Abstract
Objective
This double-blind, placebo-controlled phase 3 study was designed to compare efficacy and safety of abiraterone acetate + prednisone (abiraterone) to prednisone alone in chemotherapy-naïve, asymptomatic or mildly symptomatic metastatic castration-resistant prostate cancer (mCRPC) patients from China, Malaysia, Thailand and Russia.
Methods
Adult chemotherapy-naïve patients with confirmed prostate adenocarcinoma, Eastern Cooperative Oncology Group (ECOG) performance status (PS) grade 0–1, ongoing androgen deprivation (serum testosterone <50 ng/dL) with prostate specific antigen (PSA) or radiographic progression were randomized to receive abiraterone acetate (1000 mg, QD) + prednisone (5 mg, BID) or placebo + prednisone (5 mg, BID), until disease progression, unacceptable toxicity or consent withdrawal. Primary endpoint was improvements in time to PSA progression (TTPP).
Results
Totally, 313 patients were randomized (abiraterone: n = 157; prednisone: n = 156); and baseline characteristics were balanced. At clinical cut-off (median follow-up time: 3.9 months), 80% patients received treatment (abiraterone: n = 138, prednisone: n = 112). Median time to PSA progression was not reached with abiraterone versus 3.8 months for prednisone, attaining 58% reduction in PSA progression risk (HR = 0.418; p < 0.0001). Abiraterone-treated patients had higher confirmed PSA response rate (50% vs. 21%; relative odds = 2.4; p < 0.0001) and were 5 times more likely to achieve radiographic response than prednisone-treated patients (22.9% vs. 4.8%, p = 0.0369). Median survival was not reached. Most common (≥10% abiraterone vs. prednisone-treated) adverse events: bone pain (7% vs. 14%), pain in extremity (6% vs. 12%), arthralgia (10% vs. 8%), back pain (7% vs. 11%), and hypertension (15% vs. 14%).
Conclusion
Interim analysis confirmed favorable benefit-to-risk ratio of abiraterone in chemotherapy-naïve men with mCRPC, consistent with global study, thus supporting use of abiraterone in this patient population.
Keywords: Abiraterone, Chemotherapy-naïve, Metastatic castration-resistant prostate cancer, Prostate specific antigen, Prednisone
1. Introduction
Burden of prostate cancer is increasing worldwide and becoming the fourth most common malignancy and fifth leading cause of cancer deaths in men. The incidence of prostate cancer varies widely between different geographical areas due to differences in ethnic origin and genetic polymorphisms, incidence being highest in North America (97.2/100,000) and Northern Europe (85/100,000) and low in southeastern Asia (11.2/100,000) and eastern Asia (10.5/100,000) [1], [2]. Incidence of prostate cancer in China (47,000 cases, 2.1%) is estimated to be much lower than that in the United States (233,000 cases, 25.0%) [3]. Most Asian patients have symptomatic prostate cancer for which surgical or medical castration is the standard treatment and nearly two thirds experience biological recurrence (median follow-up: 16.8 months) [4], leading to castration-resistant prostate cancer (CRPC) and eventually to metastatic castration-resistant prostate cancer (mCRPC) [5].
Abiraterone acetate (Zytiga®), a prodrug of abiraterone, and a CYP17 specific inhibitor, targets pathogenesis of castration resistance by blocking adrenal, testicular and tumoral androgen synthesis. It is approved in over 90 countries including Europe and the United States and more recently, in Japan (July 2014). In combination with prednisone or prednisolone, abiraterone has shown clinical benefit with low toxicity in chemotherapy-naïve and docetaxel-treated mCRPC patients, and patients recruited to pivotal studies conducted in North America, European Union and Australia [6], [7]. However, limited information is available in Asian population, which constituted only a small (≤5%) percentage of these patients [8]. Extrapolating the results of studies from North America, Europe, and Australia to other ethnic populations may not be readily done. Thus, there is an unmet need for robust data in these patient populations for management of mCRPC.
Current study was designed to provide safety and efficacy of abiraterone in chemotherapy-naïve mCRPC patients from China, Malaysia, Thailand and Russia to allow bridging of clinical data on abiraterone between rest of the world and these populations. Primary objective of this study was to compare the time to prostate specific antigen (PSA) progression (TTPP) of abiraterone + prednisone versus placebo + prednisone in asymptomatic or mildly symptomatic chemotherapy-naïve mCRPC patients.
2. Patients and methods
2.1. Study population
Chemotherapy-naïve patients ≥18 years old with mCRPC and disease progression who had either asymptomatic (Brief-Pain Index-Short Form [BPI-SF] score: 0–1) or mildly symptomatic (BPI-SF score: 2–3) disease, were enrolled. These patients had confirmed adenocarcinoma of the prostate; PSA progression based on Prostate Cancer Working Group 2 (PCWG2) criteria [9] or radiographic progression as per modified Response Evaluation Criteria in Solid Tumors (RECIST); medical or surgical castration (serum testosterone levels < 50 ng/dL [<1.7 nmol/L]); Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1; and adequate hematological and biochemical aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 × upper limit of normal (ULN) indices. In patients treated with luteinizing hormone-releasing hormone (LHRH) agonists, the therapy was initiated at least 4 weeks prior to cycle 1 day 1 and was continued throughout the study.
Key exclusion criteria included: serious or uncontrolled coexistent non-malignant disease (including active and uncontrolled infection); uncontrolled hypertension; active or symptomatic viral hepatitis or chronic liver disease; brain metastasis; pituitary or adrenal dysfunction; clinically significant heart disease; gastrointestinal disorders and malignancy within the previous 5 years other than basal cell or squamous cell carcinomas of skin with a >30% probability of recurrence within 12 months. Patients previously treated for mCRPC with cytotoxic chemotherapy (docetaxel), biologic therapy, radiation (expect for primary tumor, 6 weeks prior to cycle 1 day 1) or radionuclide therapies, patients treated with opiate analgesics for cancer-related pain, ketoconazole for prostate cancer (>7 days), flutamide or azole drug within 4 weeks of cycle 1 day 1, or non-steroidal antiandrogens were also excluded from the study. Patients whose PSA did not decline for >3 months in response to anti-androgen given as a second line or later intervention required a 2-week washout prior to cycle 1 day 1.
Concomitant medications like 5α-reductase inhibitor, ketoconazole, diethylstilbestrol, PC-SPES (a herbal propriety mixture), and other treatments that would have endocrine effects on prostate cancer, radiopharmaceuticals such as strontium (89Sr) or samarium (153Sm), spironolactone, fludrocortisones, digoxin, pomegranate juice or supplements, indole-3-carbinol, flaxseed oil, black cohosh and anti-androgens both non-steroidal (bicalutamide, flutamide, nilutamide) and steroidal (megestrol acetate), cyproterone acetate and traditional Chinese medicines with an anti-cancer indication were prohibited during the study.
