Abstract
Background
Evidence-based interventions are more likely to be adopted if practitioners collaborate with researchers to develop an implementation strategy. This paper describes the steps to plan and execute a strategy, including the development of structure and supports needed for implementing proven health promotion interventions in primary and community care.
Results
Between 10 and 13 discussion and consensus sessions were performed in four highly-motivated primary health care centers involving 80% of the primary care staff and 21 community-based organizations. All four centers chose to address physical activity, diet, and smoking. They selected the 5 A’s evidence-based clinical intervention to be adapted to the context of the health centers. The planned implementation strategy worked at multiple levels: bottom-up primary care organizational change, top-down support from managers, community involvement, and the development of innovative e-health information and communication tools. Shared decision making and practice facilitation were perceived as the most positive aspects of the collaborative modeling process, which took more time than expected, especially the development of the new e-health tools integrated into electronic health records.
Conclusions
Collaborative modeling of an implementation strategy for the integration of health promotion in primary and community care was feasible in motivated centers. However, it was difficult, being hindered by the heavy workload in primary care and generating uncertainty inherent to a bottom-up decision making processes. Lessons from this experience could be useful in diverse settings and for other clinical interventions. Two companion papers report the evaluation of its feasibility and assess quantitatively and qualitatively the implementation process.
Keywords: Primary health care, Health promotion, Health education, Preventive care, Implementation strategies, Implementation research, Community of practice, Participatory action research, Learning community, Health information technologies
Background
Evidence-based interventions are more likely to be taken up if users of these interventions collaborate with researchers in the development of an effective and research-informed implementation strategy, including structure and supports that help these users to change their practice and organization to perform the proven intervention [1–5]. This type of collaborative bottom-up approach is especially necessary when implementation strategies are conceptualized not only as complex procedures but also as social processes, in which professionals take up a specific intervention or innovation if they chose to do so and creatively apply it in their setting, solving competing interests and reaching group consensus on re-design of their care delivery system [5–9]. While few studies appropriately report the details of their implementation strategies [10], there are even fewer describing the process through which these strategies were designed and tailored [11]. The report of the carrying out of this kind of collaborative experience is essential to learn from the process and to inform future refinement and replication.
Our target for improvement was the integration of healthy lifestyle promotion within primary and community healthcare. Health promotion is an excellent example of the need for implementation strategies because of the huge gap between evidence and practice in this area. Despite the sound epidemiological evidence for the impact of individual behavior on population health [12–18], we are failing to progress in the adoption of a healthy lifestyle: less than 10% of the population in developed countries do regular physical activity, follow a balanced diet, do not smoke and do not drink to excess, and the great majority have multiple behavioral risk factors [19, 20]. In addition, the current economic crisis underlines the critical role of the prevention of chronic diseases associated with these behaviors in the sustainability of healthcare systems [21] and primary care practitioners are in the best position within these systems to promote healthy behaviors among the population due to their accessibility and role in providing continuity of care [22].
Nevertheless, despite the availability of effective evidence-based interventions, healthy lifestyle promotion is far from being integrated into routine clinical practice in primary care [23–25]. For example, during the last decade our own research group has contributed with evidence-based clinical interventions for health promotion in routine primary care based on clinical trials [26–28]. Nevertheless, we have to recognize that after these trials finished participating clinicians stopped delivering the interventions [29]. The main reasons which explain this lack of sustainable integration are the inherent difficulties and complexities of changing, on the one hand, people’s lifestyles [23, 24, 30, 31], and on the other, clinical practices and the organization of primary care services [2, 23, 32, 33]. As a consequence, as in many other examples, valuable innovative initiatives fail due to implementation weaknesses [34, 35].
In accordance with the complexity of developing a targeted implementation strategy, we worked step-by-step following the UK Medical Research Council guidance for the development and evaluation of complex interventions [36]. In this paper, we describe the first step of this process, which is better visualized in relation to the extension of this guidance proposed by Pinnock et al. for phase IV implementation studies [37] (see Fig. 1). As previous preparatory work, an expert panel analyzed the causes of the implementation gap and identified roadblocks to change [23, 29]. In brief, they recommended a process of mutual adaptation of evidence-based interventions to the specific context of the primary health care (PHC) centers and, in turn, redesigning the practices and organization of these centers with the active participation of the healthcare practitioners and managers of these services, researchers and community members. They also recommended adopting the Chronic Care Model as a validated general framework to guide the redesign of primary care delivery necessary to integrate health promotion into routine practice [38–40]. Therefore, possible actions to be included in the strategy were considered at different levels: self-management support, delivery system redesign, decision support, e-health tools integrated into the clinical information systems, community resources, and health system organization.
Fig. 1.

Extended framework to include implementation research in the process of developing and evaluating complex interventions.
Modified from Pinnock et al. [37]. Reproduction authorized by the Editors
This paper describes the process of how to engage primary care staff and members of the community to reorganize primary care delivery system to optimize physical activity, healthy diet and smoking cessation interventions in primary and community healthcare. This is the companion article of two recently published in a series documenting the development and subsequent piloting of the Prescribe Healthy Life implementation strategy (PVS—from the Spanish ‘Prescribe Vida Saludable’-) (Fig. 1) [41, 42].
Methods
Action research principles were used to collaboratively model a multi-component implementation strategy for health promotion interventions [1–4]. This was a bottom-up process of dialogue, discussion and consensus among a multi-professional primary care team and community members for shared decision making on actions to be included in the implementation strategy. The study protocol has been published previously [43]. Briefly, we refer to this method as collaborative modeling, defining this as the process of redesigning the primary healthcare delivery system with a dual purpose: on the one hand, adapting available evidence-based health promotion interventions to the context of each of the collaborating PHC centers, and on the other, reaching a consensus among professionals and community members on reorganizing the practice delivery system, creating a multi-professional workforce, defining new professional roles, and redistributing tasks and workflows. Intervention mapping was used as a guide to schedule structured discussion/consensus meetings and the Institute of Medicine Plan-Do-Study-Act improvement cycles were carried out [44, 45]. The study protocol was approved by the Primary Care Research Committee of the Basque Health Service and by the Basque Country Clinical Research Ethics Committee (Ref: 06/2009).
