Abstract
PURPOSE
We report the toxicity and biochemical tumor control outcome of a prospective Phase II study using high-dose-rate brachytherapy (HDR) alone as a salvage therapy for recurrent disease after external beam radiotherapy (EBRT).
METHODS
Forty-two patients with biopsy-proven recurrence were enrolled on a Phase II study of salvage HDR monotherapy using iridium-192. Median pretreatment EBRT dose was 8100 cGy (6840–8640 cGy) and the median time from completion of EBRT to salvage HDR was 73 months. The protocol prescription dose of 3200 cGy was delivered in four fractions over 30 hours in a single insertion. Median followup after salvage HDR was 36 months (6–67 months).
RESULTS
The actuarial prostate-specific antigen biochemical relapse-free survival and distant metastases-free survival rates at 5 years were 68.5% and 81.5%, respectively. Cause-specific survival was 90.3%. Late genitourinary Grade 1and 2 toxicities were found in 38% and 48%, respectively, and one patient developed Grade 3 urinary incontinence. Late Grade 1 and 2 gastrointestinal toxicity was noted in 17% and 8% of patients, respectively. Three patients (7%) developed Grade 2 late urinary toxicity (urethral stricture), which were corrected with urethral dilatation, and one patient developed Grade 3 urinary incontinence. No Grade 4 toxicities were observed.
CONCLUSIONS
Genitourinary toxicity was the most commonly encountered toxicity observed after salvage HDR but severe toxicities were uncommon. Salvage HDR is an effective and well-tolerated modality for locally recurrent prostate cancer and should be considered even for patients who have previously been treated with ultra-high dose levels of EBRT.
Keywords: Salvage brachytherapy, High dose rate brachytherapy
Introduction
High-dose intensity-modulated radiotherapy (IMRT) has proven to be an effective treatment for localized prostate cancer (1–6). In the case of local recurrence, salvage options are limited for these patients. These patients are often not considered optimal candidates for salvage prostatectomy because of their age or medical comorbidities even if the disease presentation at the time of recurrence demonstrates localized disease only. Prior definitive dose levels of radiation to the bladder, rectal wall, and urethra place these patients at higher risk for severe complications with additional salvage therapy. High-dose-rate brachytherapy (HDR) has dosimetric and radiobiologic advantages as a salvage treatment paradigm. One recent study (7) reported 50% biochemical tumor control outcomes with salvage HDR brachytherapy when used as monotherapy. We report on the long-term results of a prospective Phase II trial where HDR brachytherapy was used as salvage therapy for localized recurrent disease after external beam radiotherapy (EBRT).
Methods and materials
Forty-two patients with biopsy-proven recurrence were enrolled on an institutional review board–approved Phase II study of salvage HDR monotherapy using iridium-192. The primary end points of the trial were toxicity, assessed with the Common Toxicity Criteria for Adverse Events version 3, as well as the International Prostate Symptom Score (IPSS), and the International Index of Erectile Function. Biochemical control was evaluated using the Phoenix definition (nadir +2). Patient accrual spanned from 2007 to 2011, and patients were followed for at least 1 year after treatment on protocol and then in routine followup thereafter. Patients were seen in followup 1 month after treatment and then at 4-month intervals.
To be eligible for the trial, patients were required to have biopsy-proven recurrence after definitive EBRT. All biopsies were confirmed by pathologic review at our institution. Patients with radiographic evidence of extraprostatic disease demonstrated on an MRI with an endorectal coil, or metastatic disease seen on bone scan, were excluded from the study. Other exclusion criteria included patients with serum prostate-specific antigen (PSA) >10 ng/mL at the time of assessment and those with a baseline total IPSS >15 before planned salvage therapy. Any patient with a history of inflammatory bowel disease or rectal surgery was also excluded from enrollment. Patients were also required to be able to tolerate general anesthesia. Those with abnormal coagulation profiles (international normalized ratio >2.5, platelet count <75,000) or liver/renal function tests >1.5 × normal were also ineligible.
