Abstract
Tuberculosis (TB) is the major cause of death from infectious diseases around the world, particularly in HIV infected individuals. TB vaccine design and development have been focused on improving Bacille Calmette-Guérin (BCG) and evaluating recombinant and viral vector expressed Mycobacterium tuberculosis (Mtb) proteins, for boosting BCG-primed immunity, but these approaches have not yet yielded significant improvements over the modest effects of BCG in protecting against infection or disease. On March 7–8, 2016, the National Institute of Allergy and Infectious Diseases (NIAID) convened a workshop on “The Impact of Mtb Immune Evasion on Protective Immunity: Implications for TB Vaccine Design” with the goal of defining immune mechanisms that could be targeted through novel research approaches, to inform vaccine design and immune therapeutic interventions for prevention of TB. The workshop addressed early infection events, the impact of Mtb evolution on the development and maintenance of an adaptive immune response, and the factors that influence protection against and progression to active disease. Scientific gaps and areas of study to revitalize and accelerate TB vaccine design were discussed and prioritized. These included a comprehensive evaluation of innate and Mtb–specific adaptive immune responses in the lung at different stages of disease; determining the role of B cells and antibodies (Abs) during Mtb infection; development of better assays to measure Mtb burden following exposure, infection, during latency and after treatment, and approaches to improving current animal models to study Mtb immunogenicity, TB disease and transmission.
Keywords: Tuberculosis, Mycobacterium tuberculosis, Vaccine, Immune evasion
1. Introduction
TB kills more people than any other single infectious disease in the world, recently surpassing HIV/AIDS. With the upsurge in multidrug resistant (MDR) and extensively drug resistant (XDR) strains of Mtb leading to a loss of therapeutic options, the lack of BCG efficacy against adult pulmonary TB, and BCG vaccination not being recommended by the World Health Organization (WHO) for children born with HIV infection, improvements in treatment, diagnosis, and identification of an effective TB vaccine are required [1]. Several of the recent vaccine candidates have not advanced through clinical development and there is a widely perceived need to step back and further analyze immune mechanisms and pathogen strategies that facilitate infection, transmission and disease by Mtb [2]. The NIAID convened a workshop on “The Impact of Mtb Immune Evasion on Protective Immunity: Implications for TB Vaccine Design” on March 7–8, 2016, with the goal of identifying novel research approaches that could help inform vaccine design and immune therapeutic interventions. Investigators from a variety of disciplines including basic and clinical TB research, immunology, microbiology and other infectious diseases were invited to participate in the workshop (Table 1). The workshop included two introductory presentations followed by three focused sessions. Session 1, which was chaired by Drs. Samuel Behar and Lalita Ramakrishnan, addressed “early infection events and the biological consequences of the host-pathogen interaction”. Session 2, which was chaired by Drs. Joel Ernst and Steven Porcelli, addressed “the impact of Mtb evolution on the development and maintenance of an adaptive immune response” and Session 3, which was chaired by Drs. Markus Maeurer and Hardy Kornfeld, discussed “factors that influence protection against or progression to active disease”.
Table 1.
List of participants.
| Name | Affiliation |
|---|---|
| Achkar, Jacqueline | Albert Einstein College of Medicine |
| Andersen, Peter | Statens Serum Institute, Denmark |
| Barber, Daniel | NIAID/NIH |
| Behar, Samuel | University of Massachusetts Medical School |
| Boom, W. Henry | Case Western Reserve University |
| Darrah, Patricia | NIAID/NIH |
| David, Sunil | University of Minnesota |
| Desvignes, Ludovic | New York University School of Medicine |
| Ernst, Joel | New York University School of Medicine |
| Hanekom, Willem | Gates Foundation |
| Hokey, David | AERAS |
| Kasmar, Anne | Gates Foundation |
| Khader, Shabaana | Washington University |
| Klein, Bruce | University of Madison-Wisconsin |
| Kornfeld, Hardy | University of Massachusetts Medical School |
| Levy, Bruce | Brigham and Women’s Hospital/Harvard Medical School |
| Lewinsohn, Deborah | Oregon Health and Science University |
| Lu, Lenette | Massachusetts General Hospital |
| Maeurer, Markus | Karolinska Institute and Karolinska Hospital, Sweden |
| Masopust, David | University of Minnesota |
| Nemes, Elisa | University of Cape Town, South Africa |
| Ottenhoff, Tom | Leiden University Medical Center, The Netherlands |
| Ouattara, Amed | University of Maryland - Baltimore |
| Porcelli, Steven | Albert Einstein College of Medicine |
| Ramakrishnan, Lalita | University of Cambridge |
| Russell, David | Cornell University |
| Schlesinger, Larry | Ohio State University |
| Schurr, Erwin | McGill University Health Centre, Canada |
| Shea, Jacqui | AERAS |
| Sher, Alan | NIAID/NIH |
| Sharpe, Arlene | Harvard Medical School |
| Tobin, David | Duke University School of Medicine |
| Urdahl, Kevin | Center for Infectious Disease Research |
To set the stage for the need for new TB vaccines and how biomedical research contributes to this endeavor, the workshop started with presentations by Drs. Jacqueline Shea and Lalita Ramakrishnan, on current challenges and strategies in vaccine research and host-Mtb interactions.
