Abstract
Hepatic encephalopathy (HE) is a condition that encompasses a range of neuropsychiatric abnormalities in patients with significant liver disease. Overt HE occurs in approximately 30% to 45% of patients with cirrhosis. This article discusses practical issues in the management of patients with overt HE and cirrhosis, including a recently developed 4-pronged approach that consists of identifying and correcting precipitating factors, recognizing and treating concomitant medical conditions, commencing empiric treatment, and caring for the unconscious patient. Following recovery from overt HE, a plan of action should be developed to prevent readmissions.
Keywords: Cirrhosis, overt hepatic encephalopathy, covert hepatic encephalopathy
Hepatic encephalopathy (HE) is a term used to describe a spectrum of neuropsychiatric abnormalities in patients with significant liver disease,1-3 and is classified as type A, B, or C based on the underlying mechanism causing the encephalopathy. Type A is caused by acute liver failure, whereas type B (for bypass) is primarily caused by portosystemic shunting of blood in the absence of liver disease.4 HE resulting from cirrhosis of the liver with portal hypertension is classified as type C.4 The symptoms of HE can be subtle; mild cognitive and attention deficits are described as covert HE, which occurs in 30% or more of patients with cirrhosis.5 There is debate regarding the absolute need for treating all patients with covert HE, although most physicians now believe that treatment is warranted based on observations of loss of quality of life, frequent falls, and progression to overt HE in patients who are not treated for covert HE.6-8 More severe symptoms, such as personality changes, intellectual impairment, and a depressed level of consciousness, characterize overt HE, which occurs in approximately 30% to 45% of patients with cirrhosis.9 Cases of overt HE account for approximately 0.33% of all hospitalizations in the United States, with an average inpatient stay of 8.5 days.10 This article discusses practical issues involved in the management of overt HE in patients with cirrhosis, including a 4-pronged treatment approach, maintenance therapy, and the role of diabetes and malnutrition in HE.
Pathogenesis
The pathogenesis of HE is believed to be due mainly to an elevated level of ammonia in the blood, along with other neuroactive substances derived from the gastrointestinal tract that are not cleared due to liver disease or portosystemic shunting.11-14 Short- and medium-chain fatty acids, benzodiazepine-like substances, mercaptans, phenols, and manganese may also contribute to neuronal changes.15-17 A synergistic role of inflammation is implicated in HE based on recent evidence.18
Clinical Features
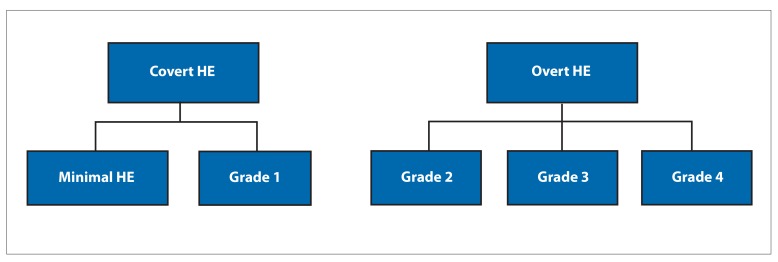
The characteristic clinical description of overt HE was originally reported in a paper by Adams and Foley, in which they described HE (then termed as hepatic coma) as a neuropsychiatric syndrome encompassing a wide spectrum of mental and motor dysfunctions.19 The authors observed a preliminary period of mild restlessness or agitation; impaired orientation; and reduced awareness of surroundings succeeded by drowsiness, stupor, and coma occurring over a period of hours to days.19 Along with changes in consciousness, rigidities, tremors, and alteration in reflexes were also observed. Asterixis (flapping tremor) was described as rhythmic bursts (3-4 per second) of flexion-extension movements of the metacarpophalangeal joints, along with side-to-side movement of fingers with the active maintenance of posture.19 These movements are also associated with flexion-extension and radial-ulnar deviation of the wrists. The presence of these clinical features in a patient with known or suspected liver disease is key to diagnosing overt HE. The West Haven grading system is currently the most commonly used scale to assess the severity of HE depending on the extent of the symptoms.20 The grading system consists of grade 0 (a lack of detectable changes in personality or behavior), grade 1 (a trivial lack of awareness, euphoria or anxiety, shortened attention span, and sleep disturbances), grade 2 (a disorientation for time, inappropriate behavior, lethargy, and asterixis), grade 3 (a gross disorientation, marked confusion, and somnolence, with response to stimuli), and grade 4 (coma). Grades 2 to 4 are considered overt HE. Grade 1 is difficult to identify objectively; therefore, grade 1 HE and minimal HE were combined into the term covert HE, which is the term now widely used (Figure).
