Abstract
Although there have been significant advances in medical therapies to treat Crohn’s disease, an estimated 50% of patients will require surgery within the first decade of disease duration. Of these patients, a substantial number will develop recurrent symptoms within the first postoperative year. To prevent disease recurrence, many physicians use postoperative prophylactic therapy. Randomized, controlled trials, although limited in number, have demonstrated that a prophylactic postoperative strategy is effective at reducing recurrence (both clinical and endoscopic) in high-risk patients. This article reviews the frequency of and risk factors for postoperative Crohn’s disease recurrence and the current evidence in favor of postoperative Crohn’s disease management strategies. Future studies must be conducted to establish a gold standard as to who should receive postoperative prophylaxis and which therapies and time course are ideal.
Keywords: Surgery, ileocecal resection, inflammatory bowel disease, biologic, prophylaxis, anastomosis
Crohn’s disease is a chronic inflammatory condition without a known cure that manifests in a variety of patterns, including intraluminal, stricturing, and penetrating phenotypes.1 Historically, medical therapy for Crohn’s disease was limited to corticosteroids and immunomodulators, and the only other option was surgical management. The advent of anti–tumor necrosis factor agents has changed the natural history of disease, and the introduction of newer biologic agents with novel mechanisms of action has increased medical options for disease control.2,3
Nonetheless, a significant proportion of patients with Crohn’s disease require surgery.4 Studies have shown that approximately 50% of patients require surgery within the first 10 years of disease onset.4-7 Surgical indications include severe pain, obstructive symptoms, hemorrhage, diarrhea, and fistula.4 Goals of surgery include symptomatic control and surgical remission. Although novel medical therapies have increased the armamentarium for treating Crohn’s disease, surgery continues to play a major role in Crohn’s disease management, with 25% and 33% of patients requiring surgery within 5 and 10 years of diagnosis, respectively, in the era of biologic agents.8-10
Postoperative Crohn’s Disease Recurrence
Although a substantial proportion of Crohn’s disease patients require surgery within their lifetime, surgery is not a cure for Crohn’s disease and postsurgical recurrence is common.4 Fifty-eight percent to 93% of patients demonstrate evidence of endoscopic recurrence within 1 year postoperatively, and 20% to 30% develop recurrent symptoms.11-14 Surgical recurrence, or the need for a subsequent surgery, has been described in 25% to 45% of patients within 10 years after an initial bowel resection.15
In a prospective cohort study, Rutgeerts and colleagues correlated endoscopic neoterminal lesions with subsequent clinical recurrence.11 As a result, a 5-point Rutgeerts scoring system was established as a way to grade endoscopic findings in the neoterminal ileum after a bowel resection and prognosticate the probability of a future clinical recurrence.11 A Rutgeerts score of i1 represents fewer than 5 aphthous ulcers in the neoterminal ileum, whereas a score of i2 represents more than 5 aphthous ulcers with normal surrounding mucosa or disease limited to the anastomosis. On the other hand, a score of i3 represents diffuse ileitis, and a score of i4 represents large ulcers and associated stenosis in addition to diffuse inflammation.16 A Rutgeerts score of i0 or i1 corresponds to endoscopic remission and correlates with a less-than-5% probability of clinical recurrence 3 years postoperatively. A score of i2 or higher corresponds to endoscopic recurrence,17 with i2 corresponding to a 15% probability of subsequent clinical recurrence and i3 and i4 corresponding to a 40% and 90% probability of clinical recurrence, respectively.17
To date, many studies in the literature have explored predictors of postoperative recurrence. Factors such as smoking tobacco, prior intestinal surgery, a penetrating disease phenotype, a short disease duration (<10 years), the presence of granulomas, and the presence of perianal disease are associated with high rates of postsurgical recurrence.4,18-21 Smoking is the most significant modifiable risk factor for surgical recurrence in patients with Crohn’s disease.22 A systematic review revealed a dose-dependent effect of tobacco smoking on postoperative recurrence with improvement in recurrence rates on smoking cessation.23 Myenteric plexitis has also been associated with recurrent disease.24 Table 1 shows the predictors of endoscopic and clinical recurrence in more detail.
Table 1.
