Abstract
Background
Imaging markers that are sensitive to parkinsonism across multiple sites are critically needed for clinical trials.
Objective
To evaluate changes in the substantia nigra using single- and bi-tensor models of diffusion magnetic resonance imaging in PD, MSA, and PSP.
Methods
The study cohort (N=425) included 107 healthy controls, 184 PD, 63 MSA, and 71 PSP patients from 3 movement disorder centers. Bi-tensor free-water, free-water corrected fractional anisotropy, free-water corrected mean diffusivity, single-tensor fractional anisotropy and single-tensor mean diffusivity were computed for anterior and posterior substantia nigra. Correlations were computed between diffusion MRI measures and clinical measures.
Results
In the posterior substantia nigra, free-water was greater for PSP than MSA, PD, and controls. PD and MSA both had greater free-water than controls. Free-water corrected fractional anisotropy values were greater for PSP when compared to controls and PD. PSP and MSA single-tensor mean diffusivity values were greater than controls and single-tensor fractional anisotropy values were lower for PSP than healthy controls. The parkinsonism effect size for free-water in the posterior substantia nigra was 0.145 and for single-tensor mean diffusivity was 0.072. The direction of correlations between single-tensor mean diffusivity and free-water values and clinical scores were similar at each site.
Conclusions
Free-water values in the posterior substantia nigra provide consistent pattern of findings across PD, MSA and PSP in a large cohort across three sites. Free-water in the posterior substantia nigra relates to clinical measures of motor and cognitive symptoms in a large cohort of parkinsonism.
Introduction
Loss of dopaminergic neurons in the substantia nigra is common to several forms of parkinsonism, including Parkinson’s disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP)1–3. Neuronal loss has been documented to have more widespread damage in PSP patients than PD or MSA patients4. In addition, several studies have indicated that MRI-derived values can provide utility in detecting structural differences between parkinsonian syndromes. A reliable and reproducible surrogate marker of degeneration within the substantia nigra on clinical MRI scanners is of great interest in terms of clinical studies and long-term management of disease severity. A recent multi-site study has shown that free-water derived from diffusion MRI can detect differences in the substantia nigra between PD and healthy controls5. However, other studies have shown across sites that diffusion MRI metrics such as fractional anisotropy may not be useful in detecting differences in the substantia nigra between parkinsonian syndromes and healthy controls6,7. The lack of convergence across studies most likely arises from differences in the pulse sequence used in the diffusion acquisition, analysis methods, diffusion metric of choice, and location of the regions within the substantia nigra where these measures are computed.
Diffusion MRI measures water motion within a voxel, which is affected by the cellular, axonal, and extracellular environment. Models of the diffusion signal help determine the underlying biological structures that determine the signal intensity. A single-tensor model approximates the data as a 3-dimensional Gaussian diffusion processes. In instances of pathology, single-tensor models cannot distinguish from the many cases of pathology. For example, crossing nerve bundles or fluid contamination may alter the diffusion signal in the same way or underestimating the unique orientation contribution of the structures comprising a particular voxel. To deal with fluid contamination, a bi-tensor model addresses the limitations of the single-tensor model to include a free-water compartment that originates in the extracellular space8. Using a bi-tensor model, it was shown that free-water (i.e. fractional volume of the free-water compartment) in the substantia nigra is increased in PD compared with control subjects in both a single site cohort and the multi-site cohort from the Parkinson’s Progressive Marker Initiative (PPMI)5. Also, it was found that free-water in the substantia nigra increases over time in PD9, and is elevated in MSA and PSP10. While there is much promise in using a bi-tensor model, these sample sizes have been small, and there has not been a study directly comparing the utility of a bi-tensor model with the single-tensor model in the substantia nigra for PD, MSA, and PSP patients across several sites. The goal of the current study was to compare directly the two diffusion MRI analysis methods, namely the conventional single-tensor model and the bi-tensor model8 in the largest cohort of subjects with parkinsonism to date. We quantified diffusion metrics in the substantia nigra across 425 individuals acquired at three imaging sites. This is a retrospective study, and the pulse sequence was not matched across sites. Each site included healthy controls (HC), PD, MSA, and PSP cohorts. We tested the following two hypotheses: 1) free-water in the substantia nigra will be elevated in the substantia nigra of Parkinsonism across sites, and 2) free-water in the posterior substantia nigra estimated with a bi-tensor model correlates with clinical symptoms across sites.