Setting and participants
Four public community PHC centers were selected for convenience by managers of the district primary care organizations on the basis of their especial motivation favorable to health promotion [42]. For a candidate center to be included, individual written commitment to the project was required by a majority of the staff within each of the professional categories (administrative and clerical staff, nurses, family physicians, pediatricians, and others), after an informative session in the center explaining the objectives of the project and the work plan. Primary care professionals of these centers, managers of the Basque Health Service, community partners, and researchers were engaged to model the implementation strategy. A local champion was identified in each of the collaborating centers and on-site supportive practice facilitation was provided by the research team.
The Basque Health Service (Osakidetza) provides universal coverage free at the point of delivery funded through regional general taxation. In Spain, primary care services are almost exclusively delivered in publically-owned centers. Each citizen is registered on the list of one family physician or pediatrician, and these clinicians work in PHC teams including nurses and administrative personnel. They provide comprehensive primary care with easy accessibility for residents in a defined geographical area (70% of the population visiting their family physician at least once a year). Healthcare staff have a civil-servant like employment status and they are paid a fixed salary with small capitation payments for physicians.
Modeling the implementation strategy
Table 1 summarizes the stages, planning and quality improvement techniques used for modeling the implementation strategy. This process was organized through discussion and consensus meetings to assess needs, prioritize areas for improvement and select common goals; provide education on evidence-based health promotion interventions and selection by PHC center staff and community members of the most appropriate clinical interventions to be implemented; make consensus on how to redesign workflows and redistribute tasks; and then brief piloting; followed by training, auditing and feedback (see Table 1). The research team acted as practice facilitators for this modeling process, including organizing and summarizing meetings, providing selected documentation and periodical activity reports. A local coordinator was selected at each center that was the liaison with the research team and leaded the process at the local level.
Table 1.
Steps in the collaborative modeling of the PVS implementation strategy under a participatory action research framework involving primary care staff and community members supported by external facilitation provided by the research team
| Implementation goals | Contents and activities | Techniques of consensus, planning, quality improvement and evaluation |
|---|---|---|
| 1st—descriptive stage (three or four 90–120 min sessions) | ||
| To obtain the commitment of the majority of the professionals to a common health promotion goal, after prioritizing which behaviors and groups to target | Presentation of PVS objectives and plan Assessment of attitudes, perceived practice and organizational climate in the primary health care center Gathering of information on general and local epidemiology of unhealthy behaviors |
Strategic evaluation of healthy lifestyle promotion practice (audit and feedback) Individual identification of areas for improvement (gaps and needs assessment) Prioritization and consensus (nominal group) |
| 2nd—creative stage (three 90–120 min sessions) | ||
| To acquire competence in planning the preliminary intervention program: specify objectives and actions, and identify agents and resources involved | Analysis of determinants of behavior Review of evidence-based interventions Tailoring of interventions to the actual context of the center Redesign of workflows and staff roles |
Educational sessions on theoretical models and health promotion interventions Group discussion—consensus and planning Specific objectives (behavior determinants) Mapping of actions and interventions Redistribution of tasks and responsibilities: what, who, how, when and where |
| 3rd—piloting stage (four to six 90–120 min sessions) | ||
| To achieve active cooperation among the multidisciplinary team within the center and with community agents, optimization of intervention components and their sustainable integration | Practical exercise of implementing intervention actions in real-world conditions, for the identification of feasible strategies Monitoring of performance measures Standardization of preliminary program on the basis of feasibility |
Brief Plan-Do-Study-Act cycles for piloting Audit and feedback in group sessions Learning sessions to identify readjustments Strategic evaluation of the center’s capability to address the planned programs and availability of resources necessary for their implementation (SWOT matrix) |
The main contribution at the management level was to ensure the availability of new information and communication technology tools, intervention materials, and other resources necessary to facilitate the organizational change at the level of the primary care system. Further, management were required to set aside time 1 day a week for the local champion of the program in each center to support and supervise implementation at local level, and on average 2 h a month for the discussion and consensus meetings within working hours, to allow participation of the entire primary care team, covering clinical and administrative tasks with additional staff. District primary care authorities also lent institutional support to the project, which helped to initiate coordination with community organizations.
At the community level, PHC center staff were asked to identify potential partners and resources in their primary care catchment area. The research team contacted these community agents by letter, informed them of the objectives of the project, and invited them to collaborate in the subsequent sessions of the collaborative modeling. Public health practitioners from the Basque Department of Health and Consumer Affairs contributed to link community organizations and the PHC centers.
Data and analyses
First, we describe actual engagement of professionals and community in the process of modeling the implementation strategy. To this end, we documented their participation in each of the steps of the implementation strategy by asking participants to sign a register at each event, keeping signed registers of attendance at meetings, and writing summary reports of each of the meeting listed in Table 1. Second, based on the abovementioned documentation, we outline the final implementation strategy designed in terms of clinical actions to be performed, distribution of tasks between participants, definition of new roles assigned to each participant, and the new organization of the health promotion delivery system. Third, we describe the experience of professionals involved. At the end of all the collaborative modeling sessions listed in Table 1, a final meeting was organized for qualitative evaluation. The nominal group technique was used to explore the opinions of primary care professionals about their experience in the process of collaborative modeling of the PVS program [46]. As a preparatory part of the nominal group technique participants were surveyed about the positive and negative aspects of the collaborative process, the implementation climate, facilitators and barriers related to the feasibility of the implementation strategy designed, and the relationship between researchers and PHC center staff. The results of the survey were summarized and reported back to the group followed by a 90-min open-group discussion session to prioritize the most relevant aspects of the process of modeling the PVS implementation strategy.
