Abstract
Food allergy, a commonly increasing problem worldwide, defined as an adverse immune response to food. A variety of immune-related effector cells such as mast cells, eosinophils, neutrophils, and T cells are involved in food-related allergic responses categorized as IgE-mediated, non-IgE mediated, and mixed (IgE and non-IgE) depending upon underlying immunological mechanisms. The dietary antigens mainly target the gastrointestinal tract including pancreas that gets inflamed due to food allergy and leads acute pancreatitis. Reports indicate several food proteins induce pancreatitis; however, detailed underlying mechanism of food-induced pancreatitis is unexplored. The aim of the review is to understand and update the current scenario of food-induced pancreatitis. A comprehensive literature search of relevant research articles has been performed through PubMed and articles were chosen based on their relevance to food allergen mediated pancreatitis. Several cases in the literature indicating that acute pancreatitis has been provoked after the consumption of mustard, milk, egg, banana, fish, and kiwi fruits. Food-induced pancreatitis is an ignored and unexplored area of research. The review highlights the significance of food in the development of pancreatitis and draws the attention of physicians and scientists to consider food allergies as a possible cause for initiation of pancreatitis pathogenesis.
Keywords: Food allergy, Pancreatitis, Eosinophils, Mast cells, Neutrophils
Introduction
The increasing prevalence of allergic diseases is an alarming issue throughout the world and food allergens are the major contributors in promoting allergic manifestations including gastrointestinal disorders [1–4] The prevalence of food allergy has been estimated about 3–8% and it significantly affects the quality of life in susceptible individuals [5]. Food allergy can be defined as a provocation of adverse allergic reaction to innocuous dietary antigens due to an overreaction of the immune system against these dietary antigens. The signs and symptoms of food allergy may range from mild to severe, and may include vomiting, diarrhea, hives, itchiness, swelling of the tongue, low blood pressure, and breathing difficulties [6]. The food induced allergic reactions typically occur within minutes to several hours of exposure and when the symptoms are severe, it can cause severe anaphylaxis [7]. The overreaction of the body’s immune system against the food antigens can affect several organs, including the respiratory and gastrointestinal (GI) tracts that lead to the development of asthma exacerbation, respiratory failure, esophagitis and gastroenteritis [2, 8, 9] Recently, food allergy has been implicated as one of the reasons that promotes acute pancreatitis [10–14]. Pancreatitis is characterized by acute or chronic inflammation which can be caused by various noxious stimuli such as gallstones, alcohol, and mutation in trypsinogen gene [15–17]. The annual incidence of acute and chronic pancreatitis varies from 13 to 45 and 4.4 to 11.9 per 100,000 people in the United States respectively [15, 18]. Moreover, various reports indicate an increased occurrence of pancreatitis in the population of Japan compared to the United States [15, 19, 20]. Unfortunately, in the current scenario, the prevalence of food-induced acute or chronic pancreatitis is still not well reported. Since 1933, food-induced allergy has been known to be a cause of acute pancreatitis in dogs [21]; furthermore, several cases of acute pancreatitis in human caused by allergy to mustard [22], milk [13, 23], cod roe [14], egg [24], bananas [10], fish [25], and kiwi fruits [26] have been reported in literature. Several studies indicate that a high dietary glycemic load [27], fat, meat, egg etc. [28] were associated with the risk of non-gallstone-related acute pancreatitis. While other studies report that vegetables and fish (including fatty and lean fish) may be associated with decreased risk of non-gallstone acute pancreatitis [29, 30]. All these studies indicate that certain foods are associated with an increased risk of acute pancreatitis while other foods might be protective.
According to clinical presentations and allergy testing, three types of food allergies have been known: IgE mediated (immediate type, type I hypersensitivity), non-IgE mediated (delayed type, cellular, type IV hypersensitivity), and mixed (IgE/Non-IgE) [31], but in the case of food-induced pancreatitis the mechanism is unknown, and this area of research is ignored and unexplored. In this review, we aim to provide the current information related to food-mediated allergic responses with special emphasis on food-induced pancreatitis. The current review also provides the platform for researchers and physicians to understand the involvement of different types of effector immune cells as well as the operational molecular mechanistic pathways that may pave the way for novel treatment therapies for food-induced pancreatitis.
Food-induced allergic disorders
Food-induced allergic disorders are increasing worldwide and certain foods have been marked as a risk factor for the development of allergic diseases [32]. The food allergy is mostly associated with an increase in Th2 cytokine, eosinophils and mast cells in response to allergens [33–36]. Food allergy has been reported in several allergic diseases including asthma [33–35], allergic rhinitis [37, 38], gastrointestinal disorders such as eosinophilic gastroenteritis [2, 39] and pancreatitis [23, 24]. Herein, we summarized the current understanding and available pieces of evidence about the development of eosinophilic gastroenteritis dependent and independent pancreatitis.
Food allergen-induced eosinophilic gastroenteritis and pancreatitis
Eosinophilic gastroenteritis (EG) is an eosinophil mediated inflammatory disease and characterized by infiltration of eosinophils in the stomach, small intestine and large intestine [2, 39]. Base line eosinophils are usually present in the gastrointestinal tract [40]; but increase in their number was observed in response to several stimuli, such as tissue injury and infections, including food allergens. These accumulated eosinophils get activated that further degranulate, and secrete various cytokines that lead to the development of EG [41]. Notably, evidences indicate that 50–75% of EG patients have personal or family history of concomitant allergies or atopic disorders [42, 43]. Food allergy has been suggested in promoting EG in these patients and an increase in total IgE and food-specific IgE provided evidence of food allergy in promoting EG in patients [44]. Cow’s milk was reported to induce erosive gastritis and its withdrawal from the diet has shown the disappearance of allergic symptoms in infants [45]. Several pieces of evidence suggest that food antigens have a critical role in the pathogenesis of eosinophilic gastroenteritis as well as colitis [46].
