Abstract
Background:
Safflower extract and aceglutamide (SA) has been used clinically for the treatment of cerebrovascular diseases such as cerebral embolism, hemorrhage, and mental deterioration. This study aimed to investigate the effect and mechanism of SA injection in the recovery of peripheral innervations of diabetic mice.
Methods:
The C57BL/6 male mice were divided into four groups: normal control group (n = 44), diabetic group (n = 44), diabetic + SA group (diabetic mice treated with SA injection, n = 44), and diabetic + SA + vascular endothelial growth factor receptor (VEGFR)1-BL group (diabetic mice treated with SA injection and VEGFR 1 blocking antibody n = 24). The streptozotocin-induced diabetic mice model and injured peripheral nerve mice model were built. The mice with injured peripheral nerves were intraperitonealy administered with SA injection for successive 21 days. The corneal sensitivity, number of corneal nerve fibers, and contents of vascular endothelial growth factor (VEGF)-B and various neurotrophic factors such as nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) in corneal tissue of four groups were observed.
Results:
The diabetic group showed decreased number of corneal nerve fibers, compared with the control group (P = 0.002). And compared with the diabetic group, the diabetic + SA group showed a significant increase in the number of nerve fibers (P = 0.024) and the contents of VEGF-B, NGF, and GDNF in the cornea (all P < 0.05). However, when the diabetic mice were treated with the blocking antibodies specialized for VEGF-B receptor, the neutralization of VEGFR-1 completely abolished the increased expression of NGF and GDNF stimulated by SA injection.
Conclusions:
SA injection could reduce the nerve injury caused by diabetic peripheral neuropathy, and its protective effect might be associated with the promotion of the expressions of VEGF-B, NGF, and GDNF.
Keywords: Glial Cell Line-derived Neurotrophic Factor, Nerve Growth Factor, Peripheral Nerve, Safflower Extract and Aceglutamide Injection, Vascular Endothelial Growth Factor
INTRODUCTION
As a kind of metabolic disease, diabetes can cause systemic injury of multiple tissues and organs. As estimated by the World Health Organization, the total number of patients with diabetes were about 170 million worldwide, which is expected to double by 2030.[1,2] Diabetic peripheral neuropathy (DPN) is one of the most common and irreversible complications in both Type 1 and Type 2 diabetes mellitus, among which the earliest alterations occur in the small unmyelinated C- and thinly myelinated Aδ-nerve fibers.[3,4] As the most densely innervated tissue in the body, the corneal nerve fibers are highly affected by hyperglycemia, such as decreased nerve fiber density, reduced length, and increased tortuosity.[5,6]
Corneal neuropathy is a part of DPN. In the recent years, many studies have mainly discussed the mechanism of the development of DPN from the angle of corneal neuropathy. Corneal nerve fibers can secrete neurotrophic factors to nourish ocular surface, and the loss of corneal nerves may lead to nutritional keratopathy and corneal ulcer. Therefore, the integrity of corneal nerve is vital to maintain the corneal functions.[7] It has always been the hotspot to research the diabetic corneal neuropathy and its hard-to-identify pathogenesis nowadays, which is significant for the diagnosis and treatment of diabetic keratopathy.
As the sterilized aqueous solution made of safflower extract of aceglutamide, safflower extract and aceglutamide (SA) injection combines the effective components of aceglutamide and safflower and effectively coordinates their functions. Administration of SA injection can improve nerve cell metabolism and brain functions, inhibit platelet aggregation, relax blood vessels, strengthen microcirculation, and resist oxygen-free radicals.[8,9]
The streptozotocin (STZ)-induced C57/BL6 diabetic mice model was adopted in this study, and the corneal epithelium scraping method was used to build the repairing model for corneal epithelium and intraepithelial nerve injury. Then, the impact of SA injection on corneal sensitivity, number of corneal nerve fibers, and contents of VEGF and various neurotrophic factors in corneal tissue were observed so as to investigate its effect and potential repairing mechanism on DPN.
METHODS
Ethical approval
The animal care and procedures of this study were conducted according to the Principles of Laboratory Animal Care. The use of animals in this study adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Streptozotocin-induced diabetic mice model
The C57BL/6 male mice at the age of 6–8 weeks in this study were purchased from Beijing HFK Bioscience Co., Ltd., (Beijing, China). The STZ was dissolved in the precooled citrate-citric acid buffer solution (pH = 4.5) with the concentration of 50 mg/kg, and intraperitoneal injection was performed for successive 5 days to induce the models of mice with Type I diabetes, and the buffer solution of the same dose was injected in the control group. The mice whose blood glucose level was higher than 3000 mg/L at the 12th weeks after the last injection of STZ were considered as the successful models of mice with diabetes and could be used for subsequent experiments.