Results
The same sequence of meetings was performed at each PHC center for collaborative modeling of the PVS implementation strategy. It required different numbers and lengths of discussion and consensus meetings at each center, ranging from 10 to 13 structured sessions lasting between 90 and 120 min. Active engagement of 71 (80%) of the 89 staff working in the four PHC centers was achieved. The highest proportion of participation was observed among family physicians (n = 24, 92% of the 26 working in the four PHC centers), followed by reception staff (n = 18, 86%), nurses (n = 23, 74%), pediatricians (n = 3, 50%), and midwives (n = 2, 50%), while the only dentist also participated. The staff participation remained above 50% in all of the discussion and consensus sessions in one center, in all but one session in two other centers, and in all but two sessions in the fourth center (Fig. 2).
Fig. 2.

Percentage of professionals who participated in each of the collaborative modeling sessions out of the total number of professionals of the primary care center
During the creative phase (Table 1), participants selected four theoretical models of behavior change as a basis for their programs, among the models most frequently used for health promotion [47]: the health belief model, the theory of planned behavior, the transtheoretical model and the social-cognitive model. The 5 A’s (Assess, Advise, Agree, Assist, and Arrange follow-up) behavioral counseling intervention was identified in all four PHC centers as the most effective and feasible evidence-based clinical intervention for the objectives set [48]. Specific tasks, goals and actions were distributed among the primary care staff as shown in Table 2 and Fig. 3. Some examples of the planned distribution of clinical intervention tasks between participants are the following. The “Assess” step was performed by receptionists before patients were seen by physicians, outside the center by school teachers, by company occupational health departments, or by individuals themselves through the Internet. The “Advice” and “Agree” steps were mainly delivered by family physicians or company doctors. The “Assist” step was mainly performed by nurses. All participants inside and outside the centers were involved in the follow-up process with particular involvement of receptionists and nurses (Table 2).
Table 2.
Targets, actions and agents of the new PVS programs to promote physical activity, healthy diet and smoking cessation in primary and community care
| Specific behavioral-cognitive objectives | Intervention actions | Who and How |
|---|---|---|
| Identify unhealthy lifestyle behavior and at-risk population Identify attitudes and intention to change to healthier lifestyles |
A1 assess: assessment of healthy lifestyle behavior and intention to change | Population self-evaluation through web-based questionnaire linked to electronic health record PVS questionnaires provided to eligible population at the health care center or through community resources (schools, sport facilities, collaborating companies, etc.) Data entry into the electronic health record by scanning by administrative staff or manually by clinicians |
| Increase perceptions of severity of risks and vulnerability associated with unhealthy lifestyles Strengthen beliefs and knowledge regarding healthy lifestyles and their positive consequences Increase intention to change behavior Strengthen positive beliefs and knowledge regarding healthy lifestyle at the community level |
A2 advise: personalized verbal advice centered on the benefits and risks of lifestyle choices A3 agree: assessment of intention to change behavior and agreement of general change goals |
Physicians or nursesa, guided by PVS software tools included in the clinical information system in routine or scheduled appointments A four-page pamphlet summarizing the abovementioned information on benefits, risks, motivation, and help offered by health care professionals Communication and diffusion of information strategies such as informal talks given by health care professionals in community settings |
| Enhance self-efficacy perception for behavior change Decrease perception of barriers to behavior change Strengthen coping skills and self-management abilities to facilitate behavior change Improve knowledge regarding community resources to facilitate and support behavior change and prevent relapse |
A4 assist: reinforcement of reasons and intention to change Identification of barriers to and solutions for behavior change Prescription of a behavior change plan through specific goal setting and action planning, including a self-monitoring log |
Nursesb assisted by PVS software, which includes tools for action planning, time management, database with contact information for community resources, and health problem-tailored information (evidence-based information on benefits related to a variety of health problems) Provision of a folder containing a brief guide to behavior change with the printed prescription attached |
| Increase reinforcement related to progress in behavior change and health improvements Strengthen perception of support for behavior change within health care, family and community contexts |
A5 arrange follow-up Review of behavior change plan, reinforcement centered on achievements, relapse prevention advice and plan re-design May include referral to community resources |
Recall system managed by administrative personnel Nursesb in scheduled appointment assisted by PVS software, which includes tools for review and re-design of behavior change plans and a database with contact information for community resources |
aIn primary health care center, although in some cases collaborating companies may also do this
bMainly nurses in the primary health care center and/or at collaborating companies, in some cases family physicians may also do this
Fig. 3.
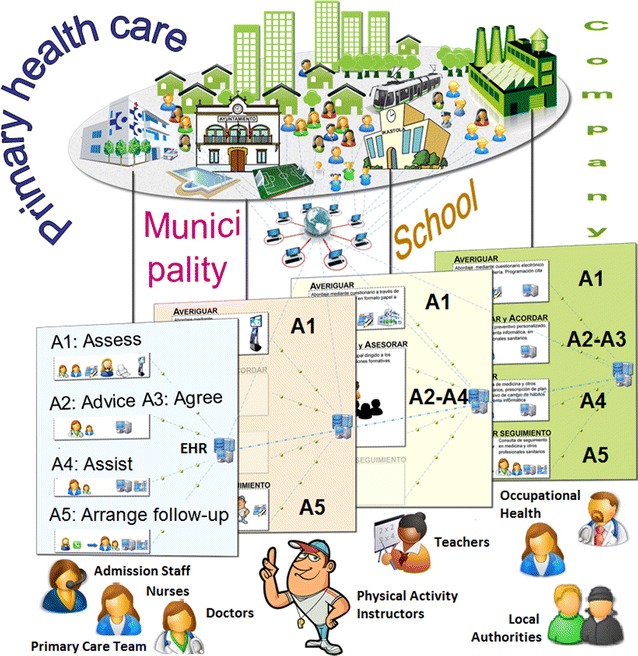
Structure and actions of the new PVS health promotion strategy
Innovative e-health tools were developed and integrated into the electronic health record (EHR) to guide PHC professionals in the process of assessment and tailored delivery of the clinical intervention for the management of healthy lifestyles (regular physical activity, adequate diet and abstinence from smoking) [41].