The method of HDR used in these patients has been previously described (8). In short, HDR catheters were placed with ultrasound guidance under general anesthesia. The entire prostate was implanted. The clinical target volume was the entire prostate, with a margin of 5 mm added around the entire gland. A dose of 800 cGy per fraction was prescribed to the periphery of the clinical target volume, except near the bladder neck, were the dose was typically 5–10% lower, at the discretion of the treating oncologist, unless tumor was thought to reside in that area. Four fractions were given a minimum of 4 hours apart, over 30 hours, in a single insertion. A genetic inverse treatment-planning algorithm was used for treatment-planning source dwell position and time optimization. The following dose–volume constraints were used for treatment planning similar to our dose thresholds used when treating non-recurrent HDR patients: minimum 95% target coverage with the prescription dose (PD), 120% of PD for maximum urethra dose, and rectal maximum dose not greater than 100% of PD. Catheter position was verified radiographically before each fraction. An iridium-192 HDR source was used for each treatment, using an afterloading technique. Table 1 summarizes key dosimetric parameters achieved for this study.
Table 1.
Dosimetry
| Dosimetry factors | Minimum | Lower- hinge |
Median | Upper- hinge |
Maximum |
|---|---|---|---|---|---|
| Prostate volume (cm3) | 17 | 28 | 33.5 | 43 | 64 |
| V100 (%) | 87 | 92 | 94.5 | 96 | 99 |
| D90 (%) | 90 | 103 | 106.5 | 108 | 119 |
| V150 (%) | 23 | 29 | 32.5 | 38 | 46 |
| Urethra Dmax | 110 | 114 | 116 | 118 | 127 |
| Urethra D5 (%) | 104 | 110 | 113 | 115 | 122 |
| Urethra D20 (%) | 100 | 108 | 110.5 | 113 | 119 |
| Rectum V100 cm3 | 0 | 0 | 0 | 0 | 0.1 |
| Rectum D1 cm3 (%) | 18 | 50 | 61.5 | 72 | 86 |
| Rectum D2 cm3 (%) | 14 | 45 | 54 | 63 | 79 |
These 42 patients had a median followup of 36 months, with a range of 6–66 months. Patient characteristics are summarized in Table 2. Median pretreatment EBRT dose was 8100 cGy (6840–8640 cGy) and the median time from completion of initial EBRT to salvage HDR was 78 months. Median presalvage PSA was 3.54 ng/mL. Eighteen patients had received androgen-deprivation therapy before salvage HDR, but androgen-deprivation therapy was discontinued after treatment in all cases.
Table 2.
Patient characteristics
| Characteristics | Patient (n = 42) I |
|---|---|
| Median months followup (range) | 36 (2–66) |
| Median patient age (range) | 72 (56–83) |
| Median cm3 pretreatment gland volume (range) | 33.5 (11–64) |
| Pretreatment PSA (%) | |
| <4.0 | 23 (55) |
| 4.0–10 | 14 (33) |
| >10 | 5 (12) |
| Gleason score (%) | |
| 6 | 3 (7) |
| 7 | 25 (60) |
| ≥8 | 14 (33) |
| Patient on ADT (%) | 18 (43) |
PSA = prostate-specific antigen; ADT = androgen-deprivation therapy.
Results
Tumor control outcomes
Ten patients developed a biochemical relapse at a median of 16.5 months from salvage treatment. The actuarial PSA relapse-free survival at 5 years was 68.5% (Fig. 1). Three patients have developed evidence of metastatic disease. The actuarial distant metastases-free survival at 5 years was 81.5% (Fig. 2), and the 5-year overall survival outcome was 79%.
Fig. 1.
PSA relapse-free survival. PSA = prostate-specific antigen; HDR = high-dose-rate brachytherapy.
Fig. 2.
Distant metastases-free survival. HDR = high-dose-rate brachytherapy.
