Abstract
Background
Campylobacter jejuni is a major cause of foodborne disease having chickens as an important reservoir. Its control at the farm would lower the contamination of the final products and therefore also lower the risk of transmission to humans. At the farm, C. jejuni is rarely found in chickens before they reach 2 weeks of age. Past studies have shown that maternal antibodies could hamper C. jejuni gut colonization. The objective of this study was to compare protocols to use in order to produce anti-C. jejuni antibodies derived from egg yolks in the perspective to be used as feed additives for the control of chicken C. jejuni colonization. Laying hens were naturally contaminated with four well-characterized strains or injected with either outer membrane proteins or formalin-killed whole bacteria derived from these same strains. Eggs were collected and IgYs present in the yolks were extracted. The amount and the specificity of the recovered antibodies were characterized.
Results
It was observed that injection yielded eggs with superior concentrations of both total and anti-C. jejuni antibodies. Equivalent performances for antibodies recovered from all protocols were observed for the ability of the antibodies to agglutinate the live C. jejuni homologous strains, to hinder their motility or to lyse the bacteria. Western blot analyses showed that proteins from all strains could be recognized by all IgY extracts. All these characteristics were strain specific. The characterization assays were also made for heterologous strains and weaker results were observed when compared to the homologous strains.
Conclusions
Based on these results, only an IgY quantitative based selection can be made in regards to which protocol would give the best anti-C. jejuni IgY enriched egg-yolks as all tested protocols were equivalent in terms of the recovered antibody ability to recognized the tested C. jejuni strains.
Keywords: Antibody, Campylobacter jejuni control, Chicken colonization, Egg yolk IgY, Immunoglobulin
Background
Campylobacter jejuni is a Gram-negative bacterium that causes campylobacteriosis in humans. It is one of the leading bacterial foodborne pathogens worldwide [1]. Poultry meat products are a major source of C. jejuni for humans [2]. Up to 108 C. jejuni colony forming units (CFU) per gram of caecal content can be found in chickens arriving at slaughter thus explaining the high rate of contaminated poultry products [3]. Lowering the level of caecal colonization may reduce the level of human exposure to this pathogen [2, 4]. To achieve this, on-farm strategies to prevent or at least to lower the bird’s intestinal colonization are required [5]. The transmission in a chicken flock is mainly horizontal [6]. Interestingly C. jejuni is usually detected in commercial chickens after the 2nd or 3rd week of age [1], which suggests the presence of natural barriers preventing C. jejuni early colonization. The presence of maternal antibodies is one mechanism that may explain that lag phase [5].
Maternal antibodies are transferred from the hens to the young poults via the egg by embryonic circulation [7, 8]. Maternal antibodies found in the young chicken can recognize C. jejuni [8–10] and limit intestinal colonization [9]. They can be detected in the first week of life, but afterwards they undergo a steady decline and are not detectable after 3 weeks of age [8]. Passively supplementing the chickens with antibodies, able to recognize C. jejuni, during the whole rearing period, could become an interesting approach to the control of colonization of chickens at the farm by this particular pathogen.
The egg can easily be used for production of such antibodies [11]. Eggs have previously been used for the production of antibodies destined to control the colonization of other pathogens with different rates of success. For example, uncharacterized egg powder from non-immunized hens showed the capacity to reduce colonization of C. jejuni, Escherichia coli and Salmonella Typhimurium [12]. Commercial egg powder derived from eggs collected from hens immunized with a live Eimeria vaccine showed a protection against a subsequent Eimeria challenge [13] while a similar approach directed against colonization of broilers by Salmonella sp. was unsuccessful [14]. For C. jejuni, different results have been achieved when using egg powder as a control strategy: a reduction of C. jejuni colonization [15] and no modification at all [16].
Almost all studies are concluding that a better characterization of the produced antibodies should be undertaken. Moreover, individually, these studies used different ways to increase the egg antibodies’ concentration and specificity. Therefore, it becomes crucial to evaluate different protocols in parallel to limit comparison bias brought by analysis of work done by multiple independent research teams. For C. jejuni specifically, several approaches can be used to immunize the laying hens and subsequently could be used to recover antibodies derived from the egg yolks. A simple approach is the natural colonization of the laying hens by C. jejuni, which increases the levels of antibodies in the recovered eggs [8, 9, 17]. On the other hands, whole C. jejuni proteins extracts [15–17], outer membrane proteins (OMP) [15, 17] and whole formalin-killed C. jejuni bacteria [17] have been used in chicken immunization trials.