Predictors of Postsurgical Recurrence
| Study | Type of Study | Type of Recurrence | Significant Predictor | Outcome |
|---|---|---|---|---|
| Cottone et al22 | Retrospective | Clinical | Tobacco smoke | HR, 1.46 (95% CI, 1.1-1.8) |
| Poggioli et al60 | Prospective, observational | Surgical | Disease duration <6 years | P=.009 |
| Bernell et al15 | Retrospective | Clinical | Perianal disease | RR, 1.6 (95% CI, 1.2-2.3) |
| McLeod et al19 | Retrospective | Endoscopic | Prior resection | OR, 1.78 (95% CI, 1.06-2.90) |
| Clinical | Prior resection | OR, 2.00 (95% CI, 1.14-3.60) | ||
| Sokol et al24 | Retrospective | Clinical | Tobacco smoke | HR, 1.94 (95% CI, 1.06-3.60) |
| Clinical | Submucosal plexitis | HR, 1.87 (95% CI, 1.00-3.46) | ||
| Simillis et al18 | Meta-analysis | Surgical | Granuloma | HR, 1.62 (95% CI, 1.19-2.21) |
| Fortinsky et al61 | Retrospective | Endoscopic | Penetrating disease | HR, 1.50 (95% CI, 1.00-2.10) |
| Clinical | Tobacco smoke | HR, 2.25 (95% CI, 1.27-4.01) | ||
| Clinical | Upper GI involvement | HR, 4.00 (95% CI, 1.82-8.33) |
GI, gastrointestinal; HR, hazard ratio; OR, odds ratio; RR, relative risk.
Early studies reported a higher rate of recurrence among women, although there is no gender disparity in more recent studies.23 Studies on family history and location and extent of disease as risk factors for postoperative recurrence are equivocal.23 Genetic factors such as the NOD2/CARD15 mutation have been evaluated for possible association with recurrent disease; however, such an association may simply be a consequence of a more aggressive disease phenotype resulting from the mutation.23 Surgical factors have also been evaluated in the prediction of recurrent Crohn’s disease. Initial studies, including a meta-analysis, demonstrated reduced recurrence rates among side-to-side anastomoses.25 However, a more recent prospective, randomized, controlled trial has suggested otherwise.19 Similar rates of postsurgical recurrence are also seen after total proctocolectomy. A retrospective study of 55 patients who underwent total proctocolectomy with definitive ileostomy revealed a 4% clinical recurrence rate at 1 year and 39% at 8 years after surgery.26 Twenty-nine percent required reoperation.26
Using the available data on predictors of postsurgical recurrence, patients can be risk stratified based upon the probability of an endoscopic or clinical recurrence. The American Gastroenterological Association (AGA) recently published a technical review on the management of Crohn’s disease after a surgical resection, in which patients were stratified into low- and high-risk groups.27 Age over 50 years, nonsmoking status, disease duration greater than 10 years, and first surgery for a short (10-20 cm) segment were considered low-risk features with a predicted 30% probability of endoscopic recurrence and 20% probability of clinical recurrence 18 months postoperatively.27 On the other hand, age less than 30 years, current tobacco use, and 2 or more prior surgeries for perforating disease were considered high-risk features with an estimated 80% probability of endoscopic recurrence and 50% probability of clinical recurrence at 18 months after a resection.27 These data can help individualize a surveillance and prevention strategy based upon recurrence risk.
Postoperative Disease Surveillance
In the recently published AGA guidelines for the management of postoperative Crohn’s disease, Nguyen and colleagues make a strong recommendation for postoperative endoscopic monitoring with ileocolonoscopy at 6 to 12 months for patients not managed with postoperative prophylaxis and a conditional recommendation to do so at the same interval in those receiving prophylactic postoperative therapy.28 Ileocolonoscopy has been the gold standard for evaluation of endoscopic postoperative activity.12 The multicenter, randomized, controlled POCER (Post-Operative Crohn’s Endoscopic Recurrence) trial demonstrated the superiority of endoscopy-directed postoperative monitoring of Crohn’s disease at 6 months as compared to standard care without endoscopic monitoring.16
Given the limitations of ileocolonoscopy in evaluating the small intestine and its invasive nature, less-invasive monitoring methods have been evaluated. Video capsule endoscopy (VCE) provides a less-invasive modality to survey for postoperative endoscopic recurrence while allowing for a more extensive evaluation of the small intestine than can be completed by ileocolonoscopy. A prospective study of 24 postoperative Crohn’s disease patients undergoing both ileocolonoscopy and VCE consecutively within 2 weeks revealed a higher diagnostic yield for endoscopic recurrence on VCE (62%) as compared to traditional ileocolonoscopy (25%).29
