Abstract
The aim of this study was to establish a fast-track protocol for bimaxillary orthognathic surgery (OGS). Fast-track surgery (FTS) is a multidisciplinary approach where the pre-, intra-, and postoperative management is focusing maximally on a quick patient recovery and early discharge. To enable this, the patients’ presurgical stress and postsurgical discomfort should be maximally reduced. Both referral patterns and expenses within the health-care system are positively influenced by FTS. University hospital-literature review through Medline, Embase, and the Cochrane Library (January 2000–July 2016) using the following words – “fast track, enhanced recovery, multimodal, and perioperative care” – to define a protocol evidence based for OGS, as well as evidenced-based medicine search of every term added to the protocol during the same period. The process has resulted in an OGS protocol that may improve the outcome of the patient through several nonoperative and operative measures such as preoperative patient education and intra/postoperative measures that should improve overall patient satisfaction, decrease morbidity such as postoperative nausea, headache, dizziness, pain, and intubation discomfort, and shorten hospital stay. A literature review allowed us to fine-tune a fast-track protocol for uncomplicated OGS that can be prospectively studied against currently applied ones.
Keywords: Orthognathic surgery, perioperative care, postoperative complications
INTRODUCTION
The terms “fast-track surgery” (FTS) and “enhanced recovery program” (ERP) were coined in 1999 by a Danish anesthesiologist Dr. Henrik Kehlet. Surgeons, like anesthesiologists, aim for a smooth and complication-free recovery after surgery. Despite existing evidence that FTS improves outcomes, there is a tendency to not question traditional beliefs and dogmas. FTS represents a global package of perioperative multidisciplinary care pathways that encompass pre-, intra-, and post-operative techniques aimed at improving patient perception, reducing hospital stay, and minimizing morbidities, thereby returning the patient to normal activities as quickly as possible while at the same time minimizing hospital expenses.
ERP has become standard practice in many surgical fields such as colorectal[1] and urological[2] surgery, but studies in cranio-maxillofacial surgery and more specifically in orthognathic surgery (OGS) are lacking.
The primary aim of this study was to review the literature on FTS and OGS. The secondary aim was to evaluate the feasibility of creating an evidence-based FTS protocol for uncomplicated OGS to minimize risks and patient discomfort and to shorten the length of hospital stay.
MATERIALS AND METHODS
The current clinical pathway in OGS was reviewed to identify procedures most amenable to improvement.
A literature review was performed using MEDLINE, EMBASE, and the Cochrane Library (January 2000–December 2015). The general search criteria were “enhanced recovery surgery” and “fast-track surgery.” A protocol was built as a “step-by-step” guide from the moment the patient first arrives at the clinic until the day the patient is discharged from the hospital. The actual clinical pathway was reviewed, and any potential change was analyzed. Every measure underwent evidence-based review. The level of evidence of every study was assessed using Oxford Centre for Evidence-Based Medicine (OCEBM) Levels I to V, with Level I reflecting the highest level of evidence (OCEBM 2011). Relative measures of effect, such as odds ratios (ORs) with 95% confidence intervals in brackets, are shown whenever possible.
A small booklet divided into phases was made in which all possible patient questions were anticipated and explained in a simple manner (http://www.mauricemommaerts.eu/files/cms1/Annex%20I%20-%20Orthognathic%20Surgery%20Brochure.pdf).
RESULTS
Preoperative stage
Counseling
During preoperative assessment, smoking cessation is advised, as this can reduce the risks of complications such as bleeding, wound infection, and cardiorespiratory complications. In a meta-analysis and systematic review focused on esthetic surgery, Pluvy et al.[3] showed that smokers have an increased risk of complications, such as postoperative cutaneous necrosis (OR, 3.60 [2.62–4.93]), wound healing delays and wound infection (OR, 2.07 [1.53–2.81]), and surgical site complications in general (OR, 1.79 [1.57–2.04]). However, the preoperative time-lapse needed for quitters to reduce postoperative risks has not been determined. Myers et al.[4] also conducted a systematic review and meta-analysis and concluded that smokers who stopped 8 weeks or less before surgery did not experience a decrease in postoperative pulmonary and wound complications. Their conclusion was based mostly on retrospective studies with OCEBM Level III evidence and thus must be interpreted cautiously. Furthermore, their study may be biased by misclassification of smoking status, as smoking cessation was not chemically validated. Although biochemical abstinence validation is the best evidence, it limits patient inclusion and increases the heterogeneity among studies. We recommend that patients stop smoking as early as possible and that smoking cessation treatment be offered to all patients.
With regard to alcohol, a Cochrane systematic review and meta-analysis (two studies at OCEBM Level II) showed that after chemical validation with tetraethylthiuram disulfide (a chemical compound that blocks an enzyme involved in metabolizing alcohol intake, producing very unpleasant side effects) for 8 weeks of preoperative alcohol cessation in drinkers of >60 g of alcohol per day, there was a decrease in postoperative complications (i.e., wound-related complications, secondary surgery, cardiopulmonary complications, and admission to intensive care; OR, 0.22 [0.08–0.61]; P = 0.004). Mortality and length of stay were unaffected (OCEBM Level III).[5]
Medication
Patients should take their usual medication according to the European Society of Anesthesiology (ESA) guidelines.[6]
The control of anxiety arising from the anticipation of surgery is a continuing challenge. Benzodiazepines, opioids, and barbiturates are among the most common drugs used in this situation. Midazolam is a benzodiazepine extensively studied and used as premedication. It is water soluble and shows high metabolic clearance, with roughly the same pharmacologic and toxicologic spectrum as diazepam. It has a rapid onset and brief half-life of 1–4 h with multiples routes of administration and few reported side effects (e.g., hiccups, coughing, nausea, and vomiting, 1%–3%).[7] An interesting randomized controlled trial (RCT)[8] (OCEBM Level II-III) showed a significant decrease in salivary cortisol levels (i.e., an endogenous glucocorticoid that increases under stress) measured during and after surgery after sublingual administration of 7.5 mg midazolam.
A Cochrane database study showed that midazolam reduced pain (midazolam mean, 2.56 (standard deviation [SD], 0.49); placebo mean, 4.62 (SD, 1.49); (P < 0.005) and anxiety (midazolam mean, 1.52 [SD, 0.3]; placebo mean, 3.97 [SD, 0.44]; P < 0.0001) in one trial with 99 patients.[9] Another Cochrane review showed that patient discharge after administration of anxiolytic premedication was not delayed compared with that in a placebo condition[10] (OCEBM Level III).
