Abstract
Prostate cancer (PCa) is the second most common cancer among men worldwide and even ranks first in Europe. Although Asia is known as the region with the lowest PCa incidence, it has been rising rapidly over the last 20 years mostly due to the introduction of prostate-specific antigen (PSA) testing. Randomized PCa screening studies in Europe show a mortality reduction in favor of PSA-based screening but coincide with high proportions of unnecessary biopsies, overdiagnosis and subsequent overtreatment. Conclusive data on the value of PSA-based screening and hence the balance between harms and benefits in Asia is still lacking. Because of known racial variations, Asian countries should not directly apply the European screening models. Like in the western world also in Asia, new predictive markers, tools and risk stratification strategies hold great potential to improve the early detection of PCa and to reduce the worldwide existing negative aspects of PSA-based PCa screening.
Keywords: Prostate cancer, Early detection, Biomarkers, Imaging, Risk prediction, Europe, Asia
1. Incidence of prostate cancer in Europe and Asia
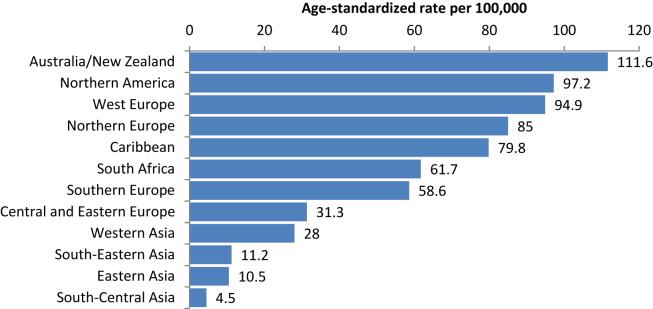
Prostate cancer (PCa) is the second most common malignancy and the fifth leading cause of cancer death in men worldwide [1]. However, the incidence differs by more than 25-fold among regions, with the highest in Australia/New Zealand and the lowest in South-Central Asia [1] (Fig. 1).
Figure 1.
Prostate cancer incidence worldwide [1]. Adapted with permission.
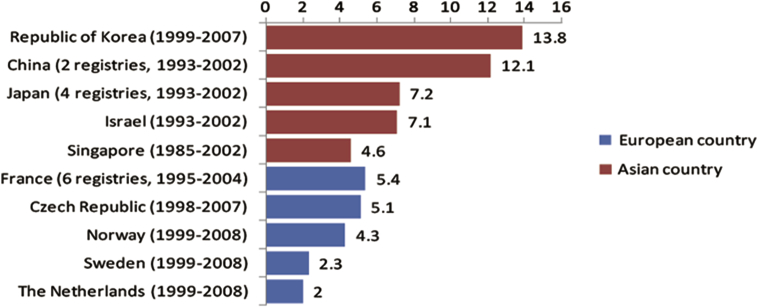
This wide variation in incidence is strongly related to the use of the prostate-specific antigen (PSA) test as a screening tool [2]. In most European countries such as France, The Netherlands, and the Czech Republic, the PCa incidence increased significantly in the early 1990s, soon after the introduction of the PSA test, and is still increasing [3], [4]. The incidence in Asian countries like China and Japan, began to increase after 1995. Although later than in Europe because of the delayed use of the PSA test as a screening tool, the increase of PCa incidence in Asian countries is more pronounced in a comparable period [3] (Fig. 2).
Figure 2.
Prostate cancer incidence trend in Europe and Asia [3]. Adapt with permission.
As said, the worldwide variation in the use of the PSA test is probably the most important reason for the variability in PCa incidence. It is interesting to note that in the early 1980s, when PSA was not yet used, a nearly 20-fold PCa incidence difference already existed (USA 91.43 vs. Japan 4.87, per 100,000) [5]. This could be explained by factors like dietary differences (e.g., a high-fat diet in the western world), the prevalence of obesity, and genetic factors [3]. A striking example is the significantly higher PCa incidence in Japanese-American men than in native Japanese men, suggesting that westernization through a high-fat diet is strongly related to the risk of having PCa [6]. Furthermore, the PCa incidence in African-American men is 2–3 times higher than in White and Asian-American men in the US, which indicates that gene and race also contribute to PCa risk [7].
2. Screening trials in Europe and Asia
The European Randomized Study of Screening for Prostate Cancer (ERSPC) is the largest randomized trial for PCa screening and is still ongoing. It started in 1993 and includes 162,338 men, aged 55–69 years at time of randomization. After 13 years of follow-up, the trial showed that PCa mortality was reduced by 21% in favor of the screening arm [8]. This finding is contrary to that of a large American randomized trial, the so-called prostate arm of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). This trial, also initiated in 1993 and in which 76,685 men were randomized showed no difference in PCa mortality between the screening and control arm with 13 years of follow-up [9]. A recent publication on the basis of the PLCO data showed a 90% PSA contamination rate in the control arm which seriously questions the value of the reported outcomes [10].
The Goteborg Randomized Prostate Cancer Screening Trial is a European prospective, randomized trial that started in 1995 and included 19,904 men, aged 50–64 years at time of randomization. After 14 years of follow-up, it showed that PCa mortality decreased by 44% in the screening group as compared to the control group [11].
It is important to note that all three PCa screening trials were based on Caucasian populations. Hence, their outcomes cannot be directly translated to an Asian population.
The Japanese Prospective Cohort Study of Screening for Prostate Cancer (JPSPC) is the only known prospective controlled PCa screening study in Asia (Table 1). It started in 2002 and ended in 2014. The aim of the study is to compare the PCa mortality between the screening and control cohort. This study comprises of 200,000 men in the age range between 50 and 79 from Hokkaido, Gunma, Hiroshima and Nagasaki prefectures. A PSA ≥3 ng/mL in men aged 50–64 years, a PSA ≥3.5 ng/mL in men aged 65–69 years and a PSA ≥4.0 ng/mL in men aged 70–79 years triggered biopsy [12]. The compliance rate of PSA testing in the Isesaki city screening cohort was about 75% over 5 years and the contamination (PCa screening) in the Kiryu city control cohort was low at 8% between 1992 and 2006 [13]. The contamination rate for the whole control cohort is expected to be considerably low, due to the absence of opportunistic PCa screening in Japan. The study outcome is eagerly awaited to show whether PSA-based screening has any potential in an Asian setting [14].