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices, applicable regulatory requirements, and in compliance with the study protocol. The study protocol was reviewed and approved by the Institutional Review Board (Appendix 1). All enrolled participants provided written consent for participation in the study.
2.2. Study design
This multinational, randomized, double-blind, placebo-controlled, phase 3 study conducted at 42 study sites in Asia (China, Malaysia, and Thailand) and Europe (Russia), consisted of screening period (within 28 days prior to cycle 1 day 1), treatment period (from the first dose of study drug to end-of-treatment evaluation; each treatment cycle was 28 days), and follow-up period (follow-up for survival every 3 months up to 60 months). The study is registered at clinicaltrials.gov (NCT01591122).
During the treatment period, eligible patients were randomized (1:1) to abiraterone 1000 mg (administered as 4 × 250-mg tablets) orally once daily or matching placebo, along with prednisone 5 mg orally twice daily. Patients continued to receive study treatment until disease progression or unacceptable toxicity. Up to two dose reductions were allowed for study drug and one dose reduction for prednisone (minimum 5 mg daily).
2.3. Efficacy
The primary efficacy endpoint was TTPP, defined as the time interval from the date of randomization to the date of PSA progression as defined by PCWG2 criteria, of abiraterone versus placebo group. Secondary endpoints included overall response rate (ORR, proportion of patients with measurable disease achieving a complete or partial response according to modified RECIST version 1.0 criteria [baseline lymph node size ≥2 cm can be considered a targeted lesion], tumor measurements [CT or MRI, bone scans, other imaging procedures] assessed at screening; tumor measurements and response evaluation assessed on day 1 of cycles 3, 5, 7, and 10; every 3 cycles beyond cycle 10; and at treatment discontinuation, if applicable); time to initiation of cytotoxic chemotherapy (docetaxel) for metastatic prostate cancer; PSA response rate (proportion of patients achieving a PSA decline ≥50% according to PCWG2 criteria); patient-reported outcome questionnaires (total score and each subscale score from FACT-P [physical well-being, social/family well-being, emotional well-being, functional well-being, and prostate cancer subscale]); time to pain intensity progression (time interval from randomization to the first date a patient experienced an increase by 2 points from baseline in the BPI-SF worst pain intensity item [item 3] observed at two consecutive evaluations ≥4 weeks apart without decrease in analgesic usage score); time to pain interference progression (time interval from randomization to the first date a patient experienced an increase of 50% the standard deviation of baseline scores from baseline in the average of BPI-SF pain interference item scores); time to deterioration in ECOG PS grade from 0–1 to ≥2; and time to analgesic progression (time interval from randomization to first date of increase in analgesic usage score ≥30% from baseline observed at two consecutive evaluations ≥4 weeks apart).
2.4. Safety
Safety assessments included monitoring for adverse events (AEs), clinical laboratory tests, vital signs, 12-lead electrocardiograms (ECGs), and physical examinations.
2.5. Statistical analysis
Considering an exponential distribution with a proportional hazards model for TTPP, with 181 PSA progression events, the study had a power of 90% at the 2-tailed significance level of 0.05 to detect a difference between a median TTPP of 4 months in the placebo group and a median TTPP of 6.5 months in the abiraterone (hazards ratio [HR] = 0.62). Assuming that 20 patients would be enrolled per month over 14.5 months and follow-up of approximately 2 months after the last patient enrollment, a total sample size of approximately 290 patients was planned for the study.
An interim analysis was planned when approximately 50% of TTPP events (91 events) were observed to allow for the early termination of the study if superiority was demonstrated. The α spending for the interim analysis was based on the Pocock boundary as implemented by Lan DeMets α spending method. The cumulative Pocock α spending was anticipated to be 0.0310 for the interim analysis and 0.0500 for the final analysis. The primary analysis of the primary and secondary efficacy time-to-event endpoints was based on the stratified log-rank test; sensitivity analyses using the nonstratified log-rank test, and Cox proportional hazards model also were performed as supportive analyses. Hypotheses testing was performed at a 2-sided overall significance level of α = 0.05.
Estimates of the time-to-event endpoints were obtained using the Kaplan–Meier methods. Response endpoints (PSA response rate, objective response rate) were summarized using descriptive statistics for categorical data by treatment group. The relative risk (treatment : control) was reported along with the associated 2-sided 95% confidence intervals (CIs). Statistical inference was evaluated using the Chi square statistic; the Fisher's exact test was used if the expected counts were small. Safety was analyzed descriptively.
All efficacy endpoints were analyzed using intent-to-treat (ITT) analysis set (all patients randomized into the study). Safety analysis set included all patients who received at least one dose of study medication. All statistical analyses were performed using SAS® version 9.1.3 (SAS Institute Inc., Cary, NC, USA). In general, all hypotheses testing were implemented at a 2-tailed significance level of α = 0.05 and interval estimations were reported using 2-tailed 95% CIs.
3. Results
3.1. Patient disposition and baseline characteristics
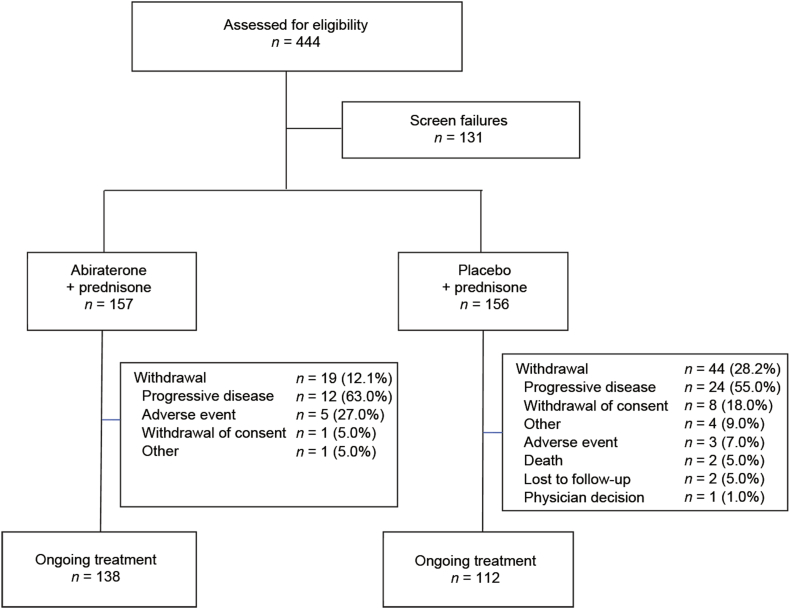
Total of 313 patients were randomized (abiraterone: n = 157; prednisone alone: n = 156) at 42 sites in China (n = 238), Thailand (n = 3), Malaysia (n = 3), and Russia (n = 69) from 27 March 2012 to 14 February 2013. At interim analysis (18 March 2013), 250 patients continued to receive study treatment. More prednisone-treated patients discontinued the study treatment (abiraterone: 12.1%; prednisone: 28.2%) with disease progression being the most common reason for withdrawal (Fig. 1).
