Abstract
Microtia is a major congenital anomaly of the external ear. It includes a spectrum of deformities from a grossly normal but small ear to the absence of the entire external ear. These deformities account for three in every 10,000 births, with bilaterally missing ears seen in fewer than 10% of all cases. Extraoral implant-retained ear prosthesis has been proven to be a predictable treatment option for rehabilitation of such congenital anomalies. This paper aims to present principles of maxillofacial implants, review of literature, advantages, disadvantages, and considerations in treatment planning and treatment phases of an implant-supported auricular prosthesis and prospective developments for ear prosthesis are also discussed. Implant supported ear reconstruction provides excellent support, good retention, and esthetically acceptable appearance to the patient.
Keywords: Ear, implant retained prosthesis, microtia
INTRODUCTION
Microtia is a major congenital anomaly of the external ear. It includes a spectrum of deformities from a grossly normal but small ear to the absence of the entire external ear. These deformities account for three in every 10,000 births, with bilaterally missing ears seen in fewer than 10% of all cases.[1] Microtia is a congenital malformation of variable severity of the external and middle ear. The microtic auricle consists of a disorganized remnant of cartilage attached to a variable amount of soft tissue lobule, which often is displaced from a position symmetrical with the opposite normal ear. The direction of displacement depends on the degree of associated facial hypoplasia. Depending on the severity of the anomaly, there may be evidence of external meatus formation. Microtia commonly involves the external canal and middle ear; hence, hearing can be affected. Microtia may present within a spectrum of branchial arch defects (hemifacial microsomia and craniofacial microsomia) or may manifest as an independent malformation. Auricular defects ranging from minor deformities to complete anotia can be due to various causes, such as congenital defects, tumor resection, inflammation of cartilage, and trauma.[2,3,4,5] The treatments of these defects are surgical reconstructions or prosthetic treatments. Although plastic surgery is capable of restoring missing tissues, it may not be the ideal choice of treatment because of the complex shape and structure of the ear. For restoration of complex organs such as ears, a prosthetic restoration is the better option to surgical restorations due to the esthetical success of them.[6] The ear prosthesis is retained by skin adhesive or the endosseous implants. There are significant disadvantages to the use of skin adhesives. The margins of the facial prosthesis may be damaged by repeated application and removal of the adhesive, and occasionally a patient will have a toxic skin reaction. The retentive capacity of adhesives may be insufficient in mobile tissues or moist environments. The presence of hair also complicates the use of skin adhesives.[7]
The use of craniofacial titanium implants for restoring auricular defects provides many benefits. The quality of retention provided far exceeds that obtained with adhesives, and skin-penetrating osseointegrated implants have demonstrated an excellent level of predictability when placed in bone in the auricular area. Extraoral implant retained prosthesis have been proven to be a predictable treatment option for maxillofacial rehabilitation.[8,9,10,11] Implant-retained auricular prosthesis provides multiple advantages for the patient convenience, security, consistent retention and positioning, elimination of the need for adhesives, and maintenance of marginal integrity and longevity.[12] We present a case of congenital microtia rehabilitated by implant retained ball and socket ear prosthesis. Advantage of ball and socket attachment for the retentive component is also described here.
CASE REPORT
A 15-year-old male patient with the right side congenital microtia was referred to our department. The patient was examined, and the presence of rudimentary cartilage was seen on right side with missing malformed ear [Figure 1]. The left side ear was normal, and there was no other congenital anomaly present except microtia. The patient was further evaluated for hearing sensation, and there was the presence of hearing loss on the right side.
Figure 1.