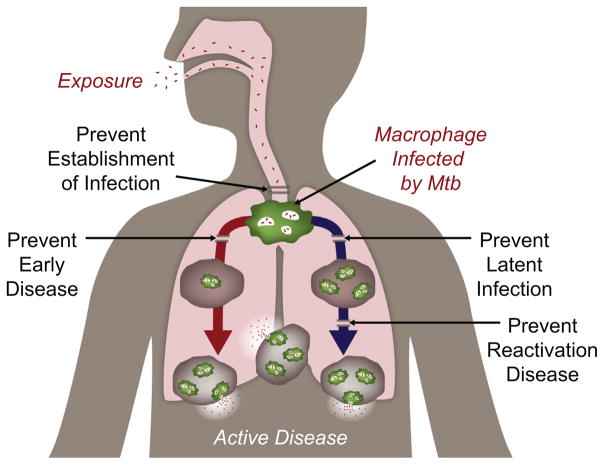
Dr. Shea provided an overview of the global TB epidemic and the clinical pipeline of TB vaccine candidates. She discussed the potential target mechanisms of action for new vaccination strategies such as preventing establishment of infection early, potentially in conjunction with preventing latent infection, or preventing disease development or its reactivation (Fig. 1). The current strategy by Aeras focuses on the development of a vaccine to prevent TB disease in adolescents and adults, since they constitute the largest reservoir of potential TB patients and the majority of transmission and prevention of active disease in these groups may have the largest impact on the epidemic. The disappointing results of the MVA85A Phase 2b trial [3] highlight the need for diversification in both the selection of types of vaccine candidates and the routes of vaccine administration, and for improvement in candidate selection through more predictive animal models. While comparing candidates in different animal models would also ensure reproducibility and robustness, the lack of adequate animal models shown to be predictive of human Mtb exposure/transmission/infection/disease continues to be a challenge. Developing an in vitro functional assay of vaccine effect, a correlate of vaccine-induced protection and a human challenge model would also help improve candidate selection. In addition, novel trial designs that incorporate smaller size, shorter duration, and decreased cost are also required to facilitate affordable clinical trials and to more rapidly identify potentially promising new approaches. Dr. Shea concluded by identifying the key scientific gaps that need to be addressed, including determining what constitutes a protective immune response, whether the response needs to be local or systemic, and whether different responses are needed at different stages of disease. She also highlighted the need to measure both the level of exposure to Mtb and the burden of Mtb during latency and to identify a true biomarker of infection and cure.
Fig. 1.
Strategies for TB vaccine development.
Dr. Ramakrishnan led the participants through a discussion on how Mtb exploits and evades both innate and adaptive immune responses (included in Table 2). Mtb’s ancient ancestor was probably a soil dwelling non-pathogenic mycobacterium that evolved to acquire virulence. She hypothesized that bacteria such as Mtb have acquired a lipid coat to help mask the bacterial pathogen-associated molecular patterns (PAMPs) and thereby avoid engagement with toll-like or other innate receptors and limit the recruitment of microbicidal macrophages. She suggested that some strains of Mtb use phenolic glycolipid (PGL) to induce the host to produce chemokines and other secreted factors that attract macrophages that are more permissive for Mtb replication, thereby enabling expansion of the Mtb niche. She cited the example of the Mtb Beijing strains, which carry PGL and are becoming more common worldwide, suggesting that they have a selective advantage over other strains.
Table 2.
Examples of Mtb immune evasion strategies discussed at the workshop.
| Host defense strategies | Mycobacterial counterstrategiesa |
|---|---|
| Recruit microbicidal macrophages to site of infection |
|
| Promote phagosome – lysosome fusion |
|
| Aggregate macrophages into epithelioid granulomas to restrict and contain mycobacteria |
|
| Processing and presentation of bacterial Ags |
|
| Activation of T cells to control infection |
|
| Increase microbicidal capacity of the granuloma by recruiting effector T cells to it |
|
Dr. Ramakrishnan then turned her focus to the formation of lung granulomas which develop as a part of the immune response to Mtb infection. She pointed out that upon traversing the epithelial barrier, mycobacteria then exploit the process of granuloma formation to expand their intracellular niche. They recruit macrophages by stimulating host pathways and then spread into these for niche expansion. She described the granuloma as a very permissive environment for bacterial replication and for the recruitment and infection of new macrophages. Granulomas with cavitary lesions are more likely to transmit bacteria to a new host, and since adolescents have fewer cavitary lesions, they are less likely to transmit bacteria. Dr. Ramakrishnan indicated that very little is known about the mechanisms that trigger the exit of Mtb from the granuloma. She described some observations that too little or too much tumor necrosis factor (TNF) may be a factor and that overall, there is a need to understand the fine balance required among macrophages, chemokines and cytokines to restrict both bacterial replication and localization to the granuloma. Dr. Ramakrishnan described strategies used by Mtb to evade the adaptive immune system. These include delaying DC migration and priming in the lymph node and inducing regulatory T cells (Tregs) that delay activation of effector T cells and their recruitment to the granuloma. All these evasive strategies will need to be considered when designing a TB vaccine. She also noted that Ab production against Mtb has long been recognized as a host response to disease progression, but it is unclear what role the humoral response plays in the immune defense against Mtb.
2. Session I: Early infection events and the biological consequences of host-pathogen interactions
Dr. Sam Behar set the stage for this session by introducing three T cell-based phases of Mtb infection: which he described as events occurring before, during or after T cell priming [4,5]. He briefly discussed how these events may affect the bacteria, whether heterogeneity of the host affects TB pathogenesis, and if early events are altered by pre-existing immunity. He highlighted how early events can be crucial in defining host immunity. These include: (1) a delay in the initiation of the T cell responses is detrimental to host defense; (2) T cell priming in the lymph node may expand T cells that cannot optimally recognize infected cells in the lung; (3) Mtb may evade T cell immunity by limiting antigen (Ag) presentation in the lung; and (4) the fate of infected cell and their cell death modality (apoptosis versus necrosis) can have an impact on T cell immunity. He also noted that T cell responses eliminate ~95% of the bacterial load (based on reduction from peak lung CFU). He suggested that heterogeneity in the type of host cells infected, in bacterial Ag expression, or adaptation of the bacteria to the intracellular niche, could impair T cell recognition of infected cells, and that these are barriers that vaccines may have to overcome [4,5].