At the community level, 30 organizations or institutions were contacted and 21 (70%) agreed to participate in the collaborative modeling process and were actively involved in the identification and prioritization of the health promotion goals of the programs. They also participated in the design of the interventions, as well as in piloting the programs. Of the 21 community participants, nine were local authority departments, six were schools, four were sports facilities and two were manufacturing companies. The community participants mostly contributed to healthy lifestyle assessment (e.g., questionnaires administered in schools), some also participated in the advice and support steps (e.g., physicians and nurses of the occupational health departments of the collaborating companies) and in arranging follow-up actions (e.g., referral to sports facilities). In three of the four neighborhoods, local PVS health promotion councils have been set under the local authority to foster and strengthen linkages between clinical practices and community organizations. The main objectives of these councils are to identify and make information available about resources and facilities for health promotion in the community, to increase communication between organizations, and to identify referral mechanisms between them.
The experiences of participants were mixed, with both positive and negative feelings. Among the positive aspects, PHC center staff agreed that the modeling process enhanced the importance of healthy lifestyle promotion in primary care. They highlighted the engagement of the entire primary care team, including reception staff and community members, in shared decision making and cooperation in a community-based program. In addition, the availability of technological tools integrated in the EHR for supporting the clinical interventions was rated positively. Lastly, they also valued the fact that the discussion and consensus meetings were held within working hours.
As for the negative aspects of the process, the PHC center staff emphasized the heavy workload associated with the new health promotion activities compounded the problem of lack of time in the routine context of primary care. They noted the “awkward” language used in the theoretical educational sessions, and, above all, the feeling of uncertainty inherent in the innovation process: “not knowing where the process will end”. Some of the participants felt that there was a hidden agenda managed by the research team to lead them to some predetermined outcome. Additionally, participants pointed out the inherent difficulty of changing behaviors, the complexity of health promotion interventions, and the difficulty to achieve short-term results.
Participants highlighted the following critical areas for optimization to enhance the feasibility and sustainability of the modeled implementation strategies for future application: (a) at the PHC center level, first of all reorganization of on-demand care to minimize work overload, to improve the coordinated flow of care to avoid extra visits by patients, with coordinated working at all professional levels, to foster communication between different tiers of professionals and to provide sufficient staff resources; (b) concerning the information system, improvement of efficiency and reliability of the information and communication tools and databases integrated into the EHR; (c) at the patient level, innovative ways of motivating patients not ready to change and ensuring continuity of care for those with intention to change behavior to minimize false expectations; and (d) at the community level, improvement of coordination with community resources to align forces and avoid duplication of efforts.
Discussion
This paper illustrates a real world example of developing an implementation strategy through a collaborative bottom-up process engaging PHC staff, community agents and researchers. The three steps followed in this process (Table 1: descriptive, creative, and piloting stages) pursue three Implementation goals needed to introduce change into an organization: first, commitment to a shared common goal; second, planning competence to tailor evidence-based interventions to the different context of each center; and third, real cooperation among the entire group of participants [49].
All the centers chose the 5 A’s clinical intervention and this is probably due to its simplicity, meaning that less time and training are required than for other interventions, and because of the strong scientific evidence available of its effectiveness in the general population [46]. We used the Chronic Care Model as a framework to guide this effort to redesign a healthcare delivery system with the goal of improving health promotion in primary and community care [38–40]. Our approach to changing and reorganizing clinical practice is consistent with newer frameworks such as the Consolidated Framework for Implementation Research, which considers five major domains that may influence successful implementation of healthcare interventions: intervention characteristics, outer setting, inner setting, characteristics of the individuals involved, and the process of implementation [50]. Such frameworks provide no specific blueprints on how they should be operationalized in practice and researchers trying to design implementation strategies for health promotion interventions need detailed examples such as that provided herein on how they should be used [51].
Cooperation among all the PHC center staff and linkage with community agents are extremely challenging and complex social processes [5–9, 52–54]. Consequently, small steps that make progress in this direction should be considered very important. In our experience, these processes present considerable challenges. Firstly, it is not easy to sustain the commitment of staff to the common goal of integrating health promotion into routine practice, over the course of the long process of modeling and implementation. Secondly, the development of useful and efficient information and communication support tools for addressing healthy lifestyle promotion in routine primary care practice should be accelerated. Thirdly, there is resistance to organizational changes, which are essential for successful cooperation among professionals in the implementation of intervention programs. Fourthly, it would be desirable to prioritize health promotion objectives, to avoid conflicts with multiple other activities in daily practice.
All these difficulties are consistent with findings in previous initiatives for integration of health promotion in primary care [55, 56]. Institutional support from managers of healthcare services, to facilitate and ensure the organization and execution of group dynamics in each center, is essential to address these difficulties. In particular, setting aside time in the agenda of practitioners and provision of substitutes to cover regular duties of all staff to free them to attend are necessary requirements to ensure participation of PHC center staff in discussion/consensus meetings.
Prescribe Healthy Life strategy (from the Spanish ‘Prescribe Vida Saludable’) was greatly influenced by previous programs such as Prescription for Health or STEP-UP, carried out in primary care practice-based research networks in the USA [55, 56]. In turn, factors associated with the successful implementation of PVS are similar to those that arose in those programs, i.e., selection of the 5 A’s intervention strategy, active participation of primary care professionals in the decision making process to adapt the intervention to a specific context, the development of innovative information and communication technologies, and linkage with community resources. The PVS project may serve as an example for other primary care services of how to do this.
The main limitation of this study is the selection of centers by convenience. It would have been desirable to measure the readiness for change of the PHC centers, a necessary condition for quality improvement, and use this information in the selection of participating centers. However, measuring organizational readiness for change is not an easy task [57]. Past performance of the organization, the main selection criteria used in this study, is probably the best predictor of successful improvements [58], along with leadership and coaching by facilitators [2, 34, 59]. The two companion papers by Sánchez et al. and Martinez et al. [submitted] evaluate quantitatively and qualitatively the feasibility/piloting of the implementation strategy (see Fig. 1). In brief, they identify a set of key factors that facilitate or hinder the PVS program implementation, show that it is feasible to improve its uptake in routine clinical practice and that contextual factors conditioned each center’s performance [41, 42].