Toxicity outcomes
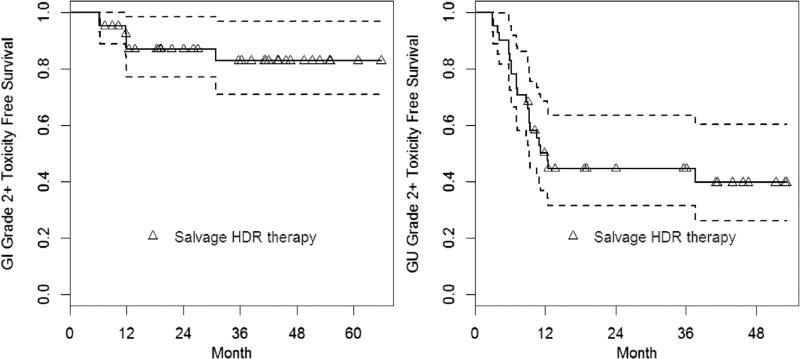
Acute genitourinary (GU) Grade 1 and 2 toxicities were noted in 38% and 40% of patients, respectively. Late GU Grade 1 and 2 toxicities were observed in 38% and 48%, respectively, and one patient developed Grade 3 urinary incontinence. Three patients developed urethral stricture (Grade 3), which were corrected with urethral dilatation. The median time to develop Grade 3 complications was 9 months (range, 9–12 months), and the median time for resolution of Grade 3 symptoms was 7 months (range, 23–21 months). No Grade 4 urinary toxicities were observed.
Baseline urinary status was found to be significantly associated with post-treatment late urinary toxicity for the development of Common Toxicity Criteria for Adverse Events Grade 2 (p = 0.008) but not for Grade 3 or higher toxicity. Figure 3 illustrates the rates of Grade 2 GU toxicity based on baseline scores. Seventy-eight percent of patients were without significant urinary symptoms (GU Grade 0–1) before the administration of salvage treatment, and 52% of these remained free of additional urinary toxicity at the time of last followup. Thus, the majority of urinary toxicity resolved to baseline. Of the three patients who developed Grade 3 urinary toxicity, two were characterized at baseline as having Grade 2 symptoms, and one patient was classified as having Grade 1 symptoms at baseline. The median IPSS at baseline was 6 (range, 1–17), and the median IPSS at last followup was 12 (range, 1–30). Resolution of an elevated IPSS was seen in 41% of patients (returned within 2 points of baseline) within a median time of 4.5 months. IPSS did not return to baseline values at the time of last followup in 24 patients, with a reported median IPSS value of 14.5 at the time of last followup (range, 5–30).
Fig. 3.
Grade 2 GI and Grade 2 GU toxicity–free toxicity survival curve. GI =gastrointestinal; GU = genitourinary; HDR = high-dose-rate brachytherapy.
Late Grade 1 and 2 gastrointestinal (GI) toxicities were noted in 43% and 14% of patients, respectively, and 83% of patients were free of Grade 2 or higher GI complications (Fig. 3). GI complications consisted almost entirely of transient rectal bleeding. No Grade 3 or higher GI complications were encountered.
The majority of patients were not sexually active at baseline. The median International Index of Erectile Function score before and after treatment was 2 and 1.5, respectively.
No dosimetric values such as V100 (volume of the prostate receiving PD) or D90 (dose to 90% of the prostate exposed to PD) were significantly associated with the risk of disease progression or any complications.
Discussion
In this prospective study of salvage HDR monotherapy, 76% of patients were able to achieve biochemical control in a patient population that is by definition radioresistant. Our data suggest that reirradiation with high-dose hypofractionation may be a rational salvage approach to eradicate tumor cells that have survived conventionally fractionated radiotherapy. We also noted an excellent tolerance profile to patients who received salvage HDR despite the high initial doses that patients had received as part of their definitive EBRT. The most frequently encountered complication was in the GU domain, with a median post-treatment IPSS score of 12, compared with a baseline IPSS of 6 for the entire cohort. Grade 3 complications were infrequent, and all such cases eventually had resolution of these effects with minor surgical intervention. It is important to note that no severe GI complications were encountered in this cohort, despite having had previous very high doses of EBRT.
Local recurrence after definitive EBRT is not infrequent. It has been estimated that nearly one-third of patients who undergo EBRT will have positive post-treatment biopsies of the prostate, and 15% of patients who received 8100 cGy were found to have positive biopsies (9). The consequences of locally recurrent disease after radiotherapy can be significant. In a report of the outcomes of locally recurrent prostate cancer after EBRT, Kuban et al. reported that nearly one-third of patients suffered from major complications associated with local recurrence (10). Locally recurrent diseases pose as well a significant risk for the development of distant metastases (1, 9, 11).