Dr. Ludovic Desvignes proposed that early immune events can shape the outcome of Mtb infection [6–8] and recommended that studies of early events include assessing: (1) cellular diversity in terms of the cells that are infected, including the alveolar epithelium and lymphatic endothelium; (2) dynamics and localization of Mtb (alveoli/parenchyma/vasculature, extracellular); (3) effects of the temporal occurrence of inflammation; (4) triangulation across models in terms of specimens studied (bronchoalveolar lavage (BAL)/lung/blood); (5) early host/bacterial factors; (6) checkpoint interventions beyond conventional T cell-derived IFN-gamma; and (7) effects of trained innate immunity. He cautioned that early post-infection immune events vary depending on the animal model being studied and suggested that researchers in various disciplines (e.g. immunology, microbiology, systems and computational biology) devise multidisciplinary approaches to study early events in Mtb infection. This would involve using different animal models and incorporating knowledge from studies of other pathogens as well as non-infectious models (e.g. inflammation).
Dr. David Russell presented the microbiological view of early events in host-pathogen interactions and addressed the need for alternative experimental approaches to understand both TB disease progression and the inability of the host to effectively clear Mtb [9]. He proposed that modern microbiological readouts of pathogen fitness (e.g. fluorescent Mtb reporter strains) can be exploited to understand bacterial replication, disease progression and acquired immunity in mice [10,11]. Advantages of this approach include: (1) they are immunologically-“agnostic,” requiring no assumptions be made about the immune pathways required for control; (2) they are host species-independent and can have equal application in murine, non-human primate, and human cell infection models; and (3) they provide information on bacterial fitness and therefore relate directly to the growth state of the bacterial populations. He concluded that when analyzing affected cells and TB pathogenesis, a loss of control or a gain of permissiveness are two ways of characterizing the disease. To dissect this, one may need more microbiological read-outs rather than an immunological focus.
Dr. Shabaana Khader discussed how the innate immune response could be targeted in vaccine design to induce sterilizing immunity. She described the delay in T cell recruitment to the lungs of mice following initial exposure to Mtb and the need for T cells to localize to ectopic lymphoid structures near infected macrophages to control Mtb [12]. Although BCG vaccination speeds up T cell recruitment to the lung, it does not significantly reduce the bacterial load [13,14]. Dr. Khader suggested that induction of helper T cells or inducible Bronchus Associated Lymphoid Tissue (iBALT) at an even earlier time point in the lung could control Mtb load and this could be achieved by targeting CD103+ lung dendritic cells (DCs). Intestinal CD103+ DCs have been shown to promote IL-22 production from Group 3 Innate Lymphoid Cells (ILC3s) [15]. In the lung, IL-22 and IL-17 production from ILC3s are required for protection against Klebsiella pneumonaie and Streptococcus pneumoniae [16,17]. Thus, Dr. Khader presented various avenues of exploration for vaccine targeting by inducing ILC3s.
Dr. Behar addressed the question of whether or not prior exposure confers an advantage against re-challenge with Mtb using data generated from naïve and previously infected mice. He noted that recall of natural memory to Mtb infection elicits an intense T cell response; however, the resulting memory response is short lived and is only partially able to control Mtb (similar to memory responses elicited by vaccination) [18]. Detailed studies tracking the development of effector T cells from naïve or memory precursors, demonstrated that both naïve and central memory CD8+ T cells are initially activated in the lymph node and not in the lung, and their recruitment to the lung have similar kinetics. Dr. Behar concluded that improving the immune response to Mtb via vaccination will be a challenge given that memory T cell responses elicited via natural immunity or currently available vaccine candidates are not very effective in mediating protection against TB [19]. Panel members discussed whether generating tissue resident memory T cells in the lung might help advance efficacy of vaccines above and beyond what can be elicited for central memory T cells. However, they acknowledged that generating lung resident T cells through adoptive transfer did not result in these T cells being initially activated in the lung (they continued to be initially activated in the lymph node). In addition, they noted that the lung does not seem to be a conducive environment for T cell activation, possibly due to defects in Ag presenting cell (APC) function.
Dr. Henry Boom addressed some of the mechanisms by which Mtb-infected APCs subvert T cell recognition and function, and potential ways that vaccines could counteract this inhibition. Earlier studies from his group have shown that Mtb through prolonged activation via TLR2 inhibits MHC-II Ag processing and presentation in macrophages [20]. He discussed more recent studies demonstrating that Mtb lipoarabinomannan (LAM) and lipomannans (LM) are released in bacterial microvesicles from infected macrophages and can directly inhibit proximal TCR signaling, and induce CD4+ T cell anergy [21,22]. These mechanisms most likely occur during the effector phase of the T cell response and may be relevant to the inability of T cells to clear Mtb during primary infection and during latent infection in lung granulomas. These findings led Dr. Steven Porcelli to suggest that secreted immunomodulatory molecules such as these might be appropriately targeted by Abs, which might facilitate their removal from the body or neutralize their immunomodulatory effects. Dr. Boom further theorized that overcoming Mtb’s ability to evade T cell immune recognition presents a substantial obstacle for new TB vaccine strategies aimed at reducing establishment of latent Mtb infection and at enhancing control/elimination of established latent Mtb infection in the lung. For example, vaccines that stimulate robust IFN-gamma and IL-2 responses and provide strong co-stimulation, may be able to overcome CD4+ T cell anergy. The large diversity of Mtb Ags/epitopes recognized by the human peripheral T cell response may not be reflective of what is actually processed and presented by latent Mtb infected cells in the lung and elsewhere. Focusing on antigens/epitopes that are expressed by MHC-I/II on these infected cells, even if at low frequency, will also increase the likelihood of producing a successful new TB vaccine.
Dr. Bruce Klein provided insights from the perspective of fungal infections [23–25] and highlighted similarities between these and TB. Like TB, fungal infections result in a spectrum of diseases, disease progression, dysregulated immunity, latency and reactivation upon immune compromise. He highlighted features of fungal diseases, including early pathogenesis and host response, immune evasion mechanisms, adaptive immunity and fungal clearance. Similarities between Blastomyces and Mtb include slow doubling times, delayed growth in the lung of mice, dissemination outside of the lung, residence inside the alveolar macrophages and reshaping of the host immune response, such as loss of IL-17 and GM-CSF producing innate lymphocytes that are involved in mediating early control.
