. Chromatographic Profile and Multivariate Analysis for Control of commercial samples of the genus Phyllanthus (quebra-pedra). Organic Chemistry D. [Google Scholar]
- 25.Kuete V, Wabo GF, Ngameni B, Mbaveng AT, Metuno R, Etoa FX, et al. Antimicrobial activity of the methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae) J Ethnopharmacol. 2007;114:54–60. doi: 10.1016/j.jep.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Ndukwe GI, Zhao Y. Pharmacological activity of 2,3,8-tri-O-methyl ellagic acid isolated from the stem bark of Irvingia gabonensis. Afr J Biotechnol. 2007;6:1910–2. [Google Scholar]
- 27.Shiota S, Shimizu M, Sugiyama J, Morita Y, Mizushima T, Tsuchiya T. Mechanisms of action of corilagin and tellimagrandin I that remarkably potentiate the activity of beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 2004;48:67–73. doi: 10.1111/j.1348-0421.2004.tb03489.x. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Luo M, Fu YJ, Zu YG, Wang W, Zhang L, et al. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phytother Res. 2013;27:1517–23. doi: 10.1002/ptr.4891. [DOI] [PubMed] [Google Scholar]


