Abstract
Benign prostatic hyperplasia (BPH) is a condition that greatly affects the quality of life of middle-aged and elderly men. Histopathologically, hyperplastic changes frequently occur in the prostate tissue of elderly men, the incidence of which has been reported to reach approximately 80% in men in their 70s. In clinical practice, approximately 25% of men with histologic BPH are assumed to experience lower urinary tract symptoms (LUTS) and receive some kind of treatment. In other words, there are some men with histologic BPH who do not exhibit LUTS. For that reason, many factors, such as the change in hormonal environment, the immune or autoimmune response, the alteration of gene expression, and so on, are thought to affect the onset and progression of LUTS in men with histologic BPH. One such factor that has long drawn attention is the presence of asymptomatic histological inflammation, which very often accompanies symptomatic BPH. Recent studies have suggested that asymptomatic histological inflammation causes repeated destruction, healing, and regeneration of the prostate tissue, leading to the enlargement of prostatic nodules, while at the same time causing stromal tissue-predominant remodeling of the prostate tissue, which can increase urination resistance and result in the condition changing from asymptomatic BPH to symptomatic BPH. In future, the biomolecular clarification of the significance of asymptomatic histological inflammation in the prostate tissue could help develop new treatment strategies for BPH accompanied by LUTS.
Keywords: Benign prostatic hyperplasia, Asymptomatic histological inflammation, Prostatitis, Lower urinary tract symptoms
1. Introduction
Benign prostatic hyperplasia (BPH) is a condition that greatly affects the quality of life (QoL) in middle-aged and elderly men. The prevalence of histologic BPH is reported to gradually increase from around 50% in men aged over 50 years to 80% in men over 70 years, indicating an increase in the incidence with age [1]. On the other hand, BPH accompanied by lower urinary tract symptoms (LUTS) poses a problem in actual clinical practice. According to one report based on 11 cross-sectional population-based studies, the incidence of at least moderate-to-severe LUTS is 29% in men in their 50s and 56% in men in their 70s [2]. These figures differ from the prevalence of histologic BPH, indicating that some patients with histologic BPH do not exhibit LUTS [3]. Furthermore, the correlation between the prostatic volume and the severity of LUTS are not so strong [4]. For that reason, many factors are thought to affect the onset and progression of LUTS in men with histologic BPH. For example, a very high incidence of inflammatory cell invasion is observed in the prostate of BPH patients with LUTS [5], we therefore believe that inflammation plays a major role in the onset of LUTS.
In recent years, several reports have revealed that inflammation contributes to the onset of LUTS in histologic BPH. To be more specific, 7.7 times more patients with BPH and LUTS have a history of prostatitis than healthy individuals [6]. In addition, approximately 20% of symptomatic BPH patients experience pain and discomfort during ejaculation; furthermore, individuals with symptoms are thought to experience more severe LUTS [7]. Like the prevalence of histologic BPH, prostatitis is also more common in elderly individuals [8]. In the present report, we discuss the mechanism underlying the onset and progression of LUTS in histologic BPH in terms of the inflammation that frequently accompanies symptomatic BPH, and review the effective mechanisms and future outlooks of symptomatic BPH treatment using drugs with an anti-inflammatory action.
2. Methods
A literature review searching PubMed was performed. The search strategy included the terms: benign prostatic hyperplasia, prostatitis, pathogenesis, progression, and LUTS. We limited out search to English-language articles published between January 2001 and December 2016. In addition, cited references from the selected articles and from review articles retrieved in our search were used to identify manuscripts that were not included in the previous search.
3. The relationship between National Institutes of Health type IV prostatitis and LUTS
Prostatitis is traditionally not viewed as a single condition but a syndrome formed from several underlying causes. In 1999, the US National Institutes of Health proposed that prostatitis be classified into types I–IV [9]. In particular, type IV is classified as asymptomatic inflammatory prostatitis. This is defined by the presence of leukocytes (white blood cells) and/or bacteria in prostate-specific samples (post-prostatic massage urine, expressed prostatic secretion, semen, and prostate biopsy) as well as the absence of subjective symptoms and is therefore diagnosed solely in the laboratory. Although the presence of such asymptomatic histological inflammation has been reported before, the absence of symptoms meant that it was not subject to treatment and thus posed no clinical problem.