The aim of this study was to compare different protocols for the production of egg yolks derived antibodies recognizing several strains of C. jejuni. Total and specific antibodies concentrations, the evaluation of their capacity to recognize C. jejuni, their capacity to block motility and to show bactericidal activity against live strains were assessed in the perspective of determining the most efficient method to produce IgYs that could eventually be used as feed additives for the on-farm control of chicken C. jejuni colonization.
Methods
Strains
Strains (C. jejuni A2008A, C. jejuni B2008A, C. jejuni G2008B, C. jejuni RM1221), previously characterized by our laboratory [18–21], were used and are referred in this study as the homologous strains. Strains A2008A and G2008B are hyper-competitive for chicken colonization while strain B2008A is a poorer competitor. Strain RM1221, isolated from a case of poultry associated campylobacteriosis, is a fully sequenced strain [22]. Control strains 81-176 [23], 81116 [24], ATCC 700819 alias NCTC11168 [25] and ATCC 33291 were used as heterologous strains.
Experimental design
All experiments were performed with the approval of the Ethics Committee (CEUA) of the Faculty of Veterinary Medicine (FMV) of the University of Montreal Canada (FMV), approval #Rech-1740. Forty specific pathogen free (SPF) White Leghorn hens acquired from the Canadian Food Inspection Agency (Ottawa, ON, Canada), of 5 weeks of age and tested negative by cloacal swabs for C. jejuni, were raised in a level two biosecurity housing facility in FMV.
During the whole study, hens were placed in individual cages and wing-tagged. All birds had access to water and were fed ad libitum. Hens received a standard commercial feed, bought from a local feed mill (Aliment Natur-Aile, COOP Fédérée, Canada) composed of 17% protein, 3.5% calcium, 3% fat, 5% fiber and 0.3 mg/kg selenium. Feed was tested by culture for the absence of C. jejuni. Upon arrival, the 40 laying hens were divided into two groups: 10 laying hens were placed in room #1 (for inoculation with C. jejuni) and 30 laying hens, housed in an independent room (room #2), were further distributed in 3 groups of 10 hens.
From day 1 to the end of the study, fresh caecal droppings were weakly tested to confirm the absence or presence of C. jejuni. At 16 weeks of age, the 10 hens of room #1 were orally inoculated with a 1 mL suspension containing 3.6 × 105 CFU of a mix of four different C. jejuni strains (GR2-INO). Also at 16 weeks of age, 10 hens from the room #2 were injected subcutaneously with 100 µg of C. jejuni OMP extracts solubilized in 1 mL of HEPES buffer (Fisher Scientific, Ottawa, ON, Canada) containing 50% of Freund’s incomplete adjuvant (Sigma-Aldrich Corporation, St. Louis, MO, USA) (GR3-OMP). Ten other hens were injected subcutaneously with 109 formalin-killed whole C. jejuni suspended a 1 mL of HEPES buffer containing 50% of Freund’s incomplete adjuvant (GR4-BACT). The antigens prepared for these injections originated from the same equivalent mix of the four strains used for the oral inoculation. The 10 remaining hens were the control group: 5 hens received an oral dose of 1 mL of tryptone salt (TS) solution (Innovation Diagnostic Inc., Montreal, QC, Canada) (GR1-CINO) or were injected with 1 mL of HEPES buffer (Fisher Scientific) containing 50% of adjuvant (GR1-CIM). These two hens group were considered being the negative control group (GR1-CTL). Injection boosters were given at 20 and 28 weeks of age (week 4 and 12 post-injection). Starting with the first egg laid and until the end of the experiment, all eggs were collected daily, identified and stored at 4 °C for egg yolks IgY extraction.
Oral inoculating suspension
Briefly, − 80 °C frozen aliquots of each C. jejuni strains were cultured on Campylobacter Blood Free Selective Medium (mCCDA) (Innovation Diagnostic Inc.) for 24 h, at 42 °C, in a microaerobic atmosphere, using CampyGen gas pack (Oxoïd, Nepean, ON, Canada). Strains were then transferred onto tryptic soy Agar (TSA) containing 5% (v/v) defibrinated sheep blood (Fisher Scientific). Each strain was suspended in 1 mL of TS (Innovation Diagnostic Inc.) to reach an optic density (630 nm) of 1.0 and subsequently diluted to obtain approximately 105 CFU/mL. The four strains were then mixed in equal volumes and 1 mL of this suspension was used to orally inoculate the GR2-INO chickens. All suspensions were enumerated by culture on Brucella Agar (Innovation Diagnostic Inc.) after 48 h incubation, at 42 °C, in a microaerobic atmosphere.