Corneal epithelial wound healing
The normal C57BL/6 mice and STZ-induced C57BL/6 mice with Type I diabetes were adopted for the experiment. For each mouse, the left eye was taken for the experiment and the right one received no treatment. Then, the indentation was made on the left cornea of the mice by the use of the corneal trephine with the diameter of 2 mm, and during this process, the trephine edge should not surpass the corneoscleral limbus. Under the ophthalmic microscope, an Algerbrush II rust ring remover (Alger Co., Lago Vista, TX, USA) was used to scrape the epithelia in the region 2 mm away from the central cornea of each mouse according to the indentation position, with the corneal epithelia at the corneal limbus left over. It should be noted that the scraping range cannot surpass the indentation made by the trephine.
The mice were divided into four groups: normal control group (n = 44), diabetic group (n = 44), diabetic + SA group (diabetic mice treated with SA injection n = 44), and diabetic + SA + vascular endothelial growth factor receptor (VEGFR)1-BL group (diabetic mice treated with SA injection and VEGFR 1 blocking antibody n = 24). The drug intervention was implemented immediately after the excochleation of corneal epithelium. Normal saline was injected intraperitoneally to the mice in the control and diabetic groups based on 1 ml/100 g, and SA injection (Tonghua Guhong Pharmacy, Meihekou, China) to the mice in the treatment group based on 1 ml/100 g. Such intraperitoneal injection was performed once every day for successive 21 days. The body weight of the mice was measured regularly and the injection dose was adjusted according to the body weight.
Corneal sensitivity
Corneal esthesiometry was carried out as previous description using a Cochet-Bonnet esthesiometer (Luneau Ophtalmologie, Chartres Cedex, France).[10,11] The nylon monofilament had a maximal extended length of 60 mm with a diameter of 0.12 mm. The central area of the cornea was touched once on each eye, beginning with the full length of nylon filament and shortened by 5 mm until a blink response was elicited. The corneal sensitivity threshold was calculated as the mean value of three longest filament lengths causing positive response. Corneal sensitivity was conducted on days 3, 6, 14, and 21.
Corneal whole-mount staining
Corneal whole-mount staining was performed as previously described.[11] The cornea of the mice was clipped along the line 1 mm away from their corneal sclera and washed with phosphate-buffered saline (PBS) for 3 times with 5 min for each time. Full-thickness corneal flat mounts were fixed for 1 h at room temperature in 4% paraformaldehyde, incubated at 37°C in 20 mmol/L ethylenediaminetetraacetic acid (Sigma-Aldrich, USA) for 30 min, and permeabilized in 10% Triton X-100 for 1 h at room temperature. All samples were incubated with 5% bovine serum albumin for 1 h. Next, the nerve staining antibody β-III tubulin (R&D system, USA) was diluted at the ratio of 1:100, and the cornea was fully covered by the antibody and then placed in the refrigerator at 4°C for an overnight. On the following day, the cornea was washed fully with PBS solution for 3 times with 5 min for each time. After being covered with a cover slip, the microscope slide was photographed under an Eclipse TE2000-U microscope (Nikon, Tokyo, Japan). The software Image J (NIH, Bethesda, MD, USA) was adopted to perform the image analysis.
Total RNA extraction and real-time-quantitative polymerase chain reaction
Total RNA extraction kit (MACHEREY-NAGEL, Germany) was used, and the cornea was placed in 350 μl TRIzol and fully cut into pieces using clean scissors. The centrifugation was conducted at 1500 ×g for 2 min after the corneal tissue was completely ground by the use of an electric burnisher. Then, the supernate was kept, and the sediments were thrown away. Next, total mRNA was extracted in accordance with the instructions, and then Eppendorf BioPhotometer® b131 (Eppendorf China Ltd., Shanghai, China) was used to determine the absorbancy of mRNA. And the PrimeScript RT kit (Takara, Japan) was used to reversely transcribe the total RNA into cDNA. The real-time-quantitative polymerase chain reaction was measured with the Synergy Brands method, including the genes such as nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophic factor 3 (NTF3), neurotrophic factor 4/5 (NTF4/5), and glyceraldehydes phosphate dehydrogenase (GAPDH), the related primers are shown in Table 1. The PCR reaction was completed with the ABI 7500 detecting system (Applied Biosystems, USA), the original data were collected and analyzed by the use of the software SDS 7500 (Applied Biosystems, Foster City, CA, USA).
Table 1.