Methods
This study includes 425 subjects across 4 groups including HC, PD, MSA, and PSP. Subjects were enrolled in studies at the Medical University Innsbruck (MUI) and Parkinson’s Disease Biomarker Program (PDBP). The PDBP for MR data includes sites at the University of Florida (UFLORIDA) and Penn State University Hershey Medical Center (PSHMC) (Table 1). Healthy controls were individuals over the age of 40 who did not have a prior history of major psychiatric or neurological disease. Patients were recruited and diagnosed by movement disorder specialists at each site using established criteria11–13. Disease severity was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) part III, the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III14, and the Hoehn-Yahr scale. UPDRS III scores were converted to MDS-UPDRS III scores15. Patients at MUI and PSHMC were examined on regular medication, whereas patients at UFLORIDA were examined after over-night withdrawal of anti-Parkinsonian medications. All individuals also underwent clinical assessment across sites that measured depression (Beck Depression Inventory16 and Hamilton Depression Rating Scale17), and cognition (Montreal Cognitive Assessment18 and Mini-mental state examination19). All healthy controls with a UPDRS part III greater than 10 or MoCA less than 23 were excluded from further analyses.
Table 1.
Study Sites
| Site | # Subjects | Age | Sex (M/F) | Cohort Enrollment | Data Obtained |
|---|---|---|---|---|---|
| Penn State Hershey Medical Center, Hershey, PA, USA (PSHMC) | 108 | 68.2 (9.5) | 75/33 | 30 HC, 45 PD, 14 MSA, 19 PSP | dMRI, MOCA, Hoehn-Yahr, HDRS1, MDS-UPDRS3 |
| University of Florida, Gainesville, FL, USA (UFLORIDA) | 149 | 65.6 (9.2) | 85/64 | 36 HC, 54 PD, 28 MSA, 31 PSP | dMRI, MOCA, Hoehn-Yahr, MDS-UPDRS3, BDI |
| University of Innsbruck, Innsbruck, Austria (MUI) | 168 | 64.4 (8.9) | 101/67 | 41 HC, 85 PD, 21 MSA, 21 PSP | dMRI, MMSE/MoCA, Hoehn-Yahr, UPDRS |
| Total | 425 | 65.8 (9.3) | 161/365 | 107 HC, 184 PD, 63 MSA, 71 PSP |
Data are either count or mean (±SD). Abbreviations: BDI = Beck Depression Inventory, dMRI = diffusion magnetic resonance imaging, F = female, HC = healthy controls, HDRS = Hamilton Depression Rating Scale, M = male, MDS-UPDRS3 = Motor section of the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale, MMSE = Mini-Mental State Examination, MoCA = Montreal Cognitive Assessment, MSA = multiple system atrophy, PD = Parkinson’s disease, PSP = progressive supranuclear palsy, UPDRS3 = Motor section of the Unified Parkinson’s Disease Rating Scale.
Data Acquisition
For the UFLORIDA cohort, diffusion-weighted images were acquired on a 3T Philips MRI scanner (Achieva, Best, Netherlands) using a 32-channel head coil. For the MUI and PSHMC cohorts, diffusion-weighted images were acquired on a 3T Siemens MR system (Verio at MUI and Trio at PSHMC, respectively; Siemens Magnetom; Erlangen, Germany) with an 8-channel head coil. The UFLORIDA imaging acquisition protocol consisted of the following scan parameters: directions=64; repetition time=7748ms; echo time=86ms; b-values: 0; 1000s/mm2; field of view = 224mmx224mm; in-plane resolutions=2mmx2mm; flip angle=90°; number of contiguous slices=60; slice thickness=2mm; acceleration factor p=2, averages=none. The MUI imaging acquisition protocol consisted of the following scan parameters: directions=20; repetition time=8200ms; echo time=83ms; b-values: 0; 1000s/mm2; field of view=230mmx230mm; in-plane resolution 2mmx2mm; number of contiguous slices=45; slice thickness=3mm; averages=none. The PSHMC imaging acquisition protocol consisted of the following scan parameters: directions=42; repetition time=8300ms; echo time=82ms; b-values: 0, 1000s/mm2; field of view=256mmx256mm, in-plane resolution=2mmx2mm, flip angle=90°, number of contiguous slices=65, slice thickness=2mm, acceleration factor p=2; and averages=7 (See Supplementary Table 1).