The first time food allergy and its association to the pancreatitis was reported in 1933 by Couvelaire and Bargeton [21], by showing food allergen-induced experimental anaphylactic shock in dogs. Several case reports of acute pancreatitis, provoked by food allergies to mustard [22], milk [13, 23], egg [24], bananas [10], fish [25] and kiwi fruits [26] in human have been published in the literature. Chronic pancreatitis persisting for as long as 8 to 10 years due to consumption of different types of food such as beef, milk, potato, fish, and eggs have also been reported [12]. Major symptoms of these patients include acute abdominal pain and elevation in serum pancreatic enzymes levels. However, abdominal sonography and CT scan result revealed edematous pancreas in only one patient. The serum IgE level was found elevated in one patient [12]. These patients were treated by cessation of ingestion of inciting food (beef, milk, potato, fish, and eggs), and simultaneously with the drug Cromoglycate, a mast cell stabilizer [47], which prevents the release of histamine from mast cells. A case of milk-associated acute pancreatitis has also been reported in a 23-year-old patient with recurrent episodes of abdominal pain, increased levels of amylase, lipase, serum IgE, beta lactoglobulin, and an enlarged pancreas on the sonographic report. The patient had recurrent episodes of acute pancreatitis accompanied by facial erythema with conjunctival injection, generalized pruritus, diarrhea, and eosinophilia after consumption of milk [13]. Inamura et al. [10] published a case of recurrent acute pancreatitis (three episodes) caused by an allergy to bananas in a 47-year-old female with past history of allergic rhinitis. The patient was treated conservatively with light diet and intravenous fluids, with resolution of clinical symptoms within few days. In addition, the elevated amylase levels returned to normal values in parallel with the clinical symptoms. The total serum IgE level was 644 IU/mL with specific IgE level to bananas was 2.18 UA/ml showing significant allergy to bananas. Gastaminza et al. [26], presented a case report of acute pancreatitis developed after consumption of kiwi fruits in a 48-year-old man with past history of duodenal ulcer and upper gastrointestinal hemorrhage. This patient also had history of rhinitis, bronchial asthma and allergies to pirazolones. The patient was reported to have increased serum amylase and leukocytosis of 835 U/I (N=60–190) and 13,000/mm2 respectively. Notably, after 48 hours of treatment with light diet, intravenous fluids, antacids, and analgesics; serum amylase and lipase levels returned back to normal. Since, the patient was a moderate drinker, he was initially diagnosed to have alcoholic pancreatitis but after about two weeks, he suffered from another episode of pancreatitis after eating kiwi fruit. Later, prick test showed allergy to kiwi fruit and he was diagnosed to have kiwi fruit-induced pancreatitis. Patient has not suffered from pancreatitis again after avoiding ingestion of kiwi fruits in the last five years as per the report. The summary of each of these case reports of food-related acute pancreatitis has been provided in Table 1. Furthermore, several large multiethnic cohort studies have indicated that high dietary glycemic load, saturated fat, cholesterol, red meat, and eggs raise the risk of non-gallstone-related acute pancreatitis [27, 28]; In contrast, recent reports have indicated that the consumption of vegetables and fish (fatty and lean fish combined) may be associated with decreased risk of non-gallstone acute pancreatitis [29, 30]. The summary of prospective cohort studies related with food-induced acute pancreatitis in human was presented in Table 2.
Table 1.
Summarized details of case reports based on food allergen-induced acute pancreatitis
| S. No. | Age (Year) | Gender | Allergic Food | Symptoms | Involved Cell type (s) | IgE | Other associated disease | References |
|---|---|---|---|---|---|---|---|---|
| 1. | 26 | F | NA | Abdominal pain, Allergic symptoms | Eosinophils | Increased serum IgE level | EP | [11] |
| 2. | 40 17 |
F M |
Beef (twice) Milk Potato Fish Egg |
Abdominal pain, Migraine, Pruritus, Elevated serum amylase, Epigastric pain associated with diffuse headache | Normal eosinophils level | Increased serum IgE level in one patient | NA | [12] |
| 3. | 23 | NA | Milk | Abdominal pain, Facial erythema with conjunctival injection, Generalized pruritus, Diarrhea, Eosinophilia, Elevated serum levels of pancreatic enzymes, Enlargement of the pancreas and edema on sonogram | Increased Eosinophils | Increased serum IgE level | NA | [13] |
| 4. | 48 | M | Kiwi | Epigastric pain, Vomiting, Diarrhea, Facial erythema, Pruritus, Elevated serum amylase | Increased Eosinophils | Increased serum total IgE 257 Ul/ml | Past history of duodenal ulcer, Upper digestive tract hemorrhage, Allergy to Pirazolones, Rhinitis, and Bronchial asthma | [26] |
| 5. | 47 | F | Banana | Epigastralgia, Nausea, Vomiting, Diarrhea, Elevated serum and urine amylase levels, Ampulla of Vater swollen and edematous | Increased Mast cells | Increased Serum total IgE (644 IU/mL), and Specific IgE to bananas (2.18 UA/ml) | NA | [10] |
| 6. | - | M | Milk | Symptoms of EG such as nausea, Vomiting, Abdominal pain, Dyspepsia, Malabsorption, Ascites and weight loss, Increased serum pancreatic enzymes and Total bilirubin | Increased Eosinophils | - | EG | [23] |
| 7. | 8 | M | codfish | Anaphylactic reaction, Abdominal pain, Elevated pancreatic enzyme serum levels, Parenchymal oedema at ultrasonography | NA | Codfish specific IgE= 1.30 kU/l | At first year of life child had severe atopic dermatitis and chronic diarrhea with mucorrhea and rectal bleeding, taking cow’s milk free diet | [25] |
| 8. | 25 | F | Egg | Vomiting, abdominal pain, diarrhea, Elevated level of lipase | Increased Eosinophils 2300–2500 Eosiophils/μl |
Total IgE = 89.7 kU/L Egg-specific IgE level =6.25 kU/L |
History of intermittent GI symptoms EG | [24] |
Abbreviations: EG= Eosinophilic gastroenteritis; NA=Not available; AP= Acute pancreatitis; EP= Eosinophilic pancreatitis
Table 2.