Primers used for polymerase chain reaction amplification
| Genes | Forward primer | Reverse primer |
|---|---|---|
| NGF | 5’-CCTGAAGCCCACTGGACTAA-3’ | 5’-TACAGTGATGTTGCGGGTCT-3’ |
| GDNF | 5’-CGGGCCACTTGGAGTTAATG-3’ | 5’-ACAGCCACGACATCCCATAA-3’ |
| BDNF | 5’-GTAAACGTCCACGGACAAGG-3’ | 5’-ATGTCGTCGTCAGACCTCTC-3’ |
| CNTF | 5’-GAGCAATCACCTCTGACCCT-3’ | 5’-CCACTGGTACACCATCCACT-3’ |
| NTF3 | 5’-CCCGGTGGTAGCCAATAGAA-3’ | 5’-CAATGGCTGAGGACTTGTCG-3’ |
| NTF4/5 | 5’-CCTGCGTCAGTACTTCTTCG-3’ | 5’-AGGACTGCTTAGCCTTGCAT-3’ |
NGF: Nerve growth factor; GDNF: Glial cell line-derived neurotrophic factor; BDNF: Brainderived neurotrophic factor; CNTF: Ciliary neurotrophic factor; NTF3: Neurotrophic factor 3; NTF4/5: Neurotrophic factor 4/5.
Enzyme-linked immunosorbent assay
Eight corneas at each time point (days 3 and 6) were homogenated in 350 μl of cold PBS and analyzed by enzyme-linked immunosorbent assay kits including VEGF (USCN Life Science Inc., Wuhan, China), NGF (Millipore, Bedford, MA, USA), and GDNF (USCN Life Science Inc.) according to the manufacturer's procedures.
Statistical analysis
Data in this study were representative of at least three different experiments and presented as the mean ± standard deviation (SD) and compared by one-way analysis of variance (ANOVA) between groups. Statistical analysis was performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
RESULTS
Safflower extract and aceglutamide injection promoting the recovery of corneal sensation of diabetic mice
The results of corneal sensitivity among the three groups revealed that the mice in the diabetic group had a significant decrease of corneal sensitivity at the 12th week since the injection of STZ, compared with the control group (F = 5.713, P = 0.031). Compared with the control group, the mice in the diabetic + SA group showed a significant decrease of corneal sensitivity on the 3rd day after the SA injection (F = 21.965, P = 0.002). However, there were no significant differences in the corneal sensitivity between diabetic + SA group and the control group on the 6th (F = 2.400, P = 0.172), 14th (F = 2.788, P = 0.147), and 21st days (F = 6.000, P = 0.050) after the injection. The sensitivities of corneal sensation in diabetic + SA group showed a statistical increase compared with the diabetic group on the 6th (F = 21.774, P = 0.003), 14th (F = 19.737, P = 0.004), and 21st days (F = 15.483, P = 0.008) after the injection [Figure 1].
Figure 1.
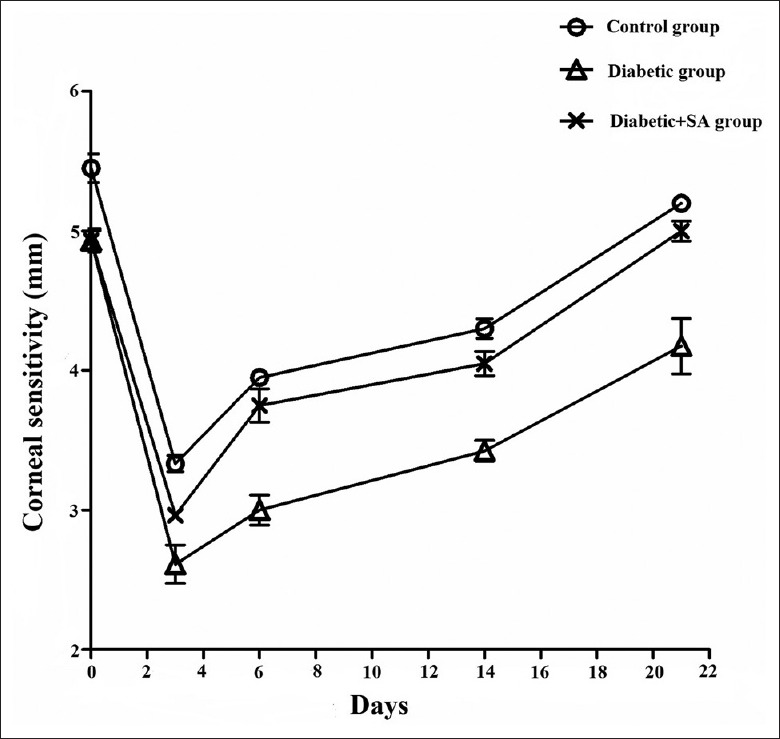
SA injection promoted improvement of corneal sensitivity in diabetic mice (n = 8 per group). The corneal sensitivity was measured on days 3, 6, 14, and 21 after injury. SA: Safflower extract and aceglutamide.
Safflower extract and aceglutamide injection promoting the repair of nerve fibers after the corneal epithelium injury
On the 7th day after the corneal epithelium scraping, the corneas were stained with β-III tubulin. The results indicated that the length and density of the nerve fibers in the corneal epithelium of the diabetic group showed a significant decrease (F = 51.020, P = 0.002), compared with the control group. Moreover, the occupied area of the corneal subbasal nerve in the diabetic + SA group showed a significant increase compared with the diabetic group (F = 11.918, P = 0.024), which was still less than that of the control group [F = 8.000, P = 0.047; Figure 2].
