Abstract
Background:
Guided bone regeneration (GBR) is the most common technique for localized bone augmentation.
Purpose:
The purpose of this review was to categorize and assess various GBR approaches for the reconstruction of human alveolar bone defects.
Materials and Methods:
Electronic search of four databases including PubMed/Medline, EMBASE, Web of Science, and Cochrane and hand searching were performed to identify human trials attempting GBR for the reconstruction of alveolar bony defects for at least 10 patients from January 2000 to August 2015. To meet the inclusion criteria, studies had to report preoperative defect dimensions in addition to outcomes of bone formation and/or resorption.
Results:
Twenty-five human clinical trials were included of which 17 used conventional technique that is the use of space maintaining membrane with bone grafting particles (GBR I). Application of block bone graft with overlying membrane and particulate fillers was reported in seven studies (GBR II), and utilizing cortical bone block tented over a defect preserving particulate fillers was reported by one study (GBR III). A wide range of initial defects’ sizes and treatment results were reported.
Conclusions:
This review introduces a therapeutically oriented classification system of GBR for treating alveolar bone defects. High heterogeneity among studies hindered drawing definite conclusions in regard to superiority of one to the other GBR technique.
Keywords: Alveolar ridge reconstruction, bone augmentation, bone grafting, implantology, nonresorbable membrane, resorbable membrane
INTRODUCTION
The irreversible process of three-dimensional (3D) alveolar bone resorption occurs as early as 6 months following tooth loss or extraction that may pose a challenge for predictable implant placement.[1,2] In addition, inadequate bone volume may jeopardize long-term prognosis of dental implants.[2,3,4] Reconstruction of resorbed alveolar ridges has been a goal and a challenge for clinicians to optimize outcomes of oral implant placement.[2,3,4]
A variety of surgical approaches have been proposed to enhance the alveolar bone volume including but not limited to ridge splitting, distraction osteogenesis (DO), and onlay or particulate bone grafts with or without membranes.[5,6,7,8] Autogenous bone, harvested from extraoral and intraoral donor sites, has been extensively used because of its osseoinductive, osseoconductive, and osteogenic properties.[9] On the other hand, high resorption rate could compromise the clinical outcomes of autogenous bone grafts.[10,11,12,13] Up to 56% autologous cortical bone graft, resorption in 4 months is reported in animal and human studies.[14,15,16,17] In addition, these grafts are associated with morbidity depending on the harvest site.[12,16]
Guided bone regeneration (GBR), by application of cell occlusive membranes that mechanically exclude nonosteogenic cell populations from the surrounding soft tissues, has become a well-documented and highly successful procedure for augmenting the height and width of the atrophic jaw before implant placement as compared to using bone grafts alone.[3,7,18,19,20,21,22,23]
Although both resorbable and nonresorbable membranes have shown clinical effectiveness, resorbable type has become the standard of care because of better soft tissue compatibility.[9,13,18] The fundamental characteristics of barrier membranes in regenerative therapy include biocompatibility, cell occlusion properties, integration by the host tissues, clinical manageability, and space-making ability.[24] It has been demonstrated that nonprotected onlay bone grafts may undergo surface resorption whereas graft resorption can be minimized with the use of membranes.[5]
Although reproducible outcomes of GBR with high implant survival and low complication rates have been demonstrated,[19,22,25] the importance of recipient-site dimensions and its features and impact on the treatment outcomes have been less investigated.[12,26] It is prudent to evaluate recipient site in addition to surgical technique and donor site to make the best treatment decision. Therefore, the aim of the present systematic review was to assess dental literature focusing on the efficacy of various GBR procedures to increase the width or height of the alveolar bone in edentulous areas before dental implant placement based on the primary defect size.
MATERIALS AND METHODS
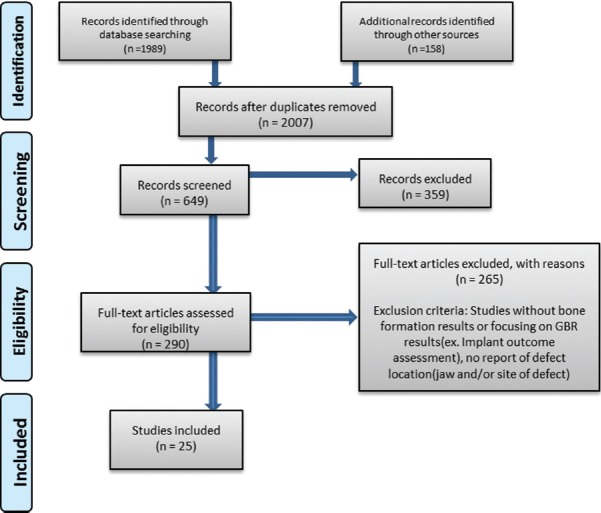
The Preferred Reporting Items for Systematic Reviews and Meta-analysis statement was used in this study[27] [Figure 1].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analysis flowchart illustrating study selection for systematic review
Inclusion criteria
Human clinical trials including case series, cohort studies, and randomized controlled trial attempting reconstruction of alveolar bone through GBR for at least 10 patients with a follow-up period of at least 6 months were included. The included studies had to report the size of the defect in one or two dimensions.
Search strategy and study selection
A search of four electronic databases namely PubMed/Medline, EMBASE, Web of Science, and Cochrane for relevant studies published in the English language from January 2000 to August 2015 was performed. The search terms used, in which mh represented the MeSH terms and tiab represented title and/or abstract, included the following: (“guided bone regeneration” [mh] OR “guided bone regeneration” [ti]) OR (“dental implantation, endosseous” [mh] OR “dental implants” [mh]) AND (“reconstruction” [tiab] OR “alveolar bone” [tiab]) AND (“treatment”[tiab] OR “therapy”[tiab] OR “therapeutics”[tiab] OR “surgery”[tiab] OR “surgical”[tiab] OR “regeneration”[tiab] OR “regenerative”[tiab] OR “guided tissue regeneration”[mh] OR “bone graft”[tiab] OR “bone graft-s”[tiab] OR “bone substitute”[tiab] OR “bone substitutes”[tiab] OR “barrier membrane”[tiab] OR “resorbable membrane”[tiab] OR “non-resorbable membrane”[tiab]). In addition, a hand search was also performed in dental- and implant-related journals from January 2000 to August 2015. Furthermore, a search in the references of included papers was conducted for publications that were not electronically identified.