3. Session II: The impact of Mtb evolution on the development and maintenance of an adaptive immune response
Dr. Joel Ernst set the stage for how to develop a better understanding of adaptive immunity to Mtb with three key considerations: (1) The presence of an adaptive immune response is not necessarily indicative of its ability to effectively control TB; (2) Mtb has approximately 4000 protein coding genes that are potentially recognized by the immune system; and (3) Mtb and humans have coevolved for millions of years, allowing the bacterium to exquisitely optimize its relationship with its host.
Dr. Ernst developed the hypothesis that human T cell recognition exerts little selection pressure on Mtb Ags based on the following findings: (1) human T cell epitopes for Mtb are evolutionarily hyper-conserved [26]; (2) Mtb T cell epitope analyses has revealed a paucity of antigenic variants and rare variable Mtb Ag [27], and sequence diversity in the pe_prgs genes—some of the most variable genes of Mtb—is independent of human T cell recognition [28]. Furthermore, Mtb manipulates Ag expression to evade T cell recognition by inducing suboptimal activation of CD4 effector cells [29] and diverting secreted antigens from the MHC class II pathway to the export pathway, avoiding CD4 T cell recognition [30].
Dr. David Masopust focused on the role of tissue-resident memory T cells. He emphasized the current limitations in measuring immunity in tissues versus peripheral blood [31] and that techniques used to enumerate and characterize immune cells in mucosal tissues have to be carefully considered since analysis of memory CD8+ T cells using disaggregated tissues results in an underestimation of cell numbers, compared to analysis via quantitative immune florescence microscopy [32]. Dr. Masopust highlighted that laboratory mice are relatively pathogen-free compared to mice obtained from pet stores or humans, making lab mice immune transcriptome more similar to the transcriptome of human cord blood-derived cells while peripheral blood mononuclear cells (PBMCs) from pet store mice behave more like human adult PBMCs. Co-housing laboratory mice with pet store mice, resulted in changes to the microbiota of the laboratory mice, altered the phenotype and distribution of CD8+ T cells, increased serum Ab titers and generated a PBMC transcriptional profile similar to that seen in human adult PBMCs [33]. Co-housed laboratory mice were also more resistance to Listeria monocytogenes infection. Dr. Masopust emphasized that improvements of mouse models, with regard to prior pathogen and commensal microbiota exposures, may make their immune response to Mtb more similar to those in humans, and thus may accelerate the evaluation of TB vaccines.
Dr. Tom Ottenhoff addressed two key challenges to TB vaccine research: the approach to Ag selection and the lack of known correlates of protection from Mtb infection or disease. He indicated that candidate TB vaccine antigens should be broadly recognized by the immune system of different human populations, and that both the immune status of different populations (e.g. adults, neonates, young children, HIV infected) as well as the diversity of circulating Mtb strains should be evaluated. To address the lack of correlates of protection, Dr. Ottenhoff suggested more comprehensive unbiased approaches, including surveys of the entire immune space during infection and disease, akin to efforts undertaken in the HIV field [34]. This would involve expanding Mtb-induced immunity studies to all immune cell populations, including mucosal/lung resident T cells, as they may have very different functional profiles compared to peripheral blood T cells; as well as investigating the role B cells and tissue resident cells, and using functional assay based approaches such as mycobacterial growth inhibition assays. Dr. Ottenhoff highlighted the need for further investigation into the role of subdominant Ags in the immune response to Mtb, since mouse models have shown their protective role in cancer and in some infectious diseases, including TB [35]. He noted that while the majority of Mtb epitopes may be hyper-conserved, recent work shows that a minority of Mtb epitopes is variable between different strains. From a vaccine standpoint, he suggested that adjuvants or immune-modulators might offer novel methods to redirect the type of immune response toward hyper-conserved or subdominant epitopes, preventing T cell exhaustion and promoting long term functional T cell memory.
As noted above for Dr. Klein’s presentation, this workshop provided an opportunity for researchers outside of the TB field to share their experience with other pathogens. Dr. Amed Ouattara described similarities and differences in the development of malaria versus TB vaccines. He described how correlates of protection are being studied in malaria by expressing stage-specific Ags and assessing their immunogenicity. Specifically, Abs derived against these stage-specific Ags are being assessed in in vitro inhibition assays. This Ag selection strategy requires that the candidate Ag be essential for parasite survival, be conserved among strains and be available to the immune system. Dr. Ouattara indicated that 3 types of malaria vaccines are being designed, one for each of the 3 malarial lifecycle stages. He also noted that malaria vaccine research has benefited from the availability of an effective human challenge model, and that a new humanized mouse model engineered to generate human red blood cells and lymphocytes may be used to identify candidate malaria antigens.
Dr. Arlene Sharpe described immunoregulatory pathways that control T cell responses and how they provide opportunities for immunotherapy against TB. Several inhibitory receptors have been discovered on T cells including CTLA4, LAG3, PD-1, and TIM3, and the functions of such co-inhibitory pathways include: (1) maintaining immune tolerance; (2) protecting tissues from damage by immune responses; (3) tuning-down the immune response after a pathogen is eliminated; and (4) promoting the resolution of inflammation [36]. Dr. Sharpe focused on the PD-1 receptor, which is upregulated on T cells upon activation, resulting in reduced T cell receptor (TCR) signaling and T cell functions such as cytokine production and cytolysis [37]. PD-1 is also expressed on B cells, macrophages, and DCs and the PD-1 pathway has been exploited by tumors and pathogenic microbes to evade immune-mediated elimination by promoting control of inflammation, inhibiting the activation of self-reactive or pathogenic effector T cells, and by inducing Tregs. Abrogation of the PD-1 pathway induces an influx of inflammatory cells into the target tissues. Dr. Sharpe added that T cells can co-express multiple inhibitory receptors, rendering them increasingly less functional [38]. Thus, co-blockade would enable better rescue of dysfunctional T cells than blockage of a single inhibitory receptor. This kind of combination therapy is being used in cancer and may prove useful in controlling Mtb.