Total protein extraction
Total protein extracts were obtained by a sonication method described by de Melo et al. [26] with minor changes. C. jejuni strains from an overnight culture on TSA blood agar were harvested and suspended in HEPES buffer (Fischer Scientific). The suspension was sonicated on ice five times (30 s each) with a 1 min cooldown period between each burst. Cell debris were removed by centrifugation at 10,000×g at 4 °C for 10 min. The total proteins were recovered in the supernatants and stored at – 20 °C until used.
OMP extractions
OMP of the homologous C. jejuni strains were obtained based the N-lauryl sarcosyl method described by Hobbs et al. [11]. After lysis, the membranes were collected by centrifugation at 100,000×g for 1 h at 4 °C. The pellet was resuspended in 2 mL of 10 mM HEPES buffer (pH 7.4) and centrifuged again for 1 h at 100,000×g. The resulting pellet was resuspended in 10 mM HEPES (pH 7.4) buffer. The protein concentration was determined by measuring the absorbance at 280 nm using a NanoDrop ND-1000. N-lauryl sarcosine (Sigma-Aldrich Cooperation, St. Louis, MO, USA) was added to the sample at a protein-to-detergent ratio of 1:4 (wt/wt) and incubated at 37 °C for 30 min with shaking. The sarkosyl-treated membranes were then centrifuged at 100,000×g for 1 h at 4 °C. The pellet was washed with 10 mL of 10 mM HEPES (pH 7.4) and centrifuged again. The pellet was resuspended in 500 µL of a 10 mM HEPES (pH 7.4), buffer. The OMP extracts were kept at – 20 °C.
Formalin-killed Campylobacter
Each strain was suspended in 1 mL TS solution (Innovation Diagnostic Inc.) to obtain an absorbance of 1.0 measured at 630 nm, corresponding to approximately 109 CFU/mL. Formalin (Sigma-Aldrich Corporation) was then added to reach a final percentage of 1%. The mixture was incubated at 4 °C for 24 h. Cells were washed four times, centrifuged at 4000xg for 10 min at 4 °C and washed with 10 mM HEPES buffer. The resulting suspension was inoculated on mCCDA (Innovation Diagnostic Inc.) for 48 h, at 42 °C, in a microaerobic atmosphere to confirm that the bacteria were no longer cultivable. Microscopic observations were also done to confirm that the cellular morphology was retained.
Antibodies extraction
A chloroform-based method described by Polson [27] with slight modification was used for the immunoglobulin extraction. After the separation from the egg albumen, the yolk was poured into a 50 mL conical tube. Twice the yolk volume of Dulbecco’s PBS (Sigma-Aldrich Corporation.) was added and the samples were mixed thoroughly by vortex. An equal volume of chloroform (Sigma-Aldrich Corporation) was added to the yolk and PBS mixture, and the contents were mixed vigorously to produce a thick emulsion. After centrifugation (16,300xg, 20 min, room temperature), the aqueous phase containing the IgY was collected, aliquoted, and stored at − 20 °C until the analysis.
Enzyme-linked immunosorbent assay
The levels of total and anti-Campylobacter IgYs in egg yolks were determined using Chicken IgG ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX, USA) following the manufacturer’s instruction. The working concentrations for the determination of the total IgY were 1:20,000 for GR2-INO and GR1-CTL while 1:10,000 was selected for the other groups.
The sample working dilution for the titration of the anti-Campylobacter antibodies was 1:75 for groups GR2-INO and GR1-CTL. A working dilution of 1:500 for groups GR3-OMP and GR4-BACT was retained. The plates were coated with 2 μg/mL of total protein extracted from an equal mix of the four C. jejuni homologous strains instead of the goat anti-chicken IgG (IgY)-Fc fragment antibody.
Agglutination assays
A bacterial suspension (OD 630 nm of 1.5) of each strain (homologous and heterologous) was obtained in a NaCl solution (0.85%, w/v) from an overnight culture grown on TSA containing 5% defibrinated sheep blood. In an agglutination plate, one drop of each suspension was added to one drop of each non-diluted IgY extracts or a drop of PBS. Agglutination was recorded within 2 min incubation at room temperature.
Immunoblot
Protein samples and molecular weight markers (Amersham High-Range Rainbow Molecular Weight Marker, GE Healthcare Bio-Sciences Corporation, Piscataway, NJ, USA) were diluted in sample buffer (0.5 M Tris (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5% mercaptoethanol). Total protein (75 µg) of each C. jejuni strain was loaded on a 10% Bis-Acrylamide (Fisher Scientific) separating gel. Gels were run at 4 °C in the running buffer (25 mM Tris, 0.2 M glycine, 0.1% SDS) at 16 mA during 16 h. After SDS-PAGE, proteins were transferred to a PVDF membrane (Bio-Rad Laboratories, Mississauga, ON, Canada) using the transfer buffer (0.125 M Tris-base, 0.1 M glycine) at 100 V for 3 h at 4 °C.