Diffusion imaging analyses
Data preprocessing was performed with the FMRIB Software Library and Unix scripts. Each brain was corrected for eddy-currents and head rotations using FSL (reference volume at 0), and gradient directions were rotated based on eddy current corrections. MUI data was resampled to a 2×2×2 resolution similar to the resolution of the other two sites. Non-brain tissue was removed from the diffusion volumes using the FSL Brain Extraction Tool (BET).
Single-tensor diffusion metrics, free-water, and free-water corrected diffusion metrics were calculated for all the diffusion imaging data. The single-tensor metrics were fractional anisotropy (FAU) and mean diffusivity (MDU). The bi-tensor metrics were free-water, corrected fractional anisotropy (FAT), and corrected mean diffusivity (MDT). Computational routines for single-tensor and bi-tensor measures have been published elsewhere8,20. A linear rigid transformation was performed between the B0 image and a T2 template in MNI space, and then a diffeomorphic non-linear transformation called the Symmetric Normalization (SyN) transformation model was performed21. Briefly, SyN uses a gradient-based iterative convergence using diffeomorphisms to converge on an optimal solution based on a similarity metric (e.g., cross-correlation). This technique has been shown to be perform greater compared to linear and other non-linear registrations. The transform matrices were then applied to all the free-water, FAu, MDu, FAT, and MDT maps.
ROI analyses
Region of interest (ROI) analyses were performed. First, the ROIs were manually drawn using an established method on the individual b-zero maps in MNI space9, blinded to the other diffusion maps and diagnosis. Four ROIs represented the left and right anterior and posterior substantia nigra. Each ROI was 8 voxels in size. The substantia nigra was identified in accordance with previous work where inter-rater reliability has been high5,10. To assess inter-rater reliability in this study, novice raters who were blind to group diagnoses drew ROIs of 10 subjects from each group across each site.
Statistical analysis
Clinical measures of age, sex, MDS-UPDRS III, cognitive status, disease duration, and Hoehn-Yahr were compared using ANOVA within each site, and after combining all data in one large cohort. The mean values for the bilateral anterior and posterior substantia nigra ROIs were calculated for free-water, FAT, MDT, FAU, and MDU across all sites. Multivariate ANOVAs were conducted on each unique ROI-dependent measure combination controlling for age, sex, and site, with group status as the fixed factor. Post-hoc tests for the group status were evaluated using Bonferroni pairwise comparisons. Inter-rater reliability for the segmentation procedure of the ROIs was determined by an intraclass correlation coefficient within site using a two-way mixed model with absolute agreement. Statistical threshold was set at p<0.05.
Correlational analyses
A priori, we decided to correlate a specific diffusion metric with MDS-UPDRS III scores, Hoehn-Yahr scores, and global cognitive status within each site, if there was a significant group effect for the specific diffusion metric. Correlation coefficients were calculated using Spearman’s rho statistic, and p-values were FDR corrected for multiple comparisons.
Results
Demographics and clinical data
Supplementary Tables 2 and 3 show the clinical and demographic data across and between sites. Across sites, there was a significant between-group effect for age, sex, MDS-UPDRS III, cognitive status, disease duration, and Hoehn-Yahr scores (p-values<0.05). Post-hoc tests revealed more males in the PD cohort and the PD cohort had longer disease duration (ie. time since diagnosis) than the MSA and PSP groups. Post-hoc test for MoCA yielded differences across groups (PSP<MSA<PD<HC) (p-values<0.05). The group effect for MDS-UPDRS III scores and Hoehn-Yahr scale scores resulted in the MSA and PSP cohorts having higher scores than the PD cohort. PD subjects also had significantly higher MDS-UPDRS III scores than HC (p-values<0.05, Supplementary Table 2).
Diffusion analyses
Intraclass correlation coefficients (ICCs) revealed strong agreement between the two raters. The average ICCs for free-water ranged from 0.88 to 0.99 (p-values<0.001), ICCs for average bi-tensor FAT ranged from 0.90 to 0.99 (p-values<0.001), ICCs for average bi-tensor MDT ranged from 0.91–0.99 (p-values<0.001), ICCs for single-tensor FAU ranged 0.87 to 0.94 (p-values<0.001), and ICCs for single-tensor MDU ranged from 0.81 to 0.99 (p-values<0.001).
Anterior ROIs
A multivariate group effect was found for anterior free-water, single-tensor FAU and bi-tensor FAT, with no other group effects found for the other diffusion metrics. Post-hoc tests revealed that this effect resulted from PSP values higher than HC and PD for free-water and FAT, and PSP with lower values than HC for single-tensor FAU (p-values<0.05, Table 2).