Conclusions
This detailed description of the design of the PVS implementation strategy can be used by implementation researchers for planning their implementation research projects and will help readers to understand the two companion papers, which describe quantitative indicators of adoption and implementation, as well as PHC center staff’s qualitative perception of the performance of the described strategy. The development of the strategy has been difficult and complex. Lessons learned will be used to improve the implementation strategy and test it in a future experimental implementation trial we are currently planning.
Authors’ contributions
GG and AS conceived the idea and are the study guarantors. They are primarily responsible for the study design and planning, project coordination and supervision, analysis and interpretation of results. GG drafted the manuscript. JMC and HP collaborated in the study design, obtained funding, and were responsible for study coordination and interpretation of the modeling process. MHC, EP, JM, and EG were local champions at the primary care centers and contributed to implementation and interpretation of the process. LB and CM were responsible for the analysis of results of the nominal groups. All contributors have critically reviewed the manuscript and approved this version submitted for publication to BMC Reseach Notes. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the large group of members of the PSV group who made the collective contribution of this action research project:
Research Team: Primary Care Research Unit of Bizkaia, Basque Health Service–Osakidetza. Principal investigator: Gonzalo Grandes; co-investigators: Álvaro Sánchez, Haizea Pombo, Josep M Cortada, Catalina Martínez, Paola Bully, and Aitor Sanz-Guinea.
Basque Health Service–Osakidetza: Carlos Sola, Deputy Director of Healthcare Services; Martín Begoña, Susana Iglesias, Maite Cuadrado, and Nuria González, from the Department of Information Technology; Teresa Garmendia, Mª Luz Jáuregui, and Amaia Hernando, from the Management Team of Goierri-Alto Urola District; Jesús Larrañaga, Maribel Romo, and Pilar Isla, from the Management Team of Bilbao District; Enrique Maíz, Cristina Domingo, and Carmen Esparta, from the Management Team of Interior District; Mª Luz Marqués, Encarnación San Emeterio, and Antón Elorriaga, from the Management of Uribe District.
Beasain Health Center (Coordinator: Justo Múgica; María Pilar Alberdi, Mª Ángeles Arrondo, Amaia Azkoitia, Xabier Epaizabal, Mª Aranzazu Echeverria, Mª Esperanza García, Mª Ángeles García, María Erkuden Imaz, Mª Antonia Iparraguirre, Mª Isabel Irizar, Mª Rosario Larrea, Mª Dolores López, Petra Pacheco, María Yolanda Porres, Begoña San Juan, Mª Aranzazu Suquia, Mª Teresa Arrospide, Carolina Díez, Miren Arantxa Igartua, Oihana Jauregui, Alazne Saizar, Mª Jose Tilves, Mª Lourdes Etxeberria, María Aurora Valdivielso, Xabier Mugica, Mª Mercedes Lasagar, Coro Zabaleta),
La Merced Health Center (Coordinator: Mª Isabel Urcelay, Mary Helen Corrales; Mª Ángeles Crespo, Javier José María Jesús de Ordozgoiti, María Iciar Elguezabal, Susana Esteban, Catalina Frau, Laura Gallo, Inés Yolanda Martín, Nerea Ordorika, José Ramón Pérez, Mª Begoña Relloso, María Soledad Sangroniz, María Iluminada Santos, Patxi Xabier Iturbe).
Matiena Health Center (Coordinator: Esther Gorostiza; Mª Esther Azpitarte, Bixente Barrutia, Amaia Bengoa, Francisco José Miguel, Ana Isabel Etxebarria, Mª Belén García, Mª Jose Ibars, Mª Jose Lasa, Mª Carmen Martínez, Maura Pernudo, Lourdes Oribe, Mª Dolores Ustarroz, Valentina Camino, Leire Corpión, Leire Ortuondo, Mª Carmen López, Rosana Abraldes, Eneko Ibarruri, Javi Alonso).
Sondika Health Center (Coordinator: Enrique de la Peña; Mª Carmen Artola, Teresa Casado, Jesús García, Mª Paz Sánchez, Luisa Santos, María Lanzarote).
Department of Health of the Basque Government: Concha Castells, Francisco Cirarda, Henar Ortuondo, Pilar Manrique, Ines Urieta, and Amaia Ajuria.
Physical activity clinical committe: Ricardo Ortega, Jesús Torcal, Mª Sol Ariestaleanizbeaskoa, Verónica Arce, Álvaro Sánchez, and Gonzalo Grandes.
Healthy diet clinical committe: Bittor Rodríguez, Pilar Amiano, Esther Gorostiza, Enrique de la Peña, Álvaro Sánchez, and Gonzalo Grandes.
Smoking cessation clinical Committe: Esther Azpitarte, Mary H. Corrales, Josep Cortada, Álvaro Sánchez, and Gonzalo Grandes.
Beasain community: Arcelormittal company (Juan Manuel Elosegui), CAF company (Iñaki Korta, Ainhoa Irastorza, Leire Makibar), Antzizar sports center (Jon Alkaiaga, Karmele Alkaiaga).
La Merced community (Bilbao): Community Health of Bilbao´s Council (Iñaki Aldamiz), School Health of Bilbao´s Council (Virginia Zelaia), Miribilla Primary Education Center (Adela Etxeberria, Itziar Basurto), Bilbao sports center (Gonzalo Casado, Maite Martínez, Alberto Díez), Municipal Office of Bilbao La Vieja, San Francisco y Zabala (Javier Rojo), “Bakuba” Community association (Sara Garteiz), “Etorkinekin Bat” Community association (Aitziber Artabe, Ainhoa Parra), “Iniciativa gitana” Community association (Marcelo Borja, Mª Carmen Jiménez).