Alpha-agonists such as clonidine reduce anxiety and provide sedation and pain relief after surgery when given in a dose of 4–5 pg/kg as part of a multimodal analgesic treatment in children.[11] Improved nonopioid pain management means less postoperative nausea and vomiting (PONV). Using the bispectral index (BIS) monitor, clonidine has been shown to reduce propofol dose requirements[12] (OCEBM Level II-III) and postoperative shivering (OCEBM Level III).[13] Furthermore, clonidine does not delay discharge for day-care surgery when given 1–2 h before surgery[10] (OCEBM Level II). Following anesthesiologist (Dr. Olivier Detriche) advice, 2 pg/kg 1 h before surgery should be sufficient to produce the desired effect.
Nutrition
Conventionally, it has been the standard to fast patients overnight; in adults, the minimum is now considered to be 6 h of fasting for liquids and solids. This was believed to minimize the risk of aspiration during the perioperative period (“Mendelsohn syndrome”). We know now that poor nutrition is of no benefit to the patient. The current ESA fasting guidelines allow adults and children 6 h for solids and up to 2 h for clear fluids (i.e., coffee, tea without milk, and pulp-free juice) before surgery (recommendation Grade 1).[14] This improves the patient's sense of well-being and attenuates catabolic responses to surgery.
Antibiotics
The American National Research Council has a widely accepted wound classification system based on the degree of expected contamination during surgery. OGS is defined as a “clean-contaminated wound,” which is an operative wound in which viscus is entered under controlled conditions without unusual contamination.[15] Without antibiotic prophylaxis, the rate of surgical site infection (SSI) in OGS was 10%–15%[16] (OECBM IV). A systematic review and meta-analysis by Tan et al. (2011)[17] showed a significant reduction in risk of SSI after administration of a single dose of antibiotics versus placebo (relative risk (RR), 0.27 [0.11–0.68]) (OCEBM Level III). Single-dose versus single-day as well as short-term versus long-term comparisons were not significant. However, the adequate antibiotic is chosen provided that oral flora such as anaerobes, enteric gram-negative bacilli, and Staphylococcus aureus are covered. The three infections reported in the study were caused by penicillin-sensitive streptococci. Therefore, we assume that penicillin or amoxicillin would suffice for prophylaxis in OGS.
Blood work
Following ESA guidelines, head and neck surgeries are classified as intermediate risk (i.e., 1%–5% cardiac risk). For healthy patients undergoing OGS with the American Society of Anesthesiologists classification I or II (i.e., healthy or with mild systemic disease), full blood count, hemostasis, kidney function, or electrocardiogram are not routinely advised. If cardiac pathology is reported, the patient should receive a separate referral to a cardiologist in addition to the anesthesiologist check-up. Treatment with beta-blockers and statins should not be interrupted for surgery (OCEBM Level I).
Physicians should explicitly inquire about and document in patient files the use of herbal drugs such as garlic, ginseng, and gingko, as they are known platelet aggregation inhibitors[18] (OECD Grade 3). ESA recommends suspension of herbal drug intake 7 days before surgery.
Patients taking anticoagulants (ACs) present a challenge for the surgeon. Temporary interruption of ACs increases the risk of thromboembolic events. If the risk is transient, such as after a recent stroke or pulmonary embolism, the best option is to delay the surgery until the risk returns to baseline.
The reasons for taking ACs depend on the risk of thromboembolic event. In patients with low thromboembolic risk, such as those with nonrheumatic atrial fibrillation and no additional risk factors (e.g., previous episode of thrombosis, diabetes mellitus, high blood pressure, age), ACs can be suspended before the procedure and resumed the night or day after surgery. Those who must maintain AC usage due to pathologies such as high risk of atrial fibrillation, prosthetic heart valve prosthesis, or history of venous thromboembolic disease are not candidates for OGS.
The duration of AC suspension depends on the type of drug taken.
Warfarin blocks the synthesis of factors II, VII, IX, and X (Vitamin K-dependent). Warfarin should be stopped 5 days before surgery, and the international normalized ratio (INR) should be checked on the day of surgery. If the INR is >1.5–2, then 1–2 mg Vitamin K should be given orally. The patient can resume warfarin 12–24 h after surgery
Dabigatran is a direct thrombin inhibitor that should be discontinued 2–3 days before surgery. Although no coagulation test is needed for monitoring, activated partial thromboplastin time can be used to check normality. As it has a rapid onset of action of 2–3 h, the patient can start the day after surgery
Rivaroxaban, apixaban, and edoxaban are direct factor Xa inhibitors that block the conversion of prothrombin to thrombin. Their management is similar to that of dabigatran.
Bridging therapy with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) is generally only used with warfarin or other vitamin K-dependent ACs. It can be administered preoperatively, postoperatively, or both depending on the risk factors and type of surgery. It usually starts 3 days before surgery when the INR has dropped below the therapeutic level and is discontinued when postsurgical hemostasis has been achieved (i.e., 24–72 h after surgery).
If severe perioperative bleeding occurs, the following treatment is advised.
Acetylsalicylic acid (ASA): Transfusion of platelets (start with two concentrates)
Warfarin: 2.5–5 mg of intravenous (IV) Vitamin K. Repeat INR after 6–12 h. Consider also fresh frozen plasma or prothrombin complex concentrates
Non-Vitamin K-dependent ACs such as davigatran, rivaroxaban, apixaban, and edoxaban: Stop or delay next dose and perform mechanical compression, fluid replacement, and blood transfusion. If uncontrolled bleeding still occurs, use activated recombinant factor VII, prothrombin coagulation complex, charcoal filtration (for dabigatran), and hemodialysis
Heparin: Stop dose. In general, coagulation should be normal 4 h after cessation. If in an emergency situation, use protamine sulfate at 1U per 1U of heparin sodium.
Prevention of deep vein thrombosis
The incidence of fatal pulmonary embolism in elective surgery without deep vein thrombosis (DVT) is estimated to be 0.1%–0.8% (and may increase to 7% for hip surgery). The current European guidelines for venous thromboembolism (VTE) prophylaxis classify head and neck surgeries with an intermediate risk of cardiac events (1%–5%). Evidence-based clinical practice guidelines (Guyatt et al. 2012)[19] recommend the following.