Figure.
The West Haven grading system assesses the severity of hepatic encephalopathy (HE) depending on the extent of the symptoms. The severity of cognitive dysfunction increases with the grade. Minimal HE and West Haven grade 1 HE are now considered covert HE, and West Haven grades 2 to 4 are considered overt HE.
Principles of Managing Overt Hepatic Encephalopathy
The basic principles involved in the management of overt HE include a 4-pronged approach: (1) identification and correction of precipitating factors (Table 1), (2) recognition and treatment of concomitant medical conditions (Table 2), (3) empiric treatment of overt HE (Table 3), and (4) care of unconscious patients. Additionally, maintenance therapy following treatment of overt HE as well as the associations between diabetes, malnutrition, and overt HE should be considered.
Table 1.
Precipitating Factors of Overt Hepatic Encephalopathy
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dehydration is often multifactorial due to diuretics, reduced intake of fluids, lactulose-related diarrhea, uncontrolled diabetes, large-volume paracentesis, and hemorrhage.
Protein intake of 1.2 to 1.5 g/kg/day is recommended.
Portal vein thrombosis is an uncommon precipitating factor but can cause portosystemic shunting.
Table 2.
Concomitant Medical Conditions That Can Affect Patients With Overt Hepatic Encephalopathy
|
|
|
|
|
|
|
|
|
|
|
|
|
Hyponatremia can cause altered mental status and precipitate hepatic encephalopathy.
Uremic encephalopathy and hepatorenal syndrome can coexist with hepatic encephalopathy.
Table 3.
Empiric Treatment of Overt Hepatic Encephalopathy
|
|
|
|
|
|
Identification and Correction of Precipitating Factors
The most crucial aspect of managing overt HE is identifying the presence of precipitating factors (Table 1), which then must be corrected before recovery from overt HE can be expected. Conn and Lieberthal described gastrointestinal bleeding, infections, a high-protein diet, constipation, dehydration, acute renal failure, neuroactive medications, hyponatremia, and hypokalemia as clinical precipitants of overt HE.21 Failure to identify and correct all of the precipitating factors can lead to episodes of prolonged HE. Often in clinical practice, multiple concurrent factors are associated with overt HE, particularly in patients with advanced liver disease.22 Dehydration and electrolyte disturbances should be corrected, and there should be a thorough search for underlying infections and gastrointestinal bleeding. Dehydration can occur in patients with cirrhosis and overt HE as a result of multiple events, including reduced fluid intake, diuretic use, and lactulose-related diarrhea. An elevated ratio of urea to serum creatinine of more than 15 (range, 10-20) may indicate underlying dehydration. However, caution should be applied in interpreting the ratio of urea to serum creatinine, as urea synthesis can be affected by liver dysfunction, and serum creatinine levels can be influenced by muscle wasting owing to malnutrition or underlying hepatorenal syndrome. In addition to dehydration, hypokalemia and hyponatremia are common electrolyte disturbances and should be corrected appropriately. Use of psychoactive medicines such as opioids and benzodiazepines should be discontinued and a urine toxic screen should be considered in all patients. Serial hemoglobin measurements and a digital rectal examination are helpful to diagnose any underlying gastrointestinal bleeding. Additionally, every patient with overt HE should be screened with a chest radiograph and urine analysis to evaluate for any evidence of underlying infection. In patients with ascites, a diagnostic paracentesis should be performed to rule out spontaneous bacterial peritonitis. Empiric antibiotics should be initiated in patients with severe overt HE pending the results of urine and blood cultures. A thorough search for the focus of infection should be carried out in all patients with overt HE, as multiple infections can occur in some patients. Portosystemic shunting resulting from a transjugular intrahepatic portosystemic shunt (TIPS) procedure represents a major risk factor for the development of overt HE in patients with cirrhosis.23-25 In patients with overt HE resulting from a recent TIPS procedure, reducing the shunt diameter can improve the HE26; however, variceal bleeding may reappear after adjusting the shunt diameter.