However, large-scale clinical trials examining the effect of drugs for symptomatic BPH, such as the Medical Therapy of Prostatic Symptoms (MTOPS) trial and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, showed the presence of asymptomatic histologic inflammation in the prostate tissue, which could be associated with the progression of BPH and LUTS. For example, in the MTOPS trial using doxazosin and finasteride [10], inflammatory findings were observed in 45% of patients who underwent prostate biopsy. Moreover, most of these patients had chronic inflammation. Meanwhile, in the REDUCE trial [11], 77.6% of patients exhibited histologic inflammation of the prostate, irrespective of the presence or absence of prostatitis. Interestingly, in the placebo group, patients with chronic inflammation of the prostate tissue exhibited a greater prostatic volume, a higher international prostate symptom score (IPSS), and a higher risk of acute urinary retention than patients without inflammation [12]. Furthermore, pathological analysis of resected tissue obtained from symptomatic BPH patients who had undergone transurethral resection of the prostate (TUR-P) for LUTS without inflammatory symptoms confirmed the presence of histological changes accompanied by inflammatory cell invasion in most patients [5], [13]. Therefore, asymptomatic histologic inflammation in the prostate very frequently accompanies histologic BPH and might in fact contribute considerably to prostate enlargement as well as the onset and progression of LUTS.
4. Prostate tissue remodeling caused by asymptomatic histological inflammation
Asymptomatic histologic inflammation in the prostate tissue is likely caused by bacterial and viral infections, the mechanism underlying allergic reactions to stimuli such as seminal fluid and urine due to poor drainage and reflux into prostate tissue, as well as estrogen-producing inflammatory cells. BPH is also considered a localized autoimmune condition. Age-related weakening of the immune system and changes in the hormonal environment reportedly result in hypofunction of suppressor cells and subsequent invasion of inflammatory cells that ultimately causes asymptomatic histological inflammation [14].
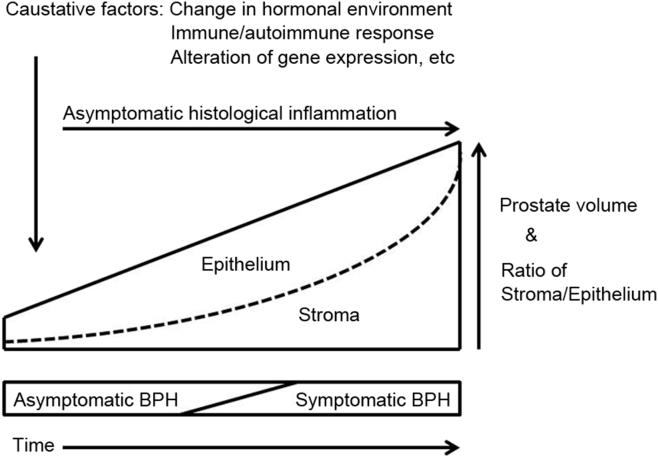
We report that asymptomatic histological inflammation causes repeated destruction, healing, and regeneration of the prostate tissue, leading to the enlargement of prostatic nodules, while at the same time causing significant morphological changes to stromal tissue (remodeling), which can increase urination resistance and result in the condition changing to symptomatic BPH [5] (Fig. 1). Furthermore, reactive oxygen species produced as a result of the low-oxygen environment of the remodeled tissue reportedly induce inflammatory and proliferative cytokine expression, which can promote a vicious cycle of remodeling [15]. The mechanism underlying prostate tissue remodeling associated with asymptomatic histological inflammation is still being clarified from a biomolecular perspective.
Figure 1.
Asymptomatic histological inflammation causes the enlargement of prostatic nodules, while at the same time causing significant morphological changes to stromal tissue (remodeling), which can increase urination resistance and result in the condition changing to symptomatic benign prostatic hyperplasia.