Figure 2.

SA injection promoted corneal innervations in diabetic mice (n = 6 per group). Representative images of subbasal nerve plexus were taken in the control group (a), diabetic group (b), and the diabetic + SA injection group (c; corneal whole-mount staining; ×200). (d) Analysis of area occupied by subbasal nerve density 7 days post wound (*P < 0.05). SA: Safflower extract and aceglutamide.
Safflower extract and aceglutamide injection promoting the vascular endothelial growth factor-B expression in the cornea of diabetic mice
The protein expression levels of the VEGF-B in the corneas of the diabetic group showed significant decreases as compared with the control group on the 3rd (F = 16.250, P = 0.017) and 6th (F = 22.303, P = 0.009) days after scraping the corneal epithelium. The protein expression levels of VEGF-B in the diabetic + SA group showed an increase compared with the diabetic group on the 3rd (F = 9.580, P = 0.036) and 6th days [F = 11.580, P = 0.027; Figure 3].
Figure 3.
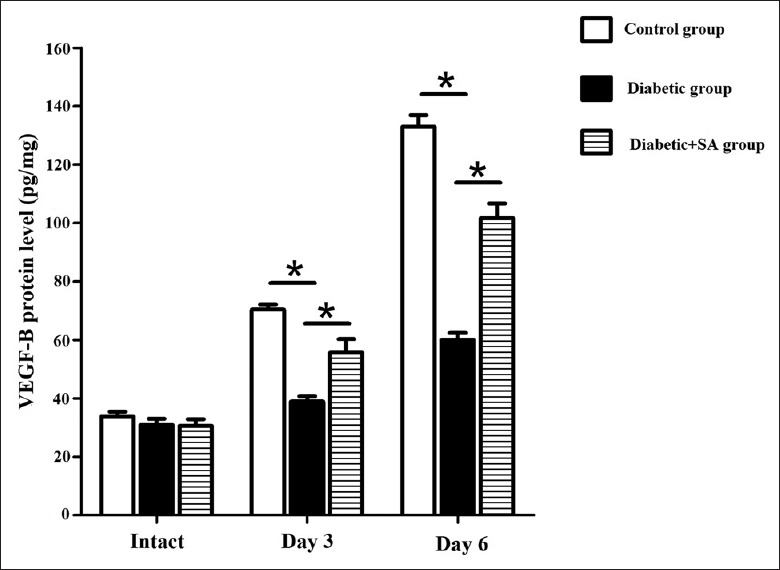
SA injection promoted the VEGF-B expression in the cornea of diabetic mice (n = 24 per group). Three and six days post wound, VEGF-B protein expression in in control group, diabetic group, diabetic + SA group were detected (*P < 0.05). VEFG: Vascular endothelial growth factor; SA: Safflower extract and aceglutamide.
Safflower extract and aceglutamide injection promoting the expressions of the neurotrophic factors such as nerve growth factor and glial cell line-derived neurotrophic factor in the cornea of diabetic mice
On the 3rd day after scraping the corneal epithelium, the mRNA and protein expression levels of NGF (mRNA: F = 10.09, P = 0.034; protein: F = 53.59, P = 0.002) and GDNF (mRNA: F = 8.639, P = 0.042; protein: F = 25.387, P = 0.007) in the corneas of the diabetic group decreased, compared with those of the control group. The mRNA and protein expression levels of the NGF (mRNA: F = 10.633, P = 0.031; protein: F = 24.341, P = 0.008) and GDNF (mRNA: F = 9.830, P = 0.035; protein: F = 10.218, P = 0.033) in the diabetic + SA group showed an increase compared with those of the diabetic group [Figures 4 and 5].
Figure 4.