Figure 1.
Patient disposition.
Baseline characteristics of all patients were similar between the two treatment groups. Median age was 71 years; at initial diagnosis, 61% of patients had metastatic disease (M1) and 55% patients had Gleason score ≥8. Mean time from initial diagnosis to first dose was similar in both treatment groups (abiraterone: 3.09 years; prednisone: 3.63 years) (Table 1).
Table 1.
Demographics and baseline characteristics (ITT analysis set).
| China |
Russia |
Overalla |
|||||
|---|---|---|---|---|---|---|---|
| Abiraterone (n = 119) | Prednisone (n = 119) | Abiraterone (n = 35) | Prednisone (n = 34) | Abiraterone (n = 157) | Prednisone (n = 156) | Total (n = 313) | |
| Age (year) | |||||||
| Mean ± SD | 70.50 ± 9.00 | 71.80 ± 8.68 | 66.70 ± 7.30 | 67.40 ± 8.03 | 69.70 ± 8.72 | 70.80 ± 8.64 | 70.30 ± 8.69 |
| <65 years, n (%) | 31 (26) | 24 (20) | 11 (31) | 16 (47) | 42 (27) | 40 (26) | 82 (26) |
| ≥65 years, n (%) | 88 (74) | 95 (80) | 24 (69) | 18 (53) | 115 (73) | 116 (74) | 231 (74) |
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 2 (2) | 0 | 1 (3) | 2 (6) | 3 (2) | 2 (1) | 5 (2) |
| Not Hispanic or Latino | 117 (98) | 119 (100) | 34 (97) | 32 (94) | 154 (98) | 154 (99) | 308 (98) |
| Race, n (%) | |||||||
| White | 0 | 0 | 35 (100) | 34 (100) | 35 (22) | 34 (22) | 69 (22) |
| Asian | 119 (100) | 119 (100) | 0 | 0 | 122 (78) | 122 (78) | 244 (78) |
| Weight (kg) | |||||||
| Mean ± SD | 69.52 ± 10.80 | 68.76 ± 10.37 | 88.76 ± 13.25 | 82.59 ± 14.03 | 73.67 ± 13.90 | 71.74 ± 12.53 | 72.71 ± 13.25 |
| Years from initial diagnosis to 1st dose | |||||||
| Mean ± SD | 2.99 ± 2.18 | 3.49 ± 3.10 | 3.31 ± 2.20 | 4.02 ± 2.67 | 3.09 ± 2.22 | 3.63 ± 3.02 | 3.36 ± 2.66 |
| Years from staging to 1st dose | |||||||
| Mean ± SD | 2.10 ± 2.94 | 3.50 ± 3.29 | 2.60 ± 3.13 | ||||
| Baseline PSA (ng/mL) | |||||||
| Median (range) | 49.11 (0.37, 12,633.69) | 53.98 (2.37, 2438.76) | 40.30 (1.44, 1554.21) | 72.12 (11.36, 2850.24) | 48.57 (0.37, 12,633.69) | 55.73 (2.37, 2850.24) | 50.89 (0.37, 12,633.69) |
| Gleason score at initial diagnosis, n (%) | |||||||
| <7 | 6 (6) | 8 (8) | 2 (6) | 5 (19) | 8 (6) | 14 (11) | 22 (8) |
| 7 | 36 (36) | 29 (30) | 17 (55) | 13 (50) | 54 (40) | 42 (33) | 96 (37) |
| ≥8 | 58 (58) | 61 (62) | 12 (39) | 8 (31) | 72 (54) | 71 (56) | 143 (55) |
| Baseline extent of disease, n (%) | |||||||
| Bone | 117 (98) | 117 (98) | 27 (77) | 28 (82) | 147 (94) | 148 (95) | 295 (94) |
| Bone only | 94 (79) | 105 (88) | 12 (34) | 9 (27) | 109 (69) | 116 (74) | 225 (72) |
| Soft tissue or node | 22 (19) | 11 (9) | 20 (57) | 22 (65) | 42 (27) | 34 (22) | 76 (24) |
| Other | 6 (5) | 4 (3) | 12 (34) | 8 (24) | 18 (12) | 12 (8) | 30 (10) |
| ECOG performance status, n (%) | |||||||
| 0 | 65 (55) | 65 (55) | 13 (37) | 13 (38) | 80 (51) | 81 (52) | 161 (51) |
| 1 | 54 (45) | 54 (45) | 22 (63) | 21 (62) | 77 (49) | 75 (48) | 152 (49) |
| Evidence of disease progression, n (%) | |||||||
| PSA only | 109 (92) | 110 (92) | 23 (66) | 26 (77) | 135 (86) | 137 (88) | 272 (87) |
| Radiographic progression | 10 (8) | 9 (8) | 12 (34) | 8 (23) | 22 (14) | 19 (12) | 41 (13) |
ECOG, Eastern Cooperative Oncology Group; ITT, intent to treat; PSA, prostate specific antigen.
Overall includes subjects from Russia, China, Malaysia, and Thailand.
All patients received prior hormonal therapy; 47% had orchiectomy, 62% had prior LHRH agonist therapy. The majority of patients (88.5%) received no opioid and non-opioid analgesics and 11.2% received non-opioid analgesics at baseline. One prednisone-treated patient received opiates for moderate pain (defined as a score of 2 on the WHO scale). Similar use of concomitant medications was found in both treatment groups. The most common concomitant medications received by patients were endocrine therapy (abiraterone: 50%; prednisone: 58%) including LHRH agonists (abiraterone: 49%; prednisone: 58%), followed by bisphosphonates (abiraterone: 26%; prednisone: 34%), calcium channel blockers (abiraterone: 22%; prednisone: 21%), and herbals and traditional medicines (abiraterone: 22%; prednisone: 17%).
Median treatment duration was 3.8 months in abiraterone group and 3.4 months in prednisone group. Overall, 68% (n = 106) patients in abiraterone group and 63% (n = 98) patients in prednisone group had initiated treatment for >4 cycles (median 4 cycles).