Very low-risk patients (<0.5%; rogers score, <7; caprini score, 0) undergoing nonorthopedic surgery (e.g., general, bariatric, urological, gynecological, plastic, or reconstructive surgery): No specific pharmacological or mechanical (i.e., intermittent pneumatic compression, graduated compression stockings, venous foot pump) prophylaxis should be used other than early ambulation (Grade 1b)
Low-risk patients (<1.5%; rogers score, 7–10; caprini score, 1–2): Mechanical prophylaxis should be used until ambulation (Grade 2c)
Moderate-risk patients (3.0%; rogers score, 10; caprini score, 3-4): LMWH or UFH is recommended (Grade 2b) over no thromboprophylaxis treatment. It is not clear whether mechanical prophylaxis improves outcomes at this risk level
Patients with very low or low risk of a cardiac event taking antiplatelet therapy such as ASA can stop 7 days prior to surgery (Grade 2c). Patients presenting moderate risk should continue to take ASA during the procedure (Grade 2c)
Patients with coronary stents receiving dual therapy should wait at least 6 months after placement of the drug-eluting stent and reassess.
Rogers et al.[20] and Caprini[21] are proponents of using standardized patient risk stratification tools for DVT that take into account characteristics of the patient (e.g., age, sex, surgical and medical history, comorbidities) and procedure (e.g., type of surgery, expected length, postoperative ambulation). Our hospital uses a modified Caprini score protocol.
Tranexamic acid
Tranexamic acid (TXA) is a synthetic lysine analog that inhibits fibrinolysis, blocking the activation of plasminogen to plasmin with a half-life of 3.1 h (Mommaerts, 2013).[22] A systematic review and meta-analysis by Ker et al.[23] shows strong evidence that TXA reduces the need for blood transfusion in surgery (RR, 0.62 [0.58–0.65]; P < 0.001) (OCEBM Level II). Several dose regimens have been proposed in the multiple RCTs published in the last 10 years. Dowd et al.[24] published a scheme that keeps TXA concentrations in plasma high enough to inhibit 80% of tissue plasminogen activator with a loading dose of 30 mg/kg and a maintenance infusion of 16 mg/kg. We prescribe 1 g oral TXA three times a day for 3 days before surgery. In cases in which above-average bleeding is expected, such as Le Fort I osteotomy with a partial turbinectomy and rhinoplasty, a 1 g intraoperative IV bolus is given, and an addition 1 g is infused during surgery. Patients with impaired kidney function will need adjustments in this dose, as 95% is excreted in the urine.
Local application of TXA over the surgical field has proven helpful in minimizing bleeding. A Cochrane meta-analysis of 29 trials with 2612 patients showed a 29% reduction in blood loss using this method (pooled ratio, 0.71 [0.69–0.72], P < 0.0001) (OCEBM Level III).[25] We use gauze strips (5 cm × 5 cm) soaked in TXA for temporarily packing the wounds after the osteotomy.
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDS) are widely used cyclooxygenase inhibitors that reduce the production of prostaglandins and decrease inflammation and pain. In a Cochrane meta-analysis of 15 studies analyzing postoperative bleeding of 1101 children under 16 years of age who underwent tonsillectomy, the funnel plot of pooled data showed no significant difference in bleeding with NSAIDS such as 5 mg/kg ibuprofen, 1 mg/kg diclofenac, or 2 mg/kg ketoprofen. However, power calculations indicated that a higher number of patients need to be included to rule out a higher risk of bleeding when patients take NSAIDS in tonsillectomies[26] (OCEBM Level II).
Another systematic review and meta-analysis performed by Kelley et al. (2015)[27] (OCEBM Level II) analyzed 384 patients in four RCTs comparing ibuprofen against other NSAIDS in a plastic surgery perioperative setting and showed no significant differences in bleeding.
Urinary catheterization
Patients may need to use a short-term urethral catheter for several reasons. In maxillofacial surgery, urinary catheterization is advocated in prolonged surgeries (i.e., more than 3 h) and/or when a large volume infusion is foreseen. Most OGS procedures are individually performed in <45 min. When a “tri-maxillary” surgery (i.e., upper and lower jaw and chin correction) is combined with other cosmetic treatments such as blepharoplasty, zygoma osteotomy, or rhinoseptoplasty, urethral catheterization is advised, but the catheter should be removed in the postanesthesia care unit. If the need for short-term catheterization appears during surgery but was not expected, there is no clear evidence regarding whether a suprapubic or urethral catheter provides the best treatment against complications such as urinary tract infections[28] (OCEBM Level III).
Intraoperative stage
Operative time
Surgery duration is considered an independent risk factor for postoperative morbidities such as SSI, dehiscence, seroma, hematoma, and delayed wounds. Hardy et al.[29] performed multivariate regression showing an increase in risk of overall complications associated with a surgical duration of more than 3 h (OR, 1.61 [1.09–2.37]; P = 0.017), reaching a peak increase after 6.77 h (OR, 4.71 [3.29–6.73]; P < 0.0001) (OCEBM Level IV). Procter et al.[30] found a linear doubling of the rate of infection at 2.1–2.5 h (OR, 1.92 [1.82–2.03]; P < 0.001). The length of hospital stay increased geometrically with surgery duration at a rate of about 6% per ½ h (0.059 per ½ h [0.058–0.060]; P < 0.001) (OCEBM Level III). Care must be taken when interpreting these results, as many confounding factors such as the presence of surgical trainees, complexity of surgery, or proficiency of the surgeon are surely present.
Single jaw OGS rarely takes longer than 35 min. When performing facial makeovers (i.e., multiple surgeries in one procedure), it is of upmost importance to employ organized and efficient teamwork to minimize the time that the patient is in the operation theater.
Fluid therapy
IV fluid therapy must be precise during surgery to maintain tissue perfusion and cellular oxygen delivery. Both hypovolemia and fluid excess may lead to complications.[1] The goal is to maintain an effective circulatory volume while avoiding interstitial overload. There are two main types of fluids that can be used: colloids and crystalloids. Colloids are human plasma derivatives (e.g., human albumin or fresh-frozen plasma) or semi-synthetic products such as dextrans, gelatins, and hydroxyethyl starch.[31] Crystalloids are solutions of electrolytes with sterile water that can be hypotonic, isotonic, or hypertonic with respect to plasma.