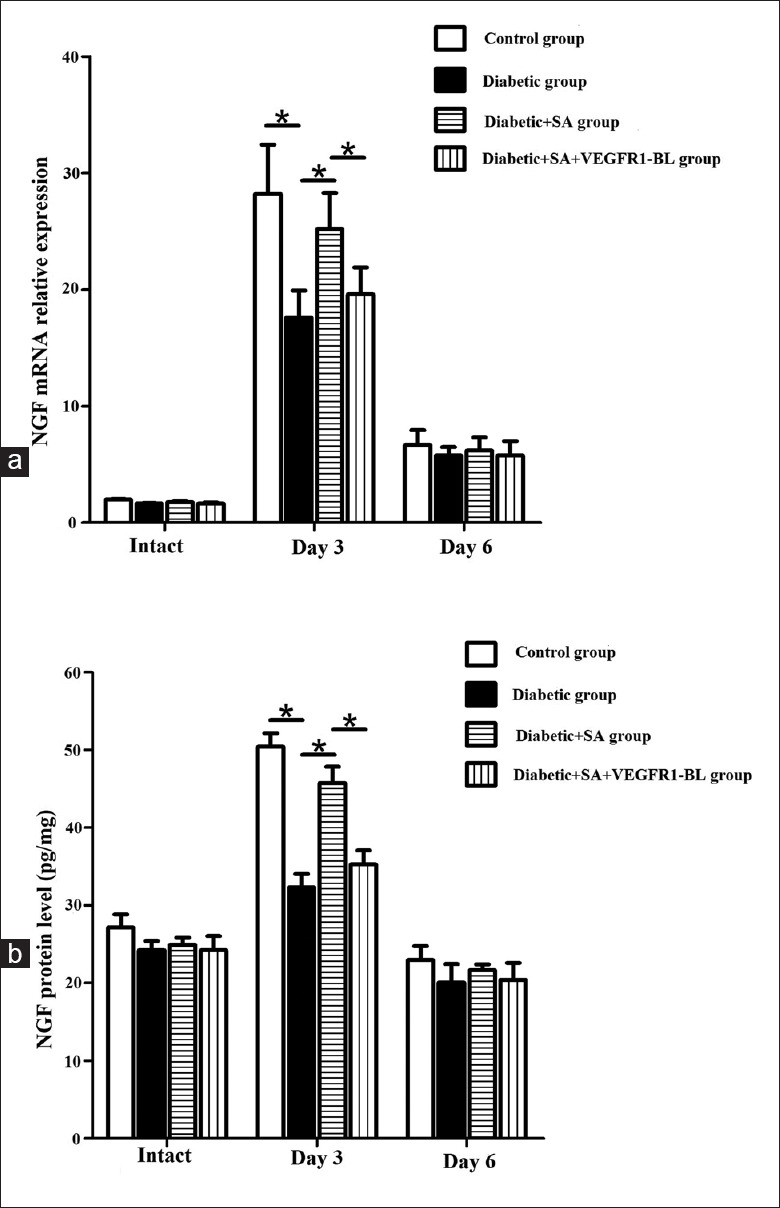
SA injection promoted the expressions of the neurotrophic factors such as NGF in the diabetic mice (n = 24 per group). Three and six days post wound, NGF mRNA and protein expression in control group, diabetic group, diabetic + SA group, and diabetic + SA + VEGFR1-BL group were detected (*P < 0.05). NGF: Nerve growth factor; SA: Safflower extract and aceglutamide; VEGFR1-BL: Vascular endothelial growth factor receptor 1-blocking antibody.
Figure 5.
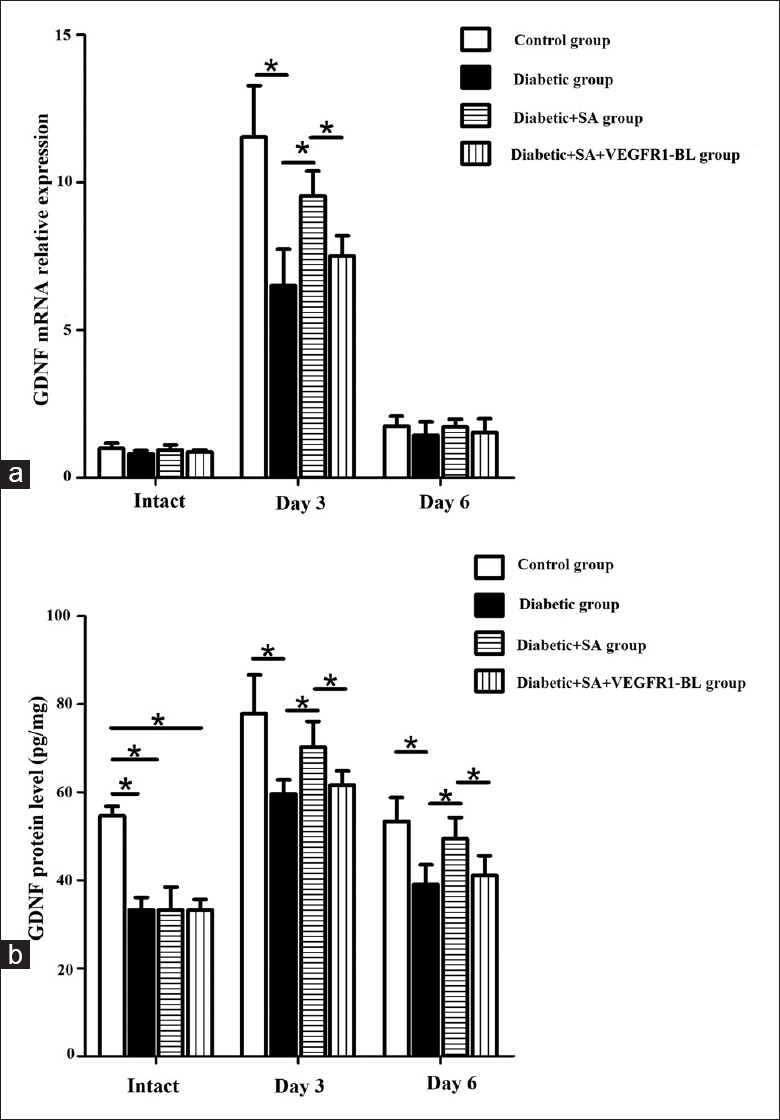
SA injection promoted the expressions of the neurotrophic factor such as GDNF in the diabetic mice (n = 24 per group). Three and six days post wound, GDNF mRNA and protein expression in control group, diabetic group, diabetic + SA group, and diabetic + SA + VEGFR1-BL group were detected (*P < 0.05). GDNF: Glial cell line-derived neurotrophic factor; SA: Safflower extract and aceglutamide; qPCR: Quantitative polymerase chain reaction; VEGFR1-BL: Vascular endothelial growth factor receptor 1-blocking antibody.