The membranes were blocked in Tris-buffered saline (TBS) supplemented with 2% (wt/vol) skim milk powder (Smucker Foods of Canada Co. Markham, ON, Canada), for 1 h at room temperature and thereafter incubated 2 h with the different IgY extracts added to the blocking buffer. The dilutions of the IgY extracts were 1:50 for GR1-CTL and GR2-INO or 1:250 for the other groups. After five washes in TBS, the blots were incubated 1 h at room temperature with a goat anti-chicken IgG (IgY)-Fc fragment antibody HRP conjugated (Bethyl Laboratories) at a concentration of 1:3000. After being washed five times, the blots were developed by incubation in a solution containing H2O2 (Fisher Scientific), methanol (Fisher Scientific) and 4-chloro-1-naphtol (Sigma-Aldrich Corporation) for 20 min.
Motility assay
Motility assays were performed as described previously [3] with slight modifications. A – 80 °C frozen aliquot of each strain was cultured onto TSA containing 5% (v/v) defibrinated sheep’s blood (Fisher Scientific). Each strain was suspended in 1 mL of TS (Innovation Diagnostic Inc.) to obtain an optic density (630 nm) of 0.18. Bacterial suspensions (1 µL) were then added to 100 µL of IgY extracts or 100 µL of PBS. The bacterial suspensions were incubated for 30 min and 10 µL was spotted onto the surface of a Brucella plate containing 0.4% agar. Motility plates were incubated for approximately 40 h at 37 °C under microaerobic conditions. The growth diameter (mm) was then measured. The experiment was repeated three times.
Bactericidal assay
The bactericidal assay was performed according to Sahin et al. [9]. Briefly, each test tubes contained 50 µL of the bacterial suspension, 50 µL of 1:5 (in PBS) complement (sera recovered from C. jejuni negative chickens) and 10 µL of antibody extracts or PBS. Control tubes included (i) bacteria + complement only; (ii) bacteria + antibody only; and (iii) bacteria + PBS only. After 1 h incubation at 37 °C, 100 µL of the bacterial suspension was enumerated onto Brucella agar plates (Innovation Diagnostic Inc.) incubated for 48 h, at 42 °C, in a microaerobic atmosphere. The percentage of reduction in the number of live C. jejuni was calculated by the following formula: [CFU (bacteria + complement only)—CFU (bacteria + antibody + complement)]/CFU (bacteria + complement only) × 100. The assay was repeated three times.
Statistical analysis
All statistical analyses were computed in GraphPad v6 (Prism, LaJolla, USA). An alpha value of 0.05 was chosen as the significance level. When more than two groups were compared, the Kruskal–Wallis test was used while two-by-two comparisons were analyzed with the Mann–Whitney test.
Results
Egg yolks total and anti-C. jejuni IgYs
Total IgY per yolk increased in time to reach a plateau at 22 weeks post-immunization (Fig. 1). A drastic increase could be seen after each reinjection. GR3-OMP and GR4-BACT eggs were the ones with the highest IgY content while GR2-INO hen’s layed eggs had similar total IgY levels as the eggs collected in group GR1-CTL.
Fig. 1.
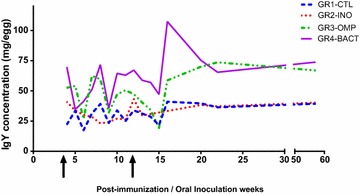
Quantification by ELISA of total IgY found in the egg yolks, post-injection of the laying hens. Each result represents the mean IgY concentrations in extracts from each laying hens group. GR2-INO (orally inoculated), GR3-OMP (injected with OMP), GR4-BACT (injected with formalin-killed whole bacteria), GR1-CTL (control groups); The arrows on the x-axis mark the boosters
The concentration of C. jejuni specific IgYs increased with time and was drastically improved by the boosters that were given 4 and 12 weeks after the first injection (Fig. 2), for GR3-OMP and GR4-BACT eggs. At 22 weeks post-injection, a maximal level of anti-C jejuni IgY was reached and remained stable until the end of the experiment. At 22 weeks, groups GR3-OMP and GR4-BACT produced significantly higher (P < 0.05 Mann–Whitney) proportion of specific IgY than group GR2-INO (1.9% of specific antibodies for GR3-OMP and GR4-BACT compared to 0.3% for GR2-INO). No C. jejuni specific IgY could be detected in the group GR1-CTL.