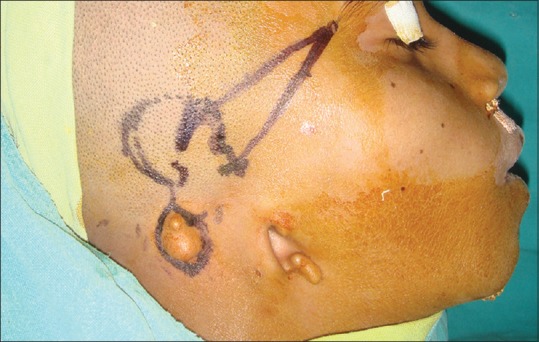
Preoperative picture of malformed ear in patient on the right side
Preliminary impressions of both the sides were taken, and the models were fabricated. Prototype of wax mimicking the left side ear was fabricated. A computed tomography (CT) scan of the middle third face in the mastoid region showed sufficient bone for the implant placement [Figure 2]. Tentative sites for implants were marked on the patient's face and transferred to diagnostic impressions. The landmarks used for selection of the implant sites were superior horizontal line through superior tarsal plate and nasion, inferior horizontal line through base of nose, anterior vertical line through outer rim of orbit and perpendicular to horizontal lines and posterior vertical line through angle of mandible and perpendicular to horizontal.
Figure 2.
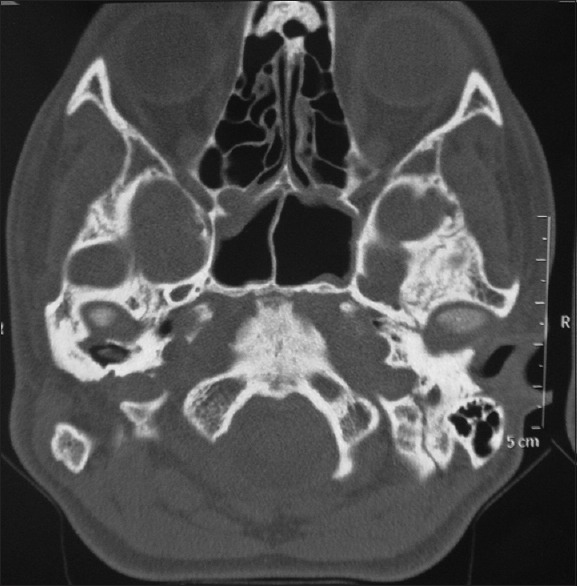
Computed tomography scan of the middle third face showing adjacent anatomy
Correlating the clinical findings, the CT diagnosis and patient expectations, it was decided to place 6-mm diameter and 6.25-mm length, internal hex implants (Adin Dental Implant System, Afula, Israel) at the 3 sites, respectively. The surgery was planned under necessary anesthesia after fitness of the patient and written informed consent. Implant sites were marked tentatively by drilling osteotomy in the prospective implant site location (transferred from the diagnostic cast at the time of diagnosis and using CT scan guide), and implants were placed in these positions [Figures 3 and 4]. Rudimentary cartilage was removed and thinning of subcutaneous tissue was done. In the second stage, uncovering of implants by removal of soft tissue and placement of secondary healing caps, 4 months after first stage surgery was carried out. Abutment placement with ball and socket attachment was done at the next stage [Figure 5]. Postoperative phase after second stage surgery was uneventful and healing was satisfactory around the abutments [Figure 6].
Figure 3.

Diagnostic impression casts used for the fabrication of prosthesis
Figure 4.
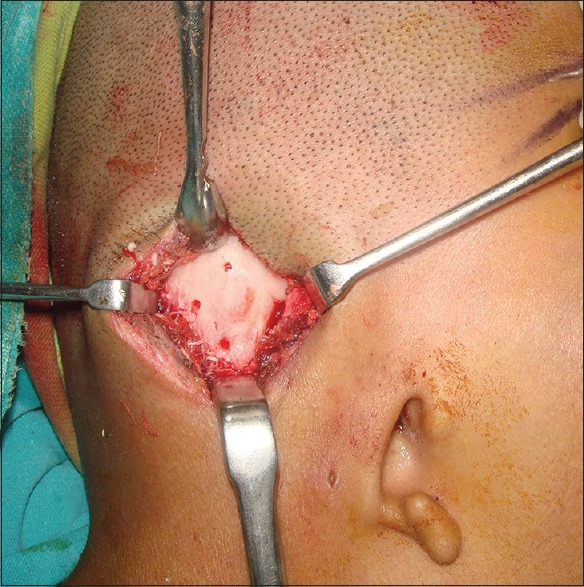
Osteotomy prepared at the prospective implant site location at first-stage surgery
Figure 5.