Table 2.
Diffusion Metrics
| Groups/Statistics | Free-Water | Bi-Tensor FA | Bi-Tensor MD | Single-Tensor FA | Single-Tensor MD |
|---|---|---|---|---|---|
| Anterior Substantia Nigra | |||||
|
| |||||
| HC | 0.19 (.006) | 0.57 (.009) | 0.56 (.005) | 0.50 (.008) | 0.76 (.015) |
| PD | 0.19 (.005) | 0.58 (.007) | 0.56 (.003) | 0.48 (.006) | 0.76 (.011) |
| MSA | 0.19 (.008) | 0.60 (.011) | 0.57 (.006) | 0.48 (.011) | 0.77 (.019) |
| PSP | 0.22 (.008) | 0.61 (.011) | 0.57 (.006) | 0.46 (.010) | 0.79 (.019) |
| Full model F statistic | 7.96 | 3.93 | 4.53 | 2.58 | 3.19 |
| Group F statistic | 3.80 | 3.59 | 2.10 | 3.55 | 0.98 |
| Partial η2 | 0.027 | 0.025 | 0.015 | 0.025 | 0.007 |
| Significant Covariate | Age, Site | Site | Site | N/A | N/A |
| Group P-value | 0.01 | 0.014 | 0.10 | 0.015 | 0.40 |
| P-value corrected | 0.02 | 0.0214 | 0.13 | 0.021 | 0.44 |
| Post Hoc | PSP > HC|PD | PSP>PD|HC | N/A | PSP<HC | N/A |
|
| |||||
| Posterior Substantia Nigra | |||||
|
| |||||
| HC | 0.19 (.007) | 0.60 (.009) | 0.57 (.005) | 0.49 (.008) | 0.79 (.013) |
| PD | 0.22 (.005) | 0.59 (.006) | 0.57 (.004) | 0.48 (.006) | 0.80 (.010) |
| MSA | 0.24 (.008) | 0.62 (.011) | 0.57 (.007) | 0.45 (.010) | 0.88 (.017) |
| PSP | 0.28 (.008) | 0.65 (.011) | 0.57 (.006) | 0.45 (.009) | 0.88 (.016) |
| Full model F statistic | 21.51 | 5.41 | 6.08 | 4.25 | 8.11 |
| Group F statistic | 23.64 | 9.26 | 0.40 | 4.11 | 10.86 |
| Partial η2 | 0.145 | 0.062 | 0.003 | 0.029 | 0.072 |
| Significant Covariate | Age, Sex, Site | Site | Site | Age | N/A |
| Group P-value | 3.75E-14 | 6E-6 | 0.75 | 0.007 | 6.98E-07 |
| P-value corrected | 3.75E-13 | 2e-5 | 0.75 | 0.018 | 3.49E-06 |
| Post Hoc | PSP>MSA| PD>HC | PSP>PD|HC | N/A | PSP|MSA<HC | PSP|MSA> PD|HC |
ROI data represent the mean (standard error) adjusted for age and sex, with the statistics for the comparisons and significant covariates listed below. Abbreviations: FA = fractional anisotropy, HC = healthy controls, MD = mean diffusivity, MSA = multiple system atrophy, PD = Parkinson’s disease, PSP = progressive supranuclear palsy.
There was a covariate effect of age for free-water (Table 2), and a site effect for free-water, bi-tensor FAT, and bi-tensor MDT (Table 2). Sex had no influence on any of the dependent metrics for the anterior ROIs (Table 2). Detailed site effects are shown in Supplemental Table 5 for each group.
Posterior ROIs
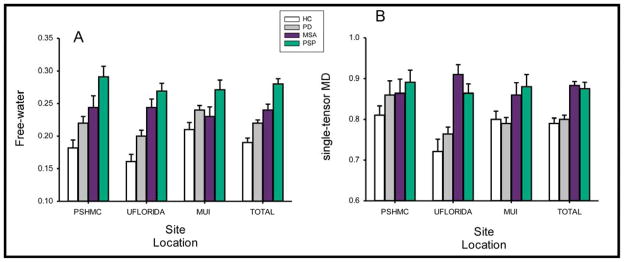
A multivariate group effect was found for free-water, bi-tensor FAT, single-tensor FAU and single-tensor MDU (p-values<0.05, Table 2). Post-hoc tests revealed PSP subjects had greater free-water than MSA, PD, and HC subjects. Although no differences were observed between PD and MSA subjects for free-water, PD and MSA subjects both had greater free-water than HC. This pattern was consistent across all sites (Figure 1). Partial η2 was 0.145 for free-water and 0.072 for single tensor MDU. Post-hoc tests also demonstrated increased bi-tensor FAT for PSP when compared to HC and PD subjects. In addition, the post-hoc tests revealed that PSP and MSA single-tensor MDU values were greater than HC (Figure 1). Post hoc tests also revealed that PSP had lower single-tensor FAU values than HC (p-values <0.05).