Recognition and Treatment of Concomitant Medical Conditions
The diagnosis of overt HE is, essentially, one of exclusion (Table 2). The level of ammonia in the blood does not aid in the diagnostic accuracy of overt HE.14 Patients with advanced liver disease may present with alcohol withdrawal and delirium tremens, which can be confused with overt HE. Alcohol withdrawal is identified by a coarse rhythmic tremor, excitability, and autonomic disturbances such as tachycardia and hypertension associated with diaphoresis.27 Intravenous thiamine should be administered to all patients with alcohol use disorder to prevent clinical expression of Wernicke-Korsakoff syndrome. Wernicke encephalopathy comprises opthalmoplegia (paralysis of horizontal or vertical conjugate gaze), gaze-evoked nystagmus, and ataxia. In contrast, Korsakoff syndrome is characterized by reduced concentration, poor perception, and irrational responses to questions, and may mimic overt HE. Fabrication of answers (confabulation) is sometimes seen in early Korsakoff syndrome; however, anterograde amnesia to words, names, and tasks is always present.28,29 Electrolyte disturbances, particularly hyponatremia, and acid-base disturbances ranging from respiratory alkalosis to high anion gap metabolic acidosis can cause reduced responsiveness in patients with decompensated cirrhosis.30 Care should be exercised in the correction of serum sodium (no more than 8 mmol/L in 24 hours) to avoid central pontine myelinolysis, especially in patients with alcohol use disorder.31 Central pontine myelinolysis can cause slurred speech; dysphagia; reduced alertness; and bilateral weakness of the face, arms, and legs due to brain stem involvement. Other causes of altered mental status, including hypothyroidism, adrenal insufficiency, and hypopituitarism, should be considered in unresponsive patients.
In patients with severe overt HE who are slow to respond to treatment, the possibility of underlying traumatic brain injury must be excluded by a careful history, neurologic examination, and brain imaging. Alcohol use and chronic liver disease can increase the risk of subdural hematoma.32,33 Acute or chronic subdural hematoma may mimic overt HE, and can be often accompanied by focal neurologic signs that may be difficult to detect in severe HE. Brain imaging such as computed tomography or magnetic resonance imaging should be considered in patients with severe overt HE; imaging is mandatory if lateralizing signs are present. In patients who are slow to recover from possible HE, an electroencephalogram analysis may help to confirm the presence of typical slow triphasic waveforms, which are associated with HE, and exclude nonconvulsive seizure activity. Meningitis and encephalitis should be included in the differential diagnosis for patients who do not respond to conventional treatment. A lumbar puncture for the analysis of cerebrospinal fluid should be approached carefully in these patients due to underlying coagulopathy.34,35
Empiric Treatment of Overt Hepatic Encephalopathy
Administration of lactulose, either orally, rectally, or via nasogastric tube, is the first-line therapeutic intervention used to reduce ammonia levels in the blood (Table 3).36 The nonabsorbable disaccharides lactulose and lactitol (not used in the United States) reduce ammonia levels through multiple mechanisms, including acidifying the colon with the resultant conversion of ammonia to ammonium, replacing urease-producing bacteria with nonurease-producing bacteria, and creating a laxative effect.37 In the initial phase of overt HE, lactulose can be administered at 20 to 30 g hourly until a laxative effect is achieved. Following the recovery of the patient, the dose should be reduced and adjusted to achieve 2 to 3 soft-formed stools daily. Lactulose dose titration is essential to avoid lactulose-related diarrhea, dehydration, and excoriation of anal skin. Although lactulose was the first-line treatment for decades, a recent meta-analysis of existing data found evidence demonstrating that the nonabsorbable disaccharides may be associated with a beneficial effect on clinical outcomes when compared