Dr. Kevin Urdahl indicated that the frequency of systemic Mtb-specific Th1 cells does not correlate with protection in the mouse model, and that the immune system is unable to eradicate Mtb despite an apparent abundance of Mtb-specific Th1 cells in the lungs. Thus, the simple strategy of increasing Th1 cell numbers will not result in protection [39]. He proposed that the rapidity of the lung Th1 response is critical and a delayed Th1 response allows Mtb to establish a lung niche. Furthermore, once Mtb establishes a lung niche within granulomas, T cell effectiveness is limited. He indicated that Ag availability restricts the protective capacity of T cells in two distinct ways. Reduced Ag expression during chronic infection limits the effectiveness of some T cells, whereas persistent Ag expression leads to functional exhaustion of other T cells. Dr. Urdahl questioned whether a single vaccine could optimally induce both rapid and durable immunity. He suggested that complementary vaccination approaches may be needed: the first to induce lung-resident T cells that act early to prevent granulomas from being established; and the second to induce central memory T cells that provide prolonged immunity and prevent progression of established infection.
Since Mtb is primarily an intracellular pathogen, the role of Abs in TB immunity remains a subject of controversy. However, recent findings have suggested that Abs may be important role in modifying or controlling Mtb infections. Dr. Jacqueline Achkar noted that Abs can effectively control intracellular bacteria, parasites and fungi [40] and in murine TB models, Mtb-specific Abs reduce pathogenicity while diminished B cell and humoral function increases susceptibility to TB [41]. In general, there is an inverse relationship between the levels and several functions of Mtb-specific Abs and susceptibility to infection and disease in humans and animals. Abs may control infection not only via neutralization but also through Fc-mediated functions like antibody-dependent cellular phagocytosis (ADCP) or antibody-dependent cell-mediated cytotoxicity (ADCC). For example, Abs targeting the Mtb glycolipid cell wall Ag LAM and/or the capsular polysaccharide AM have been associated with protection in vivo in animal models [42,43] and in in vitro studies using samples from BCG vaccinated individuals [44,45].
4. Session III: Factors that influence protection against or progression to active disease
In the introduction to this session, Dr. Markus Maeurer indicated that BCG vaccination, exposure to non-tuberculous mycobacteria (NTM) and frequent Mtb exposure in high burden countries influence protective immune responses to Mtb infections, and these influences may have biological and/or clinical consequences. He highlighted other immune modulating factors that may be influential including cross-reactivity with other pathogens, the host microbiome, and early induction of autophagy in infected cells [46–48]. He then listed key factors to consider for eliciting more effective pulmonary anti-Mtb immune responses: (1) aerosol vaccination strategies; (2) activation of early myeloid cell responses; (3) activation of resident innate immune cells; (4) induction of lung surfactants to increase phagocytosis of Mtb bacilli by myeloid cells; (5) early but controlled mucus production in the airways and lungs to help trap invading Mtb bacilli; (6) induction of lung-resident B-cell responses (Abs and cytokines); (7) induction of optimal lung-resident Ag-specific T-cell responses; and (8) induction of fatty acid metabolism in lung-resident memory CD8+ T cells, which may be needed for long-term memory. Dr. Maeurer highlighted that induction of long-term memory responses in key anatomic locations will be crucial to successful immunization against TB.
Dr. Peter Andersen noted that the requirements for preventive versus post-exposure vaccines are different and that there are many variables to consider. A preventive vaccine would largely be administered to infants who are mostly Mtb-negative, whereas, vaccines targeting adolescents would largely be post-exposure since adolescents will have already received BCG, and in high burden countries 40–60% of them will already have latent TB infection (LTBI). Data from murine models indicated that not all preventive TB vaccines have post-exposure activity [49] and vaccination doses and schema optimized for preventive TB vaccines are not suitable for post-exposure vaccination. He showed data indicating that a low concentration of Ag is more effective when used in a preventive vaccine given post exposure rather than a high dose of Ag that is optimized for a non-infected individual. Furthermore, immunization with a modified immunogen (e.g. deletion of the immunodominant region of ESAT-6) resulted in a qualitatively different immune response with an increased number of memory cells as compared to immunization with the unmodified immunogen [50]. He highlighted several research areas that need to be addressed, including a better understanding of the differences between pre- and post-exposure vaccination and Ag selection [e.g. how Ag expression is influenced by adaptive immunity in LTBI], vaccine dose and delivery systems and which features of the natural immune response to Mtb seen in LTBIs should modulated by vaccination.
Dr. Daniel Barber indicated that it is important to determine what effector molecules are produced by CD4+ T cells to control Mtb infection and whether different molecules are used at different anatomical locations. There is some evidence that CD4 T cells can restrict Mtb growth in macrophages through an IFNγ-independent mechanism. To estimate the relative contribution of IFNγ-dependent or independent mechanisms to CD4+ T cell-mediated control of Mtb, T cell-deficient mice were reconstituted with WT or IFNγ−/− CD4 + T cells. Data showed that only ~30% of T cell-dependent suppression of bacterial growth in the lung is dependent on IFNγ, in contrast to >80% in the spleen. It appears that the amount of IFN-γ produced by individual T cells is more important than the total amount of IFN-γ in the lung, and that protective effects elicited by cytokines can be organ-specific. Dr. Barber summarized these findings by stating that susceptibility to mycobacteria is extreme in the absence of IFNγ, but increasing the production of IFNγ by T helper cells can also lead to lethal disease. Thus, CD4+ T cell-derived IFNγ can be protective in one tissue, while simultaneously being detrimental in another. He noted that the presence of cytokine receptors in the local environment also needs to be considered [51].