Table 1.
PCa screening trails in Asia.
| Screening trial | Country | No. participants | Age group (years) | Follow-up time (years) | Main conclusions |
|---|---|---|---|---|---|
| JPSPC [13] | Japan | 200,000 | 50–79 | N/A | N/A |
| Kanazawa [15] | Japan | 32,769 | 55–69 | 6 | PCa incidence: 0.76% Survival rate: 97.8% |
| The Korean Heart Study [17] | South Korea | 118,665 | ≥20 | Mean 11.6 | PCa mortality: 0.047% |
| Changchun [84] | China | 12,027 | ≥50 | 3 | PCa incidence: 0.34% |
| Riyadh [19] | Saudi Arabia | 2100 | ≥50 | 1 | PCa incidence: 2.5% |
| Dharan [85] | Nepal | 1521 | ≥50 | 1 | PCa incidence: 0.73% |
| Ho Chi Minh city [86] | Vietnam | 408 | ≥50 | 1.5 | PCa incidence: 2.5% |
JPSPC, the Japanese prospective cohort study of screening for prostate cancer.
The Kanazawa population-based screening cohort study is another large PCa screening study in Japan. A total of 32,769 men aged 55–69 years participated in the program from 2000 to 2006. Contrary to the JPSPC study, the indication of biopsy varied among the different urologists participating in this study. From 2000 to 2002, all men with a PSA > 2.1 ng/mL were recommended to undergo the secondary screening (consisting of a digital rectal examination (DRE) and transrectal ultrasonography (TRUS) examination) and to consult a urologist who would decide whether or not to perform a systematic biopsy taking into account the results of the DRE and TRUS [15]. From 2003 onwards, men with PSA values 2.1–10.0 ng/mL and a free PSA/total PSA ratio (%fPSA) higher than 0.22, were not referred for further screening. 4766 men (14.9%) required secondary screening and 1041 men (3.2%) underwent prostate biopsy. A total of 249 men (0.76%) were diagnosed with PCa, of whom 231 (93.5%) were classified as clinically localized cancer. Comparing the outcomes of this screening study with those done among predominantly Caucasian men it again highlights the considerable difference in PCa incidence; 0.76% and 8.33% in this Japanese screening study and the ERSPC respectively) [8]. The percentage of localized tumors (T1,T2) in Japan was, however, remarkably higher than in the ERSPC (93.5% vs. 78.2%) [16].
A South Korean screening study focused on the relation between the PSA value and PCa mortality. It included 118,665 men from 1994 to 2004, and followed these men up to 2011. The results showed a PCa death risk of 1.0%, 1.57%, 2.41%, 4.32% and 65.0% for baseline PSA values of <1.0, 1–2, 2–4, 4–10 and ≥ 10 ng/mL respectively after adjusting for age, body mass index (BMI) and smoking status [17]. By contrast, although having a longer follow-up, in a subgroup of The Malmo Preventive Project which included 1167 men aged 60 years and who were followed up to age 85 years, the risk of dying of PCa at age 85 years was 0.9%, 2.7%, 11%, 17% and 30% for PSA levels 1.06, 1.50, 3.40, 5.17 and 14.8 ng/mL at age 60 respectively [18]. When comparing similar PSA levels, European men seem to have a higher risk of dying of PCa than Asian men.
In Saudi Arabia in West Asia, a small PCa screening study was conducted between January – December 2008 to explore the prevalence of PCa in a healthy cohort of men and to assess the feasibility of a potential screening program. The study included 2100 healthy men among whom 223 men had elevated PSA values (≥4 ng/mL) and 132 men underwent prostate biopsy. A total of 52 men were diagnosed with PCa and nearly half of the cancers were already locally advanced or metastatic [19].
Apart from the variance in incidence and mortality, differences in treatment outcome were also observed [20], [21], [22]. In an American study, for instance, of the 294,160 patients diagnosed with clinically localized PCa, 42.1% underwent surgery, 34.5% underwent radiotherapy and 23.3% underwent no treatment. The 10-year disease-specific survival rate was highest among Asian men (94.7%), as compared to White (93.5%), Hispanic (93.2%), and Black men (91.8%) [20]. A Japanese study reported that Japanese-American men had better outcomes following hormonal therapy in terms of overall and PCa-specific survival as compared to Caucasian men [21]. All these data suggest that PCa characteristics vary between races and highlight the need for the development of a population-specific guideline.
It must be noted, however, that due to considerable differences in terms of the political system, the economic climate and health policy in Asia, it will be difficult to organize a large randomised controlled PCa screening trial like the ERSPC study. It might therefore be an option to apply statistical modeling and combine results of the various Asian screening trials that are available. Whether PSA-based screening can reduce PCa mortality in Asia is currently still unknown.
3. Benefits and harms of PSA-based screening
Obviously, PCa screening can lead to the early detection of a tumor and subsequently in combination with adequate treatment can avoid cancer progression and even metastases. A British study calculated the lifetime risk of being diagnosed with and dying from PCa by different races in England between 2008 and 2010. It showed that the lifetime risks of being diagnosed with and dying of PCa were 1 in 8 and 1 in 24 respectively in White men. Lifetime risks of being diagnosed with and dying of PCa in Asian men were 1 in 13 and 1 in 44 respectively [23]. It should be noted that the risk of diagnosis in White men is 1.6 times higher than in Asian men, which is actually very close to the ratio of death risk between the two races, being 1.8. Hence, if the risk of diagnosis and death rise or decline equally, the harm-to-benefit ratio of screening will remain stable [24].
The Malmo Preventive Project study indicated that starting PSA-based screening at age 45–49 years can avoid a considerable number of men from suffering metastatic PCa compared to starting screening at age 51–55 years [25]. On the basis of this observation several guidelines currently suggest that men should start PCa screening at an early age. For instance, the European Association of Urology (EAU) recommended baseline PSA testing of men in their 40s to predict the future risk of PCa [26]. The Memorial Sloan Kettering Cancer Center (MSKCC) recommended to start screening at age 45 years [27]. In addition, since young men have a lower incidence of benign prostatic hyperplasia (BPH) that also influences the serum PSA value, an elevated PSA level at young age better reflects the presence of PCa [28].