Sondika community: Sondika Council (Gorka Carro, Bernardo Valdivielso), Commonwealth municipality of Sondika (Janire Kasuso, Esther Martin), Gorondagane Primary Education Center (Paulino Parra), Txorierri Secondary Education Institute (Loinaz Albizu), Txorierri polytechnic (Marivi Cuartango), Sondika sports center (Unai Atxa), Olarra company (Jesús Miguel Enríquez), Sondika Pharmacy (Rosario Acebal, Javier Ancel).
Matiena community: Matiena Council (José Luis Navarro, Inmaculada Zapardiez), Commonwealth municipality of Abadiño (Nerea Lejarzaburu), Traña Matiena Primary Education Center (Edurne Madariaga), Abadiño Secondary Education Institute (Eugenia Peral), Abadiño sports center (Pablo Mas)
Mutualia company (Juan Mayor), FREMAP company (Joseph Reverte), Estampaciones Metálicas company (Bernard Mandaluniz).
Others: Basque Institute for Healthcare Innovation: O + berri (Roberto Nuño), Osarean (Josu Llano), Osatek SA (Enrique Gutiérrez), University of Colorado, School of Medicine, Department of Family Medicine (Maribel Cifuentes), University of Massachusetts Boston, College of Education and Human Development (Gonzalo Bacigalupe), Laval University, Faculty of Nursing Sciences (Marie-Pierre Gagnon).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the Primary Care Research Committee of the Basque Health Service, Osakidetza, and by the Basque Country Clinical Research Ethics Committee (Ref: 6/2009). Health care professionals that committed to participate also gave written consent for the anonymous management and publication of data pertaining both to patients assigned to their practices and indicators related to their health care delivery activity.
Funding
The PVS project was funded by the Carlos III Health Institute of the Spanish Ministry of Economy and Competitiveness, co-financed by the European Regional Development Fund (PS09/01461, PI12/02113), the Health Department of the Basque Government (2009111072, 2011111145), the Basque Foundation for Social and Health Care Innovation (Grant CA-2012-086), the Basque Research Center in Chronicity-Kronikgune (Grant 11/056), and the Spanish Primary Care Research Network for Prevention and Health Promotion (redIAPP RD12/0005/0010).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 5 A’s
Assess, Advise, Agree, Assist, and Arrange follow-up
- PVS
Prescribe Healthy Life strategy (from the Spanish ‘Prescribe Vida Saludable’)
- PHC
primary health care
- EHR
electronic health record
Contributor Information
Gonzalo Grandes, Email: gonzalo.grandes@osakidetza.eus.
Alvaro Sanchez, Email: alvaro.sanchez@osakidetza.eus.
Josep M. Cortada, Email: josepmaria.cortadaplana@osakidetza.eus
Haizea Pombo, Email: haizea.pomboramos@osakidetza.eus.
Catalina Martinez, Email: catalina.martinezcarazo@osakidetza.eus.
Laura Balagué, Email: laura.balaguegea@osakidetza.eus.
Mary Helen Corrales, Email: maryhelen.corralesgarcia@osakidetza.eus.
Enrique de la Peña, Email: enrique.delapenavarona@osakidetza.eus.
Justo Mugica, Email: justo.mugicacampos@osakidetza.eus.
Esther Gorostiza, Email: mariaesther.gorostizagaray@osakidetza.eus.
on behalf of the PVS group, Email: uiap-bizkaia@osakidetza.eus.
on behalf of the PVS group:
Gonzalo Grandes, Álvaro Sánchez, Haizea Pombo, Josep M Cortada, Catalina Martínez, Paola Bully, Aitor Sanz-Guinea, Carlos Sola, Martín Begoña, Susana Iglesias, Maite Cuadrado, Nuria González, Teresa Garmendia, Mª Luz Jáuregui, Amaia Hernando, Jesús Larrañaga, Maribel Romo, Pilar Isla, Enrique Maíz, Cristina Domingo, Carmen Esparta, Mª Luz Marqués, Encarnación San Emeterio, Antón Elorriaga, Justo Múgica, María Pilar Alberdi, Mª Ángeles Arrondo, Amaia Azkoitia, Xabier Epaizabal, Mª Aranzazu Echeverria, Mª Esperanza García, Mª Ángeles García, María Erkuden Imaz, Mª Antonia Iparraguirre, Mª Isabel Irizar, Mª Rosario Larrea, Mª Dolores López, Petra Pacheco, María Yolanda Porres, Begoña San Juan, Mª Aranzazu Suquia, Mª Teresa Arrospide, Carolina Díez, Miren Arantxa Igartua, Oihana Jauregui, Alazne Saizar, Mª Jose Tilves, Mª Lourdes Etxeberria, María Aurora Valdivielso, Xabier Mugica, Mª Mercedes Lasagar, Coro Zabaleta, Mª Isabel Urcelay, Mary Helen Corrales, Mª Ángeles Crespo, Javier José María Jesús de Ordozgoiti, María Iciar Elguezabal, Susana Esteban, Catalina Frau, Laura Gallo, Inés Yolanda Martín, Nerea Ordorika, José Ramón Pérez, Mª Begoña Relloso, María Soledad Sangroniz, María Iluminada Santos, Patxi Xabier Iturbe, Esther Gorostiza, Mª Esther Azpitarte, Bixente Barrutia, Amaia Bengoa, Francisco José Miguel, Ana Isabel Etxebarria, Mª Belén García, Mª Jose Ibars, Mª Jose Lasa, Mª Carmen Martínez, Maura Pernudo, Lourdes Oribe, Mª Dolores Ustarroz, Valentina Camino, Leire Corpión, Leire Ortuondo, Mª Carmen López, Rosana Abraldes, Eneko Ibarruri, Javi Alonso, Enrique de la Peña, Mª Carmen Artola, Teresa Casado, Jesús García, Mª Paz Sánchez, Luisa Santos, María Lanzarote, Concha Castells, Francisco Cirarda, Henar Ortuondo, Pilar Manrique, Ines Urieta, Amaia Ajuria, Ricardo Ortega, Jesús Torcal, Mª Sol Ariestaleanizbeaskoa, Verónica Arce, Álvaro Sánchez, Gonzalo Grandes, Bittor Rodríguez, Pilar Amiano, Esther Gorostiza, Enrique de la Peña, Álvaro Sánchez, Gonzalo