Figure 1.
Free-water and single-tensor mean diffusivity across sites. The bar graphs indicate the levels of free-water and single-tensor mean diffusivity for healthy controls, Parkinson’s disease (PD), multiple systems atrophy (MSA), and progressive supranuclear palsy (PSP) subjects across all sites and within each site.
A significant covariate effect of age was found for free-water and single-tensor FAU (p-values<0.05) (Table 2). A site covariate effect was found for free-water, bi-tensor FAT, and bi-tensor MDT (Table 2). Supplementary Table 5 shows detailed site effects for each group. A sex covariate effect was found for free-water (Table 2).
Correlation analyses
Correlation analyses revealed that posterior free-water was significantly correlated with MDS-UPDRS III, Hoehn-Yahr, and cognitive status at all sites (Table 3). The direction of the correlation was positive for MDS-UPDRS III and Hoehn-Yahr and negative for cognitive status. Posterior single-tensor MDU was significantly correlated with MDS-UPDRS III and Hoehn-Yahr at UF and PSHMC sites; however there were no significant correlations at the MUI site for posterior single-tensor MDU. The anterior nigra free-water values for the MUI site were significantly correlated with motor severity and Hoehn-Yahr scores. Posterior FAT correlations also revealed significant moderate correlations across clinical scores for the UF and MUI sites, but not the PSHMC site.
Table 3.
Correlations between diffusion metrics and clinical scores of all parkinsonian patients across sites
| Variable | PSHMC | UFLORIDA | MUI | |||
|---|---|---|---|---|---|---|
|
| ||||||
| UPDRS-III | rho (ρ) | P-value | rho (ρ) | P-value | rho (ρ) | P-value |
|
| ||||||
| aSN FW | 0.15 | 0.12 | 0.14 | 0.10 | 0.22 | 0.004 |
| aSN FAU | −0.17 | 0.07 | −0.18 | 0.025 | −0.20 | 0.01 |
| pSN FAU | −0.14 | 0.14 | −0.28 | 0.001 | 0.09 | 0.27 |
| pSN FAT | 0.11 | 0.26 | 0.23 | 0.005 | 0.03 | 0.67 |
| pSN FW | 0.40 | 1.6E-5 | 0.57 | 4.8E-15 | 0.18 | 0.02 |
| pSN MDU | 0.24 | 0.012 | 0.50 | 1.05E-10 | 0.11 | 0.18 |
|
| ||||||
| Hoehn-Yahr | ||||||
|
| ||||||
| aSN FW | 0.17 | 0.07 | 0.12 | 0.15 | 0.32 | 2E-5 |
| aSN FAU | −0.17 | 0.09 | −0.22 | 0.008 | −0.24 | 0.002 |
| pSN FAU | −0.12 | 0.23 | −0.29 | 2.9E-4 | −0.12 | 0.14 |
| pSN FAT | 0.15 | 0.13 | 0.23 | 0.005 | 0.21 | 0.005 |
| pSN FW | 0.40 | 2E-5 | 0.58 | 1.2E-14 | 0.28 | 2.4E-4 |
| pSN MDU | 0.20 | 0.03 | 0.53 | 2.9E-12 | 0.30 | 7E-6 |
|
| ||||||
| Cognitive Status | ||||||
|
| ||||||
| aSN FW | −0.02 | 0.81 | −0.07 | 0.37 | −0.08 | 0.30 |
| aSN FAU | 0.02 | 0.84 | 0.13 | 0.11 | 0.18 | 0.02 |
| pSN FAU | 0.18 | 0.06 | 0.27 | 0.001 | 0.03 | 0.72 |
| pSN FAT | −0.17 | 0.08 | −0.17 | 0.04 | −0.23 | 0.002 |
| pSN FW | −0.38 | 6.3E-5 | −0.46 | 4.8E-9 | −0.14 | 0.07 |
| pSN MDU | −0.29 | 0.002 | −0.42 | 8.2E-8 | −0.14 | 0.06 |
The correlation coefficient (rho) and the corresponding p value are listed for each association. Cognitive status was measured by MoCA and MMSE. Abbreviations: aSN = anterior substantia nigra, FAT = bi-tensor fractional anisotropy, FAU = uni-tensor fractional anisotropy, FW = free-water, MDU = single-tensor mean diffusivity, pSN = posterior substantia nigra.