Initial screening of titles and abstracts was carried out based on the inclusion and exclusion criteria. Experiments that used animal model and did not determine the size of the defect were excluded. Full texts of all eligible studies were obtained.
Two reviewers (LKH and SRM) extracted and processed data for analysis. In case of any disagreement, an agreement was obtained following a discussion. Characteristics of the included studies and summary of the regenerative outcomes of the studies were extracted and are presented in Tables 1-5.
Table 1.
Guided bone regeneration Type I studies using resorbable membrane
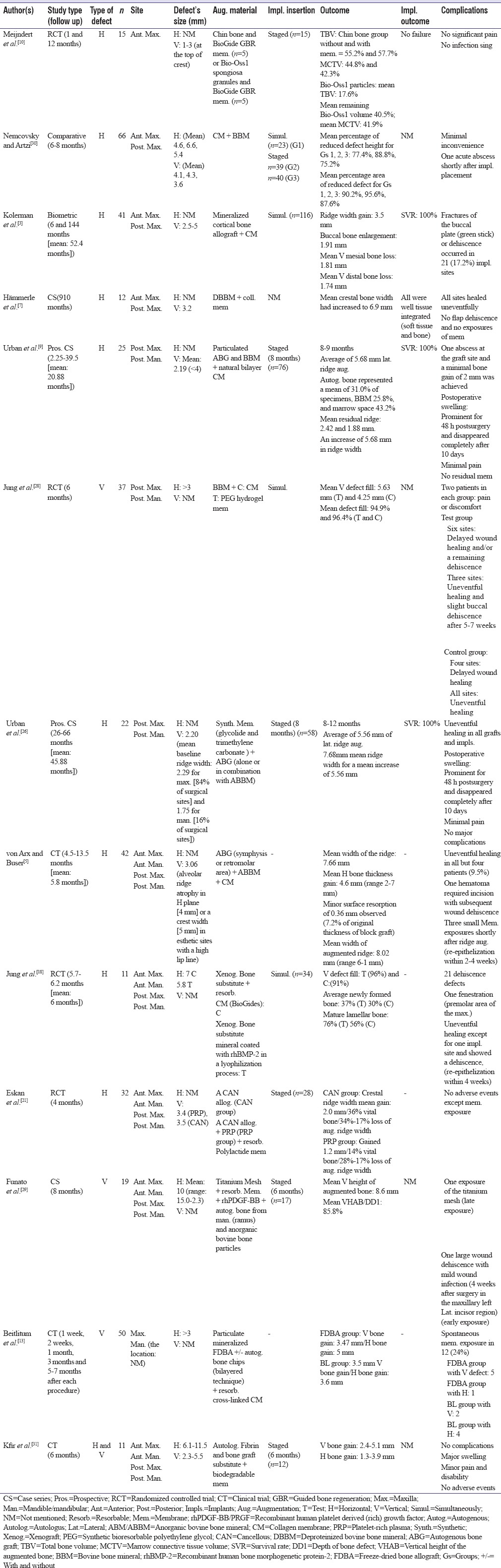
Table 5.
Guided bone regeneration Type III study
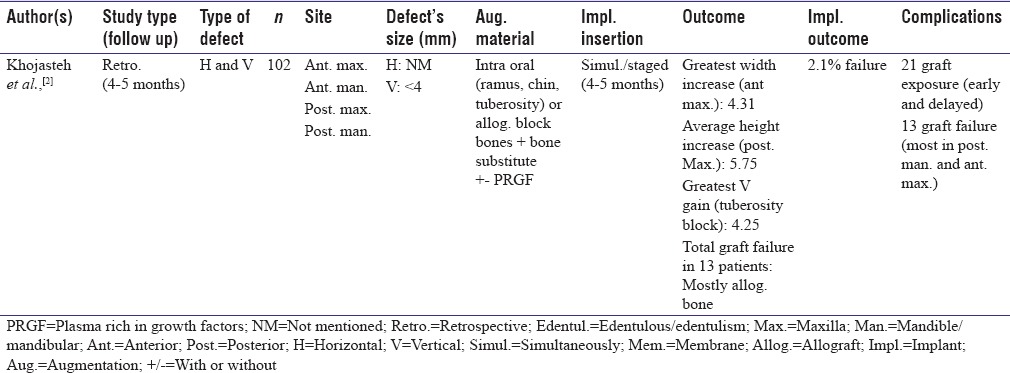
Table 2.
Guided bone regeneration Type I studies using nonresorbable membrane
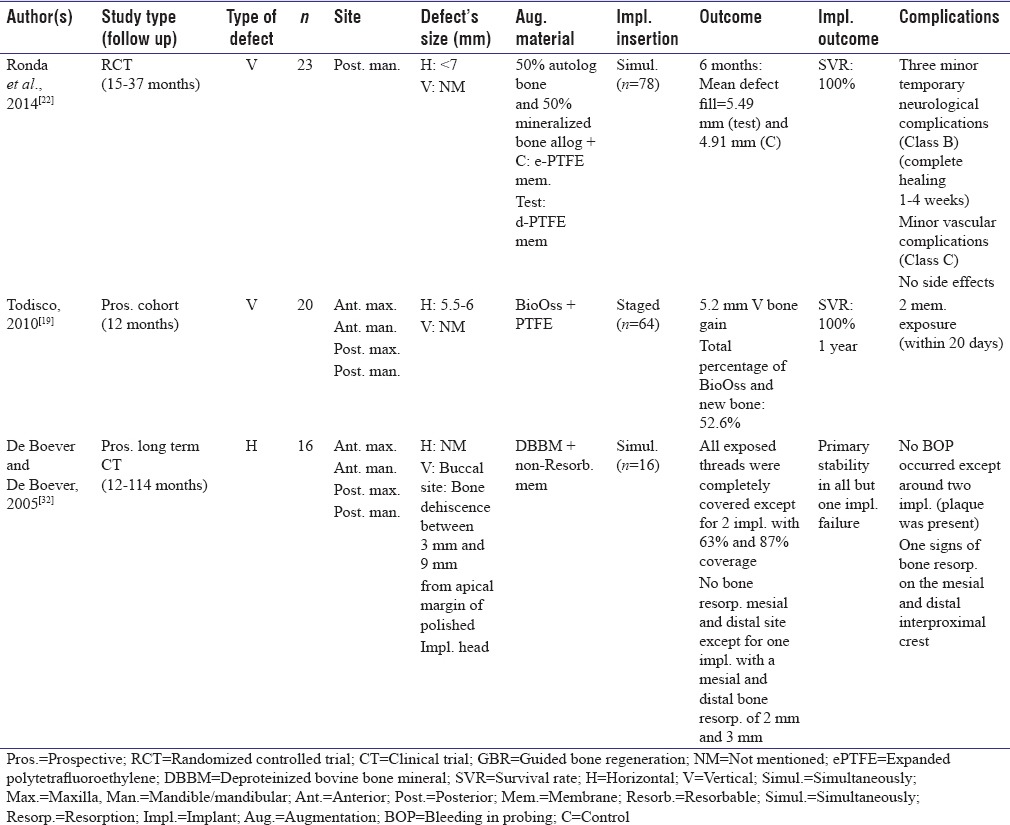
Table 3.