For most healthy adults who undergo minimally to moderately invasive surgery and are not expected to have significant fluid shifts or blood loss, there is moderate evidence that favors buffered solutions over saline-based fluids. Brief postoperative statistically significant metabolic differences have been described such as metabolic acidosis and hyperchloremia in patients given saline-based fluids (95% CI [0.04–0.08], P < 0.00001, I2 = 74%).[32] Regarding the volume of fluid, the current guidelines recommend replacement of perioperative fluid loss due to nasogastric aspirate, blood loss from the surgical site, urine, and insensible losses that occur via the skin and lungs at a ratio of 1:1.[33] One to two liters of balanced electrolyte solution (e.g., Ringer B, Braun, Melsungen, Germany) should be passed in 30 min to 2 h during the intervention to provide a safe volume margin.[34] An interesting alternative is to directly measure the blood volume needed (called the “goal-directed approach”).[35] This can be achieved through esophageal Doppler-guided fluid boluses. It allows individualized goal-directed fluid management guided by an algorithm that enhances the ventricular stroke volume while avoiding any risk of fluid overload. Although it is probably the most accurate method, it has also been noted to be inconvenient for head and neck surgery, as it requires occasional repositioning of the probe, and the diathermy interferes with the signal.[36] Therefore, this method seems unnecessary for OGS, as neither significant blood loss nor volume shifts are expected.
Nasogastric tubes and throat packs
The nasogastric tube was first introduced by Levin in 1921[37] and has since become very popular. As it is uncomfortable for the patient, we introduce it after intubation and remove it before extubation in theater when bilateral sagittal split osteotomy (BSSO) or genioplasty is performed. In upper jaw surgeries, we leave it in place until the first ward round (roughly 3–6 h after the operation) to ensure no posterior active bleeding. It is beneficial for aspirating unconsciously swallowed blood and rinsing solution coming from the paranasal sinuses and hence reduces PONV. We do not use throat packs because, in our experience, these produce a variable degree of pharyngeal edema, trauma, and sore throat and increase the risk of complications such as foreign body aspiration (OCEBM Level III).[38,39]
Surgical stress
There are two main responses to surgical stress. One is an endocrine-metabolic response, which starts as a catabolic state and leads to increased cardiovascular demand. The other is an imbalance between pro-inflammatory and anti-inflammatory cytokines.[40] Although the presence of pro-inflammatory cytokines is helpful for wound healing and resistance to infection, they are also linked to an increased pain response.[41] The most effective corticoid dose is debated, but a Cochrane review by Da Silva et al.[42] showed moderate benefit in terms of edema and ecchymosis when corticoids (i.e., 250 mg methylprednisolone) are administrated prior to the intervention (OCEBM Level III).
Temperature
General anesthesia, low temperatures in theaters, and cold IV fluids impair natural thermoregulatory control. Unless externally warmed, the patient will almost surely experience a certain degree of hypothermia. This situation is related to sympathetic response, cardiac arrhythmia, increased blood loss, negative nitrogen balance, postanesthetic shivering, and wound infection. Vasoconstriction decreases the subcutaneous oxygen tension and impairs T-cell-mediated antibody production and bacterial killing by neutrophils[43] (OCEBM Level III). A core temperature <34.7°C (i.e., 1.4°C below normal) may increase discomfort, wound infection, and hospital stay[44] (OCEBM Level II-III).
Prewarmed IV fluids and forced air-warmers distributing heated air through a blanket will keep the patient at an appropriate temperature.
General anesthesia
While a complete and detailed discussion of the general anesthesia requirements for OGS is beyond the scope of this paper, certain key factors should be mentioned as a basic part of ERP.
Optimal anesthesia in FTS involves local anesthesia infiltration aided with nerve blocks in combination with short-acting anesthetics such as propofol and analgesics such as ketamine (OCEBM Level IV).[1] Ketamine was introduced as an anesthetic agent in 1964. It acts on n-methyl-D-aspartate receptors in the spinal cord and midbrain. A 50 mg dose 10 min before local anesthesia produces a dissociative state that lasts 10–20 min[45] (OCEBM Level IV). NSAIDs such as acetaminophen and glucocorticoids have been shown to have a synergistic effect, minimizing postoperative pain and other side effects[46] (OCEBM Level III).
Other less-soluble anesthetics and opioids such as fentanyl are associated with higher rates of respiratory depression and PONV. An exception to this may be remifentanil, a short-acting opioid that provides deep stable analgesia with rapid offset when desired, allowing fast extubation and lowered anesthetic doses[47] (OCEBM Level III). The entire process of anesthesia can be performed with individually titrated doses controlled with a BIS monitor following the “Goldilocks” method.[45,48] BIS is a two-lead electroencephalographic-derived index that is sensitive to drugs that act primarily on the cerebral cortex such as thiopental, propofol, and halogenated inhalational agents. Opioids, benzodiazepines, ketamine, and N2O act mostly on lower levels and hence are not well measured by BIS. On a scale from 0 to 100 (with 100 being fully awake), 40–60 is considered general anesthesia. BIS-guided anesthesia reduces anesthetic doses and intraoperative awareness and improves postoperative recovery (OCEBM Level II).[49] However, based on our anesthesiologist's experience, there seems to be a time gap between the actual level of anesthesia and the BIS readings that must be taken into account.
Local anesthesia
Infiltration of local anesthetic in the surgical incision has been associated with a significant opioid-sparing effect and reduced hospital stay[40] (OCEBM Level IV). A bilateral regional blockade of the third branch of the trigeminal nerve with a long-lasting anesthetic such as ropivacaine (7.5 mg/ml) before the sagittal split decreases intraoperative opioid consumption (Van Lancker et al., 2003)[50] (OCEBM Level III). Tumescent anesthesia for the buccal cortex is given with a blunt needle (Whitacre spinal, pencil pointed needle 25 GA, 3.50 in 0.5 mm × 90 mm) to minimize bruising and hematomas (Level III OCEBM).[51]
Topical nasal vasoconstrictors
In patients with maxillary impactions larger than 5 mm in whom partial turbinectomy is planned or a facial makeover procedure will include rhinoseptoplasty, the use of a topical nasal vasoconstrictor is common practice. It decreases bleeding and improves visibility. A prospective nonrandomized study compared a 4% cocaine chlorhydrate solution with another solution containing epinephrine at a 1:1000 dilution. Although both treatments worked well, bleeding and field visibility were reported to be better when the solution with cocaine was used[52] (OCEBM Level III). We use soaked neurosurgical pads with 2% cocaine and 1:1000 epinephrine solution, squeezing out the excess to reduce the likelihood of postoperative headache.