Overall, 19% of patients (30/157) in abiraterone group and 12% (18/156) in prednisone group had ≥1 dose interruptions of study drug. AE was the most common reason for abiraterone/placebo dose interruption (abiraterone: 7%; prednisone: 5%) or dose reduction (abiraterone: 4%; prednisone: 1%), and for prednisone dose reduction in nine patients (abiraterone: 1%; prednisone: 1%) or dose modification/interruption (abiraterone: 3%; prednisone: 4%). One prednisone-treated patient had increased prednisone dose due to inadvertent self-administration of three 5-mg prednisone tablets.
3.2. Primary efficacy
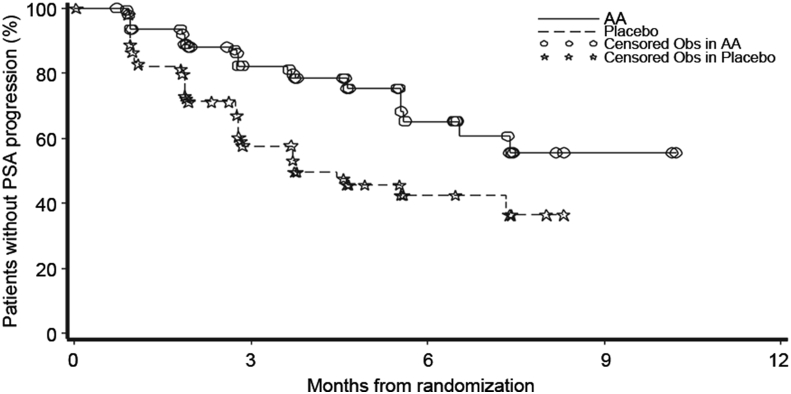
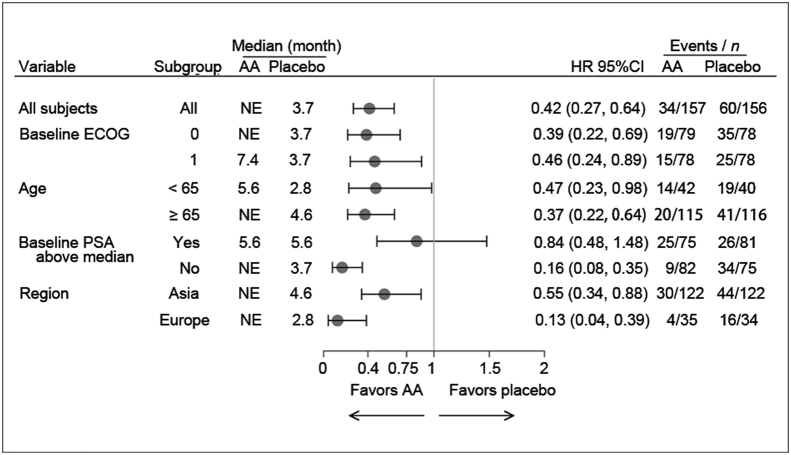
Interim analysis was conducted with 94 TTPP events (abiraterone: n = 34; prednisone: n = 60); treatment with abiraterone significantly decreased the risk of PSA progression by 58% compared with prednisone alone (HR = 0.42; 95%CI: 0.27, 0.65; p < 0.0001). With a median follow-up of 3.9 months, the median TTPP was not reached in abiraterone group while it was 3.8 months in prednisone group (Fig. 2). The p value (p < 0.0001) crossed a pre-planned Pocock stopping boundary (nominal significance level of 0.0310). Sub-group analysis demonstrated that treatment effect of abiraterone on TTPP was favorable across all sub-groups (Fig. 3).
Figure 2.
Time to prostate specific antigen progression. AA, abiraterone acetate; Obs, observation.
Figure 3.
Time to PSA progression based on PCWG2 criteria, forest plot. AA, abiraterone acetate; ECOG, Eastern cooperative oncology group; NE, not estimable; PCWG, prostate cancer working group; PSA, prostate specific antigen.
3.3. Secondary efficacy
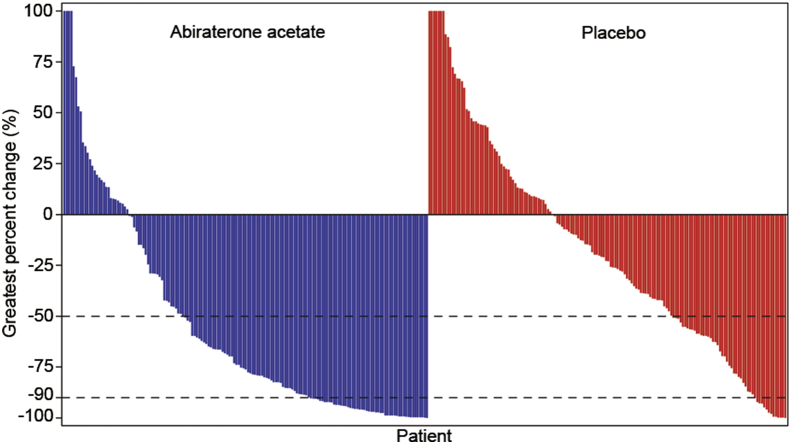
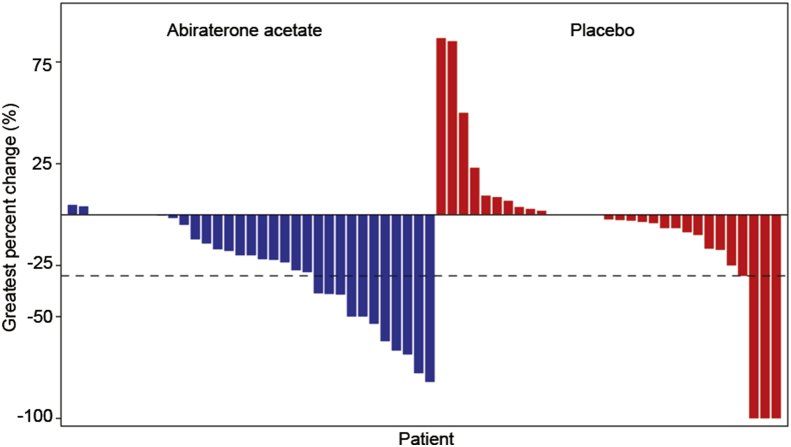
Overall, 50% of patients in abiraterone group and 21% in prednisone group had a confirmed PSA response (relative risk = 2.4; p < 0.0001) (Fig. 4). The majority of patients (87%) had only PSA progression as evidence of disease progression at baseline; hence, the number of patients with measurable disease at baseline was low (n = 77; abiraterone: n = 35; prednisone: n = 42). Of these, 10 patients (abiraterone: 22.9%; prednisone: 4.8%) had an objective response (relative risk = 4.8, p = 0.0369) (Fig. 5). The best overall responses were partial responses; no patient achieved a complete response (Table 2). At clinical cut-off, 11 (3.5%) patients died (abiraterone: n = 6; prednisone: n = 5). The median time to initiation of cytotoxic chemotherapy was not reached either due to low number of events.