Dr. Bruce Levy discussed general concepts of fatty acid-derived lipid mediators, their role in Mtb pathogenesis and their potential for regulating the progression or resolution of Mtb infection. Several lines of evidence suggest lipid mediators play a pivotal role in cytokine regulation, mechanisms of macrophage death, T cell responses, and bacterial replication [52]. Among the molecules involved are arachidonic acid-derived mediators, such as Prostaglandin E2, Leukotriene B4 (LTB4), and Lipoxin A4 (LXA4) as well as are other specialized pro-resolving mediators derived from omega-3 fatty acids (e.g. resolvins, protectins and maresins) [53,54]. Studies in mouse models have shown that interference of the leukotriene pathway by using clinically available drugs (e.g. zileuton) had a neutral or slightly positive effect on the control of Mtb infection, although LTB4 was not effective. Furthermore he noted that administration of LXA4 in too high amounts or too early, led to a poor disease outcome in the TB mouse model. Lipid mediators can also modulate vaccine efficacy as they are involved in the regulation of DC function and they have effects on CD4 T cells, B cells, NK cells, ILC2s and regulatory T cells [55–57].
Dr. Deborah Lewinsohn’s presentation focused on infant TB immunity and described the benefits of focusing on infants as the target population for an improved TB vaccine to protect infants, children, and adults. The burden of childhood TB is estimated at 1,000,000 cases and 140,000 deaths per year [1]. TB is the cause of 50% of pneumonia deaths in children from TB endemic areas and those children who survive TB suffer from significant morbidity. Dr. Lewinsohn indicated that studying infants with primary Mtb infection is important for the design of effective vaccines and immunodiagnostics for infants and children, as well as for understanding the immune response to primary infection in adults. There is also a need to have an animal model of infant TB, such as an infant macaque model, to model the human infant TB immunity. A number of vaccine studies from South Africa have shown that one can elicit robust innate and adaptive immune responses in children, although their T cell responses are less robust than those of adult T cells [58]. Dr. Lewinsohn commented that this finding highlights the importance of conducting phase 1 trials in the population of interest.
Dr. Erwin Schurr provided an overview of the current understanding of the genetics of host protection from Mtb infection following prior exposure to mycobacteria or BCG vaccination. A combination of genome-wide approaches to assess the contribution of host genetics to immunity has shown that there is a high level of inheritability of protection (25–90%) [59]. Epidemiological studies found that individuals with LTBI or with TST-positivity were protected against secondary infection and progressive disease. Furthermore, TB patients who have been cured of TB have a five-fold higher risk for reinfection and development of TB compared to the general population [60]. Overall, the genetic loci for TB susceptibility that have been identified thus far are not classical immune response genes and he noted that the genetic and/or epigenetic basis for these types of important observations will need to be determined through prospective cohort studies [61]. Dr. Schurr stated that for all types of studies, whether genetic or immunologic, having robust data on phenotype definition is key (dose, age, gender), and for genetic studies, research on extreme phenotypes may provide the most useful information.
5. Conclusion
The workshop concluded with a vigorous discussion highlighting a number of scientific opportunities to gain greater insights into the impact of Mtb immune evasion on protective immunity and their implications for TB vaccine design (see Table 3). There was recognition that it is both important and challenging to improve our understanding of innate and adaptive immune responses to Mtb in the lung. More studies are needed to assess the role of B cells and functional Abs, unconventional T cells, trained immunity and the microbiome following Mtb infection and vaccination. Developing improved animal models (that more closely mimic human Mtb infection) as well as functional assays for studying the immune response to Mtb, will be crucial for effectively guiding future vaccine design. An additional focus on Ag selection and an increased understanding of the impact of prior Mtb exposure on vaccine responses are also needed. Investigational vaccine studies in humans need to be designed to not only evaluate the candidate vaccine but to understand fundamental immunological questions. It was noted that a survey of the entire immune space akin to efforts that have been made in HIV might also help break through the barrier in identifying correlates of protective immunity in TB. In summary, to significantly advance our efforts in the design and development of improved TB vaccines and vaccination strategies, novel research approaches, including those that focus on Mtb immune evasion strategies and protective immunity need to be designed, supported, and undertaken.
Table 3.
Scientific gaps and opportunities in TB research.
Immunology
|
Model/assay development
|
Human studies
|
Acknowledgments
The authors gratefully acknowledge the contributions of all the workshop speakers and attendants for their presentations and constructive discussions; all the other members of the NIAID TB Vaccine Working Group (Elizabeth Adams, Alison Deckhut Augustine, Alan Embry, Lakshmi Jayashankar, Peter Kim, Linda Lambert, Wolfgang Leitner, Makhene Mamodikoe, Sarah Read, Roxana Rustomjee and Christine Sizemore) for their critical review and editing of the manuscript and to Karen Thiebes (www.simplifiedsciencepublishing.com) for graphic design. The meeting was supported by NIAID through the HHSN272201100001G – Research Support Services for the Division of AIDS, HHSN272201200011C – Integrated Research Mission Support Services, Palladian Partners Inc. for Division of Allergy, Immunology and Transplantation, and HHSN263201200022I – EDJ Associates under the NIHCATSII task order system for the Division of Microbiology and Infectious Diseases.
Footnotes
Author contributions
The authors discussed and wrote the manuscript in collaboration.
Conflict of interest statement
The authors have no conflicts of interest associated with this publication.
References
- 1.World Health Organization. [accessed 3/15/2017];Global TB report 2016. < http://www.who.int/tb/publications/global_report/gtbr2016_main_text.pdf?ua=1>.