Prospective cohort studies related with food-induced acute pancreatitis
| S. no. | Age years | Gender | Food type | Incidence rates | Findings | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| 1. | 45–84 | M-44,791 F-36,309 |
High dietary glycemic factors | 49 cases per 100,000 person-years in the highest quartile of glycemic load and 33 cases per 100,000 person-years in the lowest | 364 cases of incident non-non-GS-AP (236 in men and 128 in women) | diets with high glycemic lo ad are associated with an increased risk non-GS-AP | [27] |
| 2. | 45–84 | M-39,267 F-32,191 |
Fatty fish and lean fish | Fish consumption ≤2.0–3.0 servings/week was associated with a significantly decreased risk of the disease. | 320 cases (209 cases in men and 111 cases in women) of incident non-GS-AP | Consumption of total fish (fatty fish and lean fish combined) may be associated with decreased risk of non-GS-AP | [30] |
| 3. | 46–84 | 80,019 women and men, | Vegetable, fruits | 17% risk reduction with vegetable consumption | 320 incident cases (216 men and 104 women) with non-GS-AP | Vegetable consumption, but not fruit consumption, may play a role in the prevention of non-GS-AP | [29] |
| 4. | 45–75 | 145,886 Men and Women | Dietary Factors: fat, egg, red meat, milk, coffee etc. | Higher rate of associations between dietary factors and pancreatitis were observed mainly for GS-AP | GS- AP (n = 1210), AP not related to gallstones (n = 1222), or recurrent AP or suspected chronic pancreatitis (n = 378). | Saturated fat, cholesterol sources, such as red and eggs associated positively with GS- AP. Vitamin D from milk, coffee, Fiber intake protect from GS-AP. |
[28] |
Abbreviations: AP= Acute pancreatitis; GS-AP=gallstone-related Acute pancreatitis;
The association of EG with pancreatitis is also well reported in the literature and the major cause of EG is food allergy [23, 24, 48]. Numerous case reports indicate that EG can occur due to cow’s milk [23] and egg allergy, [24] and the severe EG symptoms may exacerbate acute pancreatitis. Suzuki et al. [23] reported a case of milk allergy associated pancreatitis, which rapidly improved after withdrawing the milk from diet and corticosteroids treatment. Patient showed decrease in serum titers of pancreatic enzymes and total bilirubin with normalization of blood eosinophil count. Patient remained symptom-free for more than 20 months following the elimination of cow’s milk from his diet. Thus, adult-onset cow’s milk allergies are rare; but they may pose severe complications such as anaphylaxis, gastroenteritis, and pancreatitis.
Tse and coworkers [24] presented the first report of egg allergy associated pancreatitis in a 25-year-old non-atopic female who developed abdominal pain radiating to the left upper quadrant and chest, within 30 minutes of eating an egg. Laboratory testing revealed serum lipase level of 2400 U/L, and peripheral eosinophilia of 23.3% (2300 eosinophils/μl), which remained elevated, increasing up to 26% (2500 eosinophils/μl) in the following week. Further diagnostic imaging such as ultrasound, abdominal CT, and MRCP did not reveal biliary dilatation or obstruction, which could suggest the presence of gallstones. Other common causes of pancreatitis such as alcohol and medications were also ruled out. Upper endoscopy was normal. In-vitro allergy testing confirmed positive egg-specific IgE level at 6.25 kU/L, whereas, all other tested foods were reported negative, and total IgE 89.7 kU/L was observed. However, duodenal biopsy showed 80 eosinophils/high powered field and analysis of stool sample for ova and parasite was negative except for the presence of Charcot Leyden crystals, suggesting an underlying eosinophilic gastrointestinal process. In this patient, the EG was severe enough to have likely caused acute pancreatitis. A possible mechanism predicted for food hypersensitivity mediated, and EG associated acute pancreatitis, is due to obstruction of the pancreatic duct from local duodenal inflammation [49].