Guided bone regeneration, Type I comparing the usage of resorbable and nonresorbable membranes
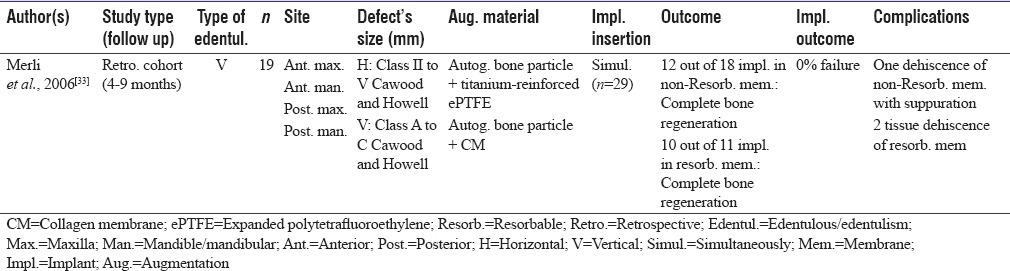
Table 4.
Guided bone regeneration Type II studies

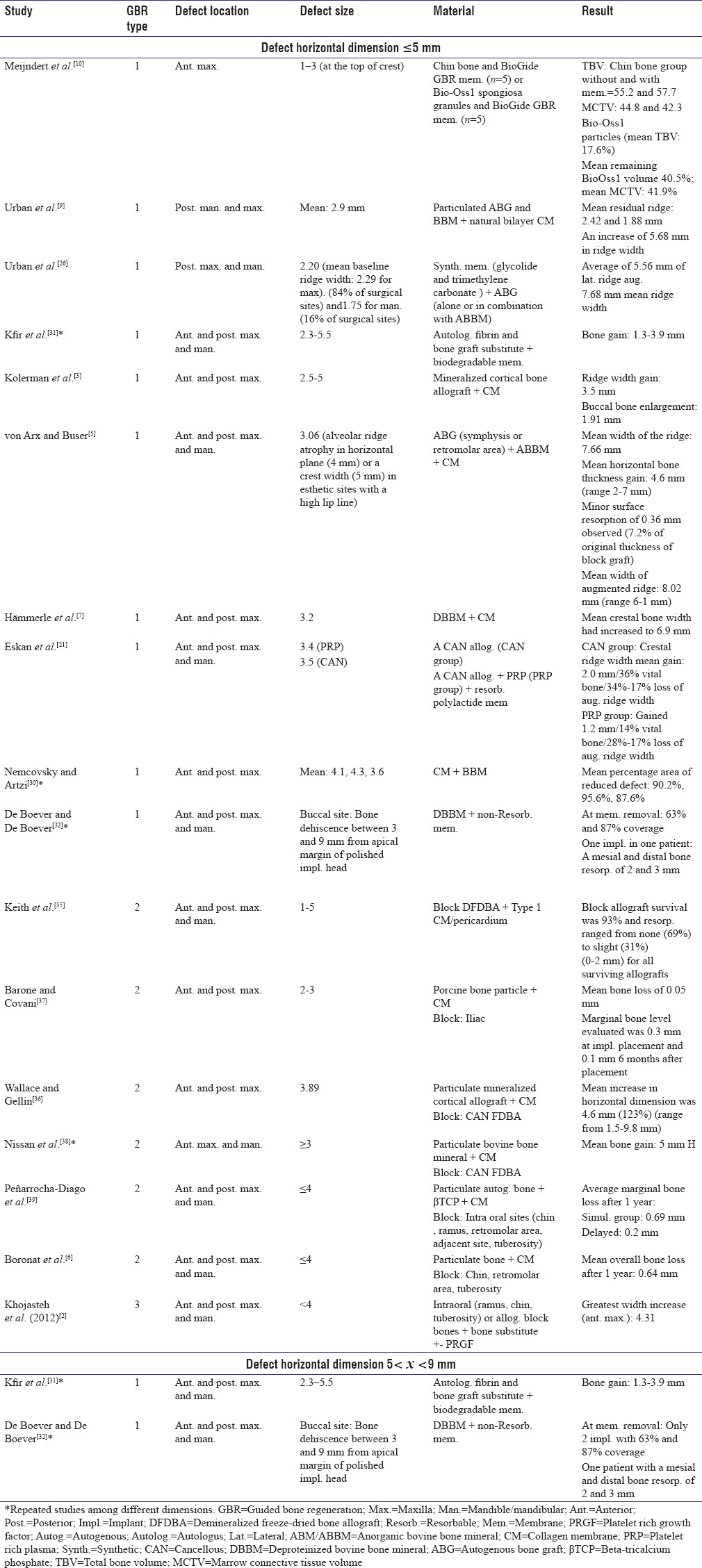
Since the focus of the present review was on defects’ features, vertical and/or horizontal dimensions of defects were extracted and reported separately [Tables 1-5]. As depicted in Tables 1-5, the defect size was reported mostly in one dimension. In addition, studies were categorized based on primary defect dimension [Tables 6 and 7]. Most vertical defects were 3–7 mm and most horizontal defects were <5 mm.