- 2.Kaufmann SH, Weiner J, von Reyn CF. Novel approaches to tuberculosis vaccine development. Int J Infect Dis. 2017;56:263–7. doi: 10.1016/j.ijid.2016.10.018. http://dx.doi.org/10.1016/j.ijid.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4. http://dx.doi.org/10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol. 2014;12(4):289–99. doi: 10.1038/nrmicro3230. http://dx.doi.org/10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behar SM, Carpenter SM, Booty MG, Barber DL, Jayaraman P. Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: immunity interruptus. Semin Immunol. 2014;26(6):559–77. doi: 10.1016/j.smim.2014.09.003. http://dx.doi.org/10.1016/j.smim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbian S, Bandyopadhyay N, Tsenova L, O’Brien P, Khetani V, Kushner NL, et al. Early innate immunity determines outcome of Mycobacterium tuberculosis pulmonary infection in rabbits. Cell Commun Signal. 2013;11:60. doi: 10.1186/1478-811X-11-60. http://dx.doi.org/10.1186/1478-811X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179(4):2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 8.Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2(1):29–39. doi: 10.1016/j.chom.2007.06.004. http://dx.doi.org/10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Tan S, Huang L, Abramovitch RB, Rohde KH, Zimmerman MD, et al. Immune activation of the host cell induces drug tolerance in Mycobacterium tuberculosis both in vitro and in vivo. J Exp Med. 2016;213(5):809–25. doi: 10.1084/jem.20151248. http://dx.doi.org/10.1084/jem.20151248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S, Sukumar N, Abramovitch RB, Parish T, Russell DG. Mycobacterium tuberculosis responds to chloride and pH as synergistic cues to the immune status of its host cell. PLoS Pathog. 2013;9(4):e1003282. doi: 10.1371/journal.ppat.1003282. http://dx.doi.org/10.1371/journal.ppat.1003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukumar N, Tan S, Aldridge BB, Russell DG. Exploitation of Mycobacterium tuberculosis reporter strains to probe the impact of vaccination at sites of infection. PLoS Pathog. 2014;10(9):e1004394. doi: 10.1371/journal.ppat.1004394. http://dx.doi.org/10.1371/journal.ppat.1004394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123(2):712–26. doi: 10.1172/JCI65728. http://dx.doi.org/10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagranderie MR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64(1):1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 15.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276–87. doi: 10.1016/j.immuni.2011.12.011. http://dx.doi.org/10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG. Innate lymphocyte/Ly6C(hi) monocyte crosstalk promotes klebsiella pneumoniae clearance. Cell. 2016;165(3):679–89. doi: 10.1016/j.cell.2016.03.017. http://dx.doi.org/10.1016/j.cell.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, et al. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis. 2014;210(3):493–503. doi: 10.1093/infdis/jiu106. http://dx.doi.org/10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter SM, Nunes-Alves C, Booty MG, Way SS, Behar SM. A Higher activation threshold of memory CD8+ T cells has a fitness cost that is modified by TCR affinity during tuberculosis. PLoS Pathog. 2016;12(1):e1005380. doi: 10.1371/journal.ppat.1005380. http://dx.doi.org/10.1371/journal.ppat.1005380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes-Alves C, Booty MG, Carpenter SM, Rothchild AC, Martin CJ, Desjardins D, et al. Human and murine clonal CD8+ T Cell expansions arise during tuberculosis because of TCR selection. PLoS Pathog. 2015;11(5):e1004849. doi: 10.1371/journal.ppat.1004849. http://dx.doi.org/10.1371/journal.ppat.1004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8(4):296–307. doi: 10.1038/nrmicro2321. http://dx.doi.org/10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahon RN, Sande OJ, Rojas RE, Levine AD, Harding CV, Boom WH. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 2012;275(1–2):98–105. doi: 10.1016/j.cellimm.2012.02.009. http://dx.doi.org/10.1016/j.cellimm.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sande OJ, Karim AF, Li Q, Ding X, Harding CV, Rojas RE, et al. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis induces CD4+ T Cell Anergy via GRAIL. J Immunol. 2016;196(2):691–702. doi: 10.4049/jimmunol.1500710. http://dx.doi.org/10.4049/jimmunol.1500710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterkel AK, Lorenzini JL, Fites JS, Subramanian Vignesh K, Sullivan TD, Wuthrich M, et al. Fungal mimicry of a mammalian aminopeptidase disables innate immunity and promotes pathogenicity. Cell Host Microbe. 2016;19(3):361–74. doi: 10.1016/j.chom.2016.02.001. http://dx.doi.org/10.1016/j.chom.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterkel AK, Mettelman R, Wuthrich M, Klein BS. The unappreciated intracellular lifestyle of Blastomyces dermatitidis. J Immunol. 2015;194(4):1796–805. doi: 10.4049/jimmunol.1303089. http://dx.doi.org/10.4049/jimmunol.1303089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma A, Wuthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5(3):a019612. doi: 10.1101/cshperspect.a019612. http://dx.doi.org/10.1101/cshperspect.a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42(6):498–503. doi: 10.1038/ng.590. http://dx.doi.org/10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coscolla M, Copin R, Sutherland J, Gehre F, de Jong B, Owolabi O, et al. M. tuberculosis T Cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe. 2015;18(5):538–48. doi: 10.1016/j.chom.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copin R, Coscolla M, Seiffert SN, Bothamley G, Sutherland J, Mbayo G, et al. Sequence diversity in the pe_pgrs genes of Mycobacterium tuberculosis is independent of human T cell recognition. MBio. 2014;5(1) doi: 10.1128/mBio.00960-13. http://dx.doi.org/10.1128/mBio.00960-13 [e00960-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7(5):e1002063. doi: 10.1371/journal.ppat.1002063. http://dx.doi.org/10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava S, Grace PS, Ernst JD. Antigen export reduces antigen presentation and limits T cell control of M. tuberculosis. Cell Host Microbe. 2016;19(1):44–54. doi: 10.1016/j.chom.2015.12.003. http://dx.doi.org/10.1016/j.chom.