Radiographic methods, such as contrast ultrasonography and computed tomography (CT) or magnetic resonance (MR) enteroclysis, provide noninvasive options for disease surveillance. A study comparing ileocolonoscopy to VCE and contrast ultrasonography showed equivalent diagnostic yields for endoscopic disease recurrence.30 CT and MR enteroclysis have also been studied, although they are not predominant in general practice.31,32 Both CT and MR enteroclysis are cross-sectional modalities for evaluating the small intestine mucosa and require infusion of an enteric contrast solution via a nasojejunal tube to distend the small intestine.32
In MR enteroclysis, a score of MR0 or MR1 represents low-grade inflammation and a score of MR2 or MR3 represents high-grade inflammation. A prospective study comparing the efficacy of ileocolonoscopy to MR enteroclysis for identification of disease recurrence among 30 postoperative Crohn’s disease patients revealed good interobserver variability with a kappa of 0.493 for the anastomosis, a kappa of 0.795 for the neoterminal ileum, and a kappa of 0.673 overall.32 Unlike MR enteroclysis, CT enteroclysis is limited by radiation exposure. A similarly designed prospective study compared CT enteroclysis to ileocolonoscopy in a postoperative Crohn’s disease cohort, and demonstrated a mean kappa between 0.88 and 1.00 in a majority of categories.31 In this study, CT enteroclysis was also able to differentiate fibrostenotic from active inflammatory disease.31
Clinical markers of disease recurrence, such as the Crohn’s Disease Activity Index or C-reactive protein, poorly correlate with disease recurrence.33 On the other hand, fecal calprotectin, a marker of bowel inflammation, correlates well with disease activity.34 A small, prospective, longitudinal study revealed normalization of fecal calprotectin and fecal lactoferrin within 2 months postsurgery, with fecal calprotectin being able to discriminate between subsequent active and inactive Crohn’s disease.34 The optimal role of these less-invasive modalities in postoperative disease surveillance has yet to be defined.
Prevention of Postoperative Crohn’s Disease Recurrence
Given the rates of postoperative Crohn’s disease recurrence, various medical strategies have been considered for postsurgical disease prevention (Table 2).35 Early studies focused on 5-aminosalicylic acids (5-ASAs) and showed superiority over placebo in the prevention of postoperative recurrence.36,37 Multiple randomized, controlled trials evaluating 5-ASAs have also been performed, with a meta-analysis finding a 13% reduction in risk of postoperative recurrence.38 However, a more recent multicenter, double-blind, randomized, controlled trial showed no difference in clinical or endoscopic remission in patients treated with 5-ASAs postoperatively compared to placebo.39
Table 2.
Postoperative Preventive Strategies
| Prophylactic Strategy | Study Design | Comparison | Endoscopic Recurrence | Clinical Recurrence |
|---|---|---|---|---|
| Mesalamine | ||||
| Caprilli et al36 | Randomized, controlled trial | Mesalazine 2.4 g/d or placebo | 52.0% vs 85.0% (24 months)a | 18.0% vs 41.0% (24 months)a |
| McLeod et al37 | Randomized, controlled trial | Mesalamine 3.0 g/d or placebo | Relative risk, 0.654 (24 months)a | 31.0% vs 41.0% (72 months)a |
| Lochs et al39 | Randomized, controlled trial | Mesalamine 4.0 g/d or placebo | 66.0% vs 50.0% (18 months) | 24.5% vs 31.4% (18 months) |
| Antibiotic | ||||
| Rutgeerts et al43 | Randomized, controlled trial | Metronidazole 20.0 mg/kg or placebo | 52.0% vs 75.0% (12 weeks) | 4.0% vs 25.0% (12 months)a |
| Rutgeerts et al44 | Randomized, controlled trial | Ornidazole 1.0 g/d or placebo | 53.6% vs 78.8% (12 months)a | 7.9% vs 37.5% (12 months)a |
| Immunomodulator | ||||
| Reinisch et al47 | Randomized, controlled trial | Mesalazine 4.0 g/d or AZA 2.0-2.5 mg/kg/d | N/A | 0.0% vs 10.8% (12 months)a |
| Hanauer et al48 | Randomized, controlled trial | 6-MP 50 mg or placebo | 43.0% vs 64.0% (24 months)a | 50.0% vs 77.0% (24 months)a |
| Mowat et al49 | Randomized, controlled trial | 6-MP 1.0 mg/kg or placebo | 43.0% vs 49.0% (36 months) | 27.0% vs 36.0% (36 months) |
| Biologic agent | ||||
| Regueiro et al33 | Randomized, controlled trial | Infliximab 5.0 mg/kg or placebo | 9.1% vs 84.6% (12 months)a | 0.0% vs 38.5% (12 months)a |
| Regueiro et al56 | Randomized, controlled trial | Infliximab 5.0 mg/kg or placebo | 30.6% vs 60.0% (76 weeks)a | 12.9% vs 20.0% (76 weeks) |
| De Cruz et al52 | Nonrandomized subgroup analysis | Adalimumab or AZA 2.0 mg/kg/d (6-MP 1.5 mg/kg/d) | 21.0% vs 45.0% (6 months)a | 18.0% vs 22.0% (6 months) |
6-MP, 6-mercaptopurine; AZA, azathioprine; d, day.