Fig. 2.
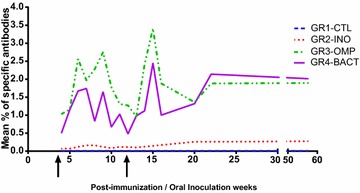
Quantification by ELISA of C. jejuni specific IgY found in the egg yolks, post-injection of the laying hens. Each result represents the mean IgY concentrations in extracts from each laying hens group. GR2-INO (orally inoculated), GR3-OMP (injected with OMP), GR4-BACT (injected with formalin-killed whole bacteria), GR1-CTL (control groups); The arrows on the x-axis mark the boosters
Immunoblot protein recognition patterns
Antibodies recovered from GR1-CTL did not recognize any proteins from tested strains (data not shown). Antibodies recovered from the egg yolks of groups GR2-INO, GR3-OMP and GR4-BACT were able to recognize proteins from both homologous and heterologous strains (Fig. 3). The C. jejuni total protein recognition pattern for GR2-INO derived antibodies was different from the ones observed for GR3-OMP and GR4-BACT, while recognition patterns from both immunized groups looked similar. Moreover, this comparison established that recognition patterns appeared strain-dependant.
Fig. 3.

Immunoblot analysis of egg-yolks derived IgY recognition profiles of total proteins of different C. jejuni strains. No proteins were recognized by group GR1-CTL egg yolks extracts. Lane 1: ladder, Lane 2: total proteins of the strain tested stained with Coomassie blue; Lanes 3 to 5, respectively: proteins recognized by the IgY extract from GR2-INO (orally inoculated), GR3-OMP (OMP) and GR4-BACT (formalin-killed whole bacteria); (a–d) Homologous C. jejuni strains: A2008A, B2008A, G2008B, RM 1221; (e–h) = Heterologous C. jejuni strains: 81116, 81-176, ATCC 700819, ATCC 33291
Agglutination
No agglutination reaction could be observed for group GR1-CTL yolk extracts while a positive reaction toward the homologous strains was noted for all other extracts (Table 1). With the exception of strain 81116 for GR2-INO extracts, no agglutination was noted for the heterologous strains.
Table 1.
Agglutination reaction after contact of each IgY extract with a suspension of different C. jejuni strain
| C. jejuni strain | GR1-CTL IgY extract | GR2-INO IgY extract | GR3-OMP IgY extract | GR4-BACT IgY extract |
|---|---|---|---|---|
| A2008A | None | Positive | Positive | Positive |
| B2008a | None | Positive | Positive | Positive |
| G2008B | None | Positive | Positive | Positive |
| RM1221 | None | Positive | Positive | Positive |
| 81116 | None | Positive | None | None |
| 81-176 | None | None | None | None |
| ATCC 700819 | None | None | None | None |
| ATCC 33291 | None | None | None | None |
None = no observed reaction; Positive = observed reaction
Homologous C. jejuni strains: A2008A, B2008A, G2008B, RM 1221
Heterologous C. jejuni strains: 81116, 81-176, ATCC 700819, ATCC 33291
GR2-INO (orally inoculated), GR3-OMP (immunized with OMP), GR4-BACT (immunized with formalin-killed whole bacteria), GR1-CTL (control group)
Motility and bactericidal assays
For the motility assay, no difference was observed. On the other hand, the bactericidal assay clearly demonstrated a loss of cultivability of C. jejuni associated with the extracts from GR2-INO, GR3-OMP and GR4-BACT (Table 2). The mean cultivability reduction of all the homologous strains tested was higher for all groups compared to the group GR1-CTL. The same result was observed for two of the heterologous strains: C. jejuni ATCC 700819 and C. jejuni ATCC 33291. The mean percentage reductions were overall higher for the homologous strains than the heterologous strains. Only strain RM1221 showed a difference in the IgY bactericidal activity depending on the chicken group. In this case, GR3-OMP and GR4-BACT IgY extracts induced a higher bactericidal effect than the extracts from group GR2-INO.
Table 2.