Figure 4.
Waterfall plot of maximum change in PSA from baseline. Shown are the greatest percentage changes in PSA for each patient. A patient must have both baseline and at least one post-baseline assessment to be included in this graph. For patients with more than 100% change, the changes are truncated at 100% in both Y axes. PSA, prostate specific antigen.
Figure 5.
Waterfall plot of maximum change in the sum of longest diameter of target lesions from baseline in patients with measurable disease at baseline. Shown are the greatest percentage changes in the sum of longest diameter of target lesions for each patient. A patient must have both baseline and at least one postbaseline assessment to be included in this graph. For patients with more than 100% change, the changes are truncated at 100% on both Y axes.
Table 2.
Best overall response rate based on modified RECIST criteria in patients with measurable disease at baseline (ITT analysis set).
| Abiraterone (n = 157) | Prednisone (n = 156) | |
|---|---|---|
| Total no. patients with measurable disease at baseline, n (%) | 35 (100) | 42 (100) |
| Responders | 8 (22.9) | 2 (4.8) |
| Non-responder | 27 (77.1) | 40 (95.2) |
| p value | 0.0369 | |
| Relative risk (95%CI) | 4.8 (1.1, 21.2) | |
| Best overall response, n (%) | ||
| CR | 0 | 0 |
| PR | 8 (22.9) | 2 (4.8) |
| SD | 24 (68.6) | 22 (52.4) |
| PD | 1 (2.9) | 6 (14.3) |
| NE | 2 (5.7) | 12 (28.6) |
CR, complete response; ITT, intent-to-treat; NE, not evaluable; PD, progressive disease; PR, partial response; RECIST, response evaluation criteria in solid tumors; SD, stable disease.
CR or PR is defined as having a confirmed CR or PR response. Responder is defined as having a confirmed CR or PR response; p value is from Fisher exact test; relative risk >1 favors abiraterone.
The mean FACT-P total score rose from 116.65 (cycle 1 day 1) (n = 157) to 120.18 (cycle 7 day 1) (n = 45) in the abiraterone group and from 116.18 (cycle 1 day 1) (n = 156) to 117.85 (cycle 7 day 1) (n = 30) in the prednisone group. The numerical increase in the FACT-P total mean score was larger for the abiraterone group, and more patients in abiraterone group (n = 18, 11.5%) than in prednisone group (n = 10, 6.4%) reached 10 point improvement in FACT-P total score at cycle 7 day 1 visit compared with baseline. Similarly, the mean FACT-P prostate cancer score rose from 34.25 (cycle 1 day 1) to 35.28 (cycle 7 day 1) in the abiraterone group and from 32.96 (cycle 1 day 1) to 34.12 (cycle 7 day 1) in the prednisone group. The numerical increase in FACT-P prostate cancer score was similar between two groups. Totally, 25 patients abiraterone: n = 10; prednisone: n = 15 experienced a pain intensity progression event; the median time to pain intensity progression was not reached in either treatment group due to a low number of events. The risk of pain interference progression was significantly reduced in abiraterone-treated patients (38%) versus prednisone (HR = 0.620; 95%CI: 0.384, 1.000; p = 0.0481). The median time to pain interference progression was not reached for the abiraterone group and was 7.79 months for the prednisone group (Table 3). The median time to deterioration in ECOG PS grade and the median time to analgesic progression were not reached due to the low number of events.
Table 3.
All secondary endpoints seen in each group (ITT analysis set).
| Abiraterone (n = 157) | Prednisone (n = 156) | p-Value and HR (95%CI) | |
|---|---|---|---|
| Time to initiation of cytotoxic chemotherapy, stratified analysis | |||
| Event, n (%) | 1 (0.6) | 2 (1.3) | |
| Censored, n (%) | 156 (99.4) | 154 (98.7) | 0.3908 0.361 (0.032, 4.069) |
| Range, month | (1.0+, 11.1+) | (0.7+, 11.2+) | |
| 6-month event-free rate, % (95%CI) | 100 (100, 100) | 98.5 (94.0, 99.6) | |
| Time to pain intensity progression, stratified analysis | |||
| Event, n (%) | 10 (6.4) | 15 (9.6) | |
| Censored, n (%) | 147 (93.6) | 141 (90.4) | |
| 25th percentile, month (95%CI) | NE (NE, NE) | NE (6.47, NE) | 0.2228 0.608 (0.272, 1.363) |
| Range, month | (0.0+, 11.1+) | (0.0+, 11.1+) | |
| 6-month event-free rate, % (95%CI) | 92.1 (85.5, 95.8) | 87.7 (76.8, 93.7) | |
| Time to pain interference progression, stratified analysis | |||
| Event, n (%) | 31 (19.7) | 42 (26.9) | |
| Censored, n (%) | 126 (80.3) | 114 (73.1) | |
| 25th percentile (95%CI) | 5.59 (2.79, NE) | 2.83 (1.91, 4.57) | 0.0481 0.620 (0.384, 1.000) |
| Median, month (95%CI) | NE (8.35, NE) | 7.79 (5.55, NE) | |
| 75th percentile, month (95%CI) | NE (8.35, NE) | NE (NE, NE) | |
| Range, month | (0.0+, 11.1+) | (0.0+, 11.1+) | |
| 6-month event-free rate, % (95%CI) | 73.7 (63.3, 81.6) | 60.8 (48.8, 70.8) | |
| Time to deterioration in ECOG PS grade from 0–1 to ≥2, stratified analysis | |||
| Event, n (%) | 5 (3.2) | 13 (8.3) | |
| Censored, n (%) | 152 (96.8) | 143 (91.7) | |
| 25th percentile, month (95%CI) | NE (NE, NE) | NE (5.55, NE) | 0.0274 0.328 (0.116, 0.928) |
| Range, month | (0.0+, 11.1+) | (0.0+, 11.1+) | |
| 6-month event-free rate, % (95%CI) | 95.1 (88.3,98.0) | 81.5 (67.0,90.1) | |
| Time to analgesic progression, stratified analysis | |||
| Event, n (%) | 5 (3.2) | 7 (4.5) | |
| Censored, n (%) | 152 (96.8) | 149 (95.5) | |
| 25th percentile, month (95%CI) | NE (7.43, NE) | NE (NE, NE) | 0.3860 0.599 (0.186, 1.929) |
| Range, month | (0.0+, 11.1+) | (0.0+, 11.1+) | |
| 6-month event-free rate, % (95%CI) | 96.4 (90.4, 98.7) | 92.4 (83.6, 96.5) | |
+, censored observation; HR, hazard ratio; ITT, intent to treat; NE, not estimable.
p value is from a log-rank test stratified by region (Asia or Europe) and ECOG PS grade (0 or 1). HR from stratified proportional hazards model. HR < 1 favors abiraterone.