Statistically significant.
Budesonide has been studied for postoperative prevention in 2 prospective trials. One study compared low-dose (3 mg) budesonide to placebo and showed a statistically insignificant difference in recurrence rate, with 57% in the budesonide arm and 70% in the placebo arm at 1 year after surgical resection.40 The other study compared ileal-release budesonide at 6 mg to placebo and similarly showed no significant difference in recurrence rates at 1 year postoperatively.41 Of note, both studies demonstrated a high withdrawal rate. When analyzing the combined data in a systematic review, Doherty and colleagues confirmed that budesonide is ineffective in preventing postsurgical Crohn’s disease recurrence, with an odds ratio of 0.87 (95% CI, 0.50-1.49).42
Antibiotics have also been studied in postoperative prophylaxis. A randomized, controlled trial comparing metronidazole for 3 months postsurgery to placebo showed a reduction in endoscopic and clinical recurrence at 1 year.43 However, no significant difference in recurrence rates persisted at 3 years postoperatively.43 Similarly, a randomized, controlled trial of ornidazole for 1 year postsurgery demonstrated its efficacy in preventing clinical and endoscopic disease recurrence as compared to placebo.44 However, patients treated with ornidazole also had significantly higher rates of medication withdrawal secondary to adverse effects.44 Given the side-effect profile and the lack of evidence supporting long-term efficacy, the role of antibiotics in postoperative prophylaxis remains unclear.
Multiple studies have investigated the effectiveness of a variety of probiotic species in postoperative disease prevention. In a randomized prospective trial, Van Gossum and colleagues compared Lactobacillus johnsonii to placebo after ileocecal resection and found no significant difference in endoscopic recurrence at 12 weeks (21% vs 15% severe endoscopic recurrence, respectively; P=.33).45 In a similar study, Marteau and colleagues evaluated endoscopic recurrence at 6 months and reported no difference between the Lactobacillus johnsonii arm and placebo (49% and 64%, respectively; P=.15).46 In a systematic review and meta-analysis, Doherty and colleagues combined data for available probiotic studies, evaluating clinical recurrence with a risk ratio of 1.41 (95% CI, 0.59-3.36) and any endoscopic recurrence with a risk ratio of 0.98 (95% CI, 0.74-1.29).42 The study concluded that probiotics were ineffective in preventing both endoscopic and clinical recurrence postoperatively.42
On the contrary, thiopurines have shown superior efficacy to placebo, 5-ASAs, and antibiotics in preventing postoperative clinical recurrence.47 A multicenter double-blind study by Hanauer and colleagues compared 6-mercaptopurine to mesalamine and placebo, and showed superiority of 6-mercaptopurine to placebo for preventing endoscopic and clinical recurrence 2 years postoperatively.48 In a Cochrane systematic review, thiopurine use was associated with less severe endoscopic and clinical recurrence with a number needed to treat of 4 and 7, respectively.42 Mesalamine was inferior to thiopurine with a relative risk of 1.45 for endoscopic recurrence, but with a more favorable side-effect profile.42 Most recently, Mowat and colleagues described a prospective, randomized, controlled trial comparing azathioprine to placebo with a reduction in clinical recurrence postoperatively, but this difference was only significant in smokers (hazard ratio, 0.13).49
With the advent of biologic therapies, the clinical course of postoperative Crohn’s disease has changed dramatically. The first use of a biologic agent to prevent postoperative recurrence was reported in 2006.50 Subsequently, a small, prospective, multicenter, observational study of 29 high-risk Crohn’s disease patients using adalimumab (Humira, AbbVie) prophylactically revealed that 13.7% of patients developed clinical recurrence and 20.7% developed endoscopic recurrence at 12 months postsurgery.51 Additionally, adalimumab was not associated with adverse events, suggesting that it was safe in the postoperative setting.51