5. The relationship between BPH-associated prostate inflammation and metabolic syndrome
Recent meta-analysis reveals that the metabolic syndrome was associated with a greater prostate volume increment [16]. In addition, other report suggests that patients with metabolic syndrome presented higher obstructive urinary symptoms score and lower uroflowmetric parameters as compared with those without metabolic syndrome [17], [18]. In this context, oxidized low-density lipoprotein led to the high secretion of IL-6, IL-8, and bFGF, which are surrogate markers of prostate inflammation on human prostatic myofibroblastic cells [19]. It is also known that metabolic syndrome is associated with increased levels of C-reactive protein, IL-6, IL-8, and TNF-α [20]. Metabolic syndrome might be correlated with prostate inflammation, followed by wound-healing and consequent proliferation of prostatic tissues. Furthermore, inflammatory infiltrates score in prostatectomy specimens associated with hypogonadism [19]. Recently, metabolic syndrome and hypogonadism has been recognized as a key factor bridging LUTS and BPH-associated prostate inflammation.
6. The involvement of macrophage inhibitory cytokine-1 in the mechanism underlying manifestations of BPH symptoms
To date, we have analyzed gene expression profiles associated with human BPH and have consequently found enhanced expression of inflammatory promoters and reduced expression of inflammatory inhibitors among other characteristics in symptomatic BPH [21]. Furthermore, among several genes with reduced expression, we focused on macrophage inhibitory cytokine-1 (MIC-1) and examined its relationship with histologic inflammatory changes in symptomatic BPH. MIC-1 belongs to the transforming growth factor-β/bone morphogenetic protein superfamily and has been found to show the expression in the prostate and placenta [22]. Moreover, MIC-1 protein has an inhibitory effect on macrophage activity, such as lipopolysaccharide-induced inhibition of the release of TNF from macrophages [23].
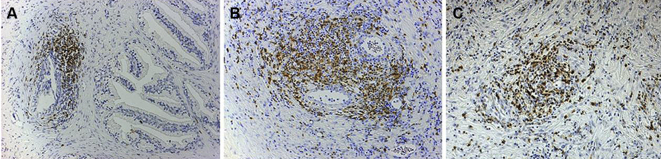
We performed hematoxylin and eosin staining and immune histological staining using anti-T cell, anti-B cell, and anti-macrophage antibodies of the prostate tissue obtained from symptomatic BPH patients who underwent transurethral resection of the prostate (TUR-P) and asymptomatic BPH patients in whom histologic BPH was found after undergoing cystoprostatectomy for muscle invasive bladder cancer. The severity and extent of inflammatory changes were scored based on a report by Nickel et al. [13], and the stroma/epithelium ratio was also found (Fig. 2). In addition, prostatic volume, uroflowmetry, and disease-specific scales such as the IPSS and a QoL index were calculated, and the MIC-1 mRNA expression level was measured using real-time polymerase chain reaction, after which its relationship with each score was examined [5]. The severity and extent of inflammatory changes was significantly higher in the symptomatic BPH group, and a strong correlation was observed with the stroma/epithelium ratio. Although MIC-1 expression was rarely observed in the prostate tissue of symptomatic BPH patients, a high level of expression was observed in the prostate tissue of asymptomatic BPH patients. Furthermore, the group with reduced MIC-1 expression had a significantly lower maximum urinary flow rate and tended to be younger at the time of undergoing TUR-P. These results suggest that in the prostate tissue, the invasion of inflammatory cells, including T-cells and macrophages, causes destruction of the glandular structure, which subsequently changes to a stromal structure and ultimately leads to LUTS. With respect to the mechanism underlying the appearance of symptoms in histologic BPH, it is thought that reduced MIC-1 gene expression is a key genetic change.
Figure 2.
The glandular/periglandular inflammatory changes from grade 1 to grade 3 (A–C) shown by immunostaining using anti-T cell (CD45RO Ab-2) as a primary antibody, reduced from × 100.