with placebo and no intervention.38 For patients who are not responding to initial therapy with correction of precipitating factors and administration of lactulose, it is important to consider the possibility of other non-identified precipitants, such as deep-seated infections (eg, empyema). If lactulose is not causing adequate bowel movements, treatment will be ineffective. Administration of the osmotic laxative polyethylene glycol (GoLYTELY, Braintree Laboratories, Inc) should then be considered after excluding bowel obstruction.39 Rifaximin (Xifaxan, Salix) or neomycin can be added to the lactulose if the bowel movements are adequate and if there is no clinical response in mental status after a few hours. Although rifaximin is not approved by the US Food and Drug Administration (FDA) for the treatment of overt HE, a randomized, controlled trial demonstrated improved treatment outcomes and a mortality benefit in patients on rifaximin combined with lactulose.40 Neomycin is a poorly absorbed aminoglycoside that is approved by the FDA for use in acute overt HE; however, the evidence for neomycin efficacy is weak,41 and prolonged use is associated with a risk of ototoxicity and nephrotoxicity.42 The antibiotics metronidazole and oral vancomycin have shown some benefit in the treatment of overt HE. The risk of neurotoxicity with metronidazole and colonization with vancomycin-resistant enterococci are the primary constraints to using these agents routinely for the treatment of overt HE.43-45
Care of Unconscious Patients
Patients with West Haven grade 3 or 4 HE require closer monitoring in an intensive care unit (ICU). These patients cannot protect their airways due to depressed consciousness; thus, endotracheal intubation and mechanical ventilation are required. Lactulose (15-30 mL) is administered every 1 to 2 hours through a nasogastric tube until 3 to 4 loose stools are passed in 24 hours. These patients should be kept in an appropriate posture in order to prevent aspiration. If nasogastric tube access is not available, or administration is ineffective, then 300 mL of lactulose mixed with 700 mL of water can be given as an enema and repeated as necessary. Rifaximin along with lactulose should be administered to all patients with grade 3 or 4 HE. Occasionally, patients with acute-on-chronic liver failure may develop cerebral edema and increased intracranial pressure. These patients should have the head of the bed elevated to 30 degrees.46,47 Patient-ventilator dyssynchrony should be controlled with the use of short-acting sedatives.48,49 Induction of transient hypocapnia (ie, a decrease of 10 mm in carbon dioxide levels) reduces intracranial pressure by inducing cerebrospinal fluid alkalosis and resulting in precapillary vasoconstriction. Other ICU interventions for cerebral edema include administration of mannitol and hypertonic saline infusion, both of which have variable effects.50,51 Some patients with severe recurrent overt HE may have large congenital or acquired portosystemic shunts that may be amenable to occlusion. These shunts should be suspected particularly when recurrent HE is occurring in patients with relatively preserved liver function. A large shunt, if identified, should be closed by radiologic techniques in patients with refractory HE and a Model for End-Stage Liver Disease (MELD) score of less than 15.52
Additional treatment options include intravenous branched-chain amino acids and L-ornithine L-aspartate (neither available in the United States). These treatment modalities are not supported with convincing data.53-56 Zinc deficiency is common in patients with cirrhosis. Urea synthesis from ammonia by ornithine transcarbamylase enzyme in the liver and glutamine formation from ammonia by glutamine synthetase in the skeletal muscle are impaired by zinc deficiency. Long-term zinc treatment has been shown to enhance the formation of urea from ammonia and amino acids.57,58 A 2010 study by Takuma and colleagues reported evidence that zinc supplementation is effective in treating HE and consequently in improving the health-related quality of life of the patient.59 However, there are no data regarding the optimal dose of zinc. Recently, extracorporeal albumin dialysis (ECAD) using the molecular adsorbent recirculating system has been used for the treatment of overt HE with an acceptable safety profile.60,61 The ECAD treatment showed a significant dialysis effect, with improvement in serum bilirubin levels, serum creatinine levels, and the severity of overt HE.60,61 A convincing beneficial effect on survival has not yet been demonstrated by ECAD therapy. Patients with significant liver dysfunction (MELD score >15) and recurrent severe HE are better treated with liver transplantation.62