In 2009, a landmark randomized, controlled trial by Regueiro and colleagues compared postoperative infliximab (Remicade, Janssen) 5 mg/kg starting 4 weeks after surgery to placebo and found endoscopic recurrence rates of 9.1% in the infliximab group at 1 year compared to 84.6% in the placebo group.33 Furthermore, in the POCER trial, while high-risk postoperative patients were managed with prophylactic azathioprine or 6-mercaptopurine, those who were thiopurine-intolerant received adalimumab induction and maintenance. In this subgroup, adalimumab use led to a significantly lower rate of endoscopic recurrence (21%) as compared to the thiopurine arm (45%).16,52
Additional research has shown the effectiveness of anti–tumor necrosis factor agents in preventing postoperative recurrence over a longer period of follow-up.53 A case-control trial of high-risk Crohn’s disease patients undergoing bowel resection showed a significant reduction in surgical recurrence in the infliximab maintenance arm as compared to matched controls by 3 years postsurgery.54 Follow-up to the initial 2009 trial by Regueiro and colleagues showed persistent efficacy at 5 years postsurgery in an open-label study.33,55 In this trial, a longer duration of infliximab exposure was associated with postoperative remission.55
More recently, Regueiro and colleagues published a prospective, multicenter, randomized, controlled trial comparing infliximab to placebo in 297 Crohn’s disease patients undergoing a surgical resection.56 The study participants included patients who were at higher risk for a postoperative recurrence, as defined by a history of intra-abdominal resection within 10 years, 2 or more previous resections, perianal fistulizing disease, active tobacco use (at least 10 cigarettes for the last year), and perforating disease as the indication for surgery.56 The primary endpoint in this study was clinical recurrence by week 76, with 12.9% achieving this outcome in the infliximab arm as compared to 20.0% in the placebo arm (P=.97).56 Although no significant difference in clinical recurrence was seen, there was a significant reduction in endoscopic recurrence in the infliximab arm (30.6% compared to 60.0% in the placebo arm; P<.001).56 On subsequent follow-up at week 104, the similarity in clinical recurrence in both arms persisted (17.7% and 25.3% in the infliximab and placebo arms, respectively; P=.098).56
A technical review of postsurgical treatment strategies revealed superiority of a prophylactic strategy over an endoscopy-guided treatment strategy with a low quality of evidence.27 With this in mind, the AGA has made a conditional recommendation to manage postoperative Crohn’s disease patients with prophylactic pharmacologic therapy over endoscopic monitoring, favoring the use of anti–tumor necrosis factor agents or thiopurines over 5-ASAs, corticosteroids, or probiotics.28
Little is known about the efficacy of newer biologic agents. A case series looking at vedolizumab (Entyvio, Takeda) as a postoperative prophylactic agent found a higher frequency of surgical site infections. These adverse effects need to be further investigated prior to considering anti-integrins for postoperative prophylaxis.57 The efficacy of novel agents, such as ustekinumab (Stelara, Janssen), also needs to be defined.
Although overall small in numbers, well-designed trials have demonstrated the safety and efficacy of postoperative prophylactic strategies in preventing recurrence.16,33,47-49,52,56 As cost-effectiveness research grows more popular and both payers and society focus on cost-utility, it is important to consider the cost-effectiveness of postoperative Crohn’s disease prophylaxis. In 2012, Doherty and colleagues created a decision tree using 4 prophylactic strategies: mesalamine, azathioprine, infliximab, and no prophylaxis.58 Using a 1-year time horizon and an endpoint of clinical recurrence, azathioprine was the most cost-effective strategy with an incremental cost-effectiveness ratio of $299,188/quality-adjusted life-year (QALY) as compared to $1,831,912/QALY for infliximab.58 This model did not account for changing health states or long-term costs, limiting its generalizability. Subsequently, Schneider and colleagues performed a cost-utility Markov model using varying time horizons to address these limitations.59 Infliximab was the most cost-effective strategy over immunomodulators and no prophylaxis.59
Conclusion
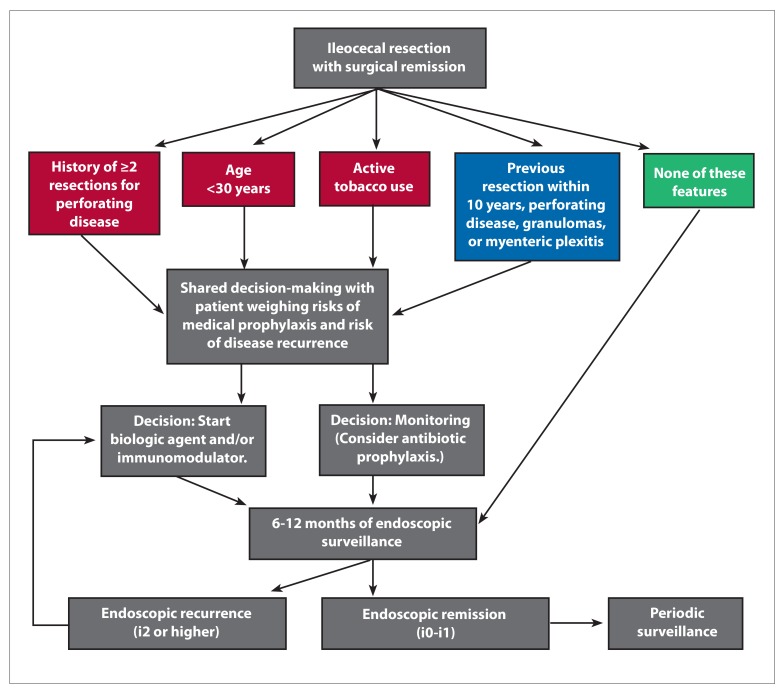