7. The evaluation of anti-inflammatory agents using a nonbacterial prostatitis rat model
A reported experimental model of prostatitis involves castrating rats and subsequently creates the same histological changes as nonbacterial prostatitis (NBP) in humans by administering estradiol [24]. Accordingly, we castrated 40-week-old Wistar rats and subsequently administered daily subcutaneous injections of 17β-estradiol (0.25 mg/kg) for 30 days to create an NBP rat model. MIC-1 was increased by castration, and the stroma/epithelium ratio in prostate tissue increased with the administration of estradiol (Fig. 3) [25].
Figure 3.
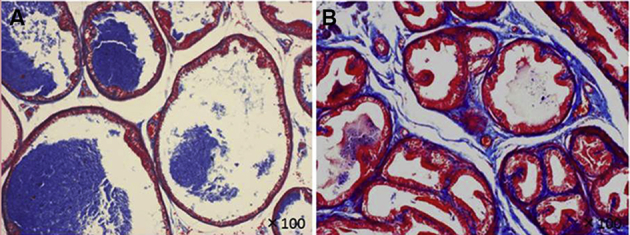
The stroma/epithelium ratio in prostate tissue of nonbacterial prostatitis (NBP) rats was increased with the administration of estradiol, which was estimated by Masson's trichrome staining, reduced from × 100. A: Control; B: NBP rat.
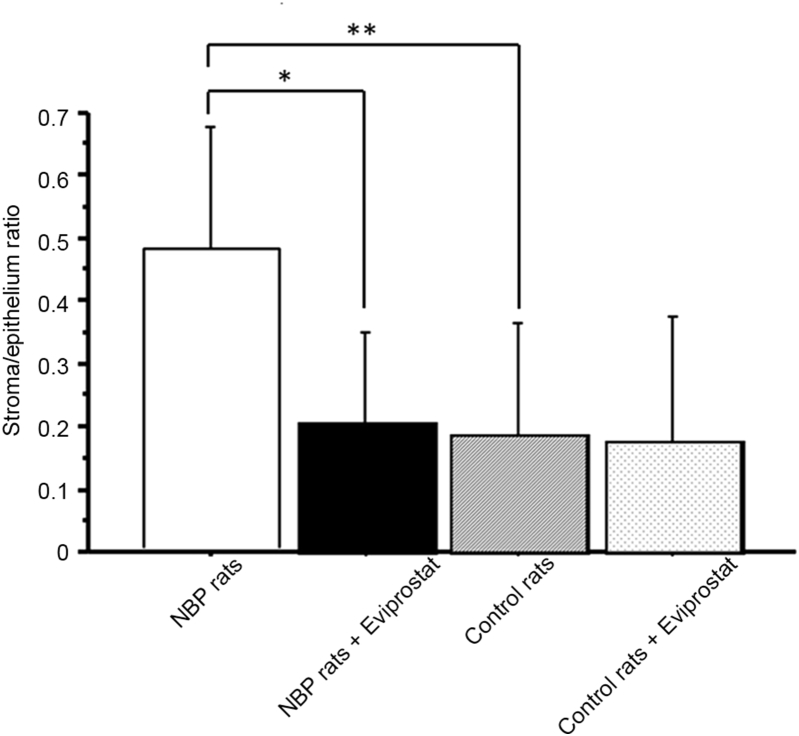
The anti-inflammatory agents seem to be obvious considering the inflammation as a new target in the treatment of symptomatic BPH. We focused on Eviprostat, a plant extract with anti-inflammatory and antioxidant properties. Eviprostat is one agent used in the treatment of BPH and is administered to approximately 280 000 patients per year. The effect of Eviprostat was examined by administering Eviprostat in the NBP rat model and then examining morphological changes in addition to measuring inflammatory cytokines and chemokines in the prostate tissue and urine [26], [27], [28]. The anti-inflammatory effect of Eviprostat inhibited the increase in the stroma/epithelium ratio (Fig. 4) and the macrophage invasion in addition to other actions. Furthermore, upon measuring inflammatory cytokine concentrations in the prostate tissue and urine, we found that the concentrations of IL-1β and TNF-α in the prostate tissue were significantly increased in the NBP group compared with those in the control group, and this increase was significantly inhibited by Eviprostat. Similarly, compared with the control group, the concentrations of the chemokines C—C motif ligand 2 (CCL2) and CCL3 in the prostate tissue were significantly increased in the NBP group, and this increase was found to be significantly inhibited by Eviprostat. Examination of urinary concentrations revealed significantly elevated urinary CCL2 levels in the NBP group, and this elevated concentration was significantly inhibited by Eviprostat. Similarly, the urinary C—X—C motif ligand (CXCL) was significantly elevated in the NBP group, and this increase was also significantly inhibited by Eviprostat. The above results suggest that anti-inflammatory agents such as Eviprostat could be effective by inhibiting inflammation, which is one cause of symptomatic BPH.