3.4. Safety
The most common (≥10% of patients) AEs in either treatment group were bone pain, arthralgia, back pain, pain in extremity, increased ALT, and hypertension (Table 4); most events were grade 1 or 2. Overall, 17% of patients in abiraterone group and 21% in prednisone group had grade 3 or 4 AEs. Most frequently (≥2% patients) reported grade 3 or 4 AEs in either treatment groups included hypertension (3% vs. 4%), increased alkaline phosphatase (3% vs. 2%), bone pain (0% vs. 3%), increased ALT (3% vs. 0%), and anemia (0.6% vs. 2.6%).
Table 4.
Most common adverse events (AEs) seen in each group (safety analysis set), n (%).
| Abiraterone (n = 157) | Prednisone (n = 156) | |
|---|---|---|
| Total no. of patients with ≥1 AEs | 103 (66) | 114 (73) |
| Total no. of patients with ≥1 serious AEs | 6 (4) | 11 (7) |
| Total no. of patients with ≥1 grade 3–4 serious AEs | 4 (3) | 9 (6) |
| Total no. of patients with ≥1 grade 3/4 AEs | 26 (17) | 33 (21) |
| No. of patients with ≥1 AEs leading to treatment discontinuation | 5 (3) | 8 (5) |
| No. of patients with ≥1 AEs leading to progressive disease related AE leading to discontinuation of study medication | 0 | 3 (2) |
| No. of patients with ≥1 AEs leading to dose modifications or interruption of abiraterone or prednisone | 13 (8) | 8 (5) |
| No. of patients with ≥1 AEs leading to dose modifications or interruption of prednisone | 9 (6) | 7 (5) |
| No. of patients with ≥1 AEs leading to hospitalization | 7 (5) | 13 (8) |
| No. of patients with ≥1 AEs leading to death | 4 (3) | 6 (4) |
| All deaths within 30 days of last dose | 4 (3) | 4 (3) |
| AE | 2 (1) | 3 (2) |
| Death due to prostate cancer | 2 (1) | 0 |
| Most common (in >5% patients) AEs | ||
| Hypertension | 23 (15) | 22 (14) |
| Arthralgia | 16 (10) | 13 (8) |
| Alanine aminotransferase increased | 15 (10) | 10 (6) |
| Hypokalemia | 12 (8) | 7 (5) |
| Bone pain | 11 (7) | 22 (14) |
| Back pain | 11 (7) | 17 (11) |
| Aspartate aminotransferase increased | 10 (6) | 10 (6) |
| Pain in extremity | 9 (6) | 18 (12) |
| Constipation | 8 (5) | 5 (3) |
| Fatigue | 8 (5) | 3 (2) |
| Musculoskeletal pain | 7 (5) | 8 (5) |
| Anemia | 7 (5) | 9 (6) |
Four (3%) patients in each treatment group died within 30 days of last dose. Overall incidence of serious AEs was low (abiraterone: 4%; prednisone: 7%) (Table 4).
Amongst AEs of special interest, more patients in abiraterone group reported cardiac disorders (10% vs. 4%) and hypokalemia (8% vs. 5%) compared with the prednisone-group; hepatotoxicity (19% vs. 20%), hypertension (15% vs. 14%), and anemia (6% vs. 8%) were similar between the two treatment groups. Most of the events were grade 1 or 2 in severity. Four abiraterone-treated patients and one prednisone-treated patient had serious adverse events (SAEs) (arrhythmias [n = 1 in each treatment group], ischemic heart disease [n = 2, abiraterone], and cardiac failure [n = 1, abiraterone]). One patient (abiraterone-treated) experienced an SAE of grade 4 congestive cardiac failure secondary to grade 3 lung infection and died with a primary cause of multiorgan failure (considered as not related to the study drug); this patient also had two reported events of grade 2 atrial flutter (with recovery) before the lung infection occurred.
In abiraterone group, deaths were due to prostate cancer (n = 2) or AE (metastasis to the central nervous system [n = 1] and multiorgan failure [n = 1]). In prednisone group, deaths were due to colorectal cancer (n = 1); duodenal ulcer hemorrhage, lung infection and peptic ulcer (n = 1), acute renal failure (n = 1) and sudden death (n = 1). Four patients in abiraterone group and three in prednisone group had grade 3 hypertension while two abiraterone treated patients had grade 3 hypokalemia and one had grade 4 hypokalemia; no grade 3–4 hypokalemia was reported in prednisone alone-treated patients. No SAEs related to hypertension or hypokalemia or peripheral edema, were reported and there were no deaths in either treatment group due to any of these events. One abiraterone-treated patient and four prednisone alone-treated patients had grade 3 anemia; no grade 4 anemia was reported in either treatment group. No osteoporosis and osteoporosis-related AEs were reported in the abiraterone group. One prednisone alone-treated patient experienced an SAE of grade 4 thoracic vertebral fracture, which along with grade 4 paraplegia and grade 2 urinary incontinence led to discontinuation of study drug. No cataract or sexual dysfunction-related AEs were reported.
4. Discussion
The current study was conducted to provide clinical data on the safety and efficacy of abiraterone when co-administered with prednisone in patients with mCRPC from China, Malaysia, Thailand and Russia who had progressive disease after androgen deprivation therapy (ADT), to allow extrapolation of the clinical data generated in the Western countries to these patients. To this end, the study was designed to be as similar as possible to permit the highest comparability with the global study conducted in asymptomatic or mildly symptomatic chemo-naïve patients with mCRPC from North America, Europe and Australia [8].