Discussion
Our findings in the largest cohort of parkinsonism patients to date demonstrate the utility of a bi-tensor model for detecting differences in the substantia nigra in parkinsonism. We examined the diffusion properties of the substantia nigra in 425 subjects and determined differences among healthy controls and patients with PD, MSA, and PSP. We found that free-water values derived from the bi-tensor model were elevated in subjects presenting with parkinsonism, and the key observation was the same pattern (PSP>MSA>PD>HC) at each site (Figure 1). The effect size for distinguishing between healthy controls and neurodegenerative parkinsonism of the bi-tensor free-water measure was 0.145 and the effect size of the single-tensor MDU measure was 0.072. The direction of correlations between clinical measures and free-water and single-tensor MDU were similar. Therefore, the results provide direct evidence that bi-tensor metrics for diffusion-weighted imaging are able to detect changes in parkinsonian substantia nigra across sites.
The finding for elevated free-water in the substantia nigra of PD, MSA, and PSP subjects across multiple sites extends prior work that detected microstructural changes between healthy controls and PD patients in a single-site and multi-site study5. We also found decreased single-tensor FA and increased bi-tensor FAT in PSP when compared to other groups. Further, free-water elevation was more pronounced in PSP than PD and MSA. This pattern of results extends a previous single-tensor model analysis at a single-site22. PD and MSA free-water values were also greater than healthy controls. Previous reports have shown that putaminal MDU can be useful to distinguish the parkinsonian variant of MSA from PD but not from PSP23. Diffusivity changes of the middle cerebellar peduncle, however, may provide additional information10,22. Our findings of changes in the posterior nigra are in line with pathological studies that report degeneration in the ventro-lateral tier of the substantia nigra in patients with degenerative parkinsonism1.
The posterior substantia nigra contains cells that progressively degenerate as one ages or presents with parkinsonian symptoms. In order to detect in-vivo changes, one must delineate this region using indirect landmarks to outline or find the substantia nigra24,25. This may be the reason why some voxel-based analyses fail to detect changes in this region when examining changes between PD patients and healthy controls7. The hand-segmented ROI approach used here seems to provide utility in detecting changes in single- and bi-tensor diffusion metrics for the substantia nigra6,7,25. Some studies have not found changes in single-tensor FA in the substantia nigra, whereas other studies have6,24. The single-tensor FA measure reflects numerous mechanisms. When more unconstrained diffusion is in the voxel, this drives the single-tensor FAU measure lower. In contrast, high myelination and densely packed axons will lead to higher single-tensor FAU values. Since single-tensor FAU can be increased due to gliosis but reduced due to inflammation or fluid in the extracellular space, these mechanisms may cancel each other out on the single-tensor FAU measure. The bi-tensor derived measures can provide more information because the bi-tensor model provides separate measures for the extracellular space (i.e., free-water) and tissue-specific measures (e.g., bi-tensor FAT).
Increased bi-tensor FAT and decreased single-tensor FAU was found for both the anterior and posterior substantia nigra regions in the PSP cohorts. Previous reports have found altered diffusion metrics in the substantia nigra of PSP 26–29; single-tensor FAU was increased in one study 29 and reduced in another 28. Increased bi-tensor FAT has been suggested to reflect gliosis, where initially FAU was low in the single-tensor model30,31. At pathology, PSP patients have increased gliosis and inclusion body pathology in the substantia nigra compared to PD patients32. We found decreased FA in the single-tensor and bi-tensor models for PSP patients. Increased bi-tensor FAT when single-tensor FAU is originally low has been suggested to reflect cellular shrinkage or atrophy33. It is unclear whether the increased FAT findings for PSP from the current study reflect increased glial pathology, cellular swelling, or other pathologies.