Maintenance Therapy After Treating Overt Hepatic Encephalopathy
After treating overt HE, patients with Child-Pugh class B and C cirrhosis generally require lactulose maintenance therapy. However, the data for secondary prevention of overt HE with lactulose are limited.63 Patients who recover from grade 3 or 4 overt HE should be kept on maintenance treatment with lactulose and rifaximin, the latter of which is FDA-approved for secondary prevention of overt HE. Patient and family education regarding lactulose dosing to achieve 2 to 3 soft-formed stools daily is an important part of management. A randomized, controlled trial of rifaximin vs placebo in patients with MELD scores of less than 25 reported a 50% reduction in hospitalizations for the rifaximin group.64 In another study, rifaximin was shown to be effective and well tolerated for long-term maintenance of remission from overt HE.65
Role of Diabetes and Malnutrition in Overt Hepatic Encephalopathy
There is evidence of an association between uncontrolled diabetes and malnutrition with liver decompensation and precipitation of overt HE in patients with cirrhosis.66,67 A study by Sigal and colleagues showed that diabetic patients experience severe HE at earlier stages of biochemical decompensation and portal hypertension.68 The control of diabetes may help to delay the onset of overt HE in patients with cirrhosis. Apart from the liver, skeletal muscle plays a role in ammonia detoxification, and malnutrition and muscle wasting may result in precipitation of HE in cirrhosis.68-73 Nutritional status assessment is challenging in patients with cirrhosis due to low albumin levels, fluid retention, and ascites, making the interpretation of weight and body mass indices difficult. Advocating a malnutrition risk assessment tool may be useful to identify patients at risk of HE.74 Recent guidelines from the International Society for Hepatic Encephalopathy and Nitrogen Metabolism recommend 1.2 to 1.5 g/kg of protein daily in patients with cirrhosis and small, evenly distributed, frequent meals and late-night snacks of complex carbohydrates to prevent negative nitrogen balance.75 Branched-chain amino acid supplements in patients with cirrhosis may improve nutritional status and muscle mass in the long term and, therefore, may help prevent development of overt HE.76
Summary
The diagnosis of HE is based on clinical criteria and is not only related to ammonia levels in the blood. Management of overt HE includes a 4-pronged approach, especially in patients with grade 3 or 4 HE. These strategies include identification and correction of precipitating factors, recognition of concomitant medical conditions and their treatment, empiric treatment of HE, and care of patients with altered consciousness. Recently, the importance of recognizing the full spectrum of precipitating factors has been emphasized.22 In certain circumstances, it is important to identify the concomitant conditions that can mimic overt HE, primarily because of the difference in the approach to management with these disorders. A fairly effective therapy for overt HE is currently available for treatment. Polyethylene glycol is a new addition to the armamentarium of treatment for overt HE. A more rapid recovery from overt HE can be anticipated with this therapy, which needs formal approval from the FDA. ECAD has been used with some improvement in overt HE, but has not shown a clear effect on improving mortality. Whether further refinement of ECAD will result in better outcomes remains to be established.