Table 6.
Vertical augmentation with guided bone regeneration techniques
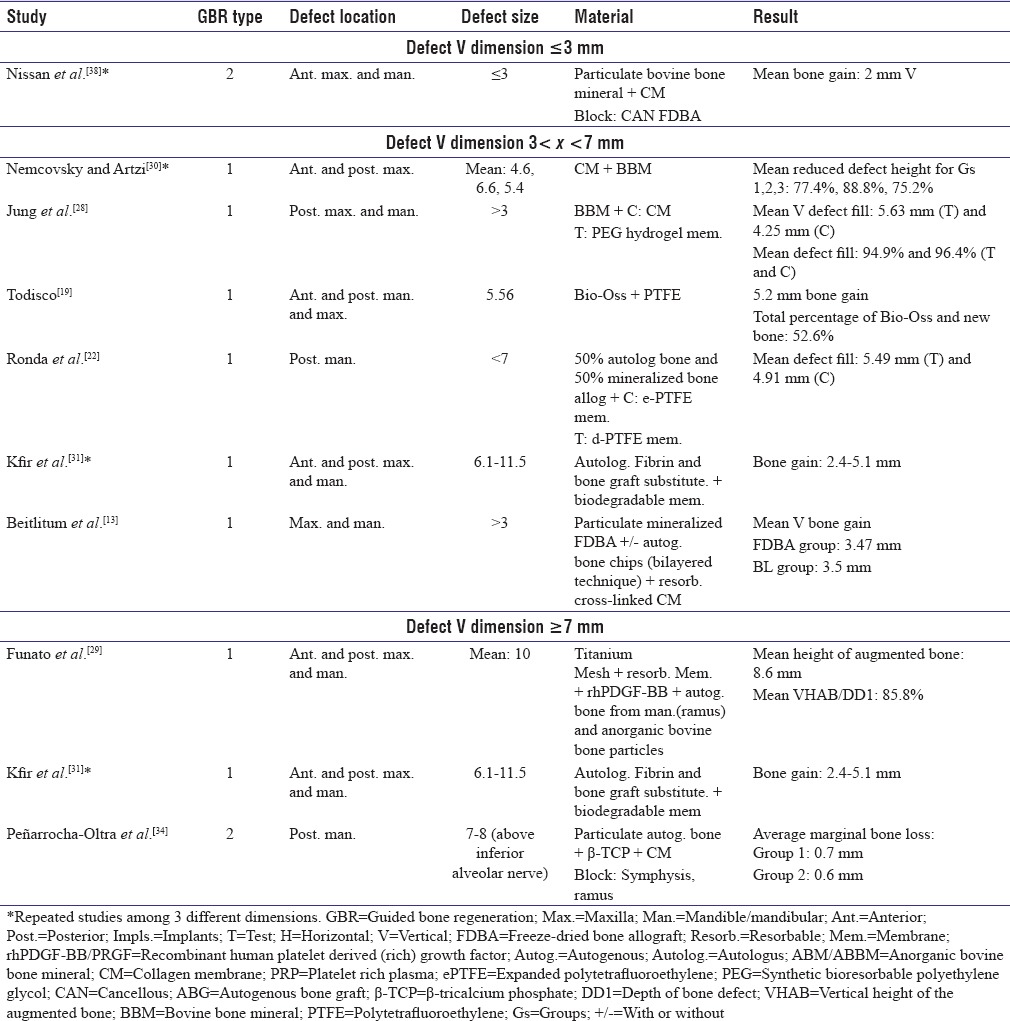
Table 7.
Horizontal augmentation with the use of guided bone regeneration
The results of bone formation reported in different ways as amount of bone gain, percentage of bone formation, or bone resorption information were extracted as well. Implant survival or success rate and the approach of implant placement (simultaneously or staged) were evaluated if applicable. Two out of 25 studies only focused on bone regeneration outcomes with no report on subsequent implant placement.[5,13]
Classification
In the current review, GBR is classified into three categories based on the used materials and techniques; Type I is the use of space maintaining membrane with particulate fillers, Type II is application of block bone graft and particulate fillers with overlying membrane, and Type III is cortical bone block tenting over a defect preserving particulate fillers.
For further evaluation of the outcomes, the data were classified based on the defect size ranges. This classification was aimed to compare the postoperative results regarding the primary defects’ features.
RESULTS
Initial search retrieved a total of 2007 citations. Following initial screening of titles and abstracts and final screening of full texts, 25 studies met the inclusion criteria and included for the final evaluation [Figure 1]. Due to wide variation of study designs regarding the size and location of defects, the results were categorized based on GBR type. Included studies were classified as GBR Type I using resorbable membrane, GBR Type I using nonresorbable membrane, GBR Type I comparing the use of resorbable and nonresorbable membrane, GBR Type II, and GBR Type III.