Even though the number of available agents to treat Crohn’s disease has continued to grow, surgery continues to play an important role in Crohn’s disease management. The risk of postoperative recurrence differs and is influenced by both modifiable and nonmodifiable factors. Immunomodulators and anti–tumor necrosis factor agents have demonstrated efficacy in preventing disease recurrence. Furthermore, proactive endoscopic surveillance is important regardless of treatment strategy. The Figure presents a potential stratified approach to postsurgical management, considering the current available evidence and previously published algorithms.
Figure.
A proposed algorithm for postsurgical management. Based on American Gastroenterological Association Institute guidelines, red signifies the highest risk for recurrence, blue signifies moderate to high risk for recurrence, and green signifies low risk for recurrence.
Stratifying patients by risk of postoperative recurrence is a step toward personalizing therapy, but further data are necessary on the optimal approach. With the increasing number of novel therapies, comparative effectiveness studies on these agents are essential. Further evidence on the optimal time frame for initiating biologic therapy and the most appropriate use of therapeutic drug monitoring in the postsurgical setting is crucial in reducing clinical and endoscopic recurrence.
References
- 1.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Reinisch W, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Feagan BG, Rutgeerts P, et al. GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 5.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13(4):481–489. doi: 10.1002/ibd.20036. [DOI] [PubMed] [Google Scholar]
- 6.Solberg IC, Vatn MH, Høie O, et al. IBSEN Study Group. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5(12):1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Aniwan S, Park SH, Loftus EV., Jr Epidemiology, natural history, and risk stratification of Crohn’s disease. Gastroenterol Clin North Am. 2017;46(3):463–480. doi: 10.1016/j.gtc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60(9):1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135(5):1493–1499. doi: 10.1053/j.gastro.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 11.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99(4):956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 12.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33(3):331–335. doi: 10.1136/gut.33.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascua M, Su C, Lewis JD, Brensinger C, Lichtenstein GR. Meta-analysis: factors predicting post-operative recurrence with placebo therapy in patients with Crohn’s disease. Aliment Pharmacol Ther. 2008;28(5):545–556. doi: 10.1111/j.1365-2036.2008.03774.x. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25(6):665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87(12):1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 16.De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385(9976):1406–1417. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 17.El-Hachem S, Regueiro M. Postoperative Crohn’s disease: prevention and treatment. Expert Rev Gastroenterol Hepatol. 2009;3(3):249–256. doi: 10.1586/egh.09.21. [DOI] [PubMed] [Google Scholar]
- 18.Simillis C, Jacovides M, Reese GE, Yamamoto T, Tekkis PP. Meta-analysis of the role of granulomas in the recurrence of Crohn disease. Dis Colon Rectum. 2010;53(2):177–185. doi: 10.1007/DCR.0b013e3181b7bfb0. [DOI] [PubMed] [Google Scholar]
- 19.McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M Investigators of the CAST Trial. Recurrence of Crohn’s disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum. 2009;52(5):919–927. doi: 10.1007/DCR.0b013e3181a4fa58. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Allan RN, Keighley MR. Long-term outcome of surgical management for diffuse jejunoileal Crohn’s disease. Surgery. 2001;129(1):96–102. doi: 10.1067/msy.2001.109497. [DOI] [PubMed] [Google Scholar]
- 21.Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008;103(1):196–205. doi: 10.1111/j.1572-0241.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 22.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology. 1994;106(3):643–648. doi: 10.1016/0016-5085(94)90697-1. [DOI] [PubMed] [Google Scholar]