Sutures
Running polyglactin 910 sutures (Vicryl rapide 4–0 16 mm, 3/8c, Johnson and Johnson, USA) are used for intraoral mucosa closure. They provide good knot security (when four knots are made), wound tensile strength, and low tissue reactivity. They do not need to be removed and therefore save office time as well as anxiety for the patient.
A Cochrane review comparing continuous versus interrupted skin sutures showed no significant differences between groups. A slight decrease in wound dehiscence was noticed in the continuous suture group (RR, 0.08 [0.02–0.35]), although the quality of the evidence was low (OCEBM Level IV).[53]
Fibrin sealant
Fibrin sealant (Tisseel Baxter Healthcare Corporation, USA) is applied after mucosal closure in BSSO and genioplasty to minimize postoperative hematomas. Care must be taken to avoid intravascular application. A recent meta-analysis showed a significant reduction in postoperative total blood loss and drop in hemoglobin without an increase in DVTs (OCEBM Level III).[54]
Screws
A systematic review and meta-analysis by Al-Moraissi and Al-Hendi[55] compared five studies using locking plates and bicortical screws for BSSO advancement and found no significant difference in skeletal stability in horizontal (fixed: skeletal muscle density [SMD], 0.255 mm [0.558–0.048]; P = 0.099) or vertical (fixed: SMD, 0.023 mm [0.326–0.280]; P = 0.882) measurement (OCEBM Level III). The biomechanical behavior of the aforementioned techniques was analyzed in a study by Sato et al.[56] using a finite element analysis system, which suggests that applying mechanical stress to a bicortical inverted “L” screw arrangement provides more stress dissipation and stability.
Postoperative stage
Pain
Proper analgesia is vital for ERP, as it increases patient comfort and allows for faster discharge. Opioids are often used for moderate to severe postoperative pain, but their side effects such as PONV, urinary retention, and respiratory depression often delay discharge (OCEBM Level III).[57]
Multimodal opioid-sparing analgesia involves the use of more than one NSAID. Fortunately, pain is rarely a concern in postoperative BSSO patients. One study shows that 1000 mg paracetamol and 400 mg ibuprofen every 8 h for 3 days provides appropriate pain control (OCEBM Level I).[58]
Postoperative nausea and vomiting
PONV is a common problem in anesthesia. It is estimated to have an incidence of 25%–30% in all surgical patients and can be as high as 70% in high-risk populations (e.g., women, nonsmokers, strabismus surgery, laparoscopic procedures).[59] Specific PONV prophylaxis is generally not performed to avoid side effects. Methylprednisolone has shown to help late PONV in doses of 40 mg IV[59,60] (OCEBM Level IV). Midazolam at a dose of 2 mg IV also decreases PONV when given postoperatively, especially when combined with 5 μg ramosetron, a 5-HT3 antagonist (OCEBM Level II).[61]
Cryotherapy and head elevation
Postoperative local intermittent compression with cold packs reduces the requirement for analgesia and has a positive outcome in terms of pain, edema, and ecchymosis after craniotomy according to a RCT[62] (OCEBM Level III). Edema probably behaves differently depending on the type of surgery performed. The former study described a progressive volume increase of the soft tissues across 3 days, whereas in rhinoplasty studies, the edema peak is achieved 1 h after the intervention (OCEBM Level III).[63] The duration of exposure to low temperatures and the objectivity of measurement are of key importance. The intense vascularization of facial tissues makes it difficult to obtain low temperatures. van der Westhuijzen et al.[64] kept ice packs in the mandibular area after third molar surgery for a period longer than 100 min and measured a mean skin temperature of 21°C, falling intraorally from 35.5°C to 35°C (i.e., only 0.5°C). This study did not find a significant improvement in pain or edema when using adjuvant cryotherapy for wisdom tooth extraction (OCEBM Level III).
Elevation of the head of the bed by 30° or more without rotation creates a favorable pressure gradient for lymph and venous flow by intravascular gravitational force.[65]
Nasal management
Le Fort I osteotomy with partial turbinectomy (and rhinoseptoplasty) may block the nasal airway. Saline solution spray (Sterimar®) is given to patients after surgery, who are instructed to use the spray as many times as needed to moisturize and clear the nasal passage.
Topical corticoids such as budesonide and mometasone help decrease postoperative symptoms such as altered sense of smell, nasal itching, sneezing, runny nose, and nasal blockage (SMD, 1.35 [2.05–0.64]; P = 0.0002; three trials; 137 patients), demonstrating their use as a safe postoperative management therapy[66] (OCEBM Level II). A dose of 64 μg budesonide (32 μg per nostril) once a day for 1 week relieves postoperative discomfort.
Oral hygiene
A meta-analysis by Caso et al.[67] showed that intraoral rinsing with 0.12% chlorhexidine during surgery and the following days (the exact number of days could not be determined) decreases the risk of alveolar osteitis after third molar removal (OCEBM Level II-III). We have not found literature to confirm this effect in OGS, but it seems a reasonable measure that we also apply to our patients. Gentle rinses with 0.12% chlorhexidine four times per day and soft tooth-brushing three times per day over the reachable areas will lower the chance of infection.
The key elements of the EBSSO FTS protocol are shown in Table 1. Text formatted in italics are recommendations derived from evidence-based medicine.
Table 1.