The variability in the methods used to analyze diffusion MRI data for PD and other movement disorders range from ROI approaches to voxel-based statistics. Further, the type of scanner, field strength, and scan sequence used also vary depending on the study7. Multi-site studies are important for increasing the number of subjects, and to generalize clinical findings that are not dependent on site. We have found in the current paper that the site effect for the diffusion metrics was greatest for the controls and PD patients across sites and this may be due to clinical information, pulse sequence, and/or head coil differences at each site. Some multi-site studies have tried to minimize the variance due to type of scanner, although this has not led to promising results to detect differences between healthy controls and PD patients using single-tensor FA6. The current paper has shown that the bi-tensor calculation of free-water in the substantia nigra distinguishes healthy controls from parkinsonism patients regardless of site or scanner, even when the pulse sequence is not matched. Clearly, matching the pulse sequence is preferred and should be done in future large-scale multi-site studies, albeit the pattern of change in Figure 1 demonstrates that effects are robust even with different hardware and pulse sequences. Single-tensor MDU also was able to distinguish between PD and MSA and healthy controls, but did not differ for PD versus controls. Due to the similarity in the pattern of correlations with single-tensor MDU, free-water and clinical scores, it would seem that changes in the extracellular space are a factor influencing diffusivity changes reported in the literature.
A potential limitation in this study could be that patients were on medication at the Innsbruck and Pennsylvania sites but off medication at the Florida site. Despite this limitation, we still observed similar patterns of elevated free-water in the substantia nigra across parkinsonism at each site (Figure 1). The medication differences could also be a reason why the pattern of correlation results are not similar across sites. Since this was a retrospective study, the bi-tensor model was estimated from single-shell b-value data. The accuracy of the bi-tensor fit may be improved when multiple b-values are acquired34. This may improve precision and accuracy of the metrics for classification between parkinsonian disorders.
In conclusion, the present study demonstrates that free-water is elevated in the posterior substantia nigra of PD, MSA, and PSP patients when compared to healthy controls. This extends previous work that has shown utility of free-water in detecting PD from healthy controls in multi-site cohorts. Further, free-water in substantia nigra relates to clinical measures of motor and cognitive symptoms in a large cohort of parkinsonism. This is the first study to directly compare efficacy of single-tensor and bi-tensor metrics for parkinsonism. Our current findings, in combination with recent work9, suggest that bi-tensor metrics may provide utility in monitoring disease severity in multi-site studies.
Supplementary Material
Acknowledgments
We would like to thank all the participants for their time and efforts.
Funding/Support: Supported by National Institutes of Health (R01 NS052318, R01 NS075012, T32 NS082169, U01 NS082151, R01 MH108574, P41 EB015902).
Footnotes
Author Contributions: Drs. Ofori, Vaillancourt, Seppi, Huang, and Du had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ofori, Krismer, Huang, Seppi, and Vaillancourt.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Study supervision: Vaillancourt, Huang, and Seppi.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Obtained funding: Vaillancourt, Huang, and Seppi.
Administrative, technical, or material support: Ofori, Vaillancourt, Du, and Krismer.
Conflict of Interest Disclosures:
Dr. Edward Ofori – Receives salary support from NIH.
Dr. Florian Krismer – Receives grant support from MSA Coalition, the International Parkinson’s Disease and Movement Disorder Society, and the Austrian Parkinson’s Disease Society and non-financial support from Fight MSA
Dr. Roxana G. Burciu – Receives salary support from NIH.
Dr. Ofer Pasternak – Receives grant support from NIH. He previously received grant support from the NIH, Department of Defense, and the Brain and Behavior Research Foundation, and consulted for projects at Florida University and the Laureate Institute for Brain Research.
Ms. Johanna McCracken – Reports no disclosures.
Dr. Mechelle Lewis – Received grant support from NIH during the conduct of the study, and NIH and the MJFF unrelated to the submitted work.
Dr. Guangwei Du – Received grant support from NIH during the conduct of the study, and NIH and the MJFF unrelated to the submitted work.
Dr. Nikolaus R. McFarland – Reports grants from the NIH and the Michael J. Fox Foundation. He has received personal honoraria from the NIH and the American Academy of Neurology.
Dr. Michael S. Okun – Serves as consultant for the National Parkinson’s Foundation, and has received research grants from the National Institutes of Health, National Parkinson’s Foundation, Michael J. Fox Foundation, Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and UF Foundation. Dr. Okun has previously received honoraria, but in the past > 60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, Quantia, Henry Stewart, and the Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
Dr. Xuemei Huang – Received grant support from NIH during the conduct of the study, and NIH, the MJFF, and GE LifeSciences unrelated to the submitted work. Dr. Huang also serves as a consultant to NIEHS.