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–22. doi: 10.1038/nprot.2014.005. http://dx.doi.org/10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161(4):737–49. doi: 10.1016/j.cell.2015.03.031. http://dx.doi.org/10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–6. doi: 10.1038/nature17655. http://dx.doi.org/10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manrique A, Adams E, Barouch DH, Fast P, Graham BS, Kim JH, et al. The immune space: a concept and template for rationalizing vaccine development. AIDS Res Hum Retroviruses. 2014;30(11):1017–22. doi: 10.1089/aid.2014.0040. http://dx.doi.org/10.1089/AID.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodworth JS, Andersen P. Reprogramming the T Cell response to tuberculosis. Trends Immunol. 2016;37(2):81–3. doi: 10.1016/j.it.2015.12.009. http://dx.doi.org/10.1016/j.it.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955–72. doi: 10.1016/j.immuni.2016.05.002. http://dx.doi.org/10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45. doi: 10.1038/ni1443. http://dx.doi.org/10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 38.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–73. doi: 10.1146/annurev-immunol-032414-112049. http://dx.doi.org/10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 39.Urdahl KB. Understanding and overcoming the barriers to T cell-mediated immunity against tuberculosis. Semin Immunol. 2014;26(6):578–87. doi: 10.1016/j.smim.2014.10.003. http://dx.doi.org/10.1016/j.smim.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev. 2015;264(1):167–81. doi: 10.1111/imr.12276. http://dx.doi.org/10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe. 2013;13(3):250–62. doi: 10.1016/j.chom.2013.02.009. http://dx.doi.org/10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95(26):15688–93. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138(1):30–8. doi: 10.1111/j.1365-2249.2004.02593.x. http://dx.doi.org/10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73(10):6711–20. doi: 10.1128/IAI.73.10.6711-6720.2005. http://dx.doi.org/10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, et al. Association of human antibodies to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis. 2016;214(2):300–10. doi: 10.1093/infdis/jiw141. http://dx.doi.org/10.1093/infdis/jiw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schito M, Migliori GB, Fletcher HA, McNerney R, Centis R, D’Ambrosio L, et al. Perspectives on advances in tuberculosis diagnostics, drugs, and vaccines. Clin Infect Dis. 2015;61(Suppl 3):S102–S1018. doi: 10.1093/cid/civ609. http://dx.doi.org/10.1093/cid/civ609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zumla AI, Schito M, Maeurer M. Advancing the portfolio of tuberculosis diagnostics, drugs, biomarkers, and vaccines. Lancet Infect Dis. 2014;14(4):267–9. doi: 10.1016/S1473-3099(14)70028-3. http://dx.doi.org/10.1016/S1473-3099(14)70028-3. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, et al. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med. 2014;2(4):301–20. doi: 10.1016/S2213-2600(14)70033-5. http://dx.doi.org/10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 49.Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, et al. ESAT-6 (EsxA) and TB10. 4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS ONE. 2013;8(12):e80579. doi: 10.1371/journal.pone.0080579. http://dx.doi.org/10.1371/journal.pone.0080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol. 2014;192(7):3247–58. doi: 10.4049/jimmunol.1300283. http://dx.doi.org/10.4049/jimmunol.1300283. [DOI] [PubMed] [Google Scholar]
- 51.Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, et al. CD4 T cell-derived IFN-gamma plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 2016;12(5):e1005667. doi: 10.1371/journal.ppat.1005667. http://dx.doi.org/10.1371/journal.ppat.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10(8):899–906. doi: 10.1038/ni.1758. http://dx.doi.org/10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115(6):1601–6. doi: 10.1172/JCI23949. http://dx.doi.org/10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205(12):2791–801. doi: 10.1084/jem.20080767. http://dx.doi.org/10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herb F, Thye T, Niemann S, Browne EN, Chinbuah MA, Gyapong J, et al. ALOX5 variants associated with susceptibility to human pulmonary tuberculosis. Hum Mol Genet. 2008;17(7):1052–60. doi: 10.1093/hmg/ddm378. http://dx.doi.org/10.1093/hmg/ddm378. [DOI] [PubMed] [Google Scholar]
- 56.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148(3):434–46. doi: 10.1016/j.cell.2011.12.023. http://dx.doi.org/10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140(5):717–30. doi: 10.1016/j.cell.2010.02.013. http://dx.doi.org/10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewinsohn DA, Lewinsohn DM. Immunologic susceptibility of young children to Mycobacterium tuberculosis. Pediatr Res. 2008;63(2):115. doi: 10.1203/PDR.0b013e3181652085. http://dx.doi.org/10.1203/PDR.0b013e3181652085. [DOI] [PubMed] [Google Scholar]
- 59.Abel L, El-Baghdadi J, Bousfiha AA, Casanova JL, Schurr E. Human genetics of tuberculosis: a long and winding road. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130428. doi: 10.1098/rstb.2013.0428. http://dx.doi.org/10.1098/rstb.2013.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54(6):784–91. doi: 10.1093/cid/cir951. http://dx.doi.org/10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox GJ, Orlova M, Schurr E. Tuberculosis in newborns: the lessons of the “Lubeck Disaster” (1929–1933) PLoS Pathog. 2016;12(1):e1005271. doi: 10.1371/journal.ppat.1005271. http://dx.doi.org/10.1371/journal.ppat.1005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–66. doi: 10.1038/nri3211. http://dx.doi.org/10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 63.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12(8):581–91. doi: 10.1038/nri3259. http://dx.doi.org/10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 64.Goldberg MF, Saini NK, Porcelli SA. Evasion of innate and adaptive immunity by Mycobacterium tuberculosis. Microbiol Spectr. 2014;2(5) doi: 10.1128/microbiolspec.MGM2-0005-2013. http://dx.doi.org/10.1128/microbiolspec.MGM2-0005-2013. [DOI] [PubMed] [Google Scholar]