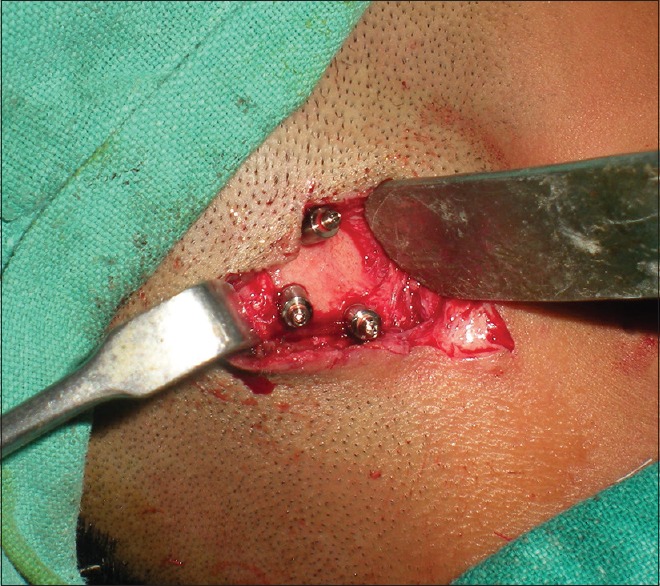
Abutment placement with ball and socket attachment at second-stage surgery
Figure 6.
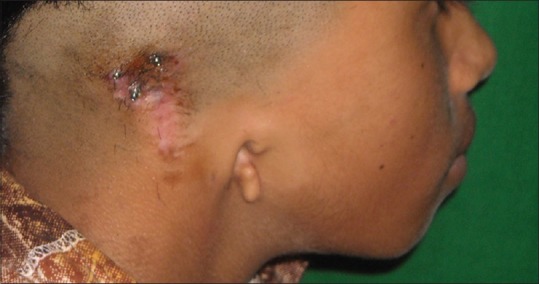
Postoperative soft-tissue healing before prosthesis placement
Fabrication of ear prosthesis using ball and socket attachment was done 3 weeks after the second stage surgery. The next step was making impression of implant positions and soft tissues together. For this, the guidelines to assist in wax-up of prosthesis were drawn on left side of face. Irreversible hydrocolloid, silicone impression material was used for making impressions.
The next prosthetic step was fabrication of an acrylic resin substructure. The acrylic resin substructure is an acrylic plate fabricated in clear resin that provides a backing for silicone and carries retentive component in its tissue surface. Its superior surface priming was done to create adhesion of acrylic with silicone. Wax up of the prosthesis was then done followed by trial on the patient. This was followed by three-part flasking of the wax up with formation of the channels for silicone escape followed by curing at room temperature overnight. Shade matching was done according to surrounding anatomic regions on patients face. Final prosthesis was retrieved and then placed on patient's face [Figure 7]. Maintenance of the peri-implant tissue was explained to the patient as it is imperative for the long-term success of prosthesis. Mechanical cleaning with interdental brush around abutments and with gauze soaked in peroxide 3% solution and saline in (1:1) ratio or soap water is advised to the patient. As it is a removable appliance, the soft-tissue cuff around the implant needs to be maintained, and for this purpose, the patient was demonstrated for the regular cleaning and maintenance of the implantation site.
Figure 7.