- 23.De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18(4):758–777. doi: 10.1002/ibd.21825. [DOI] [PubMed] [Google Scholar]
- 24.Sokol H, Polin V, Lavergne-Slove A, et al. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn’s disease. Gut. 2009;58(9):1218–1225. doi: 10.1136/gut.2009.177782. [DOI] [PubMed] [Google Scholar]
- 25.Simillis C, Purkayastha S, Yamamoto T, Strong SA, Darzi AW, Tekkis PP. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn’s disease. Dis Colon Rectum. 2007;50(10):1674–1687. doi: 10.1007/s10350-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 26.Amiot A, Gornet JM, Baudry C, et al. Crohn’s disease recurrence after total proctocolectomy with definitive ileostomy. Dig Liver Dis. 2011;43(9):698–702. doi: 10.1016/j.dld.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute Technical Review on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017;152(1):277–295.e3. doi: 10.1053/j.gastro.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen GC, Loftus EV, Jr, Hirano I, Falck-Ytter Y, Singh S, Sultan S AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the management of Crohn’s disease after surgical resection. Gastroenterology. 2017;152(1):271–275. doi: 10.1053/j.gastro.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 29.Pons Beltrán V, Nos P, Bastida G, et al. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66(3):533–540. doi: 10.1016/j.gie.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 30.Biancone L, Calabrese E, Petruzziello C, et al. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn’s disease. Inflamm Bowel Dis. 2007;13(10):1256–1265. doi: 10.1002/ibd.20199. [DOI] [PubMed] [Google Scholar]
- 31.Soyer P, Boudiaf M, Sirol M, et al. Suspected anastomotic recurrence of Crohn disease after ileocolic resection: evaluation with CT enteroclysis. Radiology. 2010;254(3):755–764. doi: 10.1148/radiol.09091165. [DOI] [PubMed] [Google Scholar]
- 32.Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008;18(11):2512–2521. doi: 10.1007/s00330-008-1034-6. [DOI] [PubMed] [Google Scholar]
- 33.Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136(2):441–450.e1. doi: 10.1053/j.gastro.2008.10.051. quiz 716. [DOI] [PubMed] [Google Scholar]
- 34.Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96(6):663–674. doi: 10.1002/bjs.6593. [DOI] [PubMed] [Google Scholar]
- 35.Buisson A, Chevaux JB, Bommelaer G, Peyrin-Biroulet L. Diagnosis, prevention and treatment of postoperative Crohn’s disease recurrence. Dig Liver Dis. 2012;44(6):453–460. doi: 10.1016/j.dld.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Caprilli R, Andreoli A, Capurso L, et al. Oral mesalazine (5-aminosalicylic acid; Asacol) for the prevention of post-operative recurrence of Crohn’s disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC) Aliment Pharmacol Ther. 1994;8(1):35–43. doi: 10.1111/j.1365-2036.1994.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 37.McLeod RS, Wolff BG, Steinhart AH, et al. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn’s disease. Gastroenterology. 1995;109(2):404–413. doi: 10.1016/0016-5085(95)90327-5. [DOI] [PubMed] [Google Scholar]
- 38.Cammà C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn’s disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113(5):1465–1473. doi: 10.1053/gast.1997.v113.pm9352848. [DOI] [PubMed] [Google Scholar]
- 39.Lochs H, Mayer M, Fleig WE, et al. Prophylaxis of postoperative relapse in Crohn’s disease with mesalamine: European Cooperative Crohn’s Disease Study VI. Gastroenterology. 2000;118(2):264–273. doi: 10.1016/s0016-5085(00)70208-3. [DOI] [PubMed] [Google Scholar]
- 40.Ewe K, Böttger T, Buhr HJ, Ecker KW, Otto H German Budesonide Study Group. Low-dose budesonide treatment for prevention of postoperative recurrence of Crohn’s disease: a multicentre randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. 1999;11(3):277–282. doi: 10.1097/00042737-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Hellers G, Cortot A, Jewell D, et al. The IOIBD Budesonide Study Group. Oral budesonide for prevention of postsurgical recurrence in Crohn’s disease. Gastroenterology. 1999;116(2):294–300. doi: 10.1016/s0016-5085(99)70125-3. [DOI] [PubMed] [Google Scholar]