Among the 25 included studies, 17 studies[3,5,7,9,10,13,18,19,21,22,26,28,29,30,31,32,33] had applied GBR I, seven applied GBR II,[6,34,35,36,37,38,39] and one used GBR III.[2]
Guided bone regeneration Type I
Utilization of resorbable membrane
Thirteen studies used different types of resorbable barrier membranes. Eight selected collagen membrane;[3,5,7,9,13,18,28,30] two assessed the use of synthetic membrane (BioGide and glycolide/trimethylene carbonate).[10,26] In another experiment, resorbable polylactic membrane was the choice.[21] Two studies did not mention the exact type of the biodegradable membrane they used.[29,31] In one study, polyethylene glycol (PEG) hydrogel membrane was utilized.[28]
In 11 out of 13 studies, implants were inserted; seven by staged approach[9,10,21,26,29,30,31] and four simultaneously;[3,18,28,30] however, only six studies reported the implants’ outcomes.[3,7,9,10,18,26]
Horizontal defect dimensions were reported in eight experiments ranging from 1 to 5.5 mm.[3,5,7,9,10,18,21,30] Urban et al.[26] demonstrated 5.56 mm of new bone formation with glycolide and trimethylene carbonate after 8–12 months. Similar outcome was reported in another study by Urban et al.,[9] in which a mixture of particulate autogenous bone graft and bovine bone mineral was covered with bilayer collagen membrane resulting in 5.68 mm ridge width augmentation during 8–9 months. In another study using collagen membrane and demineralized bovine bone mineral, mean crestal bone width increased from 3.2 mm to 6.9 mm after 9–10 months.[7]
Three experiments defined preoperative defects’ height.[13,28,29] One study reported mean vertical defect fill of 5.63 mm in the group reconstructed with bovine bone mineral and collagen membrane and 4.25 mm in bovine bone mineral plus PEG hydrogel membrane group,[28] and another study reported 8.6 mm vertical gain after the usage of ramus bone, bovine bone particles, and recombinant human platelet-derived growth factor.[29] Another experiment demonstrated mean vertical bone gain of 3.47 mm in defects with initial height of >3 mm and horizontal bone gain of 5 mm for freeze-dried bone allograft (FDBA) group and 3.5 mm vertical bone gain and 3.6 mm in tented group.[13]
Utilization of nonresorbable membrane
Among total 17 studies in GBR Type 1 classification, three utilized nonresorbable membrane.[19,22,32] Two used expanded polytetrafluoroethylene (ePTFE)[19,22] and one did not mention the exact material of membrane.[32] All three were prospective clinical trials. One focused on horizontal augmentation of defects ranging from 3 to 9 mm,[32] while the other two treated vertical defects.[19,22] In one study, using ePTFE and autologous bone plus allograft resulted in 4.91 mm bone formation and dense polytetrafluoroethylene (dPTFE) plus the same bone substitutes showed 5.49 mm vertical bone gain.[22] All experiments reported implant placement; two simultaneously[22,32] and one staged[19] and one implant failure was reported in one study.[32]
Comparison of resorbable and nonresorbable membranes
One study reported preoperative defect dimensions based on Cawood and Howell classification which compared collagen membrane with ePTFE (resorbable membrane vs. nonresorbable).[33] Implants were inserted simultaneously without failure.[33]
Guided bone regeneration Type II
Initial defect's width was reported in five studies.[6,35,36,37,39] The defects’ horizontal width ranged from 1 to 5 mm. Using cancellous FDBA block bone and particulate mineralized cortical allograft plus collagen membrane led to mean horizontal bone gain of 4.6 mm (123%).[36] In all experiments reporting preoperative horizontal dimension, implants were placed; three staged,[35,36,37] one simultaneously,[6] and one used both techniques.[39] Only one study reported survival rate of implants (100%).[36]
One experiment measured both dimensions and reported 5 mm mean horizontal and 2 mm mean vertical bone augmentation.[38]
Collagen membrane was the only type of membrane used in all seven studies.[6,34,35,36,37,38,39] The block bone grafts were harvested from symphysis,[6,34,39] ramus,[34,39] demineralized FDBA (DFDBA),[35] FDBA,[36,38] iliac,[37] retromolar area,[6,39] and tuberosity.[6,39]
Guided bone regeneration Type III
One study implemented GBR III approach.[2] Khojasteh et al. reported both vertical and horizontal bone gain; 4.31 mm as the greatest horizontal augmentation and 4.25 mm as greatest vertical bone gain.[2] In their study, bone blocks were obtained from ramus, chin, and tuberosity in addition to allograft bone blocks (AlloOss).[2] They reported 2.1% of implant failure.[2]
The following classification is proposed in this study for easier comparison of outcomes reported on new bone formation:
Vertical augmentation
Comparison of guided bone regeneration I results in vertical defects <3 mm and >7 mm
Nissan et al.[38] showed 2 mm mean vertical bone gain in vertical defects of smaller than 3 mm in the anterior region of both jaws which consisted of FDBA block graft, particulate bovine bone mineral, and collagen membrane, while another study with 7–8 mm bone above inferior alveolar nerve augmented with the use of ramus and symphysis block grafts, particulate autogenous bone, and collagen membrane demonstrated average marginal bone loss of 0.7 mm using autogenous block bone graft and 0.6 mm for implant placement in native bone[34] [Table 6].
Comparison of guided bone regeneration I results in vertical defects 3< × <7 mm and >7 mm
Nemcovsky and Artzi used GBR I method with resorbable membranes and reported 75.2%– 88.8% reduced defect height in the reconstruction of vertical defects larger than 3 mm and smaller than 7 mm.[30] Another study on the same defect dimensions and by utilization of collagen membranes reported new bone formation of 3.47 mm in group treated with FDBA and 3.5 mm in group that bilayer technique was used.[13] On the other hand, 2.4–5.1 mm bone gain was reported in vertical defects larger than 7 mm using nonresorbable membrane (ePTFE) in GBR Type I technique.[31] Todisco reported 5.2 mm bone formation for 5.56 mm vertical deficiencies with the same method.[19] In addition, Ronda et al. reported mean defect fill of 5.49 mm using dPTFE and 4.91 mm with ePTFE in similar bony defect size.[22] The bone substitute in their study was a combination of autogenous and allogenic grafts[22] [Table 6].
Horizontal augmentation
Comparison of guided bone regeneration I results in horizontal defects <5 mm and 5< × <9 mm
All three GBR approaches were performed for horizontal ridge augmentation smaller than 5 mm. Utilization of resorbable membrane in GBR Type I for horizontal defects ranging from 2.5 to 5 mm led to 3.5 mm ridge width gain in either anterior or posterior parts of the maxilla.[3] von Arx and Buser showed 4.6 mm mean horizontal bone gain (ranging from 2 to 7 mm) with the same method (GBR I with resorbable membrane) using collagen membrane and autogenous bone graft for horizontal bone defects of 3.06 mm in anterior and posterior regions of both jaws.[5] In the aforementioned study, mean width of augmented ridge was 8.02 mm.[5] In addition, Kfir et al. reported bone gain of 1.3–3.9 mm in defects larger than 5 mm and smaller than 9 mm[31] [Table 7].
Comparison of guided bone regeneration II and guided bone regeneration III results in horizontal defects <5 mm
Barone and Covani[37] used iliac block graft and porcine bone particles in the anterior and posterior maxillary defects smaller than 5 mm and showed 0.05 mm mean bone loss, while Boronat et al. chose intraoral block bone and particulate bone for similar bony defect sizes in anterior and posterior locations of jaws which resulted in 0.64 mm mean bone resorption during the 1st year.[6] 0–2 mm bone resorption was reported in horizontal defects ranged from 1 to 5 mm in the anterior and posterior maxilla and mandible with the use of DFDBA block bone, Type 1 collagen membrane, and pericardium.[35] GBR Type III in the same defect size group demonstrated 4.31 mm width increase[2] [Table 7].