Bactericidal efficiency of IgY extracts
| Strain | GR1-CTL Reduction |
GR2-INO Reduction |
GR3-OMP Reduction |
GR4-BACT Reduction |
|---|---|---|---|---|
| Homologous | ||||
| A2008A | 17.35a (6.53) | 57.57b (3.39) | 50.30b (4.39) | 53.20b (17.13) |
| B2008A | 29.57a (12.60) | 95.12bc (5.50) | 60.45bd (17.78) | 68.20bd (8.05) |
| G2008B | 21.50a (14.73) | 99.61bc (0.44) | 48.24bd (26.87) | 80.08bd (13.22) |
| RM 1221 | 22.34a (12.52) | 49.71c (20.42) | 84.11bd (9.79) | 64.75b (20.98) |
| Mean homologous | 22.62a (12.41) | 79.92bc (24.67) | 60.03bd (21.76) | 67.35b (17.21) |
| Heterologous | ||||
| 81116 | 34.92 (21.48) | 39.07 (22.01) | 33.34 (8.97) | 33.46 (16.54) |
| 81-176 | 34.92 (21.48) | 39.07 (22.01) | 33.34 (8.97) | 33.46 (16.54) |
| ATCC 700819 | 14.42a (5.04) | 37.10b (7.76) | 42.89b (33.36) | 46.31b (3.08) |
| ATCC 33291 | 6.54a (5.17) | 26.30b (10.00) | 31.53b (12.54) | 28.76b (10.96) |
| Mean heterologous | 20.95a (18.58) | 34.86b (14.78) | 35.55b (9.65) | 35.79b (12.88) |
Mean reduction percentage of the initial count for the homologous and heterologous strains
() the standard deviation for 3 biological replicates
Homologous C. jejuni strains: A2008A, B2008A, G2008B, RM 1221
Heterologous C. jejuni strains: 81116 and ATCC 33291
On a same row a different than b and c different than d, P < 0.05, Kruskal–Wallis test followed by pairs of Mann–Whitney tests
GR2-INO (orally inoculated), GR3-OMP (immunized with OMP), GR4-BACT (immunized with formalin-killed whole bacteria), GR1-CTL (control group)
Discussion
Different protocols designed to induce a strong anti-C. jejuni IgY response in the egg yolks of laying hens were compared based on the fine characterization of the egg yolks antibody content. This study also used different strains as source material for the induction of the specific anti-C. jejuni IgY in an attempt to allow the produced IgY to recognize a wide array of strains. Specific antibodies against C. jejuni were produced after an oral inoculation of live C. jejuni (mimicking natural colonization) or after an injection with C. jejuni OMP or formalin-killed whole bacteria.
This study is reporting the levels of antibody found in term of concentrations while most studies are reporting titers [15] or optic densities [8, 9, 14, 16]. Reporting the actual concentration of IgY is more practical to compare different studies. In our study, there were strong differences in IgY concentration depending on the chicken groups, as the oral inoculation stimulated a lesser IgY production compared to GR3-OMP and GR4-BACT.
Immunoblots showed that an oral inoculation or both injections (OMP or formalin-killed C. jejuni) induced the production of antibodies able to recognize a large pattern of proteins from both homologous and heterologous strains (Fig. 3). Different recognition patterns by IgY extracts for different C. jejuni strains were also observed by previous studies [8, 10].
The IgY extracts were also able to recognize and agglutinate the live homologous strains. The addition of the different IgY extracts failed to induce agglutination of most heterologous strains. Since the immunoblot results confirmed the recognition of both homologous and heterologous strains by the antibodies in a strain dependant manner (Fig. 3) this difference in agglutination between homologous and heterologous strains could indicate greater antibody avidity for homologous strains or that the recognized heterologous strain proteins do not offer binding sites that promote agglutination.
The IgY extracts were also tested for their ability to hinder C. jejuni motility as it was demonstrated that motility is required for the full colonization of chickens [28]. In this experiment conditions, none of the IgY extracts were able to block or reduce the motility of C. jejuni despite the fact that whole bacteria can be agglutinated by these same IgY extracts. In a previous study [10], it was showed that a serum with antibodies against C. jejuni was able to reduce the motility of C. jejuni homologous strains. On the other hand, in a recent study, IgY derived from hyperimmune egg yolks were not able to reduce C. jejuni motility but were able to affect C. jejuni colonization of chickens [15] thus illustrating a great discrepancy between published results.
The second in vitro characterization assay on live bacteria was the analysis of the bactericidal effect of the IgY extracts. The results showed a diminution of the number of bacteria after the co-incubation of the antibodies and the complement, which suggests the capacity of the specific IgYs to promote lysis of the homologous as well as some heterologous strains. Bactericidal assays against C. jejuni were performed by other research groups using sera as a source of antibody against C. jejuni. Maternal antibodies contained in a 2-day-old chick sera were able to reduce the CFU count for homologous but not heterologous strains tested [9] while day-old chick sera reduced the CFU count of two heterologous strains with a higher reduction for one strain than the other [8]. Our results also showed that the intensity of the bactericidal effect is strain dependent and could not be related to a specific IgY production protocol.