References
- 1.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 2.Riggio O, Efrati C, Catalano C, et al. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42(5):1158–1165. doi: 10.1002/hep.20905. [DOI] [PubMed] [Google Scholar]
- 3.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(1 (suppl)):S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10(11):1208–1219. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Morgan MY, Amodio P, Cook NA, et al. Qualifying and quantifying minimal hepatic encephalopathy. Metab Brain Dis. 2016;31(6):1217–1229. doi: 10.1007/s11011-015-9726-5. [DOI] [PubMed] [Google Scholar]
- 8.Waghray A, Waghray N, Mullen K. Management of covert hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S75–S81. doi: 10.1016/j.jceh.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(suppl 1):3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 10.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–1041.e1. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth RF. Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis. 2016;31(6):1211–1215. doi: 10.1007/s11011-015-9746-1. [DOI] [PubMed] [Google Scholar]
- 12.Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17(4):221–227. doi: 10.1023/a:1021989230535. [DOI] [PubMed] [Google Scholar]
- 13.Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24(1):169–181. doi: 10.1007/s11011-008-9122-5. [DOI] [PubMed] [Google Scholar]
- 14.Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193. doi: 10.1016/s0002-9343(02)01477-8. [DOI] [PubMed] [Google Scholar]
- 15.Mullen KD, Martin JV, Mendelson WB, Bassett ML, Jones EA. Could an endogenous benzodiazepine ligand contribute to hepatic encephalopathy? Lancet. 1988;1(8583):457–459. doi: 10.1016/s0140-6736(88)91245-7. [DOI] [PubMed] [Google Scholar]
- 16.Mullen KD, Szauter KM, Kaminsky-Russ K. “Endogenous” benzodiazepine activity in body fluids of patients with hepatic encephalopathy. Lancet. 1990;336(8707):81–83. doi: 10.1016/0140-6736(90)91594-z. [DOI] [PubMed] [Google Scholar]
- 17.Riordan SM, Williams R. Gut flora and hepatic encephalopathy in patients with cirrhosis. N Engl J Med. 2010;362(12):1140–1142. doi: 10.1056/NEJMe1000850. [DOI] [PubMed] [Google Scholar]
- 18.Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5(suppl 1):S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams RD, Foley JM. The neurologic changes in the more common types of severe liver disease. Trans Am Neurol Assoc. 1949;74:217–219. [Google Scholar]
- 20.Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72(4 Pt 1):573–583. [PubMed] [Google Scholar]
- 21.Conn HO, Lieberthal MM. The Hepatic Coma Syndromes and Lactulose. Baltimore, MD: Williams and Wilkins; 1979. Management of acute portosystemic encephalopathy; pp. 189–219. [Google Scholar]
- 22.Pantham G, Post A, Venkat D, Einstadter D, Mullen KD. A new look at precipitants of overt hepatic encephalopathy in cirrhosis. Dig Dis Sci. 2017;62(8):2166–2173. doi: 10.1007/s10620-017-4630-y. [DOI] [PubMed] [Google Scholar]
- 23.Orloff MJ, Orloff MS, Orloff SL, Rambotti M, Girard B. Three decades of experience with emergency portacaval shunt for acutely bleeding esophageal varices in 400 unselected patients with cirrhosis of the liver. J Am Coll Surg. 1995;180(3):257–272. [PubMed] [Google Scholar]
- 24.Riggio O, Angeloni S, Ridola L. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: still a major problem. Hepatology. 2010;51(6):2237–2238. doi: 10.1002/hep.23707. [DOI] [PubMed] [Google Scholar]
- 25.Salerno F, Cammá C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133(3):825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Fanelli F, Salvatori FM, Rabuffi P, et al. Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglass-shaped balloon-expandable stent-graft. AJR Am J Roentgenol. 2009;193(6):1696–1702. doi: 10.2214/AJR.09.2968. [DOI] [PubMed] [Google Scholar]