Consistent with the global study results which demonstrated a median TTPP of 11.1 months for abiraterone group versus 5.6 months for prednisone group [8], the current study also demonstrated that abiraterone effectively achieved a significant improvement in TTPP. The median TTPP was not reached in the abiraterone group (lower bound of 95%CI for median was 6.54 months) while it was 3.8 months in the prednisone group. This is consistent with another study with similar study design conducted in Asian patients with mCRPC who have failed docetaxel-based chemotherapy, in which the TTPP was found to be 5.55 months following abiraterone treatment versus 2.76 months following prednisone treatment alone [10]. Other secondary endpoints like PSA response rate and ORR were also significantly higher in abiraterone group versus prednisone group. These findings are also comparable with reported response rates in the phase 3 global study [8]. However, TTPP and objective responses by RECIST appear numerically inferior to those of the global study. This may be due to the much shorter duration of treatment in this study. The current study was designed as a bridging study to the global 302 study with major design elements being similar, except that the current study used TTPP as primary endpoint only while the 302 study had radiographic progression free survival (rPFS) and overall survival (OS) as primary endpoints. TTPP was one of the major secondary endpoints for the global study, with the same definition in this bridging study. Furthermore, the frequency of serial PSA assessments was monthly in the current study versus every 3 months in the global 302 study. Since the current study was a bridging study, sample size was 313 patients versus 1088 patients in the global 302 study. The study passed the stopping criteria at the preplanned interim analysis when approximately 50% of TTPP events (91 out of 181 events) were observed and IDMC members recommended to unblind the treatment arm and patients in the placebo group were crossed over to the abiraterone acetate treatment. Interim analysis results are presented in this paper. In contrast, the results of the global study were based on the second interim analysis performed when approximately 40% of OS events (333 events) were observed. This difference led to the much shorter median follow-up time of 3.9 months in the current study versus 22.2 months in the global 302 study. This explains the short treatment duration of the current study versus the global study.
The HR of TTPP in the current study is comparable to the global study (0.418 vs. 0.488); the median TTPP was not reached in the current study whereas it was 11.1 months in the global study. The median TTPP in the current study was 3.8 months in control group versus 5.6 months in the control group of global 302 study. This numerical difference between the median TTPPs of two studies may be due to more frequent PSA assessment in the current study versus the global study as mentioned previously and also the fact that the patients in the current study had more advanced disease at baseline (51% and 49% of patients in the current study had a stratification ECOG PS grade of 0 or 1, respectively, compared with 76% and 24% of patients, respectively in global study; 61% of patients in the current study had M1 at diagnosis versus 26% in the global 302 study; the median time from initial diagnosis to first dose of study drug was 2.7 years in the current study [2.6 years for the China subgroup] versus 5.3 years in the global study) and the shorter duration of follow-up compared with global study. The latter also explains the difference in overall response rate (ORR) observed between the two studies, as the data were not mature enough for meaningful analyses, compared with global study. In contrast to the PSA response rate which was comparable between both the study (69% vs. 67%), the ORR was higher in the global study than the current (23% vs. 36%); no complete response (CR) was observed in the current study at the time of interim analysis, while 11% of patients treated with abiraterone acetate plus prednisone achieved a complete response in the global study [8]. This may be attributed to the early analysis of data at 94 events where 30% of the study population had PSA progression event compared with the global study in which data were analyzed much later, when 66% of the population had a PSA progression event [8]. Results of time to initiation of cytotoxic chemotherapy and time to ECOG PS deterioration could not be evaluated due to shorter follow-up and low number of events at the time of interim analysis. However, a similar study in Japanese men demonstrated the ECOG PS scores for most patients in abiraterone group were maintained at 0 or 1 throughout the 12-week treatment period [11].
It should be noted that though this was a multicenter study conducted in different countries (China, Malaysia, Thailand and Russia), data obtained for primary endpoint (serial PSA concentrations to calculate TTPP) were measured centrally to ensure single methodology for all patients with a single assay and reference values. Response tracking was also centrally managed in order to ensure a consistent application of PCWG2 criteria. The study confirms that androgen synthesis blocking action of abiraterone produces tumor responses in these CRPC patients from Asia and Russia who do not respond to standard hormonal therapies. Based on the observed outcomes at the preplanned interim analysis, the IDMC unanimously recommended unblinding of the study.
The safety profile of abiraterone was also consistent with that of the Japanese study [11] as well as the global population [8]. Incidence of AEs was generally lower in the current study compared with both global (99%) [8] as well as the study conducted in Japanese men (96%) [11], thus reflecting the shorter duration of follow-up in this study and a potential impact of regional differences in reporting of AE. The observed median duration of treatment (abiraterone: 3.8 months; prednisone: 3.4 months) and median follow-up (3.9 months) was much lower compared with the global study (median treatment duration: abiraterone: 13.8 months, prednisone: 8.3 months; median follow-up: 22.2 months).
The most frequently reported AEs included bone pain, arthralgia, back pain, pain in extremity, and hypertension. These AEs were similar with those reported in previous global studies [12]. The frequencies of AEs leading to death and deaths within 30 days of last dose were low and consistent with previous studies [12]. Most common AEs in abiraterone group versus prednisone group were usually related to underlying disease or were related to the mechanism of action (mineralocorticoid-related) or known to be associated with abiraterone (hepatotoxicity). Discontinuations due to AEs were reported at a higher incidence in the prednisone-treated (5%) versus abiraterone-treated (3%), and were generally associated with disease progression. Overall, these data when combined with the data from previous global clinical studies suggest a consistent safety profile of abiraterone.
5. Conclusion
This interim analysis confirms a favorable benefit to risk ratio of abiraterone in men with mCRPC from China, Malaysia, Thailand and Russia who were asymptomatic or mildly symptomatic. While cytotoxic chemotherapy (docetaxel or mitoxantrone) is ordinarily reserved for patients with symptomatic or rapidly progressive cancer [13], abiraterone, however, showed a clear benefit for typical clinical parameters like TTPP and route of administration in these patients. The results from this bridging study can potentially change the landscape of treatment of mCRPC patients in these countries and make abiraterone readily accessible to the urologists and oncologists for routine use.
Conflicts of interest
The study was funded by Janssen Research & Development, LLC, who was responsible for study design and data collection, analysis, and its interpretation. The sponsor was also responsible for deciding to publish the data and provided a formal review of the manuscript.
Acknowledgements
The authors thank all the patients for their participation in this study and acknowledge the collaboration and commitment of all investigators and their staff. The authors also thank Dr. Shruti Shah (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Dr. Namit Ghildyal (Janssen Research & Development, LLC.) for providing additional editorial support for this manuscript. The authors are solely responsible for its content.
Footnotes
Peer review under responsibility of Second Military Medical University.