On the 6th day after scraping the corneal epithelium, the mRNA and protein expression levels of the NGF among the control, diabetic, and the diabetic + SA groups showed no statistical difference [Figure 4]. And, the mRNA levels of the GDNF in the control, diabetic, and the diabetic + SA groups also showed no statistical difference. The protein expression level of the GDNF in the corneas of the diabetic group showed a decrease compared with the control group (F = 10.618, P = 0.031). The protein expression level of the GDNF in the diabetic + SA group showed an increase (F = 12.886, P = 0.023), compared with the diabetic group [Figure 5].
However, when the diabetic + SA group was treated with the blocking antibodies specialized for VEGF-B receptors, the neutralization of VEGFR-1 partially inhibited SA injection-stimulated NGF (mRNA: F = 9.386, P = 0.038; protein: F = 14.696, P = 0.019) and GDNF (mRNA: F = 8.499, P = 0.044; protein: F = 8.782, P = 0.041) expression in diabetic cornea on day 3, and also inhibited SA injection-stimulated GDNF protein expression in diabetic cornea on day 6 [F = 12.003, P = 0.026; Figures 4 and 5].
On the 3rd day after scraping the corneal epithelium, the mRNA expression levels of the BDNF, CNTF, NTF3, and NTF4/5 among the control, diabetic, and the diabetic + SA groups showed no statistical difference [all P > 0.05; Figure 6].
Figure 6.
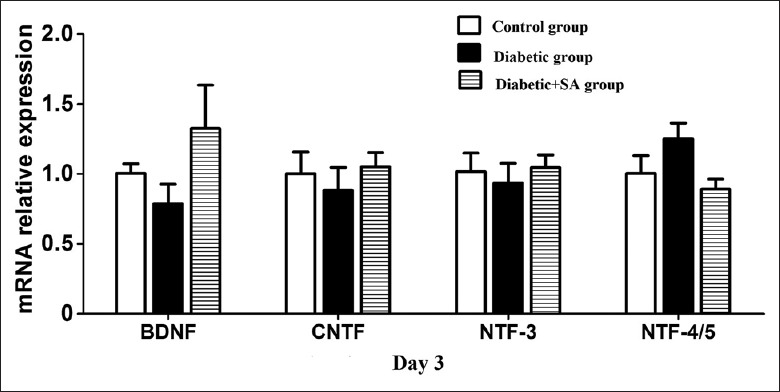
SA injection had no effect on the expressions of the neurotrophic factors such as BDNF, CNTF, NTF-3, and NTF-4/5 in the diabetic mice (n = 6 per group). Three and six days post wound, the mRNA expressions of BDNF, CNTF, NTF-3, and NTF-4/5 in control group, diabetic group, diabetic + SA group were detected (P > 0.05). BDNF: Brain-derived neurotrophic factor; CNTF: Ciliary neurotrophic factor; NTF: Neurotrophic factor; qPCR: Quantitative polymerase chain reaction; SA: Safflower extract and aceglutamide.