While these data show the potential of PSA-based risk stratification, it is crucial to acknowledge that a purely PSA-based screening algorithm also results in large numbers of unnecessary biopsies and the detection of potentially indolent PCa. This so-called overdiagnosis often leads to overtreatment [29]. In the ERSPC study, up to 75.9% men with an elevated PSA value (≥3 ng/mL) had a benign biopsy result, and 72.2% of the PCa detected in the screening arm had low-risk disease (Gleason score 6) [30]. In ERSPC Rotterdam, as many as 58.1% men with low-risk PCa underwent aggressive treatment [31].
In another smaller Korean population screening study, 3943 men ≥55 years of age were included. It showed that among 719 men with PSA values ≥3 ng/mL 71.6% of the biopsies showed a benign result and were unnecessary at that point in time. In addition, 53.9% of the PCa detected had a Gleason score of 2–6 [32]. In a relatively small two-country (Japan, China) PCa screening study of 5778 men, a PSA value of 4.1 ng/mL was used as cut-off for prostate biopsy, 73.4% of the biopsies had a benign result [33].
In 2012, having reviewed all the available data on prostate cancer screening, the United States Preventive Services Task Force (USPSTF) recommended against PSA-based screening for PCa [34]. Recently the first studies reported on the consequences of this recommendation. In general there is a decreases in PSA testing rates [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. However, while screening rates in men over 75 continued to decline, a decline was also noted in younger men for whom PSA screening has demonstrated benefit. In addition, men at high risk for developing PCa also received less screening [38], [46], [50]. However, it is still unclear whether unnecessary testing and over diagnosis has actually decreased [51].
In conclusion, it is unclear whether PSA based screening results in a PCa mortality reduction in Asian countries. Results of the JPSPC study are awaited and can hopefully give more insight. There is however reason to assume that the harm-to-benefit ratio of PSA-based screening in Asian men is comparable to that in the western world. This implies that also in Asia, optimization of PCa screening algorithms has to be based on introducing new biomarkers, risk prediction tools and individual risk based screening strategies.
4. Application of new markers and tools in Europe and Asia
4.1. PSA subforms
PSA circulates in the serum in two forms, the complex form (PSA binded to α1-antichymotrypsin) and the free form known as free PSA (fPSA). proPSA is a molecular subform of fPSA and has three known forms: [-2], [-4], and [-5/-7] proPSA, in which [-2]proPSA (p2PSA) is the most stable one [52], [53].
The so-called Prostate Health Index (PHI) combines three PSA-based biomarkers using the following formula: (p2PSA/fPSA) × PSA½. It was developed by Beckman Coulter, Inc in cooperation with the NCI Early Detection Research Network and then approved by U.S. Food and Drug Administration (FDA) in 2012 [54].
In a multicenter European study, a total of 883 men with elevated PSA values and/or suspicious DRE results underwent prostate biopsy. Total PSA (tPSA), fPSA, p2PSA levels were measured upfront and the %fPSA and PHI were calculated. On the basis of biopsy outcome (PCa detected yes or no), PHI showed the best discrimination of PCa with areas under the curve (AUC) of 0.68 as compared to tPSA (AUC 0.51) and %fPSA (AUC 0.64) [55]. In another five-center European study of 646 biopsied men with PSA levels 2–10 ng/mL, PHI also showed better performance than tPSA, fPSA, %fPSA or p2PSA in predicting PCa with Gleason ≥7 (AUC's of 0.65 vs. 0.54, 0.56, 0.59, 0.54, respectively) [56].
A study from Hong Kong included 230 biopsied men with PSA levels 4–10 ng/mL and confirmed that PHI achieved the highest predictive value for predicting PCa (AUC 0.781) as compared to %fPSA (AUC 0.654) and PSA (AUC 0.547) [57]. In another Chinese study of 636 biopsied men from Shanghai, the AUC of PHI for predicting PCa was as high as 0.88 [58].
These data, although being small in size suggest that PHI has more discriminatory capability (higher AUC) in Asian populations as compared to European populations. In addition, although the mean and median PSA values of men were similar in the Asian and European studies (mean PSA 6.29 ng/mL (range 4.0–9.5) vs. median PSA 6.39 ng/mL (range 0.5–19.9)) [55], [57], the cancer detection rate differed remarkably (9.13% vs. 41.3%), suggesting that these European and Asian studies might not be comparable.
This difference in cancer detection is further confirmed by the fact that when comparing the Chinese study [58] to the European study [55], it was shown that the positive biopsy rate was comparable: 43.1% vs. 41.3% respectively. However, the median PSA value of cancer cases was significantly different (31.78 vs. 9.36 ng/mL).
The available data on PHI in Asian men suggest that PHI has a good, perhaps even better discriminative capability than PSA. It is, however, important to realize that the Asian and western study populations are not directly comparable and more data are needed to assess the value of PHI in predicting biopsy outcome in Asian men.
4.2. Genetic markers
Next to PSA subforms, gene-based tests have been developed with the aim to improve the prediction biopsy outcome. Well-known tests are the prostate cancer gene 3 (PCA3) and the transmembrane protease, serine 2 (TMPRSS2):v-ets erythroblastosis virus E26 oncogene homolog (ERG) tests [59]. Since these tests are urine-based, they are noninvasive and as such convenient in daily clinic practice [60]. The clinical benefits of PCA3 and TMPRSS2:ERG will have to be assessed further in Asian men as well as its cost-effectiveness. In the UK it has been shown that in an NHS-setting PCA3 was not cost-effective, but this may be different for an Asian population of change in the future due to technological improvements [61].
PCA3 is a non-coding RNA that was found to be highly overexpressed in PCa cells as compared to normal prostate tissue. In 2012, PCA3 was approved by the FDA for PCa detection [62], [63].
A multicenter study, with the aim to test the added value of PCA3 in pre-biopsy risk stratification was conducted in Europe and North America and included 809 men [64]. The median PSA level of the study cohort was 6.3 ng/ml and PCa was detected in 319 men (39.1%). PCA3 showed a higher discriminative capability than PSA (AUC 0.679 vs. 0.527) [64].