Fast-track procedures for orthognathic surgery
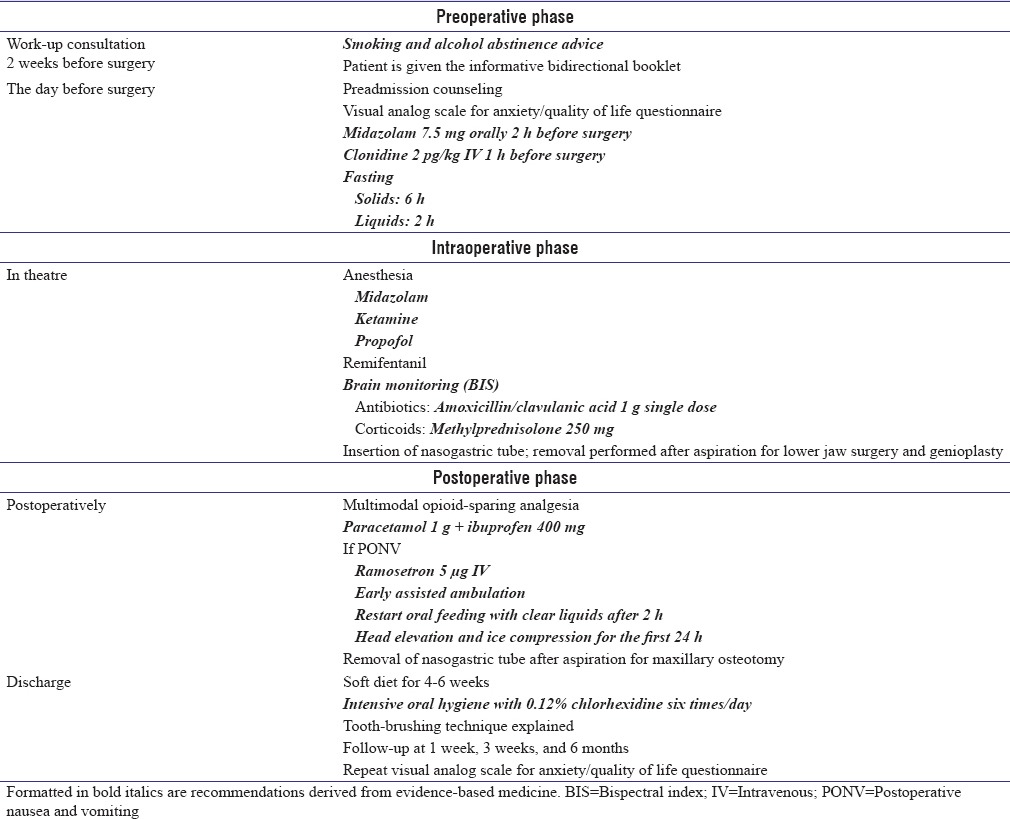
DISCUSSION
FTS protocols aim to enhance patient surgical experience while also lowering hospital expenses. Significant amounts of data collected during the last decade show improvements in quality of care and economic benefits due to shortened hospital stays. Nicholson et al.[68] reviewed almost forty trials (mainly in colorectal and urology surgery) showing a 20% reduction in the length of stay (roughly 1 day shorter) as well as reduced risks of all complications within 30 days (RR, 0.71 [0.60–0.86]).
Most interventions described in this article have been proven effective through well-documented data, but when lacking conclusive studies, we have applied our own professional experience.
More than 60 g of alcohol per day increases the risk of postoperative complications.[5] Midazolam and clonidine reduce anxiety when given before surgery[8] (Lambert et al., 2014). Beta-blockers and statins should not be interrupted prior to surgery. Patients with low thromboembolic risk pathologies can suspend AC use before the procedure (ESA guidelines). A single dose of an appropiate antibiotic in a clean-contaminated wound reduces the risk of SSI to acceptable levels.[17] VTE prophylaxis is not needed in standard OGS.[67] TXA (local and IV) reduces intraoperative bleeding.[24] We prescribe 1 g oral TXA three times a day for 3 days before surgery. In cases in which above-average bleeding is expected, such as a Le Fort I osteotomy with partial turbinectomy and/or rhinoplasty, a 1 g intraoperative IV bolus is given, and another 1 g is infused during surgery (patients with impaired kidney function will need an adjusted dose, as 95% is excreted in the urine). Locally, gauze strips (5 cm × 5 cm) soaked in TXA can be temporarily packed in the wounds after the osteotomy is performed.
Regional anesthesia minimizes postoperative pain and lessens both intra-and postoperative opioid requirements and their side effects.[41] Patient-specific titrated anesthesics such as propofol monitored with BIS will avoid over-or under-dosing and minimize many common postoperative side effects including PONV,[50] allowing patients to ambulate earlier and reducing their length of stay.[47] Fibrin sealant can decrease postoperative bleeding and hematoma formation.[52] Multimodal opioid-sparing analgesia with paracetamol and ibuprofen should be used in standard orthognathic uncomplicated procedures.[58]
The impacts of certain interventions are more difficult to quantify. We know that tobacco increases complications after surgery, but it is not clear how far in advance of surgery we should advise smoking cessation to produce a benefit. Most studies do not chemically test patients for nicotine levels, so a measurement bias is present. We recommend quitting at the first visit and insist on smoking cessation 2 weeks before surgery.
Nasogastric tubing provides clear discomfort to patients and represents, in our daily practice, one of the most common complaints during ward rounds. Nasogastric tubes are kept for 6-8 h only in patients who undergo Le Fort I-related procedures to rule out postoperative bleeding. Measuring the effect of cryotherapy, such as facial cold packs, can be challenging because most studies in this area have focused on minor oral procedures that typically do not cause severe swelling. We have noticed less edema and pain using cold packs as soon as the patient is shifted to the postoperative recovery area combined with head elevation, especially if the surgery is performed in a timely manner.
We do not use throat packs because these produce a variable degree of pharyngeal edema, trauma, and sore throat and increase the risk of complications such as foreign body aspiration[39,40] (OCEBM Level III).
The booklet given to patients is a bidirectional tool assessed by the surgeon during the next three postoperative controls that provides clear instructions and a walkthrough of the patient's anticipated experience (http://www.mauricemommaerts.eu/files/cms1/Annex%20I%20-%20Orthognathic%20Surgery%20Brochure.pdf).
Most of these techniques are simple and inexpensive. Used supplementary to an adequate surgical technique, analgesics and anti-inflammatory medications will prove helpful in minimizing morbidities such as discomfort, pain, and edema.
CONCLUSION
Simple measures can make significant improvements in patient outcome. In an evidence-based environment, every detail applied to a protocol should not be overlooked. From the patient's first appointment until the last postoperative follow-up, every procedure must be carefully studied before its implementation.