Figure 4.
The anti-inflammatory effect of Eviprostat inhibited the increase in the stroma/epithelium ratio in nonbacterial prostatitis rats. *p < 0.005; **p < 0.0001.
8. The potential role of anti-inflammatory agents for symptomatic BPH
Drugs currently investigated for the treatment of prostatic inflammation include the hexaniclipidosterolic extract of Serenoa repens, nonsteroidal anti-inflammatory drugs, and so on [15]. In particular, recent reports have stated that the anti-inflammatory effect of phosphodiesterase type 5 inhibitors (PDE5i) is useful for the treatment of BPH [29], [30]. It is demonstrated that PDE5i leads to an increased testosterone/estradiol ratio [31]. In an experimental study in the rabbit, PDE5i reduces prostate inflammation, fibrosis, and hypo-oxygenation [32]. However, the clinical guidelines published by the American Urological Association and those of the European Association of Urology [33], [34] do not mention anti-inflammatory agents. Although further evidence is needed to support this, the underlying mechanism is currently being elucidated, indicating that it is a promising agent.
Regarding Eviprostat used in our study, in a randomized controlled trial of naftopidil, the Eviprostat group showed no significant improvement in symptoms or urodynamic testing [35], which led to the conclusion that the evidence supporting the effectiveness of Eviprostat for BPH was not strong, as reported in the clinical guidelines for male LUTS published by the Japanese Urological Association [36]. On the other hand, our study suggested that the use of anti-inflammatory agents could help prevent or treat histological changes in the prostate associated with inflammation [26], [27], [28]. Naturally, the mechanism underlying the onset and progression of BPH cannot be explained by chronic inflammation alone. However, there is no doubt that chronic inflammation plays a key role.
We believe that the effect of anti-inflammatory agents could be limited depending on the patient being treated and the timing of the administration. Urinary and prostatic fluid levels of CCL2 and the IL-8 concentration in the seminal fluid could serve as markers to estimate the timing for the administration of anti-inflammatory agents [20]. In future, we hope to further clarify the mechanism underlying the effectiveness of agents with anti-inflammatory action in the treatment of symptomatic BPH to enable more efficient tailor-made treatments and help implement more effective prevention.
9. Conclusion
In LUTS associated with BPH, the symptoms do not always coincide with the extent of hyperplasia. We believe that the asymptomatic histological inflammation that often accompanies symptomatic BPH is an important factor underlying this discrepancy. Inflammation-induced changes in the prostatic environment lead to various changes in gene expression and subsequently result in chronic inflammation. This suggests that asymptomatic histological inflammation that progresses to a chronic condition contributes to changes in the prostatic structure and the appearance of symptoms. In future, further biomolecular clarification of the significance of inflammation in the prostate tissue might help in the development of new treatment strategies for LUTS associated with BPH.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Berry S.J., Coffey D.S., Walsh P.C., Ewing L.L. The development of human benign prostatic hyperplasia with age. J Urol. 2015;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Wein A.J., Kavoussi L.R., Novick A.C., Partin A.W., Peters C.A. 10th ed. vol. 3. Elsevier; 2011. p. 2585. (Campbell-Walsh urology). [Google Scholar]
- 3.Roehrborn C.G. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;(Suppl. 3):S11–S18. doi: 10.1038/ijir.2008.55. [DOI] [PubMed] [Google Scholar]