To date, a Japanese multicenter study conducted between 2009 and 2011 is the only large Asian study that assessed the value of PCA3. It comprised of 647 men with PSA values considered to be elevated (median PSA 7.6 ng/ml) and/or abnormal DRE. All men underwent prostate biopsy and 264 patients (41.7%) were diagnosed with PCa. PCA3 significantly outperformed total PSA in predicting biopsy outcome; with AUC's of 0.748 and 0.583 respectively [65].
TMPRSS2:ERG is a fusion gene that is present in approximately 50% of PCa cases [62]. In a European study including 443 men, TMPRSS2:ERG (cut-off ≥10 copies mRNA) showed a specificity of 93.2% and a sensitivity of 24.3% for clinically significant PCa defined as clinical stage ≥ T2, Gleason score ≥7, PSA density >0.15, and >33% positive cores. In contrast, when using a PCA3 cut-off of ≥35, a specificity of 58.3% and a sensitivity of 68.4% were found [66]. TMPRSS2:ERG showed lower AUC's as compared to PCA3 and PSA (AUC's 0.59 vs. 0.72 vs. 0.67) due to its low sensitivity [66].
In a Japanese study of 102 men, TMPRSS2:ERG (cut-off ≥20 copies mRNA) also showed a very high specificity of 93.5%, and a low sensitivity of 27.5% for PCa. The AUC of TMPRSS2:ERG, PCA3 and PSA were comparable with the European study mentioned above (0.604, 0.824 and 0.691, respectively). It should be noted that the cut-off of TMPRSS2:ERG differed between the two studies [62]. Because of the low sensitivity of TMPRSS2:ERG, combining it with other markers, e.g., TMPRSS2:ERG with PSA or TMPRSS2:ERG with PSA and PCA3, is likely to improve test performance [67]. The European study mentioned above showed that TMPRSS2:ERG (cut-off ≥10) plus PCA3 (cut-off ≥25) increased sensitivity from 24.3% to 88.1%, while a decrease in specificity from 93.2% to 49.6% was seen [66].
Based on the existing data, PCA3 and TMPRSS2:ERG seem to have similar performance between European and Asian populations. However, and this applies for the European, but certainly also for the Asian setting, due to limited availability and unknown clinical effectiveness, PSA remains the most widely used marker for the detection of PCa.
4.3. Multiparametric MRI (mpMRI)
In Europe MRI has been used in the diagnosis of PCa since the 1980s. At first, only T1-weighted (T1W) and T2-weighted (T2W) pulse sequences were available, limiting the ability to distinguish benign prostate nodes from PCa [68]. mpMRI is a new technique combining anatomic T2W with functional and physiological assessments, including diffusion-weighted imaging (DWI) with apparent-diffusion coefficient (ADC) maps and dynamic contrast-enhanced (DCE) MRI. MpMRI not only improves detection of PCa, but in addition, as is shown in several studies can differentiate between indolent and clinically significant PCa [68], which is crucial in avoiding overdiagnosis of PCa.
A five-point scale system which is known as the Prostate Imaging-Reporting and Data System (PI-RADS) has been developed to classify suspicious lesions on prostate mpMRI with points ranging from 1 (no suspicion) to 5 (high suspicion) [69]. In 2015, the PI-RADS system has been updated (PI-RADS 2.0) and extensive information was added on how to acquire, interpret and report mpMRI of the prostate [70]. In addition to having the availability of anatomical/structural and functional imaging of the prostate, advances in technology have led to the development of the so-called MRI-targeted prostate biopsy. This technique comprises of MRI in-bore-guided biopsy, MRI visual estimation-guided biopsy and MRI/TRUS fusion-guided biopsy [71], [72].
In a European systematic review, 1926 men with a positive MRI from 16 studies were included with the aim to compare MRI-targeted biopsy (including in-bore, visual and fusion-guided biopsy) with transrectal ultrasound (TRUS)-targeted biopsy in detecting PCa and significant PCa [73]. Analyses showed that both modalities had similar performance in overall PCa detection (sensitivity: MRI-targeted biopsy 85%, TRUS-targeted biopsy 81%), but MRI-targeted biopsy had remarkably higher sensitivity in significant PCa detection (91% vs. 76%) and lower sensitivity in insignificant PCa detection (44% vs. 83%). In the subgroup of men with an initial biopsy, the two approaches had similar results in overall PCa detection and a small difference in significant PCa detection. However, the differences were most obvious in men with a previous negative biopsy, either on overall PCa detection (sensitivity: 88% vs. 54%) or significant PCa detection (sensitivity: 87% vs. 56%) [73]. On the basis of the available data, the EAU recommends mpMRI and MRI-targeted biopsy in men with a previous negative biopsy [26].
Next to the European review, which mostly included European and US based studies (two Korean small-sample studies were also included), a Japanese study evaluated the value of mpMRI in the detection PCa and significant PCa in 288 men [74]. In this study, all men underwent a mpMRI scan and a 14-core systematic biopsy. In addition, 2 MRI-targeted biopsy cores were added for each suspicious or equivocal lesions seen on mpMRI. Although PI-RADS system was not yet available when the biopsies were taken, in retrospect a single experienced uroradiologist reviewed all MRI lesions according to the PI-RADS scoring system 2.0. It showed that the PI-RADS score was a strong predictor for the presence of PCa as compared to PSA and TRUS outcome (AUC 0.835 vs. 0.622 and 0.543 respectively) [74].
In a Korean study of 76 men with a PSA level <10 ng/mL, MRI-targeted biopsy showed a remarkable higher significant PCa detection rate than TRUS-targeted biopsy (74.1% vs. 35.1%). In addition, the positive prostate biopsy rate per biopsy core was 46.6% and 8.4% for MRI- and TRUS-targeted biopsy respectively. This finding translates into a potentially considerable reduction of unnecessary biopsies, biopsy cores, as well as patients' harm and costs [75].
Although the available Asian data on mpMRI and MRI-targeted biopsy are currently limited, both in Asia and Europe mpMRI holds great potential in improving the selective-detection of clinically significant PCa and as such reduce unnecessary biopsies and overdiagnosis. At present time MRI-targeted biopsy is only available in a few Asian centers and is not (yet) included in the guidelines of most Asian Urological Associations. It is likely that for MRI becoming more widespread available, visual (cognitive) MRI-targeted biopsy holds the most potential in Asia, due to costs and the relatively shorter learning curve.