DISCUSSION
Diabetes is a kind of metabolic disease involving various organs of the whole body. For the eyes, more studies focused on diabetic retinopathy and diabetic cataract; however, research on diabetic keratopathy is easy to be neglected. The relevant data showed that 47–64% of patients with diabetes might suffer from primary keratopathy, and its clinical features mainly include decreased corneal sensitivity, delayed epithelium healing, neurotrophic corneal ulcer, and other symptoms and it even causes blindness.[12,13,14] Primary keratopathy in patients with diabetes is mainly associated with the change in the components of corneal epithelial basement membrane, decrease in the junction number of hemidesmosome, accumulation of advanced glycated end products, change in growth factors, corneal nerve-ending injury, oxidative stress, and other abnormalities under diabetic conditions.[15,16]
Cornea is the tissue with the densest distribution of human nerves, whose nerve fibers are mainly from trigeminal ganglion. Corneal nerves have an important regulating effect on maintaining blink reflex, corneal epithelial cell metabolism, and production and secretion of tears.[17,18] Herpes simplex virus, trigeminal ganglion injury, and diabetes can cause decreased density of corneal nerve and corneal sensation, chronic inflammation, delayed healing, and even nonhealing of corneal epithelium injury.[19,20] Normal corneal nerves are important in maintaining the homeostasis of corneal epithelium, but their injury can cause corneal dystrophy, which may be one pathogenesis for diabetic keratopathy.[13]
In the recent years, the mechanism of the occurrence and development of diabetic corneal neuropathy has been researched and discussed in many studies from the perspectives of the expression and abnormal function of NGFs. The NGFs include NGF, BDNF, and neurotrophins-3, 4/5, and 6. The studies on the growth factor-mediated nervous signaling pathways indicated that the DPN derives from oxidative stress, and the lack of neurotrophic factors affects the normal neurological functions and the repair and regeneration capacity after injury.[21,22]
Decrease in corneal sensation of patients with diabetes is a part of diabetic systemic and multiple peripheral neuropathies. Currently, no effective method is available to treat severe diabetic keratopathy, and such patients cannot achieve an ideal vision improvement even if their ocular fundus lesions are repaired by operation, which is also the reason for exploring drugs with special curative effect to treat diabetic keratopathy. It has been proved by lots of clinical trials that SA injection could effectively treat the acute ischemic stroke and had a good effect on treating DPN.[23,24,25]
The results in this study revealed the decreases in the corneal sensitivity and number of corneal nerve fibers of the diabetic group as compared with the control group. Compared with the diabetic group, the corneal sensitivity, number of corneal nerve fibers, and contents of such neurotrophic factors as VEGF-B, NGF, and GDNF in the corneal tissue in diabetic + SA group showed a significant increase, indicating that SA injection had a certain neurotrophic effect, which could enhance the repairing capacity of the corneal nerves after injury by promoting the expression levels of the NGF and GDNF.
VEGF and its receptor family play an important role in regulating and maintaining the avascular state of cornea; meanwhile, VEGF has the nutritional and protective effect on central and peripheral nerves.[26,27,28] In the recent years, studies have showed that VEGF-B could not only protect central nerves, but also promote the injury repair of peripheral nerves. By VEGFR1 and NRP1 receptors, VEGF-B could promote the growth and branching of the cell axons of trigeminal ganglion, VEGF-B is the factor needed by normal nerve regeneration, mice with VEGF-B deficiency could show the injured nerve repair accompanied by nutritional function abnormality, and exogenous administration of VEGF-B could significantly promote the regeneration of corneal nerve endings and restore the corneal sensation and neurotrophic functions.[29] In addition, it could selectively promote the regeneration of injured nerves but not lead to neovascularization. In addition, VEGF-B might be a novel target for treating corneal nerve injury and promoting corneal nerve regeneration.
The results of the existing research showed that the VEGF expression in the rat's cortex decreased on the 14th day after the cerebral ischemia reperfusion and increased after the administration of SA injection.[30] Our study achieved the similar result in the repair process of the corneal nerve injury of the mice. Compared with the diabetic group, the VEGF content in corneal tissue showed a statistical increase in the diabetic + SA group. SA injection could promote the VEGF expression of corneal and peripheral nerves when injured, thereby enhancing the repairing capacity after the corneal nerve injury.
This study investigated the effect of SA on diabetic corneal neuropathy, and this protective effect might be achieved by promoting the expression of VEGF-B and regulating the expression of NGF and GDNF. However, there might be other mechanisms of action that need to be discovered. In addition, this study only discussed the effect of SA on diabetic keratopathy. Moreover, whether SA has a protective effect on central nervous system injury needs further investigation.
Corneal sensitivity is the most commonly used index for detecting corneal nerve functions. The results in this study revealed a significant decrease in the corneal sensitivity of the mice in diabetic group, and the diabetic + SA group showed a significant improvement compared with the diabetic group. The detection result under the fluorescence microscope indicated a significant improvement in the number of nerve fibers of the corneal nerve in diabetic + SA injection group. In summary, SA injection had the protective effect on the diabetic corneal neuropathy caused by diabetes, by promoting the expressions of VEGF and neurotrophic factors such as NGF and GDNF.