In this study, all evaluated protocols yielded egg yolk derived IgY that were able to recognize the tested C. jejuni strains, with best results achieved against the homologous strains compared to the heterologous ones. This illustrates that in vivo trials aimed at determining the efficiency of hyperimmune egg powders for the control of C. jejuni chicken colonization should include both homologous and heterologous strains as chickens raised on commercial farms are likely to be exposed to numerous C. jejuni strains.
Higher concentration of IgY could be recovered in extracts derived from GR3-OMP and GR4-BACT eggs. On the other hand, when comparing the different extracts ability to recognize C. jejuni, it is surprising that the antibodies obtained from colonized hens, that mimic the production of maternal antibodies, gave results similar to the other two groups despite a lower concentration of both total and anti-C. jejuni IgYs in the egg yolks. It can be hypothesized that the in vivo colonization process induced the expression of colonization factors different than what is produced in vitro [29], creating an immune response more efficient to recognize C. jejuni. This clearly illustrates that quantity alone is insufficient to assess the effectiveness of a given protocol to produce IgY enriched egg yolks for an eventual use to block colonization of food animals by the foodborne pathogen C. jejuni. It also illustrates the need for a better understanding of C. jejuni proteome expressed during colonization in order to stimulate in vitro the production of proteins more relevant to colonization which would induce a much better production of antibody by the chickens.
Conclusions
The inoculation of OMP or bacterine induced the greatest concentration of anti-C. jejuni IgYs in the corresponding chicken egg yolks but on the other hand, all protocols, including natural colonization, yielded equivalent results when the recovered antibodies were further characterized. This is needed to be taken into account when trying to select for the best protocols to use for the further development of egg-yolk derived antibody as an in-feed control strategy for C. jejuni chicken colonization.
Authors’ contributions
AT wrote the manuscript, participated in the elaboration of the experimental design and reviewed the manuscript; AP realized all experiments, first-drafted the article, participated in the elaboration of the experimental design and reviewed the final manuscript; PF, SL and AL participated in the elaboration of the experimental design and reviewed the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank all the staff of the housing facility (Centre de recherche avicole) for their excellent technical assistance during the whole experiment.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
All experiments were performed with the approval, located in Saint-Hyacinthe, QC, Canada, approval #Rech-1740.
Funding
We acknowledge the following agencies for their financial supports: the Centre de Recherche en Infectiologie Porcine et aviaire (CRIPA), NSERC Industrial Research Chair in Meat safety (IRCPJ/412247-2010) and its financial partners.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- OMP
outer membrane proteins
- CEUA
ethics committee if the faculty of veterinary medicine
- FMV
Faculty of Veterinary Medicine
- SPF
specific pathogen free, i.e. free from any chicken infection as well as Salmonella and Campylobacter
- TS
tryptone salt
- mCCDA
Campylobacter Blood Free Selective Medium
- TSA
tryptic soy Agar
- ELISA
enzyme-linked immunosorbent assay
- TBS
tris-buffered saline
Contributor Information
Alexandre Thibodeau, Email: alexandre.thibodeau@umontreal.ca.
Philippe Fravalo, Email: philippe.fravalo@umontreal.ca.
Audrey Perron, Email: audrey.perron01@gmail.com.
Sylvette Laurent- Lewandowski, Email: sylvette.laurent-lewandowski@umontreal.ca.
Ann Letellier, Email: ann.letellier@umontreal.ca.