To our knowledge, this is the first fast-track evidence-based program in OGS and should answer the majority of questions that a surgeon in our field may have. We expect that this protocol will provide similar benefits as those observed in other surgical specialties and set the basis for an OGS fast-track guideline.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Counihan TC, Favuzza J. Fast track colorectal surgery. Clin Colon Rectal Surg. 2009;22:60–72. doi: 10.1055/s-0029-1202888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urol Assoc J. 2011;5:342–8. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pluvy I, Panouillères M, Garrido I, Pauchot J, Saboye J, Chavoin JP, et al. Smoking and plastic surgery, part II. Clinical implications: A systematic review with meta-analysis. Ann Chir Plast Esthet. 2015;60:e15–49. doi: 10.1016/j.anplas.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: A systematic review and meta-analysis. Arch Intern Med. 2011;171:983–9. doi: 10.1001/archinternmed.2011.97. [DOI] [PubMed] [Google Scholar]
- 5.Oppedal K, Møller AM, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. 2012;7:CD008343. doi: 10.1002/14651858.CD008343.pub2. [DOI] [PubMed] [Google Scholar]
- 6.De Hert S, Imberger G, Carlisle J, Diemunsch P, Fritsch G, Moppett I, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684–722. doi: 10.1097/EJA.0b013e3283499e3b. [DOI] [PubMed] [Google Scholar]
- 7.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–24. [PubMed] [Google Scholar]
- 8.Jerjes W, Jerjes WK, Swinson B, Kumar S, Leeson R, Wood PJ, et al. Midazolam in the reduction of surgical stress: A randomized clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:564–70. doi: 10.1016/j.tripleo.2005.02.087. [DOI] [PubMed] [Google Scholar]
- 9.Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Database Syst Rev. 2016;5:CD009491. doi: 10.1002/14651858.CD009491.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker KJ, Smith AF. Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev. 2009;4:CD002192. doi: 10.1002/14651858.CD002192.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert P, Cyna AM, Knight N, Middleton P. Clonidine premedication for postoperative analgesia in children. Cochrane Database Syst Rev. 2014;1:CD009633. doi: 10.1002/14651858.CD009633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris J, Acheson M, Reeves M, Myles PS. Effect of clonidine pre-medication on propofol requirements during lower extremity vascular surgery: A randomized controlled trial. Br J Anaesth. 2005;95:183–8. doi: 10.1093/bja/aei172. [DOI] [PubMed] [Google Scholar]
- 13.Lewis SR, Nicholson A, Smith AF, Alderson P. Alpha-2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database Syst Rev. 2015;8:CD011107. doi: 10.1002/14651858.CD011107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Søreide E, et al. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 15.Mangram AJ, Teresa C, Pearson HM, Silver LC, Jarvis WR. A brief overview of the 1999 CDC guideline for the prevention of surgical site infection. Am J Infect Control. 1999;27:97–134. [PubMed] [Google Scholar]
- 16.Peterson LJ. Antibiotic prophylaxis against wound infections in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1990;48:617–20. doi: 10.1016/s0278-2391(10)80477-x. [DOI] [PubMed] [Google Scholar]
- 17.Tan SK, Lo J, Zwahlen RA. Perioperative antibiotic prophylaxis in orthognathic surgery: A systematic review and meta-analysis of clinical trials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:19–27. doi: 10.1016/j.tripleo.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208–16. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers SO, Jr, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: Results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1211–21. doi: 10.1016/j.jamcollsurg.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 21.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–8. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Mommaerts MY. A comprehensive guide to orthofacial surgery. The Surgical Art of Facial Makeover: Planning and Operative Techniques. Vol. 1. Sint-Martens-Latem, Belgium: Orthoface R & D GCV. 2013. [Last accessed on 2017 Aug 24]. pp. 472–3. Available from: http://www.facialmakeover.info/.http://www.facialmakeover.info/
- 23.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowd NP, Karski JM, Cheng DC, Carroll JA, Lin Y, James RL, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002;97:390–9. doi: 10.1097/00000542-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. 2013;7:CD010562. doi: 10.1002/14651858.CD010562.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev. 2013;7:CD003591. doi: 10.1002/14651858.CD003591.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley BP, Bennett KG, Chung KC, Kozlow JH. Ibuprofen Does Not Increase Bleeding in Plastic Surgery Patients. Plast Reconstr Surg. 2015;136(4 Suppl):156–7. [Google Scholar]
- 28.Kidd EA, Stewart F, Kassis NC, Hom E, Omar MI. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev. 2015;12:CD004203. doi: 10.1002/14651858.CD004203.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy KL, Davis KE, Constantine RS, Chen M, Hein R, Jewell JL, et al. The impact of operative time on complications after plastic surgery: A multivariate regression analysis of 1753 cases. Aesthet Surg J. 2014;34:614–22. doi: 10.1177/1090820X14528503. [DOI] [PubMed] [Google Scholar]
- 30.Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60–5.e1-2. doi: 10.1016/j.jamcollsurg.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Van Aken H, Westpha ML. Relevance of non-albumin colloids in intensive care medicine. Best Pract Res Clin Anaesthesiol. 2009;23:193–212. doi: 10.1016/j.bpa.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Burdett E, Dushianthan A, Bennett-Guerrero E, Cro S, Gan TJ, Grocott MP, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev. 2012;12:CD004089. doi: 10.1002/14651858.CD004089.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Soni N. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP): Cassandra's view. Anaesthesia. 2009;64:235–8. doi: 10.1111/j.1365-2044.2009.05886_1.x. [DOI] [PubMed] [Google Scholar]
- 34.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 35.Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93:1069–76. doi: 10.1002/bjs.5454. [DOI] [PubMed] [Google Scholar]
- 36.Coyle MJ, Main B, Hughes C, Craven R, Alexander R, Porter G, et al. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol. 2016;41:118–26. doi: 10.1111/coa.12482. [DOI] [PubMed] [Google Scholar]
- 37.Levin AL. A new gastroduodenal catheter. JAMA. 1921;76:1007–7. [Google Scholar]