Dr. Christoph Mueller – received grant support from the Oesterreichische Nationalbank
Dr. Elke R. Gizewski – Does not have any disclosures.
Dr. Michael Schocke - Does not have any disclosures.
Dr. Christian Kremser - Does not have any disclosures.
Dr. Hong Li – Does not have any disclosures.
Dr. Klaus Seppi – Received honoraria or consulting fees from Astra Zeneca, Teva, UCB, Boehringer-Ingelheim, Lundbeck, AOP Orphan Pharmaceuticals AG, Novartis, Movement Disorder Society and grant supports from Medical University Innsbruck, Oesterreichische Nationalbank, FWF Austrian Science Fund, Michael J. Fox Foundation, Movement Disorder Society, Boehringer-Ingelheim.
Dr. David E. Vaillancourt – Reports grants from NIH, Bachmann-Strauss, and Tyler’s Hope Foundation during the conduct of the study, and personal honoraria from NIH, National Parkinson’s Foundation, UT Southwestern Medical Center, and Northwestern University unrelated to the submitted work.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s Disease: Substantia Nigra Regional Selectivity. Brain : a journal of neurology. 1991;114(5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. 1999;122(8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 4.Hardman CD, Halliday GM, McRitchie DA, Cartwright HR, Morris JGL. Progressive supranuclear palsy affects both the substantia nigra pars compacta and reticulata. Experimental Neurology. 1997;144(1):183–192. doi: 10.1006/exnr.1997.6415. [DOI] [PubMed] [Google Scholar]
- 5.Ofori E, Pasternak O, Planetta PJ, et al. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiology of Aging. 2015;36(2):1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuff N, Wu IW, Buckley S, et al. Diffusion imaging of nigral alterations in early Parkinson’s disease with dopaminergic deficits. Mov Disord. 2015;30(14):1885–1892. doi: 10.1002/mds.26325. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clinical. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine. 2009;62(3):717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 9.Ofori E, Pasternak O, Planetta PJ, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain : a journal of neurology. 2015;138(Pt 8):2322–2331. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain : a journal of neurology. 2016;139(Pt 2):495–508. doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP International Workshop*. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. 1992. Neurology. 2001;57(10 Suppl 3):S34–38. [PubMed] [Google Scholar]
- 13.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson’s disease rating scale scores to Movement Disorder Society-unified Parkinson’s disease rating scale scores. Mov Disord. 2012;27(10):1239–1242. doi: 10.1002/mds.25122. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Chiro GD. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 21.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prodoehl J, Li H, Planetta PJ, et al. Diffusion tensor imaging of Parkinson’s disease, atypical parkinsonism, and essential tremor. Mov Disord. 2013;28(13):1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schocke MF, Seppi K, Esterhammer R, et al. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58(4):575–580. doi: 10.1212/wnl.58.4.575. [DOI] [PubMed] [Google Scholar]
- 24.Burciu RG, Ofori E, Shukla P, et al. Free-water and BOLD imaging changes in Parkinson’s disease patients chronically treated with a MAO-B inhibitor. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focke NK, Helms G, Pantel PM, et al. Differentiation of typical and atypical parkinson syndromes by quantitative MR imaging. American Journal Of Neuroradiology. 2011;32:2087–2092. doi: 10.3174/ajnr.A2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knake S, Belke M, Menzler K, et al. In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: a DTI study using TBSS. Movement Disorders. 2010;25:1232–1238. doi: 10.1002/mds.23054. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa K, Nakata Y, Yamada K, Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry. 2004;75(3):481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Wai Y, Lin WY, et al. Microstructural changes in patients with progressive supranuclear palsy: a diffusion tensor imaging study. Journal of Magnetic Resonance Imaging. 2010;32:69–75. doi: 10.1002/jmri.22229. [DOI] [PubMed] [Google Scholar]
- 30.Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N-k, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouix S, Pasternak O, Rathi Y, Pelavin PE, Zafonte R, Shenton ME. Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PLoS One. 2013;8(6):e66205. doi: 10.1371/journal.pone.0066205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17(1):74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasternak O, Koerte IK, Bouix S, et al. Hockey Concussion Education Project, Part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. Journal of Neurosurgery. 2014;120(4):873–881. doi: 10.3171/2013.12.JNS132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.