Even though, most cases of food-induced pancreatitis showed intestinal wall edema and duodenal inflammation, but CT scan reported as a normal pancreas [10, 23, 24, 26]. This indicates imaging of pancreas is not sufficient to diagnose pancreatitis. The revised Atlanta classification of pancreatitis state that two out of three following criteria should be considered to diagnose pancreatitis, such as classical abdominal pain, approximately 3 times induced levels of amylase or lipase in the blood, and imaging show inflammatory changes in pancreas. Further, Balthazar’s index and CT severity scoring system should also be considered to diagnose acute pancreatitis as Grade A and score of 0 respectively because pancreatitis can occur even without any visible morphological changes following CT imaging [50]. However, there are some case reports that indicate abdominal pain and lipase elevations greater than 3 times normal limit and do not have pancreatitis. Even though, they meet the clinical criteria for acute pancreatitis but they may have intestinal ulcers or bowel wall edema. In the clinical circumstances, where there is doubt about the validity of an elevated lipase, clinicians will commonly get a CT/MRI to verify interstitial edema/inflammation of the pancreas. When it is present, then there is no question that pancreatitis occurred. When it is absent, while clinical criteria have been met, remains doubtful whether pancreatitis truly occurred. Therefore, some of these cases may not be pancreatitis, but that at a minimum they meet the current conventional clinical criteria.
Mechanism associated with food-induced pancreatitis
Inamura et al. (2005) proposed that the reflux of bile into the pancreatic duct due to obstruction of the ampulla of Vater and improper activation of zymogens could be the culprit of food-induced pancreatitis [10]. They found inflammatory changes to the ampulla of Vater in endoscopic exam and pathology showed accumulation of a large number of mast cells in the mucosa and submucosa on immunohistochemical staining against human mast cell tryptase. In addition, mast cells play a pivotal role in pancreatitis [51, 52]. This patient had eaten bananas each time before the attacks of recurrent acute pancreatitis and had specific IgE to bananas of 2.18 UA/1, a low positive. Another pancreatitis patient associated with milk allergy had a specific IgE to cow milk of 2.17 KU/1 [13]. Therefore, hypersensitivity to a food substance could also lead to the obstruction of the ampulla of Vater, causing reflux of bile into the pancreatic duct and inappropriate activation of pancreatic enzymes leading to an attack of pancreatitis.
Food allergen-induced IgE has a role in induction of pancreatitis
IgE-mediated food allergy has been triggered when IgE antibodies bind with FcεRI (Fragment, crystallizable epsilon region R1) receptors present on mast cells and basophils causing the release of biologically active mediators and subsequent development of hypersensitivity responses against ingested food proteins [1]. Typically, IgE-mediated food allergy follows two steps i.e. sensitization and degranulation. The primary exposure to allergen creates sensitization and secondary exposure to the same allergen causes degranulation of immune cells. When primary exposure to food allergens occurs, these allergens were captured and processed into small peptide fragments by antigen presenting cells (APCs) with the help of proteasome complex. Later, these processed peptide fragments were presented along with major histocompatibility complex class-II (MHC-II) by APCs to naive CD4+ T cells. These naive CD4+ T cells differentiate into Th2 cells in the presence of IL-4 and produce Th2 specific cytokines such as IL-5 and IL-13 [53]. These released Th2 cytokines, especially IL-4 and IL-13, were responsible for the cross talk between T and B cells and induce differentiation of B cells into the IgE-producing plasma cells. These food allergen-specific IgE antibodies bind to FcεRI surface receptors on mast cells and basophils [54]. This stage is commonly known as the ‘sensitization phase,’ and now the sensitized immune cells get ready to provoke a stronger allergic response upon re-exposure to the same food allergen. Secondary exposure to the same food allergen causes crosslinking of allergen-specific IgE antibodies to FcεRI receptors on mast cells and/or basophils. This event, in turn, induces the degranulation of mast cells or basophils releasing several allergic mediators such as histamine, prostaglandin D2 etc. leading to the development of characteristic symptoms of allergy [55].
Additionally, IgE has been reported in pancreatitis and several studies indicate that chronic pancreatitis patients have high serum levels of IgE, especially during acute inflammatory episodes, or if the patient has taken alcohol [56, 57]. Furthermore, an earlier case report has also shown increased IgE levels in a patient with recurrent pancreatitis lasting several years which suggests food-mediated allergy as a cause of recurrent pancreatitis [12]. Additionally, milk protein allergy was associated with acute pancreatitis and the serum levels of IgE specific to milk proteins and beta-lactoglobulin was increased in patients with acute pancreatitis [13]. Furthermore, even fruit allergies that include bananas [10] and kiwi fruit [26] were also reported in the induction of acute pancreatitis. These patients showed no recurrence of pancreatitis following the avoidance of responsible food [26]. Additionally, increased dietary glycemic load [27], intakes of saturated fat, cholesterol, red meat, and eggs [28] promote the risk of non-gallstone-related acute pancreatitis. In a large population-based prospective cohort of 80,019 Swedish people (aged 46–84 years), Oskarsson and coworker showed that vegetable consumption might play a role in the prevention of non-gall stone-related acute pancreatitis [26]. Similarly, a case of a 26-year-old woman with a large number of eosinophils in the resected pancreas was reported who was suspected to have eosinophilic pancreatitis. Interestingly, she had repeatedly normal eosinophil count, histologically normal gastroduodenal, splenic and celiac lymph node biopsies. It was suggested that it could be due to a rare manifestation of patient’s past history of allergic manifestations and increase in serum IgE levels [11]. These studies indicate that some foods can induce an episode of pancreatitis, whereas some foods minimize the risk of development of pancreatitis.