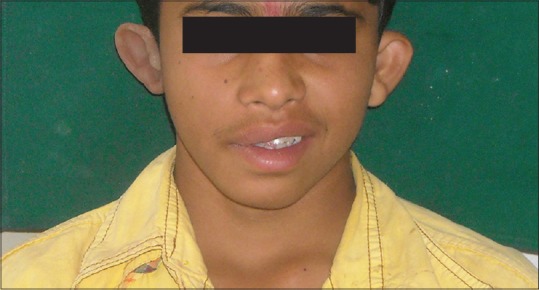
Postoperative picture of patient with ear prosthesis in place
DISCUSSION
Reconstruction of the ear, whether it be for microtia or major acquired deformities, has long been recognized as a demanding undertaking with well-recognized shortcomings, even in the hands of some very accomplished surgeons. Some of the shortcomings are inherent in the underlying tissue shortages, some are in the limitations of the donor tissues, and some are specifically operator-dependent. Even more demanding is the reoperation for a previously unsatisfactory result, as the recipient bed is now scarred, the donor tissues more limited, and the expertise of the operating surgeon even more critical.
Maxillofacial defects can prevent a patient from returning to normal daily activities. Many patients with these defects have been rehabilitated successfully with prosthetic restorations. Secondary mechanical factors (tissue undercuts), skin adhesives, and implants can provide retention. The use of craniofacial implants for retention of extraoral prosthesis offers excellent support and retentive abilities and improves a patient's appearance and quality of life.[13,14] However, a satisfactory outcome may only be achieved by careful planning in terms of the number and position and orientation of the implants and the proper connection of the auricular prosthesis to implant retention structure.[15]
It has been shown in clinical and biomechanical studies that two implants are sufficient to retain an auricular prosthesis. Osseointegrated implants placed in the mastoid temporal region are used to retain attachments for ear prosthesis. Magnet and bar-clip retention are the two primary forms of retention used in the auricular region.[16] The bar-clip system provides good retention for the prostheses. However, bars may limit access for performing hygiene procedures and make it difficult to insert and remove the prosthesis. Magnetic retention can be selected because of hygiene, mechanical, and esthetic considerations. Individual magnets provide ease for cleansing.[17]
Implant survival rate in mastoid region of 100%– 95.7% has been reported, which has been attributed to dense cortical bone in mastoid region and vasculature, which ensures maintenance of a bone implant interface adequate to support functional loads. However, meticulous planning and postoperative care can prolong the life and success of prosthesis.[5]
In this reported case, osseointegrated implants were placed in the mastoid temporal region and were used to retain attachments for ear prosthesis. A ball and socket retained acrylic resin-based keeper, to which silicone ear prosthesis was attached. The keeper provided vertical support for the prosthesis and facilitated orientation for the prosthesis insertion. Stability and retention were provided without the use of adhesives. Thus, in this case, quality of retention provided far exceeds that obtained with adhesives, and skin-penetrating osseointegrated implants have demonstrated an excellent level of predictability when placed in bone in the auricular area.
Advantages of implants include a short and straightforward surgery, which can be performed either as a two-stage or a single-stage procedure under local anesthesia or general anesthesia, esthetics being much similar to normal, a remake is possible, implants enables early detection of recurrence in cancer patients.[18] Disadvantages of the implant-supported prosthesis are inability to maintain peri-implant hygiene leads to peri-implantitis and other soft-tissue changes, which may result in misfit of the prosthesis if ignored or even failure of implants.[18]
There are various methods for manufacturing an auricular prosthesis. These are (a) hand sculpturing method, (b) impression of a similar ear and wax modeling with this impression, and (c) rapid prototyping techniques.[19,20] Conventional prosthetic treatment is done by taking the impression with irreversible hydrocolloid or silicone, and afterward, forming the model using hard dental stone. Wax model is prepared on this cast, and the final prosthesis is processed with silicone material. This method may discomfort the patient, and underlying tissues may distort and cause difference between the cast and the patient's tissues. Besides, the conventional method needs more experience and more time for the laboratory work.[21,22] Although there are various rapid prototyping techniques, expensiveness of each promotes the investigators to find cheaper, and that has wider field of usage. Besides all, this technology is insufficient for the production of maxillofacial prosthesis. Because taking the output of prosthesis directly from the silicone material is not yet possible and coloring is still a problem for clinicians.[23,24,25]
In this case, we used conventional method of fabrication of ear implant using ball and socket preventative attachments. The use of ball and socket was more convenient for us to take the impressions and maintenance of peri-implant area is easier than bar and clip type of prosthesis.