References
- 1.Young KT, Davis LM, DiRita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007 doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 2.Rosenquist H, Nielsen NL, Sommer HM, Norrung B, Christensen BB. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol. 2003 doi: 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Gerwe TJ, Bouma A, Jacobs-Reitsma WF, Klinkenberg D, Stegeman JA, Heesterbeek JA, van den Broek J. Quantifying transmission of Campylobacter spp. among broilers. Appl Environ Microbiol. 2005;71:5765–5770. doi: 10.1128/AEM.71.10.5765-5770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Barrios P, Hempen M, Messens W, Stella P, Hugas M. Quantitative microbiological risk assessment (QMRA) of food-borne zoonoses at the European level. Food Control. 2013 [Google Scholar]
- 5.Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, et al. Colonization factors of Campylobacter jejuni in the chicken gut. Vet Res. 2011 doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Deun K, Pasmans F, Ducatelle R, Flahou B, Vissenberg K, Martel A, et al. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet Microbiol. 2008 doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Hamal KR, Burgess SC, Pevzner IY, Erf GF. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poultry Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- 8.Sahin O, Zhang QJ, Meitzler JC, Harr BS, Morishita TY, Mohan R. Prevalence, antigenic specificity, and bactericidal activity of poultry anti-Campylobacter maternal antibodies. Appl Environ Microb. 2001 doi: 10.1128/AEM.67.9.3951-3957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin O, Luo ND, Huang SX, Zhang QJ. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl Environ Microb. 2003 doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoaf-Sweeney KD, Larson CL, Tang XT, Konkel ME. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl Environ Microb. 2008 doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schade R, Staak C, Hendriksen C, Erhard M, Hugl H, Koch G, et al. The production of avian (egg yolk) antibodies: IgY—the report and recommendations of ECVAM Workshop 21. Atla-Altern Lab Anim. 1996;24:925–934. [Google Scholar]
- 12.Kassaify ZG, Mine Y. Nonimmunized egg yolk powder can suppress the colonization of Salmonella Typhimurium, Escherichia coli O157: H7, and Campylobacter jejuni in laying hens. Poultry Sci. 2004;83:1497–1506. doi: 10.1093/ps/83.9.1497. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Lillehoj HS, Park DW, Jang SI, Morales A, Garcia D, et al. Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet Parasitol. 2009 doi: 10.1016/j.vetpar.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Chalghoumi R, Marcq C, Thewis A, Portetelle D, Beckers Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poultry Sci. 2009 doi: 10.3382/ps.2009-00173. [DOI] [PubMed] [Google Scholar]
- 15.Hermans D, Van Steendam K, Verbrugghe E, Verlinden M, Martel A, Seliwiorstow T, et al. Passive immunization to reduce Campylobacter jejuni colonization and transmission in broiler chickens. Vet Res. 2014 doi: 10.1186/1297-9716-45-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul NC, Al-Adwani S, Crespo R, Shah DH. Evaluation of passive immunotherapeutic efficacy of hyperimmunized egg yolk powder against intestinal colonization of Campylobacter jejuni in chickens. Poultry Sci. 2014 doi: 10.3382/ps.2014-04234. [DOI] [PubMed] [Google Scholar]
- 17.de Zoete MR, van Putten JPM, Wagenaar JA. Vaccination of chickens against Campylobacter. Vaccine. 2007 doi: 10.1016/j.vaccine.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Thibodeau A, Fravalo P, Garneau P, Masson L, Laurent-Lewandowski S, Quessy S, et al. Distribution of colonization and antimicrobial resistance genes in Campylobacter jejuni isolated from chicken. Foodborne Pathog Dis. 2013 doi: 10.1089/fpd.2012.1271. [DOI] [PubMed] [Google Scholar]
- 19.Thibodeau A, Fravalo P, Taboada EN, Laurent-Lewandowski S, Guevremont E, Quessy S, et al. Extensive characterization of Campylobacter jejuni chicken isolates to uncover genes involved in the ability to compete for gut colonization. BMC Microbiol. 2015 doi: 10.1186/s12866-015-0433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibodeau A, Fravalo P, Yergeau E, Arsenault J, Lahaye L, Letellier A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS ONE. 2015 doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibodeau A, Letellier A, Yergeau E, Larriviere-Gauthier G, Fravalo P. Lack of evidence that selenium-yeast improves chicken health and modulates the caecal microbiota in the context of colonization by Campylobacter jejuni. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, Ravel J, et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 2005 doi: 10.1371/journal.pbio.0030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poly F, Threadgill D, Stintzi A. Genomic diversity in Campylobacter jejuni: identification of C-jejuni 81-176-specific genes. J Clin Microbiol. 2005 doi: 10.1128/JCM.43.5.2330-2338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson BM, Gaskin DJH, Segers RPAM, Wells JM, Nuijten PJA, van Vliet AHM. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828) J Bacteriol. 2007 doi: 10.1128/JB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaynor EC, Cawthraw S, Manning G, MacKichan JK, Falkow S, Newell DG. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J Bacteriol. 2004 doi: 10.1128/JB.186.2.503-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demelo MA, Pechere JC. Identification of Campylobacter-jejuni surface-proteins that bind to eukaryotic cells-invitro. Infect Immun. 1990;58:1749–1756. doi: 10.1128/iai.58.6.1749-1756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polson A. Isolation of Igy from the yolks of eggs by a chloroform polyethylene-glycol procedure. Immunol Invest. 1990;19:253–258. doi: 10.3109/08820139009041840. [DOI] [PubMed] [Google Scholar]
- 28.Hendrixson DR, DiRita VJ. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol. 2004 doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 29.Malik-Kale P, Parker CT, Konkel ME. Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J Bacteriol. 2008 doi: 10.1128/JB.01736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