Food allergen-induced non-IgE mediated pancreatitis
Non-IgE mediated food allergies were caused by components of the immune system other than IgE antibodies. In non-IgE mediated food allergy, the cellular immune response was engaged, with the primary effect on the GI mucosa [58]. Eosinophilic infiltration and T cell involvement have been reported in non-IgE mediated gastrointestinal food allergic disorders (non-IgE- EGID) such as food protein-induced enterocolitis syndrome (FPIES) and food protein-induced enteropathy (FPE) [2, 59]. Further, the activation and recruitment of eosinophils by a number of cytokines including IL-3, IL-5, and GM-CSF as well as chemokines leads to eosinophil degranulation. The degranulation of eosinophils causes the release of several pro-inflammatory mediators, including granule-stored cationic proteins, and many cytokines such as TGF-β, IL-3, IL-4, IL-5, IL-8, IL-13, IL-18, and TNF-α that were responsible for tissue damage at allergic site [2, 15]. Several case reports have been cited in the literature indicating the accumulation of eosinophils in pancreatitis patients and termed this condition as “Eosinophilic Pancreatitis (EP)” [11, 60]. Collectively, it can be speculated that food-specific non-IgE mediated reactions might have a crucial role in induction of pancreatic inflammation but further effort is needed to establish this phenomenon.
Role of inflammatory cells in food-allergen mediated pancreatitis
Several immune effector cells such as mast cells, basophils, eosinophils, neutrophils and T cells are known to have crucial roles in promoting food-mediated allergic responses in pancreatitis [61, 62]. In brief, the details of each cell have been presented below.
Mast Cells
The involvement of mast cells in food allergy-induced gastrointestinal disorders was reported during food-induced allergic responses [63, 64]. In IgE-mediated reactions, the production of allergen-specific IgE occurs and subsequent binding to FcεRI receptors on mast cells initiates allergic response via release of several mediators resulting in vasodilatation, edema, and bronchoconstriction [65]. Mast cells have a crucial and pivotal role in lethal acute [66], chronic pancreatitis [52, 67] and multiple organ failure [68]. The involvement of mast cells in the development of pancreatitis and the possibility of an allergic basis to the disease has also been identified [66].
Basophils
Basophils represent less than 1% of peripheral blood leukocytes and are involved in the development of IgE-mediated allergic diseases [1]. A recent observation based on initial findings showed that activated basophils play an important role in the pathophysiology of autoimmune pancreatitis via activation of TLR signaling pathway [69]. However, the role of basophils in food allergen-induced pathogenesis of acute and chronic pancreatitis is still not clear and needs to be explored further.
Eosinophils
Eosinophils also play a key role in food-induced allergic responses [23, 24]. Blood and tissue eosinophilia with marked degranulation have been reported in a number of allergic diseases associated with food [2]. Eotaxins are the important molecules and act as eosinophil chemo-attractant that promotes accumulation of eosinophils in the tissue [70]. Eosinophilic pancreatitis is a relatively rare disease characterized by local or diffuse infiltration of eosinophils into the pancreas [60] during acute pancreatitis and also reported in association with eosinophilic gastroenteritis [48, 71]. These observations suggest food allergen-induced allergic responses may be involved in the development of eosinophilic pancreatitis but is not yet well understood and need to be explored to better understand the mechanistic aspects of food-induced pancreatitis. Most recently, we reported an important role of eosinophils in cerulein induced chronic pancreatitis. Our report indicates that the eosinophils deficient GATA 1 mice show reduced pancreatic remodeling compared to wild type or IL-5 gene deficient mice following the 4 weeks of cerulein treatment [72].
Neutrophils
A crucial role of neutrophils has been shown in patients with food allergy [73] and well documented in acute pancreatitis [15, 74, 75]. The neutrophil mediators cause trypsin activation, inflammation and may promote pathogenesis of pancreatitis [74] via IgE independent anaphylactic reactions [76]. Neutrophil extracellular traps (NETs) are the decondensed DNA and histones that trap microbial pathogens and orchestrate initiation and resolution of inflammation [17, 77]. NETs were formed in the pancreas of mice during AP and cause induction of trypsin and promote pancreatitis pathogenesis [74]. Based on these reports, food allergen-related induction of neutrophils may be implicated in the progression of acute pancreatitis.
Lymphocytes
Several studies indicate that food allergen-specific T-lymphocytes trigger the production of IL-4 and IL-13 essential for IgE synthesis and T-lymphocytes has been well documented in the pathogenesis of acute [78, 79] and chronic pancreatitis [80]. CD4+ T-cells play a critical role in progression of disease during acute experimental pancreatitis [78]. CD40 ligand (CD40L) present on Th-cells binds with CD40 receptor present on APC and regulates events of pancreatitis [81]. In addition, T-cells are involved in both IgE and non-IgE mediated food allergic responses [82, 83] and may have a major role in regulating pathogenesis of pancreatitis. Therefore, further studies will be needed to understand in-depth molecular mechanisms involved in food allergy and pancreatitis pathogenesis.