- 27.Davidson EA, Solomon P. The differentiation of delirium tremens from impending hepatic coma. J Ment Sci. 1958;104(435):326–333. doi: 10.1192/bjp.104.435.326. [DOI] [PubMed] [Google Scholar]
- 28.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd ed. Philadelphia, PA: F. A. Davis Company; 1989. [Google Scholar]
- 29.Wijdicks EF. Hepatic encephalopathy. N Engl J Med. 2016;375(17):1660–1670. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez JV, Carrillo-Pérez DL, Rosado-Canto R, et al. Electrolyte and acid-base disturbances in end-stage liver disease: a physiopathological approach. Dig Dis Sci. 2017;62(8):1855–1871. doi: 10.1007/s10620-017-4597-8. [DOI] [PubMed] [Google Scholar]
- 31.Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10) suppl 1:S1–S42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt L, Gørtz S, Wohlfahrt J, Melbye M, Munch TN. Recurrence of subdural haematoma in a population-based cohort—risks and predictive factors. PLoS One. 2015;10(10):e0140450. doi: 10.1371/journal.pone.0140450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan LM, Kress WL, Strnad LC, et al. Low likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169. doi: 10.1016/j.cgh.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Friedman EW, Sussman Safety of invasive procedures in patients with the coagulopathy of liver disease. Clin Lab Haematol. 1989;11(3):199–204. doi: 10.1111/j.1365-2257.1989.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 35.Youssef WI, Salazar F, Dasarathy S, Beddow T, Mullen KD. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: a dual phase study. Am J Gastroenterol. 2003;98(6):1391–1394. doi: 10.1111/j.1572-0241.2003.07467.x. [DOI] [PubMed] [Google Scholar]
- 36.Blei AT, Córdoba J. Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 37.Gerber T, Schomerus H. Hepatic encephalopathy in liver cirrhosis: pathogenesis, diagnosis and management. Drugs. 2000;60(6):1353–1370. doi: 10.2165/00003495-200060060-00008. [DOI] [PubMed] [Google Scholar]
- 38.Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;4:CD003044. doi: 10.1002/14651858.CD003044.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350—electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733. doi: 10.1001/jamainternmed.2014.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 41.Strauss E, Tramote R, Silva EP, et al. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39(6):542–545. [PubMed] [Google Scholar]
- 42.Lerner SA, Matz GJ. Aminoglycoside ototoxicity. Am J Otolaryngol. 1980;1(2):169–179. doi: 10.1016/s0196-0709(80)80012-3. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23(1):1–7. doi: 10.1136/gut.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhl MD, Riely CA. Metronidazole in treating portosystemic encephalopathy. Ann Intern Med. 1996;124(4):455. doi: 10.7326/0003-4819-124-4-199602150-00015. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Yoon JH, Kim BH, et al. Enterococcus: not an innocent bystander in cirrhotic patients with spontaneous bacterial peritonitis. Eur J Clin Microbiol Infect Dis. 2009;28(1):21–26. doi: 10.1007/s10096-008-0578-3. [DOI] [PubMed] [Google Scholar]
- 46.Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54(3):593–597. doi: 10.1227/01.neu.0000108639.16783.39. [DOI] [PubMed] [Google Scholar]
- 47.Ropper AH, O’Rourke D, Kennedy SK. Head position, intracranial pressure, and compliance. Neurology. 1982;32(11):1288–1291. doi: 10.1212/wnl.32.11.1288. [DOI] [PubMed] [Google Scholar]
- 48.Tufano R. Analgesia and sedation in intensive care: a progress report. Minerva Anestesiol. 2003;69(10):735–737. [PubMed] [Google Scholar]
- 49.Sassoon CS, Foster GT. Patient-ventilator asynchrony. Curr Opin Crit Care. 2001;7(1):28–33. doi: 10.1097/00075198-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004;10(2):126–131. doi: 10.1097/00075198-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Harukuni I, Kirsch JR, Bhardwaj A. Cerebral resuscitation: role of osmotherapy. J Anesth. 2002;16(3):229–237. doi: 10.1007/s005400200030. [DOI] [PubMed] [Google Scholar]