- 4.Oesterling J.E. Benign prostatic hyperplasia: a review of its histogenesis and natural history. Prostate. 1996;(Suppl. 6):67–73. [PubMed] [Google Scholar]
- 5.Taoka R., Tsukuda F., Ishikawa M., Haba R., Kakehi Y. Association of prostatic inflammation with down-regulation of macrophage inhibitory cytokine-1 gene in symptomatic benign prostatic hyperplasia. J Urol. 2004;171:2330–2335. doi: 10.1097/01.ju.0000127760.87421.e9. [DOI] [PubMed] [Google Scholar]
- 6.Collins M.M., Meigs J.B., Barry M.J., Walker C.E., Giovannucci E., Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002;167:1363–1366. [PubMed] [Google Scholar]
- 7.Nickel J.C., Elhilali M., Vallancien G. Benign prostatic hyperplasia (BPH) and prostatitis: prevalence of painful ejaculation in men with clinical BPH. BJU Int. 2005;95:571–574. doi: 10.1111/j.1464-410X.2005.05341.x. [DOI] [PubMed] [Google Scholar]
- 8.Pontari M.A. Chronic prostatitis/chronic pelvic pain syndrome in elderly men: toward better understanding and treatment. Drugs Aging. 2003;20:1111–1125. doi: 10.2165/00002512-200320150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Krieger J.N., Nyberg L., Jr., Nickel J.C. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 10.Crawford E.D., Wilson S.S., McConnell J.D., Slawin K.M., Lieber M.C., Smith J.A. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175:1422–1426. doi: 10.1016/S0022-5347(05)00708-1. [DOI] [PubMed] [Google Scholar]
- 11.Nickel J.C., Roehrborn C.G., O'leary M.P., Bostwick D.G., Somerville M.C., Rittmaster R.S. Examination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trial. J Urol. 2007;178:896–900. doi: 10.1016/j.juro.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Nickel J.C., Roehrborn C.G., Castro-Santamaria R., Freedland S.J., Moreira D.M. Chronic prostate inflammation is associated with severity and progression of benign prostatic hyperplasia, lower urinary tract symptoms and risk of acute urinary retention. J Urol. 2016;196:1493–1498. doi: 10.1016/j.juro.2016.06.090. [DOI] [PubMed] [Google Scholar]
- 13.Nickel J.C., Downey J., Young I., Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 14.Kramer G., Mitteregger D., Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Ficarra V., Rossanese M., Zazzara M., Giannarini G., Abbinante M., Bartoletti R. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep. 2014;15:463. doi: 10.1007/s11934-014-0463-9. [DOI] [PubMed] [Google Scholar]
- 16.Gacci M., Corona G., Vignozzi L., Salvi M., Serni S., De Nunzio C. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. 2015;115:24–31. doi: 10.1111/bju.12728. [DOI] [PubMed] [Google Scholar]
- 17.Gacci M., Vignozzi L., Sebastianelli A., Salvi M., Giannessi C., De Nunzio C. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16:101–106. doi: 10.1038/pcan.2012.44. [DOI] [PubMed] [Google Scholar]
- 18.Vignozzi L., Morelli A., Corona G., Sebastianelli A., Serni S., Gacci M. Testosterone protects the lower urinary tract from metabolic syndrome-induced alterations. Horm Mol Biol Clin Investig. 2012;11:329–337. doi: 10.1515/hmbci-2012-0029. [DOI] [PubMed] [Google Scholar]
- 19.Vignozzi L., Gacci M., Cellai I., Santi R., Corona G., Morelli A. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013;73:789–800. doi: 10.1002/pros.22623. [DOI] [PubMed] [Google Scholar]
- 20.Gandaglia G., Briganti A., Gontero P., Mondaini N., Novara G., Salonia A. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) BJU Int. 2013;112:432–441. doi: 10.1111/bju.12118. [DOI] [PubMed] [Google Scholar]
- 21.Prakash K., Pirozzi G., Elashoff M., Munger W., Waga I., Dhir R. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99:7598–7603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakehi Y., Segawa T., Wu X.X., Kulkarni P., Dhir R., Getzenberg R.H. Down-regulation of macrophage inhibitory cytokine-1/prostate derived factor in benign prostatic hyperplasia. Prostate. 2004;59:351–356. doi: 10.1002/pros.10365. [DOI] [PubMed] [Google Scholar]