Grandes, Esther Azpitarte, Mary H. Corrales, Josep Cortada, Álvaro Sánchez, Gonzalo Grandes, Juan Manuel Elosegui, Iñaki Korta, Ainhoa Irastorza, Leire Makibar, Jon Alkaiaga, Karmele Alkaiaga, Iñaki Aldamiz, Virginia Zelaia, Adela Etxeberria, Itziar Basurto, Gonzalo Casado, Maite Martínez, Alberto Díez, Javier Rojo, Sara Garteiz, Aitziber Artabe, Ainhoa Parra, Marcelo Borja, Mª Carmen Jiménez, Gorka Carro, Bernardo Valdivielso, Janire Kasuso, Esther Martin, Paulino Parra, Loinaz Albizu, Marivi Cuartango, Unai Atxa, Jesús Miguel Enríquez, Rosario Acebal, Javier Ancel, José Luis Navarro, Inmaculada Zapardiez, Nerea Lejarzaburu, Edurne Madariaga, Eugenia Peral, Pablo Mas, Juan Mayor, Joseph Reverte, Bernard Mandaluniz, Roberto Nuño, Josu Llano, Enrique Gutiérrez, Maribel Cifuentes, Gonzalo Bacigalupe, and Marie-Pierre Gagnon
References
- 1.Waterman H, Tillen D, Dickson R, de Koming K. Action research: a systematic review and guidance for assessment. Health Technol Assess. 2001;5(23):III. doi: 10.3310/hta5230. [DOI] [PubMed] [Google Scholar]
- 2.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 3.Kottke TE, Solberg LI. Optimizing practice through research: a preventive services case study. Am J Prev Med. 2007;33:505–506. doi: 10.1016/j.amepre.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Leykum LK, Pugh JA, Lanham HJ, Harmon J, McDaniel RR., Jr Implementation research design: integrating participatory action research into randomized controlled trials. Implement Sci. 2009;4:69. doi: 10.1186/1748-5908-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Øvretveit J. Evaluating improvement and implementation for health. Maidenhead: Open University Press, McGraw-Hill Education; 2014. [Google Scholar]
- 6.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grol R. Has guideline development gone astray? Yes. BMJ. 2010;340:c306. doi: 10.1136/bmj.c306. [DOI] [PubMed] [Google Scholar]
- 8.Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Q. 2011;89:167–205. doi: 10.1111/j.1468-0009.2011.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittman BS. Implementation science in health care. In: Brownson RC, Colditz GA, Proctor EK, editors. Dissemination and implementation research in health: translating science to practice. New York: Oxford University Press; 2012. pp. 400–418. [Google Scholar]
- 10.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell BJ, Beidas RS, Lewis CC, Aarons GA, Aarons JC, Proctor EK, Mandell DS. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. 2017;44:177. doi: 10.1007/s11414-015-9475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2013 Risk Factors Collaborators. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Bergmann MM, Kröger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169:1355–1362. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101:1922–1929. doi: 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170:711–718. doi: 10.1001/archinternmed.2010.76. [DOI] [PubMed] [Google Scholar]
- 16.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 19.Galán I, Rodríguez-Artalejo F, Díez-Gañán L, Tobías A, Zorrilla B, Gandarillas A. Clustering of behavioural risk factors and compliance with clinical preventive recommendations in Spain. Prev Med. 2006;42:343–347. doi: 10.1016/j.ypmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors. 2001 National Health Interview Survey. Am J Prev Med. 2004;27(Suppl):18–24. doi: 10.1016/j.amepre.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Maruthappu M, Sood HS, Keogh SB. The NHS five year forward view: transforming care. Br J Gen Pract. 2014;64:635. doi: 10.3399/bjgp14X682897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandes G, Sanchez A, Cortada JM, et al. Is integration of healthy lifestyle promotion into primary care feasible? Discussion and consensus sessions between clinicians and researchers. BMC Health Serv Res. 2008;8:213. doi: 10.1186/1472-6963-8-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasgow RE, Lictenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/AJPH.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 26.Grandes G, Sánchez A, Montoya I, Ortega Sánchez-Pinilla R, Torcal J, PEPAF Group Two-year longitudinal analysis of a cluster randomized trial of physical activity promotion by general practitioners. PLoS ONE. 2011;6(3):e18363. doi: 10.1371/journal.pone.0018363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandes G, Sánchez A, Ortega R, Torcal J, Montoya I, Lizarraga K, Serra J, PEPAF Group Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomised randomized trial. Arch Intern Med. 2009;169:694–701. doi: 10.1001/archinternmed.2009.23. [DOI] [PubMed] [Google Scholar]
- 28.Grandes G, Cortada J, Arrazola A, Laka JP. Predictors of long-term outcome of a smoking cessation programme in primary care. Br J Gen Pract. 2003;53:101–107. [PMC free article] [PubMed] [Google Scholar]
- 29.Grandes G, Sanchez A, Cortada JM, Calderon C, Balague L, Millan E et al. Useful strategies for healthy lifestyle promotion in primary health care (Report in Spanish). Commissioned Research. Vitoria-Gasteiz: Departamento de Sanidad, Gobierno Vasco, Informe nº Osteba D-08-07; 2008.