4.4. Risk calculators
Crucial before deciding on a prostate biopsy, or even an mpMRI is a proper risk stratification with the main goal to avoid unnecessary interventions. In the three large PSA-based screening trials PLCO, ERSPC and the Goteborg screening trial, as well as in most Asian screening trials, every man within a certain age range and surpassing a pre-defined PSA cut-off value was biopsied resulting in large numbers of unnecessary biopsies and the detection and active treatment of many indolent tumors. An individual multivariate risk-based screening strategy is based on the concept of calculating a man's personal risk using more relevant pre-biopsy information coming from multiple sources and subsequently apply a certain PCa risk value as a threshold for prostate biopsy or subsequent screening [76].
There are a few well-known risk calculators based on western populations, such as the Prostataclass model, the Finne model, the Karakiewcz model, and the Prostate Cancer Prevention Trial (PCPT) model [77], [78], [79], [80].
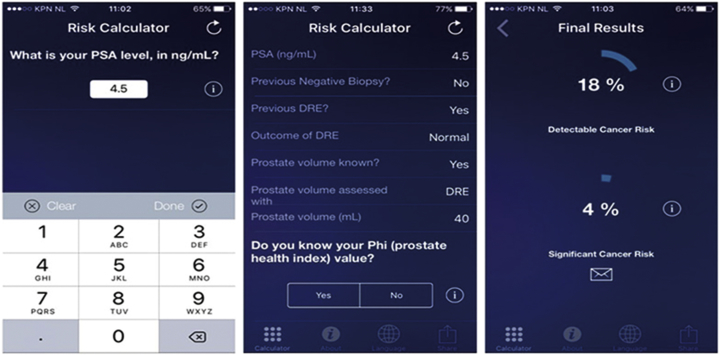
Furthermore, an often-used, externally validated risk calculator with good performance is the so-called ERSPC risk calculator. This risk calculator is developed on the Dutch data of the ERSPC trial, comprising of 19,970 men repeatedly screened and biopsied. The risk calculator consists of six different logistic regression models, each requiring different pre-biopsy information ranging from information available for lay men to information that requires a visit to the urologist. The calculator is easily accessible through the internet (www.prostatecancer-riskcalculator.com) or through a smartphone-app (App: Rotterdam Prostate Cancer Risk Calculator, Fig. 3). The risk factors included in the various prediction models are age, family history, IPSS score, PSA, ultrasound-assessed prostate volume, DRE results, TRUS outcome, and previous biopsy status [81]. The development study of risk calculator step number 3 which predicts initial biopsy outcome reached an AUC of 0.77 as compared to 0.64 for PSA alone when predicting overall PCa [81] (Fig. 4).
Figure 3.
Screenshots of the European Randomized Study of Screening for Prostate Cancer risk calculator (app).
Figure 4.
Screenshots of the Seoul National University prostate cancer calculator (Version 1.1, app).
Retrospective analyses of 1850 men biopsied in ERSPC Rotterdam at initial screening, comparing a purely PSA-based strategy (PSA ≥3 ng/mL) with a risk-based strategy combining the PSA ≥3.0 ng/mL threshold with an individually calculated risk ≥12.5%, showed that 33% of biopsies could be avoided. Although 14% of PCa would potentially be missed with the risk-based strategy, 70% of these missed cancers were classified as potentially indolent. At repeat screening 4 years later, a similar strategy would result in 37% fewer biopsies while missing 16% of PCa diagnosis of which 81% could be classified as potentially indolent. As a comparison, applying a higher PSA threshold for biopsy (≥4.0 ng/mL) could avoid similar numbers of unnecessary biopsies, but would miss larger numbers of potentially aggressive PCa's (12% vs. 10% at initial screening, 50% vs. 15% at repeat screening) [81].
In Asia, the Seoul National University Prostate Cancer Risk Calculator (SNUPC-RC) was developed. The risk calculator is based on data from 3482 Korean men who were all biopsied. In a validation cohort of 1112 Korean men, the SNUPC-RC showed a high predictive value with an AUC of 0.811, the AUC of the ERSPC risk calculator validated in the same Korean population was 0.768 [82]. When applying the 30%-threshold of the SNUPC-RC instead of a PSA level of >4 ng/mL for biopsy, 21.5% of biopsies would be avoided and only 1.4% of the PCa diagnoses would be missed [82].
When comparing the two risk calculators, it is notable that the ERSPC risk calculator was developed on 6-core biopsy scheme while the SNUPC-RC was based on a ≥12-core biopsy scheme [82]. Furthermore, Korean men have a different distribution of PSA levels than the European population [83]. External validation studies confirm the existence of racial variations and highlights that caution should be paid to using risk calculator models outside their region of origin, predominantly when proper validation studies have not been conducted.
It must however be noted that as compared to a purely PSA based strategy a risk based strategy is always better in avoiding unnecessary biopsies and reducing potential overdiagnosis, both in Europe and Asia.
5. The future of PCa screening
Since the incidence and tumor characteristics differ between European and Asian populations, it is crucial to acknowledge that European-based screening outcomes are not directly transferrable to an Asian setting. Similar concerns, e.g., unnecessary testing and overdiagnosis exist and like in the western world PSA-based population screening is not recommended (yet).
Risk stratification has proven to be of aid in identifying men at low risk of harboring aggressive disease and can as such reduce unnecessary testing and overdiagnosis. Hence, Asian countries need to develop or adapt western-based risk stratification strategies using region-specific data.
mpMRI-guided biopsy holds great potential to replace conventional systematic TRUS-guided biopsy. It can provide direct visualization of suspicious lesions and improve diagnostic accuracy for significant PCa, especially in men with a previous negative biopsy. As a new technique, however, performance and cost-effectiveness needs validation in Asian countries which is currently hampered by the availability of data.
PCa screening in most Asian countries is still in its infancy, with the priority of assessing the effect on disease specific mortality. This is contrary to most European countries, where the focus now lies in reducing overdiagnosis and overtreatment. Further research on PCa screening in Asia should however be based on the European experiences to avoid making the same mistakes. The conventional model “PSA screening-biopsy-active treatment” should not be duplicated. Instead, multivariate risk prediction, tailored to the Asian setting, followed by imaging in those considered at high risk for a potentially life threatening PC is advised, preferably in a controlled setting of a governmental regulated screening program.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics. CA Cancer J Clin. 2012;2015(65):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Attard G., Parker C., Eeles R.A., Schroder F., Tomlins S.A., Tannock I. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 3.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 4.Wong M.C., Goggins W.B., Wang H.H., Fung F.D., Leung C., Wong S.Y. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70:862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents, volumes I to IX: IARC CancerBase No. 9. Lyon, France: International Agency for Research on Cancer. World Health Organization Web site. http://ci5.iarc.fr.