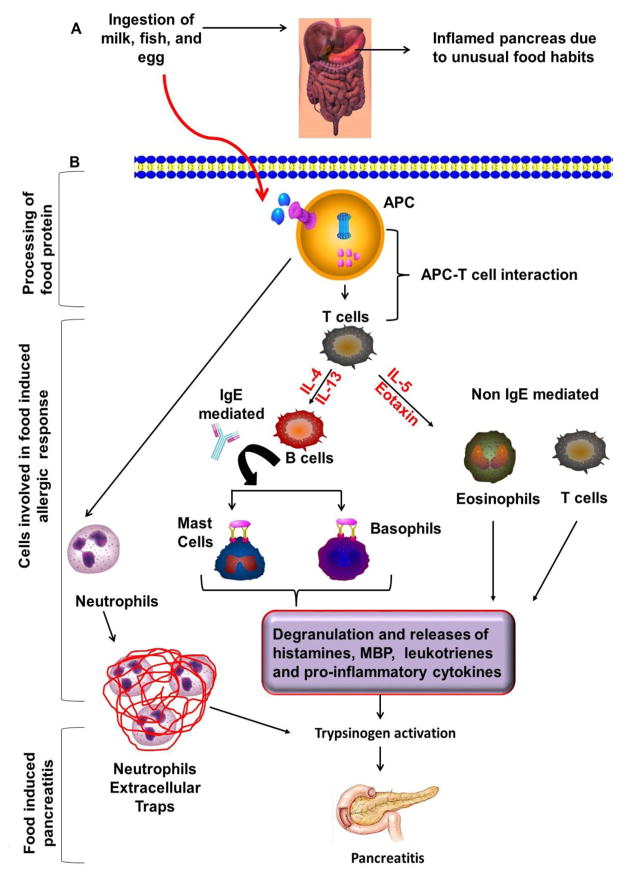
As per the understanding, it seems that IgE-mediated and non-IgE mediated allergic mechanisms may be operational. Therefore, mast cells, basophils, eosinophils, neutrophils and T-cells may be playing critical roles in food-induced pancreatitis and need to be tested in the future for the development of treatment options. A possible operational mechanism of food allergen-induced acute pancreatitis and involvement of different types of immune cells have been shown in Figure 1.
Figure 1. The possible operational mechanism in food allergen-induced acute pancreatitis.
Food allergens internalized and processed by antigen presenting cells (APCs) with the help of proteosomal complex and presented by major histocompatibility complex class-II (MHC-II) to the naive CD4+ T cells that differentiate into the Th2 cells in the presence of IL-4 and produce IL-5 and IL-13 that activate downstream allergic pathways and induces food allergen associated acute pancreatitis. The food allergen–induced allergic response in the pancreas may be IgE or non-IgE mediated. In IgE mediated response, Th2 cells secrete IL-4 and IL-13 that causes B cell activation and IgE class switching. IgE antibodies bind to FcεRI surface receptors present on mast cells and basophils that lead degranulation and release of several allergic mediators. These allergic mediators probably cause trypsinogen activation and development of pancreatitis. However, this hypothesis needs to be explored further for the designing treatment strategies. In contrast, the non-IgE mediated response may also occur that includes the role of IL-5 and eosinophil active chemokines eotaxins that recruit, activate and releases several inflammatory mediators and lead trypsinogen activation. Increased level of trypsin consecutively setup the episode of acute pancreatitis. In addition, neutrophils are forming neutrophil extracellular traps (NETs) of de-condensed DNA and histones, which is sufficient to induce trypsin activation to promote pancreatitis pathogenesis.
Knowledge gaps exist between food-induced acute pancreatitis and pathogenesis of pancreatitis
Lack to recognize the underlying mechanisms of food-induced acute pancreatitis raises concern and a great need to develop the novel clinical strategy to identify the food allergens that promote acute pancreatitis. Pancreatitis is a lethal disease with significant morbidity and mortality. Although, food-induced acute pancreatitis is a rare disorder, a clear understanding of the food-associated acute pancreatitis with the condition should alert the clinician to suitably diagnose and treat these food affected acute pancreatitis patients. Future investigations should be performed to understand molecular mechanisms of food-induced acute pancreatitis and its clinical sequelae. Furthermore, an appreciation by allergist and gastroenterologist of the food commonly linked to the pancreatitis condition will help to establish earlier diagnosis. An early diagnosis can facilitate and prompt cessation of the upsetting food, thereby reducing complications and length of hospital stay.
Efforts required in establishing food allergen-induced pancreatitis
Currently, very few case reports implicate food allergens to the induction of pancreatitis; therefore, more effort is needed by the medical community to identify the causative food for acute pancreatitis and better understand the mechanistic aspects of food-mediated pancreatitis. Additionally, there should be a great collaboration between allergist and gastroenterologist to diagnose and understand the pathogenesis of food-induced pancreatitis by developing novel and efficient approaches such as identification of a particular type of food allergen for a particular patient.
Treatment of food-induced acute pancreatitis
Food allergy-induced pancreatitis can be cured by specific diet allergen elimination; by avoiding a particular type of food. ‘Nil per Os’ is the first-line of treatment along with aggressive hydration to help the pancreas to rest and recover [24, 26]. On the basis of the underlying cause of pancreatitis, treatment options may differ to address the specific cause. Analgesics and antiemetics can be used if needed. Cromoglicic acid (mast cell stabilizer) and corticosteroids have been frequently used to treat food-induced acute pancreatitis [12, 24, 26]. Further, the use of micronutrient therapy including several vitamins, methionine, and β-carotene has very much promising results in the treatment of chronic pancreatitis. In one study, the micronutrient therapy has been prepared in a different way to supply methyl and thiol moieties, which limit the generation of reactive oxygen species, deactivate pro-inflammatory responses, and prevent mast cell degranulation [51]. Therefore, mast cell stabilizer drugs may hold a future treatment option of food-induced pancreatitis.