- 30.Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioural counselling interventions: an evidence based approach. Am J Prev Med. 2002;22:267–284. doi: 10.1016/S0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 31.Moreno-Peral P, Conejo-Cerón S, Fernández A, Berenguera A, Martínez-Andrés M, Pons-Vigués M, Motrico E, Rodríguez-Martín B, Bellón JA, Rubio-Valera M. Primary care patients’ perspectives of barriers and enablers of primary prevention and health promotion—a meta-ethnographic synthesis. PLoS ONE. 2015;10:e0125004. doi: 10.1371/journal.pone.0125004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58:107–112. doi: 10.1016/j.jclinepi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Rubio-Valera M, Pons-Vigués M, Martínez-Andrés M, Moreno-Peral P, Berenguera A, Fernández A. Barriers and facilitators for the implementation of primary prevention and health promotion activities in primary care: a synthesis through meta-ethnography. PLoS ONE. 2014;9:e89554. doi: 10.1371/journal.pone.0089554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grol R, Wensing M, Eccles M, Davis D. Improving patient care: the implementation of change in health care. 2. Chichester: Wiley Blackwell/BMJ; 2013. [Google Scholar]
- 35.Foy R, Eccles M, Grimshaw J. Why does primary care need more implementation research? Fam Pract. 2001;18:353–355. doi: 10.1093/fampra/18.4.353. [DOI] [PubMed] [Google Scholar]
- 36.Medical Research Council . Developing and evaluating complex interventions: new guidance. London: Med Res Counc; 2008. [Google Scholar]
- 37.Pinnock H, Epiphaniou E, Taylor SJ. Phase IV implementation studies. The forgotten finale to the complex intervention methodology framework. Ann Am Thorac Soc. 2014;11(Suppl 2):118–122. doi: 10.1513/AnnalsATS.201308-259RM. [DOI] [PubMed] [Google Scholar]
- 38.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4:12–25. [PubMed] [Google Scholar]
- 39.Glasgow RE, Orleans CT, Wagner EH, Curry SJ, Solerg LI. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79:579–612. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davy C, Bleasel J, Liu H, Tchan M, Ponniah S, Brown A. Factors influencing the implementation of chronic care models: a systematic literature review. BMC Fam Pract. 2015;16:102. doi: 10.1186/s12875-015-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez A, Grandes G, Cortada JM, Pombo H, Martinez C, Corrales MH, de la Peña E, Mugica J, Gorostiza E, PVS group Feasibility of an implementation strategy for the integration of health promotion in routine primary care: a quantitative process evaluation. BMC Fam Pract. 2017;18(1):24. doi: 10.1186/s12875-017-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez C, Bacigalupe G, Cortada JM, Grandes G, Sanchez A, Pombo H, Bully P, PVS group The implementation of health promotion in primary and community care: a qualitative analysis of the ‘Prescribe Vida Saludable’ strategy. BMC Fam Pract. 2017;18(1):23. doi: 10.1186/s12875-017-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez A, Grandes G, Cortada JM, Pombo H, Balagué L, Calderon C. Modelling innovative interventions for optimizing healthy lifestyle promotion in primary health care: ‘Prescribe Vida Saludable’ phase I research protocol. BMC Health Serv Res. 2009;9:103. doi: 10.1186/1472-6963-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav. 1998;25:545–563. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 45.Institute for Healthcare Improvement (IHI). The Breakthrough Series: IHI’s Collaborative model for achieving breakthrough improvement. IHI innovation series white paper. Boston: Institute for Healthcare Improvement; 2003. (http://www.IHI.org). Accessed 16 Oct 2015.
- 46.Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I. The nominal group technique: a research tool for general practice? Fam Pract. 1993;10:76–81. doi: 10.1093/fampra/10.1.76. [DOI] [PubMed] [Google Scholar]
- 47.Rimer B, Glanz K. Theory at a glance: a guide for health promotion practice. 2nd edn. National Institutes of Health, National Cancer Institute, NIH Publication No. 05-3896, Washington, DC: NIH; 2005.
- 48.Glasgow RE, Goldstein MG, Ockene JK, Pronk NP. Translating what we have learned into practice. Principles and hypotheses for interventions addressing multiple behaviors in primary care. Am J Prev Med. 2004;27(Suppl 2):88–101. doi: 10.1016/j.amepre.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Beer M, Eisenstat RA. Spector: why change programs don’t produce change. Harv Bus Rev. 1990;68:158–166. [PubMed] [Google Scholar]
- 50.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadu MK, Stolee P. Facilitators and barriers of implementing the chronic care model in primary care: a systematic review. BMC Fam Pract. 2015;16:12. doi: 10.1186/s12875-014-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porterfield D, Hinnant L, Kane H, Horne J, McAleer K, Roussel A. Linkages between clinical practices and community organizations for prevention. RTI Project Number 0210088.006 for the Agency for Healthcare Research and Quality. Research Triangle Institute, NC; 2010.
- 53.Woolf SH, Glasgow RE, Krist A, Bartz C, Flocke SA, Holtrop JS, Rothemich SF, Wald ER. Putting it together: finding success in behavior change through integration of services. Ann Fam Med. 2005;3(Suppl 2):S20–S27. doi: 10.1370/afm.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etz RS, Cohen DJ, Woolf SH. Bridging primary care practices and communities to promote healthy behaviors. Am J Prev Med. 2008;35(5S):S390–S397. doi: 10.1016/j.amepre.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Cifuentes M, Fernald DH, Green LA, Niebauer LJ, Crabtree BF, Hassmiller SB. Prescription for health: changing primary care practice to foster healthy behaviors. Ann Fam Med. 2005;3(Suppl):S4–S12. doi: 10.1370/afm.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruhe MC, Weyer SM, Zronek S, Wilkinson A, Wilkinson PS, Stange KC. Facilitating practice change: lessons from the STEP-UP clinical trial. Prev Med. 2005;40:729–734. doi: 10.1016/j.ypmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Attieh R, Gagnon MP, Estabrooks CA, Légaré F, Ouimet M, Roch G, el Ghandour K, Grimshaw J. Organizational readiness for knowledge translation in chronic care: a review of theoretical components. Implement Sci. 2013;28(8):138. doi: 10.1186/1748-5908-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gustafson DH, Quanbeck AR, Robinson JM, Ford II JH, Pulvermacher A, French MT, McConnell KJ, Batalden PB, Hoffman KA, McCarty D. Which elements of improvement collaboratives are most effective? A cluster-randomized trial. Addiction. 2013;2013(108):1145–1157. doi: 10.1111/add.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012;10:63–74. doi: 10.1370/afm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed.