Often patients who have developed locally recurrent disease after radiotherapy are not candidates for salvage prostatectomy due to age or coexisting medical comorbidities. Salvage prostatectomy also carries a significant risk of rectal injury (16–58%), and 68% of patients will require the use of at least one pad for urinary incontinence (12). Long-term followup suggests that up to 54% of patients who undergo salvage prostatectomy will achieve biochemical control of their disease (13).
The use of other salvage modalities such as cryoablation after failed radiotherapy has been reported. In a large study of quality of life, 72% of patients reported incontinence at a median of 17 months, and two-thirds of patients reported significant urinary symptoms (14). Rectal injury rates of 2% and incontinence rates between 4% and 8% have been reported. The fistula rate was 3.4% (15). In a large study of salvage cryotherapy, 17% of patients were noted to positive biopsies after treatment (16). Therefore, effective treatment options without significant morbidity in the setting of locally recurrent prostate cancer after radiotherapy are limited.
The results of low-dose-rate salvage brachytherapy have also been reported. Reports published over 10 years ago (17, 18) have indicated that while tumor control can be achieved with low-dose-rate salvage brachytherapy in 30–50% of patients, toxicity outcomes were increased possibly related to the use of less sophisticated planning techniques or the selection of less optimal patients based on the presence of baseline symptoms.
More recently Chen et al. (7) have described preliminary outcomes of MRI-based partial prostate salvage low-dose-rate brachytherapy in 15 patients with a median followup of 23 months (8–88 months), with no cases of Grade 3 or higher GU complications. Biochemical control was achieved in 73% of patients at 3 years.
Aaronson et al. have reported outcomes for 24 patients who underwent low-dose-rate brachytherapy for recurrences after 66–70.2 Gy of EBRT. Biochemical control was observed in 88% of patients with a median followup of 30 months (19). Burri et al. have reported the outcomes of 37 patients with a median followup of 87 months who underwent low-dose-rate salvage brachytherapy. At 5-year followup, 65% of patients were found to have achieved biochemical freedom from failure (Phoenix definition), and 57% remained free of biochemical failure at 10 years. Grade 3 or 4 toxicity was noted in 11% of patients, consisting of transurethral resection of the prostate (TURP) in 2 patients, hematuria requiring fulguration in one patient, and one case of prostatorectal fistula (20).
Several aspects unique to HDR brachytherapy make it ideally suited for use as a salvage procedure.
Brachytherapy delivers high-dose radiation directly to the target tissue. In the case of EBRT, radiation must pass through skin, muscle, bone, bowel, and other soft tissues to reach the prostate. Thus, in the setting of previously irradiated patients, HDR brachytherapy can deliver radiation without repeating significant exposure to these surrounding tissues.
HDR brachytherapy results in very little treatment-related uncertainty. Organ motion is eliminated with HDR brachytherapy. Because post-implant CT-based three-dimensional dosimetry is used for treatment planning, the exact position of critical structures such as the rectum, bladder, and urethra are known and predictable. Thus, there is no uncertainty regarding the dose of radiation given to these structures, as well as the prostate.
By taking advantage of the inverse dose fall-off phenomenon, areas within the prostate can be differentially treated to higher or lower doses. HDR brachytherapy uses computer-optimized treatment planning. Dose constraints can be applied to normal structures to reduce dose while emphasizing the dose to potential tumor-bearing areas.
HDR brachytherapy is well suited to hypofractionated treatment schedules. Prostate cancer is thought to respond favorably to large amounts of radiation given in a limited number of treatments.
In addition, a patient-based dosimetric comparison between stereotactic body radiotherapy and HDR brachytherapy found that EBRT was not able to achieve either the high doses to the prostate or the dose-sparing effect on normal tissues that HDR brachytherapy is able to achieve (21), underscoring the advantages of HDR over external beam techniques.
The results of the University of California San Francisco (UCSF) salvage HDR experience have been recently updated (7). In this retrospective analysis, 52 consecutive patients received 36 Gy in six fractions, given in two insertions, 1 week apart. With a median followup of 60 months, 51% of patients were found to be biochemically free of relapse at 2 years, and only two patients developed Grade 3 or higher GU toxicity using CTAE criteria. An initial report published in 2007 reported 14% Grade 3 complications in 21 patients with a median followup of 19 months (22), suggesting that, with further time, Grade 3 complications tend to improve.