Conclusion
The present review provides a comprehensive understanding of mechanisms of food allergy and associated disorders including an emphasis on food-induced pancreatitis. Food-induced acute or chronic pancreatitis poses a challenge to the scientific community as its pathogenesis is still not clear, and how the disease development/progression take place by the food antigen-activated immune components. However, there is scarcity of literature about the involvement of food in the induction of pancreatitis. In this review, we summarized the available case reports of pancreatitis associated with the food allergy and its possible mechanism. Our review of literature provokes further thinking in this direction and it will be exciting to know exactly how and why certain foods cause pancreatitis in some susceptible individuals. Improving the clinical outcomes for patients remains the focus of extensive basic science and clinical research and many of the current issues relating to food-induced pancreatitis are particularly pertinent to the clinical biochemists and to the health care providers. There is need for further research in this area of food-induced pancreatitis, and we strongly suggest that food induced pancreatitis should not be ignored and patients should be evaluated by the allergist. Therefore, we urge clinicians to be extra vigilant for food-induced pancreatitis especially in patients who are diagnosed with idiopathic pancreatitis. An extended dietary history including the recently ingested foods provoking the symptoms might lead the physicians to the diagnosis of food-induced pancreatitis.
Acknowledgments
Dr. Mishra is the Endowed Schlieder Chair; therefore, authors thank Edward G. Schlieder Educational Foundation for the support.
Footnotes
Conflict of Interest
All authors declare no conflict of interest.
References
- 1.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla A, MA, Venkateshaiah SU, Manhoar M, Mahadevappa CP, Mishra A. Elements Involved In Promoting Eosinophilic Gastrointestinal Disorders. J Genet Syndr Gene Ther. 2015;6:265. doi: 10.4172/2157-7412.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma AK, MMUVSU, Blecker U, Collins MH, Mishra A. Role of vasoactive intestinal peptide in promoting the pathogenesis of eosinophilic esophagitis (EoE) Cell Mol Gastroenterol Hepatol. 2017 Sep; doi: 10.1016/j.jcmgh.2017.09.006. Accepted, Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma AK, Manohar M, UVS, Mishra A. Endocrine cells derived chemokine vasoactive intestinal polypeptide (VIP) in allergic diseases. Cytokine Growth Factor Rev. 2017 Sep; doi: 10.1016/j.cytogfr.2017.09.002. In press. [DOI] [PMC free article] [PubMed]
- 5.Kattan J. The Prevalence and Natural History of Food Allergy. Curr Allergy Asthma Rep. 2016;16(7):47. doi: 10.1007/s11882-016-0627-4. [DOI] [PubMed] [Google Scholar]
- 6.Zukiewicz-Sobczak WA, et al. Causes, symptoms and prevention of food allergy. Postepy Dermatol Alergol. 2013;30(2):113–6. doi: 10.5114/pdia.2013.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden SR, Wesley Burks A. Anaphylaxis: a history with emphasis on food allergy. Immunol Rev. 2011;242(1):247–57. doi: 10.1111/j.1600-065X.2011.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe JL, Aceves SS. Gastrointestinal manifestations of food allergies. Pediatr Clin North Am. 2011;58(2):389–405. x. doi: 10.1016/j.pcl.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Verma AK, et al. A comprehensive review of legume allergy. Clin Rev Allergy Immunol. 2013;45(1):30–46. doi: 10.1007/s12016-012-8310-6. [DOI] [PubMed] [Google Scholar]
- 10.Inamura H, et al. Acute pancreatitis possibly caused by allergy to bananas. J Investig Allergol Clin Immunol. 2005;15(3):222–4. [PubMed] [Google Scholar]
- 11.Flejou JF, Potet F, Bernades P. Eosinophilic pancreatitis: a rare manifestation of digestive allergy? Gastroenterol Clin Biol. 1989;13(8–9):731–3. [PubMed] [Google Scholar]
- 12.Matteo A, Sarles H. Is food allergy a cause of acute pancreatitis? Pancreas. 1990;5(2):234–7. doi: 10.1097/00006676-199003000-00018. [DOI] [PubMed] [Google Scholar]
- 13.de Diego Lorenzo A, et al. Acute pancreatitis associated with milk allergy. Int J Pancreatol. 1992;12(3):319–21. doi: 10.1007/BF02924372. [DOI] [PubMed] [Google Scholar]
- 14.Iwata F, Odajima Y. Acute pancreatitis associated with food allergy. Eur J Pediatr. 1997;156(6):506. [PubMed] [Google Scholar]
- 15.Manohar M, et al. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10–25. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12(46):7421–7. doi: 10.3748/wjg.v12.i46.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manohar M, VA, Venkateshaiah SU, Sanders NL, Mishra A. Chronic Pancreatitis Associated Acute Respiratory Failure. MOJ Immunology. 2017;5(2):1–7. doi: 10.15406/moji.2017.05.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang AL, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649–56. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 20.Hirota M, et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology. 2012;12(2):79–84. doi: 10.1016/j.pan.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Couvelaire RBD. Quelques résultats expérimentaux sur la génèse des oedèmes aigus et des hémorragies pancréatiques. Les pancréatites anaphylactiques. CR Soc Biol. 1933;11:1435–1437. [Google Scholar]