- 6.Watanabe M., Nakayama T., Shiraishi T., Stemmermann G.N., Yatani R. Comparative studies of prostate cancer in Japan versus the United States. A review. Urol Oncol. 2000;5:274–283. doi: 10.1016/s1078-1439(00)00092-2. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 8.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Zappa M., Nelen V. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, Buys S.S., Chia D., Church T.R. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoag J.E., Mittal S., Hu J.C. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 11.Hugosson J., Carlsson S., Aus G., Bergdahl S., Khatami A., Lodding P. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi M., Takezawa Y., Takechi H., Ito K., Yamamoto T., Suzuki K. MP-11.12: Japanese prospective cohort study of screening for prostate cancer (JPSPC): the study concept and the first analyses on compliance and contamination for the PSA test (gunma section) Urology. 2007;70(Supplement):97. [Google Scholar]
- 13.Ito K., Kakehi Y., Naito S., Okuyama A., Japanese Urological Association Japanese Urological Association guidelines on prostate-specific antigen-based screening for prostate cancer and the ongoing cluster cohort study in Japan. Int J Urol. 2008;15:763–768. doi: 10.1111/j.1442-2042.2008.02125.x. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa Y., Namiki M. Prostate-specific antigen-based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J Androl. 2015;17:475–480. doi: 10.4103/1008-682X.143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagawa Y., Mizokami A., Nakashima K., Koshida K., Shimamura M., Miyazaki K. Clinical outcomes of prostate cancer patients detected by prostate-specific antigen-based population screening in Kanazawa City, Japan. Int J Urol. 2011;18:592–596. doi: 10.1111/j.1442-2042.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- 16.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mok Y., Kimm H., Shin S.Y., Jee S.H., Platz E.A. Screening prostate-specific antigen concentration and prostate cancer mortality: the Korean heart study. Urology. 2015;85:1111–1116. doi: 10.1016/j.urology.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickers A.J., Cronin A.M., Bjork T., Manjer J., Nilsson P.M., Dahlin A. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabah D.M., Arafa M.A. Prostate cancer screening in a Saudi population: an explanatory trial study. Prostate Cancer Prostatic Dis. 2010;13:191–194. doi: 10.1038/pcan.2009.60. [DOI] [PubMed] [Google Scholar]
- 20.Tyson M.D., 2nd, Castle E.P. Racial disparities in survival for patients with clinically localized prostate cancer adjusted for treatment effects. Mayo Clin Proc. 2014;89:300–307. doi: 10.1016/j.mayocp.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Fukagai T., Namiki T.S., Carlile R.G., Yoshida H., Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97:1190–1193. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 22.Faisal F.A., Sundi D., Cooper J.L., Humphreys E.B., Partin A.W., Han M. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology. 2014;84:1434–1441. doi: 10.1016/j.urology.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd T., Hounsome L., Mehay A., Mee S., Verne J., Cooper A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med. 2015;13:171. doi: 10.1186/s12916-015-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokhorst L.P., Roobol M.J. Ethnicity and prostate cancer: the way to solve the screening problem? BMC Med. 2015;13:179. doi: 10.1186/s12916-015-0427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers A.J., Ulmert D., Sjoberg D.D., Bennette C.J., Bjork T., Gerdtsson A. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Vickers A.J., Eastham J.A., Scardino P.T., Lilja H. The memorial sloan kettering cancer center recommendations for prostate cancer screening. Urology. 2016;91:12–18. doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalona W.J. Baseline PSA testing for men in their 40s: currently available evidence strongly supports baseline PSA measurements in this age group. Oncol Willist Park. 2014;28:154–156. [PubMed] [Google Scholar]
- 29.Loeb S., Bjurlin M.A., Nicholson J., Tammela T.L., Penson D.F., Carter H.B. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder F.H., Hugosson J., Roobol M.J., Tammela T.L., Ciatto S., Nelen V. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 31.Bokhorst L.P., Venderbos L.D., Schroder F.H., Bangma C.H., Steyerberg E.W., Roobol M.J. Do treatment differences between arms affect the main outcome of ERSPC Rotterdam? J Urol. 2015;194:336–342. doi: 10.1016/j.juro.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 32.Song C., Ahn H., Lee M.S., Park J., Kwon T.G., Kim H.J. Mass screening for prostate cancer in Korea: a population based study. J Urol. 2008;180:1949–1953. doi: 10.1016/j.juro.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 33.Kuwahara M., Tochigi T., Kawamura S., Ogata Y., Xu N., Wang H. Mass screening for prostate cancer: a comparative study in Natori, Japan and Changchun, China. Urology. 2003;61:137–141. doi: 10.1016/s0090-4295(02)02093-9. [DOI] [PubMed] [Google Scholar]
- 34.Moyer V.A., Force USPST Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 35.Abdollah F., Dalela D., Sood A., Meyer C.P., Hansen M., Han P. American Urological Association Annual Meeting San Diego 2016. 2016. The impact of 2011 United States preventive services task force panel update on PSA screening practice: a nationwide, and state-by-state level analyses. [Google Scholar]
- 36.Aslani A., Minnillo B.J., Johnson B., Cherullo E.E., Ponsky L.E., Abouassaly R. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191:1737–1742. doi: 10.1016/j.juro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Drazer M.W., Huo D., Eggener S.E. National prostate cancer screening rates after the 2012 US preventive-services task force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33:2416–2423. doi: 10.1200/JCO.2015.61.6532. [DOI] [PubMed] [Google Scholar]