- 52.Laleman W, Simon-Talero M, Maleux G, et al. EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–2457. doi: 10.1002/hep.26314. [DOI] [PubMed] [Google Scholar]
- 53.Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54(3):1063–1070. doi: 10.1002/hep.24412. [DOI] [PubMed] [Google Scholar]
- 54.Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106(6):1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- 55.Kircheis G, Nilius R, Held C, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25(6):1351–1360. doi: 10.1002/hep.510250609. [DOI] [PubMed] [Google Scholar]
- 56.Stauch S, Kircheis G, Adler G, et al. Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998;28(5):856–864. doi: 10.1016/s0168-8278(98)80237-7. [DOI] [PubMed] [Google Scholar]
- 57.Bresci G, Parisi G, Banti S. Management of hepatic encephalopathy with oral zinc supplementation: a long-term treatment. Eur J Med. 1993;2(7):414–416. [PubMed] [Google Scholar]
- 58.Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23(5):1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 59.Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32(9):1080–1090. doi: 10.1111/j.1365-2036.2010.04448.x. [DOI] [PubMed] [Google Scholar]
- 60.Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002;36(4 Pt 1):949–958. doi: 10.1053/jhep.2002.36130. [DOI] [PubMed] [Google Scholar]
- 61.Bañares R, Nevens F, Larsen FS, et al. RELIEF study group. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 62.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 63.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891.e1. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 64.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 65.Mullen KD, Sanyal AJ, Bass NM, et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12(8):1390–1397.e2. doi: 10.1016/j.cgh.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 66.Kalaitzakis E, Olsson R, Henfridsson P, et al. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27(9):1194–1201. doi: 10.1111/j.1478-3231.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 67.Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823–831. doi: 10.1002/hep.27228. [DOI] [PubMed] [Google Scholar]
- 68.Sigal SH, Stanca CM, Kontorinis N, Bodian C, Ryan E. Diabetes mellitus is associated with hepatic encephalopathy in patients with HCV cirrhosis. Am J Gastroenterol. 2006;101(7):1490–1496. doi: 10.1111/j.1572-0241.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu TL, Trogdon J, Weinberger M, Fried B, Barritt AS., IV Diabetes is associated with clinical decompensation events in patients with cirrhosis. Dig Dis Sci. 2016;61(11):3335–3345. doi: 10.1007/s10620-016-4261-8. [DOI] [PubMed] [Google Scholar]
- 70.Merli M, Iebba V, Giusto M. What is new about diet in hepatic encephalopathy. Metab Brain Dis. 2016;31(6):1289–1294. doi: 10.1007/s11011-015-9734-5. [DOI] [PubMed] [Google Scholar]
- 71.Bunchorntavakul C, Supanun R, Atsawarungruangkit A. Nutritional status and its impact on clinical outcomes for patients admitted to hospital with cirrhosis. J Med Assoc Thai. 2016;99(suppl 2):S47–S55. [PubMed] [Google Scholar]
- 72.Córdoba J, López-Hellín J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Chadalavada R, Sappati Biyyani RS, Maxwell J, Mullen K. Nutrition in hepatic encephalopathy. Nutr Clin Pract. 2010;25(3):257–264. doi: 10.1177/0884533610368712. [DOI] [PubMed] [Google Scholar]
- 74.Borhofen SM, Gerner C, Lehmann J, et al. The Royal Free Hospital-Nutritional Prioritizing Tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61(6):1735–1743. doi: 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- 75.Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 76.Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28(2):217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]