- 23.Paralkar V.M., Vail A.L., Grasser W.A., Brown T.A., Xu H., Vukicevic S. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760–13767. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 24.Kamijo T., Sato S., Kitamura T. Effect of cernitin pollen-extract on experimental nonbacterial prostatitis in rats. Prostate. 2001;49:122–131. doi: 10.1002/pros.1126. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi S., Taoka R., Inui M., Sugimoto M., Kakehi Y. Influence of inflammation and aging on macrophage inhibitory cytokine-1 gene expression in rat ventral prostate. Urology. 2009;73:410–414. doi: 10.1016/j.urology.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Tsunemori H., Sugimoto M., Xia Z., Taoka R., Oka M., Kakehi Y. Effect of the phytotherapeutic agent Eviprostat on inflammatory changes and cytokine production in a rat model of nonbacterial prostatitis. Urology. 2011;77:e15–e20. doi: 10.1016/j.urology.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto M., Oka M., Tsunemori H., Yamashita M., Kakehi Y. Effect of a phytotherapeutic agent, Eviprostat®, on prostatic and urinary cytokines/chemokines in a rat model of nonbacterial prostatitis. Prostate. 2011;71:438–444. doi: 10.1002/pros.21299. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya S., Xia Z., Sugimoto M., Ueda N., Haba R., Kakehi Y. The phytotherapeutic agent, eviprostat, suppresses stromal proliferation and inflammation even after establishment of nonbacterial prostatitis in the rat prostate. Urology. 2014;83:528–534. doi: 10.1016/j.urology.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Roumeguère T., Zouaoui Boudjeltia K., Babar S., Nuyens V., Rousseau A., Van Antwerpen P. Effects of phosphodiesterase inhibitors on the inflammatory response of endothelial cells stimulated by myeloperoxidase-modified low-density lipoprotein or tumor necrosis factor alpha. Eur Urol. 2010;57:522–528. doi: 10.1016/j.eururo.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Vignozzi L., Gacci M., Cellai I., Morelli A., Maneschi E., Comeglio P. PDE5 inhibitors blunt inflammation in human BPH: a potential mechanism of action for PDE5 inhibitors in LUTS. Prostate. 2013;73:1391–1402. doi: 10.1002/pros.22686. [DOI] [PubMed] [Google Scholar]
- 31.Vignozzi L., Filippi S., Morelli A., Comeglio P., Cellai I., Sarchielli E. Testosterone/estradiol ratio regulates NO-induced bladder relaxation and responsiveness to PDE5 inhibitors. J Sex Med. 2012;9:3028–3040. doi: 10.1111/j.1743-6109.2012.02946.x. [DOI] [PubMed] [Google Scholar]
- 32.Morelli A., Comeglio P., Filippi S., Sarchielli E., Vignozzi L., Maneschi E. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: an experimental study in the rabbit. Prostate. 2013;73:428–441. doi: 10.1002/pros.22584. [DOI] [PubMed] [Google Scholar]
- 33.American Urological Association guideline: management of benign prostatic hyperplasia (BPH). https://www.auanet.org/education/guidelines/benign-prostatic-hyperplasia.cfm.
- 34.European Association of Urology guideline: treatment of non-neurogenic male LUTS. http://uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/#1.
- 35.Yamanishi T., Yasuda K., Kamai T., Tsujii T., Sakakibara R., Uchiyama T. Single-blind, randomized controlled study of the clinical and urodynamic effects of an α-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. Int J Urol. 2004;11:501–509. doi: 10.1111/j.1442-2042.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- 36.Homma Y., Araki I., Igawa Y., Ozono S., Gotoh M., Yamanishi T. Clinical guideline for male lower urinary tract symptoms. Int J Urol. 2009;16:775–790. doi: 10.1111/j.1442-2042.2009.02369.x. [DOI] [PubMed] [Google Scholar]