DISCUSSION
Several augmentation techniques have been proposed to enhance the outcomes of atrophic jaw reconstruction; however, the recipient-site features as well as the type of bone deficiency might have an impact on the outcome of these procedures.[12] This systematic review aimed at evaluating the outcomes of different GBR modalities to identify practicable treatment protocols for various defect sizes based on the proposed classification. Due to inconsistency in methodologies and considerable heterogeneity among the included studies, for example, reporting the outcomes by different variables, conducting a meta-analysis deemed impossible.
Previously, morphologic classifications for homogenizing the future study designs on different types of defects have been carried out for peri-implant defects,[40] extraction socket defects,[41] posterior maxillary defects with sinus pneumatization,[42] and vertical alveolar defects.[43] Tinti et al. proposed a classification of defects related to immediate or staged dental implant placement which was only based on the amount of deficiency, nonetheless; complicated defects with combined deficiencies could not be evaluated by that classification.[44]
The anatomic site of the defect might influence the outcome of bone regeneration. Anterior and posterior segments of the mandible and maxilla demonstrate different bone qualities;[11,12,45] therefore, it might be prudent to select the donor site close to recipient site if applicable. It is noteworthy that measuring the preoperative defect size and also the amount of augmentation immediately after bone grafting is necessary since the area, size, and contour of the bone regeneration and bone resorption are dictated by the size and shape of the undeveloped alveolar ridge.[2,25] It has been shown that the width at the base of the defect facilitates space provision and influences bone regeneration through GBR.[46] Evidently, in small defects, the need for augmentation and therefore the expected gain are slightly smaller than in larger defects.[13] The augmentation of large defects appears to be more challenging and more technique sensitive that is mainly done with incorporation of block bone grafts.[12]
The cancellous block graft can be modified to comply with the height and width of new generated bone while contour and size of cortical bone grafts are difficult to control for their inherent shape.[25] Cortical bone block is not able to maintain long-term 3D stability since they have resorption rate up to 60% at the time of implant placement; however, cancellous block graft showed up to 10% of resorption.[25] On the other hand, there are significant differences in the healing process of autogenous cortical versus autogenous cancellous grafts also in their mechanical strength.[47] In contrast to cortical bone, cancellous bone revascularizes faster and is strengthened by creeping substitution.[47] In addition, cancellous grafts are strengthened during the repair process, whereas cortical grafts are weakened.[47]
As mentioned previously, bone augmentation with block bone graft is generally associated with some subsequent bone resorption.[38] To prevent bone resorption during healing period, membranes are useful; however, the resorption still occurs to some extent.[2,38,39] A marked resorption of 17% was reported for onlay block bone grafts used for vertical alveolar ridge augmentation.[5]
Selection of appropriate barrier membrane is essential for success in GBR. Spontaneous membrane exposure has an adverse effect on newly bone formation since at the site of exposure barrier function is lost, whereas in cases with no membrane exposure significantly, more new bone formation would be expected.[13] Larger defects might lead to greater risk of membrane exposure and they may require longer barrier function time;[2,13] therefore, GBR I might only be appropriate for localized and smaller bony defects.[2] The amount of bone fill with resorbable membrane was similar to that obtained with the ePTFE and dehiscence seems to be less frequent when using resorbable membrane compared with nonresorbable ePTFE.[7,33]
It has been shown that maintaining enough space beneath the membrane is crucial for GBR.[36] Membrane collapse comprises the outcome of GBR techniques.[36] An experimental histological study in the beagle dog has shown that a nondesired biologic effect occurs when the resorption of the degradable membrane starts, provoking resorption of the newly formed bone; therefore, the nonresorbable membrane might provide better results since it stabilizes the blood clot and bone substitute on the implant surface which is important in the early phase.[36] Previous in vitro studies demonstrated that osteoblasts can generate a harder, stiffer, and more mineralized matrix on a titanium surface compared to other bioinert materials, highlighting advantages of titanium mesh in volume maintenance as well as osteocompatibility.[33] On the other hand, soft tissue problems have encouraged the development of resorbable membranes.[9]
Success rate of implants placed in ridges following GBR procedures ranges from 61.5% to 100% and survival rate ranges from 91.7% to 100%.[25] A systematic review comparing different techniques reported implant survival rate of 95.5% for GBR, 90.4% for onlay/veneer grafting, 94.7% for DO, and 83.8% for combinations of onlay, veneer, and interpositional inlay grafting.[25] In the present study, we have reported 95%– 100% survival rate and 91.1%– 95.9% success rate of implant placement among all GBR approaches.
Some controversies still exist regarding simultaneous implant placement with GBR.[25] Simultaneous implant insertion with block grafts offers the advantages of shortened treatment time and a reduction in the required number of surgical interventions.[36] The most important issues to be addressed are primary stability and optimal positioning of the implant.[4,36] If insufficient bone remains to provide primary stability and proper implant positioning, delayed implant placement is more appropriate.[38] Although one-stage surgery reduces the surgical interventions and healing time, better results with two-staged approach have been reported compared to one-stage approach which have been associated with the revascularization process of the block grafts allowing a good integration to the recipient site.[25] Since cancellous bone grafts revascularize much more quickly while cortical bone is much stronger, combination of both promotes early vascularization and maximum graft maintenance.[25]
It is not reasonable to compare studies in which horizontal GBR has been performed with those presenting outcomes of vertical GBR. With the current systematic review, proposal of different GBR treatment options based on the defect size would mostly retort clinical outcomes than evidences. The classification was not concentrated on suggesting definite GBR treatments based on the variations in defect size. Rather, it was designed to refine the focus of prospective experiments on designing the most appropriate study to compare the results and to improve the standard of care for patients.