- 38.Jaiswal V, Bedford GC. Review of the use of throat packs in nasal surgery. J Laryngol Otol. 2009;123:701–4. doi: 10.1017/S0022215109004356. [DOI] [PubMed] [Google Scholar]
- 39.Fennessy BG, Mannion S, Kinsella JB, O’Sullivan P. The benefits of hypopharyngeal packing in nasal surgery: A pilot study. Ir J Med Sci. 2011;180:181–3. doi: 10.1007/s11845-010-0601-4. [DOI] [PubMed] [Google Scholar]
- 40.Carli F, Kehlet H, Baldini G, Steel A, McRae K, Slinger P, et al. Evidence basis for regional anesthesia in multidisciplinary fast-track surgical care pathways. Reg Anesth Pain Med. 2011;36:63–72. doi: 10.1097/AAP.0b013e31820307f7. [DOI] [PubMed] [Google Scholar]
- 41.Kehlet H. Fast-track surgery-an update on physiological care principles to enhance recovery. Langenbecks Arch Surg. 2011;396:585–90. doi: 10.1007/s00423-011-0790-y. [DOI] [PubMed] [Google Scholar]
- 42.da Silva EM, Hochman B, Ferreira LM. Perioperative corticosteroids for preventing complications following facial plastic surgery. Cochrane Database Syst Rev. 2014;6:CD009697. doi: 10.1002/14651858.CD009697.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sessler DI. Non-pharmacologic prevention of surgical wound infection. Anesthesiol Clin. 2006;24:279–97. doi: 10.1016/j.atc.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 45.Friedberg BL. Anesthesia for cosmetic facial surgery. Int Anesthesiol Clin. 2003;41:13–28. doi: 10.1097/00004311-200341030-00004. [DOI] [PubMed] [Google Scholar]
- 46.White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: From multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–96. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 47.Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: A systematic review. Anaesthesia. 2007;62:1266–80. doi: 10.1111/j.1365-2044.2007.05221.x. [DOI] [PubMed] [Google Scholar]
- 48.Friedberg B. Getting Over Going Under: Five Things You Need to Know before Anesthesia. 1st ed. Newport Beach, California: Goldilocks Press; 2010. pp. 86–9. [Google Scholar]
- 49.Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014;6:CD003843. doi: 10.1002/14651858.CD003843.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Lancker P, Abeloos JV, De Clercq CA, Mommaerts MY. The effect of mandibular nerve block on opioid consumption, nausea and vomiting in bilateral mandibular osteotomies. Acta Anaesthesiol Belg. 2003;54:223–6. [PubMed] [Google Scholar]
- 51.Yu W, Jin Y, Yang J, Ma G, Qiu Y, et al. Occurrence of bruise, hematoma, and pain in upper blepharoplasty using blunt-needle vs.sharp-needle anesthetic injection in upper blepharoplasty. JAMA Facial Plast Surg. 2017;19:128–32. doi: 10.1001/jamafacial.2016.1376. [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Cossío S, Rodríguez-Dintén MJ, Gude F, Fernández-Álvarez JM. Topical vasoconstrictors in cosmetic rhinoplasty: Comparative evaluation of cocaine versus epinephrine solutions. Aesthetic Plast Surg. 2016;40:637–44. doi: 10.1007/s00266-016-0673-2. [DOI] [PubMed] [Google Scholar]
- 53.Gurusamy KS, Toon CD, Allen VB, Davidson BR. Continuous versus interrupted skin sutures for non-obstetric surgery. Cochrane Database Syst Rev. 2014;2:CD010365. doi: 10.1002/14651858.CD010365.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Xiao L, Guo H, Zhao G, Ma J. The efficiency and safety of fibrin sealant for reducing blood loss in primary total hip arthroplasty: A systematic review and meta-analysis. Int J Surg. 2017;37:50–57. doi: 10.1016/j.ijsu.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Al-Moraissi EA, Al-Hendi EA. Are bicortical screw and plate osteosynthesis techniques equal in providing skeletal stability with the bilateral sagittal split osteotomy when used for mandibular advancement surgery? A systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2016;45:1195–200. doi: 10.1016/j.ijom.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Sato FR, Asprino L, Noritomi PY, da Silva JV, de Moraes M. Comparison of five different fixation techniques of sagittal split ramus osteotomy using three-dimensional finite elements analysis. Int J Oral Maxillofac Surg. 2012;41:934–41. doi: 10.1016/j.ijom.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: A quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–32. doi: 10.1016/j.jamcollsurg.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults-an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;9:CD008659. doi: 10.1002/14651858.CD008659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovac AL. Update on the management of postoperative nausea and vomiting. Drugs. 2013;73:1525–47. doi: 10.1007/s40265-013-0110-7. [DOI] [PubMed] [Google Scholar]
- 60.Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–53. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 61.Kim WJ, Kang H, Shin HY, Baek CW, Jung YH, Woo YC, et al. Ramosetron, midazolam, and combination of ramosetron and midazolam for prevention of postoperative nausea and vomiting: A prospective, randomized, double-blind study. J Int Med Res. 2013;41:1203–13. doi: 10.1177/0300060513485864. [DOI] [PubMed] [Google Scholar]
- 62.Shin YS, Lim NY, Yun SC, Park KO. A randomised controlled trial of the effects of cryotherapy on pain, eyelid oedema and facial ecchymosis after craniotomy. J Clin Nurs. 2009;18:3029–36. doi: 10.1111/j.1365-2702.2008.02652.x. [DOI] [PubMed] [Google Scholar]
- 63.Kargi E, Hosnuter M, Babucçu O, Altunkaya H, Altinyazar C. Effect of steroids on edema, ecchymosis, and intraoperative bleeding in rhinoplasty. Ann Plast Surg. 2003;51:570–4. doi: 10.1097/01.sap.0000095652.35806.c5. [DOI] [PubMed] [Google Scholar]
- 64.van der Westhuijzen AJ, Becker PJ, Morkel J, Roelse JA. A randomized observer blind comparison of bilateral facial ice pack therapy with no ice therapy following third molar surgery. Int J Oral Maxillofac Surg. 2005;34:281–6. doi: 10.1016/j.ijom.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Stucker FJ. Prevention of post-rhinoplasty edema. Laryngoscope. 1974;84:536–41. doi: 10.1288/00005537-197404000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Fandiño M, Macdonald KI, Lee J, Witterick IJ. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: A systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27:e146–57. doi: 10.2500/ajra.2013.27.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caso A, Hung LK, Beirne OR. Prevention of alveolar osteitis with chlorhexidine: A meta-analytic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:155–9. doi: 10.1016/j.tripleo.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101:172–88. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]