- 38.Frendl D., Epstein M., Fouyazi H., Krajenta R., Rybicki B., Sokoloff M. 2000–2014: preliminary results from the first AUA data Grant American Urological Association Annual Meeting San Diego 2016. 2016. Impact of guidelines on prostate cancer screening in a population-based setting. [Google Scholar]
- 39.Jemal A., Fedewa S.A., Ma J., Siegel R., Lin C.C., Brawley O. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 40.Li J., Berkowitz Z., Hall I.J. Decrease in prostate cancer testing following the US preventive services task force (USPSTF) recommendations. J Am Board Fam Med. 2015;28:491–493. doi: 10.3122/jabfm.2015.04.150062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller C., Kabarriti A., Pulido J., Ziemba J., Guzzo T., Wein A. American Urological Association Annual Meeting San Diego 2016. 2016. United States preventive services task force prostate cancer screening guidelines were associated with age and race dependent changes in primary care prostate specific antigen based screening at a tertiary care center. [Google Scholar]
- 42.Rezaee M.E., Ward C.E., Odom B.D., Pollock M. Prostate cancer screening practices and diagnoses in patients age 50 and older, Southeastern Michigan, pre/post 2012. Prev Med. 2016;82:73–76. doi: 10.1016/j.ypmed.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Sammon J.D., Abdollah F., Choueiri T.K., Kantoff P.W., Nguyen P.L., Menon M. Prostate-specific antigen screening after 2012 US preventive Services Task Force recommendations. JAMA. 2015;314:2077–2079. doi: 10.1001/jama.2015.7273. [DOI] [PubMed] [Google Scholar]
- 44.Sammon J.D., Dalela D., Abdollah F., Sood A., Han P., Hansen M. American Urological Association Annual Meeting San Diego 2016. 2016. Age dependent variation in the effect of physician recommendations to undergo prostate specific antigen (PSA) screening following the United States preventive services task force 2012 statement against PSA screening. [Google Scholar]
- 45.Shoag J., Halpern J.A., Lee D.J., Mittal S., Ballman K.V., Barbieri C.E. Decline in prostate cancer screening by primary care physicians: an analysis of trends in the use of digital rectal examination and prostate specific antigen testing. J Urol. 2016;196(4):1047–1052. doi: 10.1016/j.juro.2016.03.171. [DOI] [PubMed] [Google Scholar]
- 46.Turini G.I., Gjelsvik A., Golijanin D., Pareek G., Renzulli J.I. American Urological Association Annual Meeting San Diego 2016. 2016. The role of patient race and ethnicity in predicting physician recommendation of prostate-specific antigen (PSA) testing. [Google Scholar]
- 47.Zavaski M., Meyer C.P., Hanske J., Friedlander D., Cheng P., Menon M. American Urological Association Annual Meeting San Diego 2016. 2016. Differences in prostate specific antigen testing among urologists and primary care providers in the United States following the 2011 USPSTF recommendations. [Google Scholar]
- 48.Zeliadt S.B., Hoffman R.M., Etzioni R., Gore J.L., Kessler L.G., Lin D.W. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103:520–523. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 49.Cohn J.A., Wang C.E., Lakeman J.C., Silverstein J.C., Brendler C.B., Novakovic K.R. Primary care physician PSA screening practices before and after the final U.S. preventive services task force recommendation. Urol Oncol. 2014;32:e23–30. doi: 10.1016/j.urolonc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Zargar H., van den Bergh R., Moon D., Lawrentschuk N., Costello A., Murphy D. The impact of United States preventive Services Task Force (USPTSTF) recommendations against PSA testing on PSA testing in Australia. BJU Int. 2016;119:110–115. doi: 10.1111/bju.13602. [DOI] [PubMed] [Google Scholar]
- 51.Fleshner K.C.S., Carlsson S.V., Roobol M.J. A review of prostate-specific antigen screening and prostate cancer incidence patterns after the 2011–2012 United States preventive services task force recommendation. Nat Rev Urol. 2016 in press. [Google Scholar]
- 52.Mikolajczyk S.D., Grauer L.S., Millar L.S., Hill T.M., Kumar A., Rittenhouse H.G. A precursor form of PSA (pPSA) is a component of the free PSA in prostate cancer serum. Urology. 1997;50:710–714. doi: 10.1016/S0090-4295(97)00449-4. [DOI] [PubMed] [Google Scholar]
- 53.Mikolajczyk S.D., Millar L.S., Wang T.J., Rittenhouse H.G., Marks L.S., Song W. A precursor form of prostate-specific antigen is more highly elevated in prostate cancer compared with benign transition zone prostate tissue. Cancer Res. 2000;60:756–759. [PubMed] [Google Scholar]
- 54.Sartori D.A., Chan D.W. Biomarkers in prostate cancer: what's new? Curr Opin Oncol. 2014;26:259–264. doi: 10.1097/CCO.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lughezzani G., Lazzeri M., Haese A., McNicholas T., de la Taille A., Buffi N.M. Multicenter European external validation of a prostate health index-based nomogram for predicting prostate cancer at extended biopsy. Eur Urol. 2014;66:906–912. doi: 10.1016/j.eururo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Lazzeri M., Haese A., de la Taille A., Palou Redorta J., McNicholas T., Lughezzani G. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/mL: a multicentric European study. Eur Urol. 2013;63:986–994. doi: 10.1016/j.eururo.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Ng C.F., Chiu P.K., Lam N.Y., Lam H.C., Lee K.W., Hou S.S. The prostate health index in predicting initial prostate biopsy outcomes in Asian men with prostate-specific antigen levels of 4-10 ng/mL. Int Urol Nephrol. 2014;46:711–717. doi: 10.1007/s11255-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 58.Na R., Ye D., Liu F., Chen H., Qi J., Wu Y. Performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and the prostate health index (PHI) in a Chinese hospital-based biopsy population. Prostate. 2014;74:1569–1575. doi: 10.1002/pros.22876. [DOI] [PubMed] [Google Scholar]