Differences in the balance of shape, size, and position of body organs are immediately perceived as “looking wrong,” and this perception can subject the individual to significant peer ridicule and social ostracism, often expressing as intense shame and anguish in the attitude of the afflicted. The onus of the deed lies in the hands of a team that combines artistic excellence with surgical expertise, by combining the skills of anaplastologists, surgeons, and prosthodontists.[18]
In this case, the rehabilitation of the patient for microtia made a remarkable positive impact on the life of the patient and improved his self-esteem and confidence in the society.
CONCLUSION
Placement of endosseous implants in the temporal bone may overcome the apparent disadvantages of skin adhesives and skin pockets for the fixation of auricular prostheses. From the results of this study, it is obvious that osseointegrated implants have great advantages compared with skin adhesives and skin pockets to rehabilitate patients suffering from auricular defects. The major achievement of implant-supported auricular prostheses is the patient's increased comfort and confidence wearing these types of prostheses
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kumar PS, Satheesh Kumar KS, Savadi RC. Bilateral implant-retained auricular prosthesis for a patient with congenitally missing ears. A clinical report. J Prosthodont. 2012;21:322–7. doi: 10.1111/j.1532-849X.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RD, Roumanas E, Sugai T, Moy PK. Auricular prostheses and osseointegrated implants: UCLA experience. J Prosthet Dent. 1995;73:553–8. doi: 10.1016/s0022-3913(05)80115-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang RR, Andres CJ. Hemifacial microsomia and treatment options for auricular replacement: A review of the literature. J Prosthet Dent. 1999;82:197–204. doi: 10.1016/s0022-3913(99)70156-3. [DOI] [PubMed] [Google Scholar]
- 4.Visser A, Raghoebar GM, van Oort RP, Vissink A. Fate of implant-retained craniofacial prostheses: Life span and aftercare. Int J Oral Maxillofac Implants. 2008;23:89–98. [PubMed] [Google Scholar]
- 5.Aydin C, Karakoca S, Yilmaz H, Yilmaz C. Implant-retained auricular prostheses: An assessment of implant success and prosthetic complications. Int J Prosthodont. 2008;21:241–4. [PubMed] [Google Scholar]
- 6.Beumer J, Curtis TA, Marunick MT. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis: Medico Dental Media International; 1996. [Google Scholar]
- 7.Sharma A, Rahul GR, Poduval ST, Shetty K. Implant-supported auricular prosthesis – An overview? J Oral Implantol. 2012;1:58. doi: 10.1563/AAID-JOI-D-12-00058.1. doi: 10.1563/AAID-JOI-D-12-0001. [DOI] [PubMed] [Google Scholar]
- 8.Wolfaardt JF, Wilkes GH, Parel SM, Tjellström A. Craniofacial osseointegration: The Canadian experience. Int J Oral Maxillofac Implants. 1993;8:197–204. [PubMed] [Google Scholar]
- 9.Tolman DE, Desjardins RP. Extraoral application of osseointegrated implants. J Oral Maxillofac Surg. 1991;49:33–45. doi: 10.1016/0278-2391(91)90264-m. [DOI] [PubMed] [Google Scholar]
- 10.Parel SM, Tjellström A. The United States and Swedish experience with osseointegration and facial prostheses. Int J Oral Maxillofac Implants. 1991;6:75–9. [PubMed] [Google Scholar]