Appendix 1. List of institutional review boards for the study
| Country | IRB name | IRB address |
|---|---|---|
| China | Shanghai Changhai Hospital Ethics Committee | No. 168, Changhai Road, Shanghai, 200433, China |
| China | Ethics Committee of Fudan University Shanghai Cancer Center | No. 270, Dong'an Road, Shanghai, 200032, China |
| China | Renji Hospital Ethics Committee, Shanghai Jiaotong University School of Medicine | No. 1630, Dongfang Road, Shanghai, 200127, China |
| China | Ruijin Hospital Ethics Committee, Shanghai Jiaotong University School of Medicine | No. 197, Ruijin Er Road, Shanghai, 200025, China |
| China | Medical IRB of Chinese People's Liberation Army General Hospital | No. 28, Fuxing Road, Haidian District, Beijing, 100853, China |
| China | Medical Clinical Trial IRB of PUMCH | No. 41 Damuchuang, Xicheng District, Beijing, 100730, China |
| China | Ethics Committee of Peking University First Hospital | No. 8, Xishiku Street, Xicheng District, Beijing, 100034, China |
| China | IRB of Cancer Institute and Hospital, Chinese Academy of Medical Sciences | No. 17, Panjiayuan South Road, Beijing, 100021, China |
| China | IRB of Beijing Hospital | No. 1, Dongdandahua Road, Beijing, 100730, China |
| China | Medical IRB of Beijing Chaoyang Hospital | No. 8, Gongti South Road, Beijing, 100020, China |
| China | Drug Clinical Research IRB of Beijing Friendship Hospital, Capital Medical University | No. 95, Yong'an Road, Xuanwu District, Beijing, 100050, China |
| China | IEC of Sun Yat-Sen University Cancer Center | No. 651, Dongfengdong Road, Guangzhou, 510065, China |
| China | IEC of Guangzhou First Municipal People's Hospital | No. 1, Panfu Road, Guangzhou, 510080, China |
| China | IEC of Tianjin Medical University Cancer Institute and Hospital | Huxi Road, Ti Yuan Bei Huan, Hexi District, Tianjin, 300060, China |
| China | Ethics Committee of the Second Hospital of Tianjin Medical University | No. 23, Pingjiang Rd, Tianjin, 300211, China |
| China | IEC of Tongji Hospital | No. 1095, Jiefang Road, Wuhan, 430030, China |
| China | IEC of Zhongnan Hospital of Wuhan University | No. 169, Donghu Road, Wuchang District, Wuhan, 430070, China |
| China | Ethics Committee of Sichuan Provincial People's Hospital | No. 32, Section 2, West of 1st Ring Road, Chengdu, Sichuan, 610072, China |
| China | Ethics Committee of Southwest Hospital, The Third Military Medical University | No. 30, Gaotanyanzheng Street, Shapingba District, Chongqing, 400025, China |
| China | Clinical Trial Ethics Committee of Qilu Hospital of Shandong University | 1F, Peace Building, No. 107, Wenhua West Road, Jinan, Shandong, 250012, China |
| China | Clinical Trial Ethics Committee of The Second Hospital of Shandong University | 4F, Office Building, No. 247, Beiyuan Avenue, Jinan, Shandong, 250033, China |
| China | Clinical Research Ethics Committee of Second Affiliated Hospital of Zhejiang University College of Medicine | 16F, Outpatient Building, Jiefang Road, Hangzhou, Zhejiang, 310009, China |
| China | Medical Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University | 5F, The Sixth Building, Qingchun Road, Hangzhou, Zhejiang, 310002, China |
| China | Drug Clinical Research IRB of Peking University Third Hospital | No. 49, North Garden Road, Haidian District, Beijing, 100083, China |
| China | Huashan Hospital HIRB, Fudan University | No. 12, Middle Wulumuqi Road, Shanghai, 200040, China |
| China | IEC of Jiangsu Cancer Hospital | No. 42, Baiziting Road, Nanjing, Jiangsu, 210000, China |
| Malaysia | Medical Ethics Commitee University Malaya Medical Centre | University Malaya Medical Centre, Lembah Pantai, Kuala Lumpur, 59100, Malaysia |
| Malaysia | UKM Research Ethics Committee | Tingkat 1, Blok Klinikal, Pusat Perubatan Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Kuala Lumpur, 56000, Malaysia |
| Russia | The Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies | 51 Leningradsky Ave, Moscow, 125468, Russia |
| Thailand | Committee on Human Rights Ramathibodi Hospital | 270 Rama VI Road, Bangkok, 10400, Thailand |
| Thailand | Ethics Committees on Researches Involving Human Subjects | Rajvithi Hospital, Bangkok, 10400, Thailand |
References
- 1.Cullen J., Elsamanoudi S., Brassell S.A., Chen Y., Colombo M., Srivastava A. The burden of prostate cancer in Asian nations. J Carcinog. 2012;11:7. doi: 10.4103/1477-3163.94025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y.C., Wei L.J., Liu J.T., Li S.X., Wang Q.S. Comparison of cancer incidence between China and the USA. Cancer Biol Med. 2012;9:128–132. doi: 10.3969/j.issn.2095-3941.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research in Cancer, World Health Organisation; 2012. http://globocan.iarc.fr/Pages/fact_sheets [20 Oct 2014]. Available from: [Google Scholar]
- 4.Hoedemaeker R.F., Rietbergen J.B., Kranse R., Schroder F.H., van der Kwast T.H. Histopathological prostate cancer characteristics at radical prostatectomy after population based screening. J Urol. 2000;164:411–415. [PubMed] [Google Scholar]
- 5.Peyromaure M., Debre B., Mao K., Zhang G., Wang Y., Sun Z. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol. 2005;174:1794–1797. doi: 10.1097/01.ju.0000176817.46279.93. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Fizazi K., Scher H.I., Molina A., Logothetis C.J., Chi K.N., Jones R.J. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 8.Ryan C.J., Smith M.R., de Bono J.S., Molina A., Logothetis C.J., de Souza P. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2012;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubley G.J., Carducci M., Dahut W., Dawson N., Daliani D., Eisenberger M. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y., Zou Q., Sun Z., Li C., Du C., Chen Z. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: a randomized, double-blind, placebo-controlled phase 3 bridging study. Int J Urol. 2016;23:404–411. doi: 10.1111/iju.13051. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara N., Uemura H., Satoh T., Suzuki H., Nishiyama T., Uemura H. A phase 2 trial of abiraterone acetate in Japanese men with metastatic castration-resistant prostate cancer and without prior chemotherapy (JPN-201 study) Jpn J Cancer Res. 2014;44:1216–1226. doi: 10.1093/jjco/hyu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan C.J., Shah S., Efstathiou E., Smith M.R., Taplin M.E., Bubley G.J. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantoff P.W., Schuetz T.J., Blumenstein B.A., Glode L.M., Bilhartz D.L., Wyand M. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]