- 59.Leapman M.S., Carroll P.R. New genetic markers for prostate Cancer. Urol Clin North Am. 2016;43:7–15. doi: 10.1016/j.ucl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Sanguedolce F., Cormio A., Brunelli M., D'Amuri A., Carrieri G., Bufo P. Urine TMPRSS2: ERG fusion transcript as a biomarker for prostate cancer: literature review. Clin Genitourin Cancer. 2016;14:117–121. doi: 10.1016/j.clgc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson A., Mahon J., Boland A., Beale S., Dwan K., Fleeman N. The clinical effectiveness and cost-effectiveness of the PROGENSA(R) prostate cancer antigen 3 assay and the prostate health index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(i–xxxi):1–191. doi: 10.3310/hta19870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okihara K., Ochiai A., Kamoi K., Fujizuka Y., Miki T., Ito K. Comprehensive assessment for novel prostate cancer markers in the prostate-specific antigen era: focusing on Asians and Asian countries. Int J Urol. 2015;22:334–341. doi: 10.1111/iju.12701. [DOI] [PubMed] [Google Scholar]
- 63.Bussemakers M.J., van Bokhoven A., Verhaegh G.W., Smit F.P., Karthaus H.F., Schalken J.A. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 64.Chun F.K., de la Taille A., van Poppel H., Marberger M., Stenzl A., Mulders P.F. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol. 2009;56:659–667. doi: 10.1016/j.eururo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 65.Ochiai A., Okihara K., Kamoi K., Oikawa T., Shimazui T., Murayama S. Clinical utility of the prostate cancer gene 3 (PCA3) urine assay in Japanese men undergoing prostate biopsy. BJU Int. 2013;111:928–933. doi: 10.1111/j.1464-410X.2012.11683.x. [DOI] [PubMed] [Google Scholar]
- 66.Leyten G.H., Hessels D., Jannink S.A., Smit F.P., de Jong H., Cornel E.B. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–542. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Tomlins S.A., Day J.R., Lonigro R.J., Hovelson D.H., Siddiqui J., Kunju L.P. Urine TMPRSS2:ERG plus PCA3 for individualized prostate cancer risk assessment. Eur Urol. 2016;70:45–53. doi: 10.1016/j.eururo.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J. PI-RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barentsz J.O., Richenberg J., Clements R., Choyke P., Verma S., Villeirs G. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turkbey B., Choyke P.L. PIRADS 2.0: what is new? Diagn Interv Radiol. 2015;21:382–384. doi: 10.5152/dir.2015.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinto P.A., Chung P.H., Rastinehad A.R., Baccala A.A., Jr., Kruecker J., Benjamin C.J. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bjurlin M.A., Mendhiratta N., Wysock J.S., Taneja S.S. Multiparametric MRI and targeted prostate biopsy: improvements in cancer detection, localization, and risk assessment. Cent Eur J Urol. 2016;69:9–18. doi: 10.5173/ceju.2016.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoots I.G., Roobol M.J., Nieboer D., Bangma C.H., Steyerberg E.W., Hunink M.G. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 74.Washino S., Okochi T., Saito K., Konishi T., Hirai M., Kobayashi Y. Combination of PI-RADS score and PSA density predicts biopsy outcome in biopsy naive patients. BJU Int. 2016;119:225–233. doi: 10.1111/bju.13465. [DOI] [PubMed] [Google Scholar]
- 75.Lee D.H., Nam J.K., Park S.W., Lee S.S., Han J.Y., Lee S.D. Visually estimated MRI targeted prostate biopsy could improve the detection of significant prostate cancer in patients with a PSA level <10 ng/mL. Yonsei Med J. 2016;57:565–571. doi: 10.3349/ymj.2016.57.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu X., Albertsen P.C., Andriole G.L., Roobol M.J., Schroder F.H., Vickers A.J. Risk-based prostate cancer screening. Eur Urol. 2012;61:652–661. doi: 10.1016/j.eururo.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephan C., Cammann H., Semjonow A., Diamandis E.P., Wymenga L.F., Lein M. Multicenter evaluation of an artificial neural network to increase the prostate cancer detection rate and reduce unnecessary biopsies. Clin Chem. 2002;48:1279–1287. [PubMed] [Google Scholar]
- 78.Finne P., Finne R., Bangma C., Hugosson J., Hakama M., Auvinen A. Algorithms based on prostate-specific antigen (PSA), free PSA, digital rectal examination and prostate volume reduce false-positive PSA results in prostate cancer screening. Int J Cancer. 2004;111:310–315. doi: 10.1002/ijc.20250. [DOI] [PubMed] [Google Scholar]
- 79.Karakiewicz P.I., Benayoun S., Kattan M.W., Perrotte P., Valiquette L., Scardino P.T. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol. 2005;173:1930–1934. doi: 10.1097/01.ju.0000158039.94467.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson I.M., Ankerst D.P., Chi C., Goodman P.J., Tangen C.M., Lucia M.S. Assessing prostate cancer risk: results from the prostate Cancer prevention trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 81.Roobol M.J., Steyerberg E.W., Kranse R., Wolters T., van den Bergh R.C., Bangma C.H. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol. 2010;57:79–85. doi: 10.1016/j.eururo.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 82.Jeong C.W., Lee S., Jung J.W., Lee B.K., Jeong S.J., Hong S.K. Mobile application-based Seoul national university prostate cancer risk calculator: development, validation, and comparative analysis with two Western risk calculators in Korean men. PLoS One. 2014;9:e94441. doi: 10.1371/journal.pone.0094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ku J.H., Ahn J.O., Lee C.H., Lee N.K., Park Y.H., Byun S.S. Distribution of serum prostate-specific antigen in healthy Korean men: influence of ethnicity. Urology. 2002;60:475–479. doi: 10.1016/s0090-4295(02)01807-1. [DOI] [PubMed] [Google Scholar]
- 84.Gao H.W., Li Y.L., Wu S., Wang Y.S., Zhang H.F., Pan Y.Z. Mass screening of prostate cancer in a Chinese population: the relationship between pathological features of prostate cancer and serum prostate specific antigen. Asian J Androl. 2005;7:159–163. doi: 10.1111/j.1745-7262.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 85.Belbase N.P., Agrawal C.S., Pokharel P.K., Agrawal S., Lamsal M., Shakya V.C. Prostate cancer screening in a healthy population cohort in eastern Nepal: an explanatory trial study. Asian Pac J Cancer Prev. 2013;14:2835–2838. doi: 10.7314/apjcp.2013.14.5.2835. [DOI] [PubMed] [Google Scholar]
- 86.Vu Le C., Dao O.Q., Khac Tran L.N. Mass screening of prostate cancer in Vietnam: current status and our opinions. Urol Oncol. 2010;28:673–676. doi: 10.1016/j.urolonc.2009.12.008. [DOI] [PubMed] [Google Scholar]