CONCLUSIONS
This review of literature demonstrated that information regarding the characteristics of the initial dimension of defects is not incorporated in most of the studies. There is a large body of evidence demonstrating the successful use of GBR to regenerate resorbed bone at implant sites. The lack of accord with regard to determining the most efficacious procedure might rise from uneven methodology of studies. The proposed classification considers the different outcomes of vertical and horizontal bone augmentation using GBR approaches. The limited number of comparative studies does not provide sufficient evidence to select the most appropriate procedure; however, attention to this classification in the future experiments might eliminate the effect of recipient site's morphology on the accomplished results. The presented classification of bone defects is meant as a basis on which clinicians make the most appropriate decision regarding the choice of the best method.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shabestari GO, Shayesteh YS, Khojasteh A, Alikhasi M, Moslemi N, Aminian A, et al. Implant placement in patients with oral bisphosphonate therapy: A case series. Clin Implant Dent Relat Res. 2010;12:175–80. doi: 10.1111/j.1708-8208.2009.00150.x. [DOI] [PubMed] [Google Scholar]
- 2.Khojasteh A, Behnia H, Shayesteh YS, Morad G, Alikhasi M. Localized bone augmentation with cortical bone blocks tented over different particulate bone substitutes: A retrospective study. Int J Oral Maxillofac Implants. 2012;27:1481–93. [PubMed] [Google Scholar]
- 3.Kolerman R, Nissan J, Tal H. Combined osteotome-induced ridge expansion and guided bone regeneration simultaneous with implant placement: A biometric study. Clin Implant Dent Relat Res. 2014;16:691–704. doi: 10.1111/cid.12041. [DOI] [PubMed] [Google Scholar]
- 4.Shayesteh YS, Khojasteh A, Siadat H, Monzavi A, Bassir SH, Hossaini M, et al. A comparative study of crestal bone loss and implant stability between osteotome and conventional implant insertion techniques: A randomized controlled clinical trial study. Clin Implant Dent Relat Res. 2013;15:350–7. doi: 10.1111/j.1708-8208.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- 5.von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin Oral Implants Res. 2006;17:359–66. doi: 10.1111/j.1600-0501.2005.01234.x. [DOI] [PubMed] [Google Scholar]
- 6.Boronat A, Carrillo C, Penarrocha M, Pennarocha M. Dental implants placed simultaneously with bone grafts in horizontal defects: A clinical retrospective study with 37 patients. Int J Oral Maxillofac Implants. 2010;25:189–96. [PubMed] [Google Scholar]
- 7.Hämmerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: A report of twelve consecutive cases. Clin Oral Implants Res. 2008;19:19–25. doi: 10.1111/j.1600-0501.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 8.Morad G, Khojasteh A. Cortical tenting technique versus onlay layered technique for vertical augmentation of atrophic posterior mandibles: A split-mouth pilot study. Implant Dent. 2013;22:566–71. doi: 10.1097/01.id.0000433590.33926.af. [DOI] [PubMed] [Google Scholar]
- 9.Urban IA, Nagursky H, Lozada JL, Nagy K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int J Periodontics Restorative Dent. 2013;33:299–307. doi: 10.11607/prd.1407. [DOI] [PubMed] [Google Scholar]
- 10.Meijndert L, Raghoebar GM, Schüpbach P, Meijer HJ, Vissink A. Bone quality at the implant site after reconstruction of a local defect of the maxillary anterior ridge with chin bone or deproteinised cancellous bovine bone. Int J Oral Maxillofac Surg. 2005;34:877–84. doi: 10.1016/j.ijom.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Hassani A, Khojasteh A, Shamsabad AN. The anterior palate as a donor site in maxillofacial bone grafting: A quantitative anatomic study. J Oral Maxillofac Surg. 2005;63:1196–200. doi: 10.1016/j.joms.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Khojasteh A, Morad G, Behnia H. Clinical importance of recipient site characteristics for vertical ridge augmentation: A systematic review of literature and proposal of a classification. J Oral Implantol. 2013;39:386–98. doi: 10.1563/AAID-JOI-D-11-00210. [DOI] [PubMed] [Google Scholar]
- 13.Beitlitum I, Artzi Z, Nemcovsky CE. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin Oral Implants Res. 2010;21:1242–50. doi: 10.1111/j.1600-0501.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- 14.Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997;8:117–24. doi: 10.1034/j.1600-0501.1997.080206.x. [DOI] [PubMed] [Google Scholar]
- 15.Merkx MA, Maltha JC, Freihofer HP, Kuijpers-Jagtman AM. Incorporation of particulated bone implants in the facial skeleton. Biomaterials. 1999;20:2029–35. doi: 10.1016/s0142-9612(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki W, Buchman SR. Volume maintenance of onlay bone grafts in the craniofacial skeleton: Micro-architecture versus embryologic origin. Plast Reconstr Surg. 1998;102:291–9. doi: 10.1097/00006534-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Widmark G, Andersson B, Ivanoff CJ. Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int J Oral Maxillofac Surg. 1997;26:106–9. doi: 10.1016/s0901-5027(05)80827-6. [DOI] [PubMed] [Google Scholar]
- 18.Jung RE, Glauser R, Schärer P, Hämmerle CH, Sailer HF, Weber FE, et al. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14:556–68. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 19.Todisco M. Early loading of implants in vertically augmented bone with non-resorbable membranes and deproteinised anorganic bovine bone. An uncontrolled prospective cohort study. Eur J Oral Implantol. 2010;3:47–58. [PubMed] [Google Scholar]
- 20.Khojasteh A, Hassani A, Motamedian SR, Saadat S, Alikhasi M. Cortical Bone Augmentation Versus Nerve Lateralization for Treatment of Atrophic Posterior Mandible: A Retrospective Study and Review of Literature. Clin Implant Dent Relat Res. 2016;18:342–59. doi: 10.1111/cid.12317. [DOI] [PubMed] [Google Scholar]
- 21.Eskan MA, Greenwell H, Hill M, Morton D, Vidal R, Shumway B, et al. Platelet-rich plasma-assisted guided bone regeneration for ridge augmentation: A randomized, controlled clinical trial. J Periodontol. 2014;85:661–8. doi: 10.1902/jop.2013.130260. [DOI] [PubMed] [Google Scholar]