Financial support and sponsorship
This study was supported by grants from Shandong Provincial Natural Science Foundation, China (No. ZR2016HP17), the Project of Medical and Health Technology Development Program in Shandong Province (No. 2016WS0282), and Qingdao Postdoctoral Application Research Funded Project (No. 2016063).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat Rev Endocrinol. 2016;12:616–22. doi: 10.1038/nrendo.2016.105. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 3.Løseth S, Stålberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: Thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255:1197–202. doi: 10.1007/s00415-008-0872-0. doi: 10.1007/s00415-008-0872-0. [DOI] [PubMed] [Google Scholar]
- 4.Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35:591–8. doi: 10.1002/mus.20732. doi: 10.1002/mus.20732. [DOI] [PubMed] [Google Scholar]
- 5.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–22. doi: 10.1167/iovs.03-0637. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 6.He J, Bazan HE. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2012;119:956–64. doi: 10.1016/j.ophtha.2011.10.036. doi: 10.1016/j.ophtha.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelkader H, Patel DV, McGhee CN, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: A review. Clin Exp Ophthalmol. 2011;39:259–70. doi: 10.1111/j.1442-9071.2010.02435.x. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Wei WL. Protective effect of Guhong Zhusheye on ischemic/reperfusion injury in rats (in Chinese) World Latest Med Inf. 2012;12:21–2. doi: 10.3969/j.issn.1671-3141.2012.06.008. [Google Scholar]
- 9.Zhang B, Ning Y. Clinical efficacy and safety of Guhong injection in the treatment of acute cerebral infarction. Pract Pharm Clin Remedies. 2015;18:1129–32. doi: 10.14053/j.cnki.ppcr.201509033. [Google Scholar]
- 10.Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, et al. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63:4262–74. doi: 10.2337/db14-0163. doi: 10.2337/db14-0163. [DOI] [PubMed] [Google Scholar]
- 11.Di G, Zhao X, Qi X, Zhang S, Feng L, Shi W, et al. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci Rep. 2017;7:40582. doi: 10.1038/srep40582. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 13.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–99. [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:3301–8. doi: 10.1167/iovs.10-5670. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–80. doi: 10.1167/iovs.09-4569. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhou Q, Xie L. Diabetic keratopathy: New progresses and challenges (in Chinese) Chin J Ophthalmol. 2014;50:69–72. doi: 10.3760/cma.j.issn.0412-4081.2014.01.018. [PubMed] [Google Scholar]
- 17.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: Structure, contents and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. doi: 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 18.Heigle TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15:135–8. doi: 10.1097/00003226-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Okada Y, Reinach PS, Kitano A, Shirai K, Kao WW, Saika S. Neurotrophic keratopathy; its pathophysiology and treatment. Histol Histopathol. 2010;25:771–80. doi: 10.14670/HH-25.771. doi: 10.14670/HH-25.771. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53:867–72. doi: 10.1167/iovs.11-8438. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 22.Kim HC, Cho YJ, Ahn CW, Park KS, Kim JC, Nam JS, et al. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet Med. 2009;26:1228–34. doi: 10.1111/j.1464-5491.2009.02856.x. doi: 10.1111/j.1464-5491.2009.02856.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao HD, Wang XZ, Huang SL, Zhai HF. Clinical observation of Guhong injection in treatment of 60 cases with acute cerebral hemorrhage. Appl J Gen Pract. 2006;4:630–1. doi: 10.3969/j.issn.1674-4152.2006.06.006. [Google Scholar]
- 24.Wang J, Wu ZF. Effects of safflower extract and aceglutamide injection on expressions of CD62P and CRP in patients with coronary heart disease of blood stasis type. Shanghai J Trad Chin Med. 2013;47:47–8. doi: 10.16305/j.1007-1334.2013.11.027. [Google Scholar]
- 25.Ai J, Wan H, Shu M, Zhou H, Zhao T, Fu W, et al. Guhong injection protects against focal cerebral ischemia-reperfusion injury via anti-inflammatory effects in rats. Arch Pharm Res. 2017;40:610–22. doi: 10.1007/s12272-016-0835-4. doi: 10.1007/s12272-016-0835-4. [DOI] [PubMed] [Google Scholar]
- 26.Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: A new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46:1–10. doi: 10.1016/j.npep.2011.05.005. doi: 10.1016/j.npep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Pan Z, Fukuoka S, Karagianni N, Guaiquil VH, Rosenblatt MI. Vascular endothelial growth factor promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea. FASEB J. 2013;27:2756–67. doi: 10.1096/fj.12-225185. doi: 10.1096/fj.12-225185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bry M, Kivelä R, Leppänen VM, Alitalo K. Vascular endothelial growth factor-B in physiology and disease. Physiol Rev. 2014;94:779–94. doi: 10.1152/physrev.00028.2013. doi: 10.1152/physrev.00028.2013. [DOI] [PubMed] [Google Scholar]
- 29.Guaiquil VH, Pan Z, Karagianni N, Fukuoka S, Alegre G, Rosenblatt MI. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc Natl Acad Sci U S A. 2014;111:17272–7. doi: 10.1073/pnas.1407227111. doi: 10.1073/pnas.1407227111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang R, Liang Z, Yang N, Ji C, Liu YY, Zuo PP. Effects of Guhong injection on expression of vascular endothelial growth factor in cortex after cerebral ischemia-reperfusion in rats (in Chinese) Chin J Rehabil Theory Pract. 2015;7:770–2. doi: 10.3969/j.issn.1006-9771.2015.07.007. [Google Scholar]