- 11.Cheng AC, Morrison D, Cho RS, Archibald D. Vacuum-formed matrix as a guide for the fabrication of craniofacial implant tissue bar-retained auricular prostheses. J Prosthet Dent. 1998;79:711–4. doi: 10.1016/s0022-3913(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 12.Ozturk AN, Usumez A, Tosun Z. Implant-retained auricular prosthesis: A case report. Eur J Dent. 2010;4:71–4. [PMC free article] [PubMed] [Google Scholar]
- 13.McCartney JW. Osseointegrated implant-supported and magnetically retained ear prosthesis: A clinical report. J Prosthet Dent. 1991;66:6–9. doi: 10.1016/0022-3913(91)90342-t. [DOI] [PubMed] [Google Scholar]
- 14.Chung RW, Siu AS, Chu FC, Chow TW. Magnet-retained auricular prosthesis with an implant-supported composite bar: A clinical report. J Prosthet Dent. 2003;89:446–9. doi: 10.1016/s0022-3913(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 15.Gilson TD, Asgar K, Peyton FA. The quality of union formed in casting gold to embedded attachment metals. J Prosthet Dent. 1965;15:464–73. doi: 10.1016/s0022-3913(65)80014-2. [DOI] [PubMed] [Google Scholar]
- 16.Del Valle V, Faulkner G, Wolfaardt J, Rangert B, Tan HK. Mechanical evaluation of craniofacial osseointegration retention systems. Int J Oral Maxillofac Implants. 1995;10:491–8. [PubMed] [Google Scholar]
- 17.Karakoca S, Aydin C, Yilmaz H, Bal BT. Survival rates and periimplant soft tissue evaluation of extraoral implants over a mean follow-up period of three years. J Prosthet Dent. 2008;100:458–64. doi: 10.1016/S0022-3913(08)60265-6. [DOI] [PubMed] [Google Scholar]
- 18.Nanda A, Jain V, Kumar R, Kabra K. Implant-supported auricular prosthesis. Indian J Dent Res. 2011;22:152–6. doi: 10.4103/0970-9290.79983. [DOI] [PubMed] [Google Scholar]
- 19.Lemon JC, Chambers MS, Wesley PJ, Martin JW. Technique for fabricating a mirror-image prosthetic ear. J Prosthet Dent. 1996;75:292–3. doi: 10.1016/s0022-3913(96)90487-4. [DOI] [PubMed] [Google Scholar]
- 20.Penkner K, Santler G, Mayer W, Pierer G, Lorenzoni M. Fabricating auricular prostheses using three-dimensional soft tissue models. J Prosthet Dent. 1999;82:482–4. doi: 10.1016/s0022-3913(99)70038-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen LH, Tsutsumi S, Iizuka T. A CAD/CAM technique for fabricating facial prostheses: A preliminary report. Int J Prosthodont. 1997;10:467–72. [PubMed] [Google Scholar]
- 22.Bibb R, Freeman P, Brown R, Sugar A, Evans P, Bocca A, et al. An investigation of three-dimensional scanning of human body surfaces and its use in the design and manufacture of prostheses. Proc Inst Mech Eng H. 2000;214:589–94. doi: 10.1243/0954411001535615. [DOI] [PubMed] [Google Scholar]
- 23.Karatas MO, Cifter ED, Ozenen DO, Balik A, Tuncer EB. Manufacturing implant supported auricular prostheses by rapid prototyping techniques. Eur J Dent. 2011;5:472–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Goh JC, Ho NC, Bose K. Principles and applications of computer-aided design and computer-aided manufacturing (CAD/CAM) technology in orthopaedics. Ann Acad Med Singapore. 1990;19:706–13. [PubMed] [Google Scholar]
- 25.Jiao T, Zhang F, Huang X, Wang C. Design and fabrication of auricular prostheses by CAD/CAM system. Int J Prosthodont. 2004;17:460–3. [PubMed] [Google Scholar]