- 22.Ronda M, Rebaudi A, Torelli L, Stacchi C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:859–66. doi: 10.1111/clr.12157. [DOI] [PubMed] [Google Scholar]
- 23.Khojasteh A, Motamedian SR, Sharifzadeh N, Zadeh HH. The influence of initial alveolar ridge defect morphology on the outcome of implants in augmented atrophic posterior mandible: an exploratory retrospective study? Clin Oral Implants Res. 2016 doi: 10.1111/clr.12991. [Article in press] doi: 10.1111/clr.12991. [DOI] [PubMed] [Google Scholar]
- 24.Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration – Animal and human studies. Periodontol. 2000;1993;1:26–35. [PubMed] [Google Scholar]
- 25.Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(Suppl):49–70. [PubMed] [Google Scholar]
- 26.Urban IA, Nagursky H, Lozada JL. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: A prospective case series in 22 patients. Int J Oral Maxillofac Implants. 2011;26:404–14. [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009;3:123–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Jung RE, Hälg GA, Thoma DS, Hämmerle CH. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin Oral Implants Res. 2009;20:162–8. doi: 10.1111/j.1600-0501.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- 29.Funato A, Ishikawa T, Kitajima H, Yamada M, Moroi H. A novel combined surgical approach to vertical alveolar ridge augmentation with titanium mesh, resorbable membrane, and rhPDGF-BB: A retrospective consecutive case series. Int J Periodontics Restorative Dent. 2013;33:437–45. doi: 10.11607/prd.1460. [DOI] [PubMed] [Google Scholar]
- 30.Nemcovsky CE, Artzi Z. Comparative study of buccal dehiscence defects in immediate, delayed, and late maxillary implant placement with collagen membranes: Clinical healing between placement and second-stage surgery. J Periodontol. 2002;73:754–61. doi: 10.1902/jop.2002.73.7.754. [DOI] [PubMed] [Google Scholar]
- 31.Kfir E, Kfir V, Eliav E, Kaluski E. Minimally invasive guided bone regeneration. J Oral Implantol. 2007;33:205–10. doi: 10.1563/1548-1336(2007)33[205:MIGBR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.De Boever AL, De Boever JA. Guided bone regeneration around non-submerged implants in narrow alveolar ridges: A prospective long-term clinical study. Clin Oral Implants Res. 2005;16:549–56. doi: 10.1111/j.1600-0501.2005.01154.x. [DOI] [PubMed] [Google Scholar]
- 33.Merli M, Migani M, Bernardelli F, Esposito M. Vertical bone augmentation with dental implant placement: Efficacy and complications associated with 2 different techniques. A retrospective cohort study. Int J Oral Maxillofac Implants. 2006;21:600–6. [PubMed] [Google Scholar]
- 34.Peñarrocha-Oltra D, Aloy-Prósper A, Cervera-Ballester J, Peñarrocha-Diago M, Canullo L, Peñarrocha-Diago M, et al. Implant treatment in atrophic posterior mandibles: Vertical regeneration with block bone grafts versus implants with 5.5-mm intrabony length. Int J Oral Maxillofac Implants. 2014;29:659–66. doi: 10.11607/jomi.3262. [DOI] [PubMed] [Google Scholar]
- 35.Keith JD, Jr, Petrungaro P, Leonetti JA, Elwell CW, Zeren KJ, Caputo C, et al. Clinical and histologic evaluation of a mineralized block allograft: Results from the developmental period (2001-2004) Int J Periodontics Restorative Dent. 2006;26:321–7. [PubMed] [Google Scholar]
- 36.Wallace S, Gellin R. Clinical evaluation of freeze-dried cancellous block allografts for ridge augmentation and implant placement in the maxilla. Implant Dent. 2010;19:272–9. doi: 10.1097/ID.0b013e3181e5d2a1. [DOI] [PubMed] [Google Scholar]
- 37.Barone A, Covani U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: Clinical results. J Oral Maxillofac Surg. 2007;65:2039–46. doi: 10.1016/j.joms.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 38.Nissan J, Mardinger O, Strauss M, Peleg M, Sacco R, Chaushu G, et al. Implant-supported restoration of congenitally missing teeth using cancellous bone block-allografts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:286–91. doi: 10.1016/j.tripleo.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Peñarrocha-Diago M, Aloy-Prósper A, Peñarrocha-Oltra D, Guirado JL, Peñarrocha-Diago M. Localized lateral alveolar ridge augmentation with block bone grafts: Simultaneous versus delayed implant placement: A clinical and radiographic retrospective study. Int J Oral Maxillofac Implants. 2013;28:846–53. doi: 10.11607/jomi.2964. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Bogaerde L. A proposal for the classification of bony defects adjacent to dental implants. Int J Periodontics Restorative Dent. 2004;24:264–71. [PubMed] [Google Scholar]
- 41.Caplanis N, Lozada JL, Kan JY. Extraction defect assessment, classification, and management. J Calif Dent Assoc. 2005;33:853–63. [PubMed] [Google Scholar]
- 42.Wang HL, Katranji A. ABC sinus augmentation classification. Int J Periodontics Restorative Dent. 2008;28:383–9. [PubMed] [Google Scholar]
- 43.Khojasteh A, Eslaminejad MB, Nazarian H, Morad G, Dashti SG, Behnia H, et al. Vertical bone augmentation with simultaneous implant placement using particulate mineralized bone and mesenchymal stem cells: A preliminary study in rabbit. J Oral Implantol. 2013;39:3–13. doi: 10.1563/AAID-JOI-D-10-00206. [DOI] [PubMed] [Google Scholar]
- 44.Tinti C, Parma-Benfenati S, Polizzi G. Vertical ridge augmentation: What is the limit? Int J Periodontics Restorative Dent. 1996;16:220–9. [PubMed] [Google Scholar]
- 45.Sakka S, Coulthard P. Bone quality: A reality for the process of osseointegration. Implant Dent. 2009;18:480–5. doi: 10.1097/ID.0b013e3181bb840d. [DOI] [PubMed] [Google Scholar]
- 46.Polimeni G, Koo KT, Qahash M, Xiropaidis AV, Albandar JM, Wikesjö UM, et al. Prognostic factors for alveolar regeneration: Bone formation at teeth and titanium implants. J Clin Periodontol. 2004;31:927–32. doi: 10.1111/j.1600-051X.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 47.Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy) J Clin Periodontol. 2008;35:173–202. doi: 10.1111/j.1600-051X.2008.01269.x. [DOI] [PubMed] [Google Scholar]