The results of the USCF experience as well as the current report have used dose-fractionation schedules with similar biologic effective doses (170–180 Gy, assuming an α/β of 1.5 Gy) with acceptable toxicity and tumor control, suggesting that either 36 Gy in six fractions with two insertions, or 32 Gy in four fractions in a single insertion, would be appropriate fractionation schedules to consider. However, the optimal dose-fractionation schedule for salvage HDR brachytherapy has yet to be elucidated.
As illustrated in Table 3, the outcomes of the present study compare favorably with results in the literature. HDR brachytherapy is an excellent treatment for salvage because it provides the high doses for hypofractionation while maintaining low doses to the urethra, bladder, and rectum. Because HDR brachytherapy is able to provide high-dose gradients that provide meaningful sparing of normal tissue between intimately juxtaposed structures such as the prostate and rectum that even stereotactic body radiation with the latest imaging and treatment delivery techniques cannot match, treatment was well tolerated with high rates of local control.
Table 3.
Outcomes of salvage treatments
| Author | Year | Modality | N | Followup (mo) |
Outcome | Grade 3–4 toxicity |
|---|---|---|---|---|---|---|
| Vaidya (23) | 2000 | RP | 6 | 27 | 83% 3-year FFP | 0% |
| Bianco (12) | 2005 | RP | 100 | 60 | 55% 5-year FFP | 13–33% |
| Izawa (24) | 2002 | Cryotherapy | 131 | 58 | 40% 5-year bNED | NR |
| Ismail (25) | 2007 | Cryotherapy | 100 | 34 | 59% 3-year bNED | NR |
| Bahn (26) | 2012 | Cryotherapy | 73 | 44 | 75% negative biopsy | |
| Grado (17) | 1999 | LDR | 49 | 64 | 34% 5-year bNED | 3% |
| Beyer (18) | 1999 | LDR | 17 | 62 | 53% 5-year FFP | 16% |
| Wong (27) | 2006 | LDR | 17 | 44 | 75% 4-year bNED | NR |
| Allen (28) | 2007 | LDR | 12 | 45 | 63% 4-year bNED | 47% |
| Nguyen (29) | 2007 | LDR | 25 | 47 | 70% 4-year bNED | 0% |
| Lee (30) | 2008 | LDR | 21 | 36 | 38% 5-year bNED | 30% |
| Aaronson (19) | 2009 | LDR | 24 | 30 | 88% 3-year bNED | 0% |
| Burri (20) | 2009 | LDR | 37 | 86 | 54% 10-year bNED | 11% |
| Chen (7) | 2013 | HDR | 52 | 60 | 51% 5-year bNED | 4% |
| Present series | 2013 | HDR | 42 | 38 | 70% 3-year bNED | 8% |
RP = radical prostatectomy; FFP = freedom from progression; bNED = biochemical non-evidence of disease; LDR = low-dose-rate brachytherapy; NR = not reported.
Conclusion
This prospective study of HDR salvage monotherapy demonstrates that it is an effective and well-tolerated treatment paradigm for patients who develop locally recurrent prostate cancer EBRT. HDR brachytherapy should be considered in the local management of recurrent prostate cancer, even in patients who have been previously heavily treated with ultra-high-dose EBRT.
Footnotes
Disclosures: The authors have no disclosures.