- 42.Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst Rev. 2009;(4):CD006873. doi: 10.1002/14651858.CD006873.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108(6):1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 44.Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128(4):856–861. doi: 10.1053/j.gastro.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Van Gossum A, Dewit O, Louis E, et al. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after lleo-caecal resection. Inflamm Bowel Dis. 2007;13(2):135–142. doi: 10.1002/ibd.20063. [DOI] [PubMed] [Google Scholar]
- 46.Marteau P, Lémann M, Seksik P, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55(6):842–847. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinisch W, Angelberger S, Petritsch W, et al. International AZT-2 Study Group. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut. 2010;59(6):752–759. doi: 10.1136/gut.2009.194159. [DOI] [PubMed] [Google Scholar]
- 48.Hanauer SB, Korelitz BI, Rutgeerts P, et al. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127(3):723–729. doi: 10.1053/j.gastro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Mowat C, Arnott I, Cahill A, et al. TOPPIC Study Group. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection (TOPPIC): a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2016;1(4):273–282. doi: 10.1016/S2468-1253(16)30078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorrentino D, Terrosu G, Avellini C, Beltrami CA, Bresadola V, Toso F. Prevention of postoperative recurrence of Crohn’s disease by infliximab. Eur J Gastroenterol Hepatol. 2006;18(4):457–459. doi: 10.1097/00042737-200604000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Aguas M, Bastida G, Cerrillo E, et al. Adalimumab in prevention of postoperative recurrence of Crohn’s disease in high-risk patients. World J Gastroenterol. 2012;18(32):4391–4398. doi: 10.3748/wjg.v18.i32.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Cruz P, Kamm MA, Hamilton AL, et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients—a POCER study analysis. Aliment Pharmacol Ther. 2015;42(7):867–879. doi: 10.1111/apt.13353. [DOI] [PubMed] [Google Scholar]
- 53.Sorrentino D. State-of-the-art medical prevention of postoperative recurrence of Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2013;10(7):413–422. doi: 10.1038/nrgastro.2013.69. [DOI] [PubMed] [Google Scholar]
- 54.Araki T, Uchida K, Okita Y, et al. Impact of postoperative infliximab maintenance therapy on preventing the surgical recurrence of Crohn’s disease: a singlecenter paired case-control study. Surg Today. 2014;44(2):291–296. doi: 10.1007/s00595-013-0538-0. [DOI] [PubMed] [Google Scholar]
- 55.Regueiro M, Kip KE, Baidoo L, Swoger JM, Schraut W. Postoperative therapy with infliximab prevents long-term Crohn’s disease recurrence. Clin Gastroenterol Hepatol. 2014;12(9):1494–1502.e1. doi: 10.1016/j.cgh.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 56.Regueiro M, Feagan BG, Zou B, et al. PREVENT Study Group. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150(7):1568–1578. doi: 10.1053/j.gastro.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 57.Lightner AL, Raffals LE, Mathis KL, et al. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. J Crohns Colitis. 2017;11(2):185–190. doi: 10.1093/ecco-jcc/jjw147. [DOI] [PubMed] [Google Scholar]
- 58.Doherty GA, Miksad RA, Cheifetz AS, Moss AC. Comparative cost-effectiveness of strategies to prevent postoperative clinical recurrence of Crohn’s disease. Inflamm Bowel Dis. 2012;18(9):1608–1616. doi: 10.1002/ibd.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider Y, Cohen-Mekelburg SA, Saumoy M, Gold S, Scherl E, Steinlauf AF. A cost-effectiveness analysis of post-operative prevention strategies for patients with Crohn’s disease utilizing a Markov model with varying time horizons. Gastroenterology. 2017;152(5):S588. [Google Scholar]
- 60.Poggioli G, Laureti S, Selleri S, et al. Factors affecting recurrence in Crohn’s disease. Results of a prospective audit. Int J Colorectal Dis. 1996;11(6):294–298. doi: 10.1007/s003840050065. [DOI] [PubMed] [Google Scholar]
- 61.Fortinsky KJ, Kevans D, Qiang J, et al. Rates and predictors of endoscopic and clinical recurrence after primary ileocolic resection for Crohn’s disease. Dig Dis Sci. 2017;62(1):188–196. doi: 10.1007/s10620-016-4351-7. [DOI] [PubMed] [Google Scholar]