References
- 1.Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: Impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71:1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 2.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahlon O, Zelefsky MJ, Shippy A, et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Chen CP, Weinberg V, Shinohara K, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-Year outcomes. Int J Radiat Oncol Biol Phys. 2013;86:324–329. doi: 10.1016/j.ijrobp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Bhatia S, Zaider M, et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy. 2006;5:157–164. doi: 10.1016/j.brachy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuban DA, el-Mahdi AM, Schellhammer PF. Prognosis in patients with local recurrence after definitive irradiation for prostatic carcinoma. Cancer. 1989;63:2421–2425. doi: 10.1002/1097-0142(19890615)63:12<2421::aid-cncr2820631208>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: A comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco FJ, Jr, Scardino PT, Stephenson AJ, et al. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:448–453. doi: 10.1016/j.ijrobp.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Paparel P, Cronin AM, Savage C, et al. Oncologic outcome and patterns of recurrence after salvage radical prostatectomy. Eur Urol. 2009;55:404–410. doi: 10.1016/j.eururo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Perrotte P, Litwin MS, McGuire EJ, et al. Quality of life after salvage cryotherapy: The impact of treatment parameters. J Urol. 1999;162:398–402. [PubMed] [Google Scholar]
- 15.Bahn DK, Lee F, Silverman P, et al. Salvage cryosurgery for recurrent prostate cancer after radiation therapy: A seven-year follow-up. Clin Prostate Cancer. 2003;2:111–114. doi: 10.3816/cgc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 16.Chin JL, Pautler SE, Mouraviev V, et al. Results of salvage cryoablation of the prostate after radiation: Identifying predictors of treatment failure and complications. J Urol. 2001;165:1937–1941. doi: 10.1097/00005392-200106000-00022. discussion 1941–1942. [DOI] [PubMed] [Google Scholar]
- 17.Grado GL. Benefits of brachytherapy as salvage treatment for radiorecurrent localized prostate cancer. Urology. 1999;54:204–207. doi: 10.1016/s0090-4295(99)00175-2. [DOI] [PubMed] [Google Scholar]
- 18.Beyer DC. Permanent brachytherapy as salvage treatment for recurrent prostate cancer. Urology. 1999;54:880–883. doi: 10.1016/s0090-4295(99)00241-1. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson DS, Yamasaki I, Gottschalk A, et al. Salvage permanent perineal radioactive-seed implantation for treating recurrence of localized prostate adenocarcinoma after external beam radiotherapy. BJU Int. 2009;104:600–604. doi: 10.1111/j.1464-410X.2009.08445.x. [DOI] [PubMed] [Google Scholar]
- 20.Burri RJ, Stone NN, Unger P, et al. Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:1338–1344. doi: 10.1016/j.ijrobp.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 21.Spratt DE, Scala LM, Folkert M, et al. A comparative dosimetric analysis of virtual stereotactic body radiotherapy to high-dose-rate monotherapy for intermediate-risk prostate cancer. Brachytherapy. 2013;12:428–433. doi: 10.1016/j.brachy.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee B, Shinohara K, Weinberg V, et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: The University of California-San Francisco experience. Int J Radiat Oncol Biol Phys. 2007;67:1106–1112. doi: 10.1016/j.ijrobp.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya A, Soloway MS. Salvage radical prostatectomy for radiorecurrent prostate cancer: Morbidity revisited. J Urol. 2000;164:1998–2001. [PubMed] [Google Scholar]
- 24.Izawa JI, Madsen LT, Scott SM, et al. Salvage cryotherapy for recurrent prostate cancer after radiotherapy: Variables affecting patient outcome. J Clin Oncol. 2002;20:2664–2671. doi: 10.1200/JCO.2002.06.086. [DOI] [PubMed] [Google Scholar]
- 25.Ismail M, Ahmed S, Kastner C, et al. Salvage cryotherapy for recurrent prostate cancer after radiation failure: A prospective case series of the first 100 patients. BJU Int. 2007;100:760–764. doi: 10.1111/j.1464-410X.2007.07045.x. [DOI] [PubMed] [Google Scholar]
- 26.Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012;62:55–63. doi: 10.1016/j.eururo.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Wong WW, Buskirk SJ, Schild SE, et al. Combined prostate brachytherapy and short-term androgen deprivation therapy as salvage therapy for locally recurrent prostate cancer after external beam irradiation. J Urol. 2006;176:2020–2024. doi: 10.1016/j.juro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Allen GW, Howard AR, Jarrard DF, et al. Management of prostate cancer recurrences after radiation therapy-brachytherapy as a salvage option. Cancer. 2007;110:1405–1416. doi: 10.1002/cncr.22940. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen PL, D’Amico AV, Lee AK, et al. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: A systematic review of the literature. Cancer. 2007;110:1417–1428. doi: 10.1002/cncr.22941. [DOI] [PubMed] [Google Scholar]
- 30.Lee HK, Adams MT, Motta J. Salvage prostate brachytherapy for localized prostate cancer failure after external beam radiation therapy. Brachytherapy. 2008;7:17–21. doi: 10.1016/j.brachy.2007.11.002. [DOI] [PubMed] [Google Scholar]