- 22.Carrilllo T, AM, Gusmán TM, Vilardell VU, Goiri IM. Pancreatitis aguda como complicación de anaphilaxia por mostaza. Rev Esp Alergol Immunol Clin. 1987;2:388–390. [Google Scholar]
- 23.Suzuki S, et al. Eosinophilic gastroenteritis due to cow’s milk allergy presenting with acute pancreatitis. Int Arch Allergy Immunol. 2012;158(Suppl 1):75–82. doi: 10.1159/000337782. [DOI] [PubMed] [Google Scholar]
- 24.Tse KY, Christiansen SC. Eosinophilic gastroenteritis due to egg allergy presenting as acute pancreatitis. Allergy Rhinol (Providence) 2015;6(1):80–1. doi: 10.2500/ar.2015.6.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrino K, et al. Severe reaction in a child with asymptomatic codfish allergy: food challenge reactivating recurrent pancreatitis. Ital J Pediatr. 2012;38:16. doi: 10.1186/1824-7288-38-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gastaminza G, Bernaola G, Camino ME. Acute pancreatitis caused by allergy to kiwi fruit. Allergy. 1998;53(11):1104–5. doi: 10.1111/j.1398-9995.1998.tb03824.x. [DOI] [PubMed] [Google Scholar]
- 27.Oskarsson V, et al. High dietary glycemic load increases the risk of non-gallstone-related acute pancreatitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12(4):676–82. doi: 10.1016/j.cgh.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 28.Setiawan VW, et al. Dietary Factors Reduce Risk of Acute Pancreatitis in a Large Multiethnic Cohort. Clin Gastroenterol Hepatol. 2017;15(2):257–265 e3. doi: 10.1016/j.cgh.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oskarsson V, et al. Vegetables, fruit and risk of non-gallstone-related acute pancreatitis: a population-based prospective cohort study. Gut. 2013;62(8):1187–92. doi: 10.1136/gutjnl-2012-302521. [DOI] [PubMed] [Google Scholar]
- 30.Oskarsson V, et al. Fish consumption and risk of non-gallstone-related acute pancreatitis: a prospective cohort study. Am J Clin Nutr. 2015;101(1):72–8. doi: 10.3945/ajcn.113.076174. [DOI] [PubMed] [Google Scholar]
- 31.Ho MH, Wong WH, Chang C. Clinical spectrum of food allergies: a comprehensive review. Clin Rev Allergy Immunol. 2014;46(3):225–40. doi: 10.1007/s12016-012-8339-6. [DOI] [PubMed] [Google Scholar]
- 32.Beausoleil JL, Fiedler J, Spergel JM. Food Intolerance and childhood asthma: what is the link? Paediatr Drugs. 2007;9(3):157–63. doi: 10.2165/00148581-200709030-00004. [DOI] [PubMed] [Google Scholar]
- 33.Bihouee T, et al. Food allergy enhances allergic asthma in mice. Respir Res. 2014;15:142. doi: 10.1186/s12931-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt EB, et al. Experimental gastrointestinal allergy enhances pulmonary responses to specific and unrelated allergens. J Allergy Clin Immunol. 2006;118(2):420–7. doi: 10.1016/j.jaci.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Cho KA, et al. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L429–40. doi: 10.1152/ajplung.00252.2011. [DOI] [PubMed] [Google Scholar]
- 36.Bihouée T, BG, Chesné J, Lair D, Rolland-Debord C, Braza F, Cheminant MA, Aubert P, Mahay G, Sagan C, Neunlist M, Brouard S, Bodinier M, Magnan A. Food allergy enhances allergic asthma in mice. Respir Res. 2014;30(15):142. doi: 10.1186/s12931-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tariq SM, et al. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol. 2000;11(3):162–7. doi: 10.1034/j.1399-3038.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 38.James JM, Bernhisel-Broadbent J, Sampson HA. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med. 1994;149(1):59–64. doi: 10.1164/ajrccm.149.1.8111598. [DOI] [PubMed] [Google Scholar]
- 39.Torpier G, et al. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin Exp Immunol. 1988;74(3):404–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra A, et al. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–27. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uppal V, Kreiger P, Kutsch E. Eosinophilic Gastroenteritis and Colitis: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;50(2):175–88. doi: 10.1007/s12016-015-8489-4. [DOI] [PubMed] [Google Scholar]
- 42.Khan S, Orenstein SR. Eosinophilic gastroenteritis: epidemiology, diagnosis and management. Paediatr Drugs. 2002;4(9):563–70. doi: 10.2165/00128072-200204090-00002. [DOI] [PubMed] [Google Scholar]
- 43.Alfadda AA, Storr MA, Shaffer EA. Eosinophilic colitis: epidemiology, clinical features, and current management. Therap Adv Gastroenterol. 2011;4(5):301–9. doi: 10.1177/1756283X10392443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaffe JS, et al. Evidence for an abnormal profile of interleukin-4 (IL-4), IL-5, and gamma-interferon (gamma-IFN) in peripheral blood T cells from patients with allergic eosinophilic gastroenteritis. J Clin Immunol. 1994;14(5):299–309. doi: 10.1007/BF01540983. [DOI] [PubMed] [Google Scholar]
- 45.el Mouzan MI, al Quorain AA, Anim JT. Cow’s-milk-induced erosive gastritis in an infant. J Pediatr Gastroenterol Nutr. 1990;10(1):111–3. doi: 10.1097/00005176-199001000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Bischoff SC. Food allergy and eosinophilic gastroenteritis and colitis. Curr Opin Allergy Clin Immunol. 2010;10(3):238–45. doi: 10.1097/ACI.0b013e32833982c3. [DOI] [PubMed] [Google Scholar]
- 47.Valle-Dorado MG, et al. The mast cell stabilizer sodium cromoglycate reduces histamine release and status epilepticus-induced neuronal damage in the rat hippocampus. Neuropharmacology. 2015;92:49–55. doi: 10.1016/j.neuropharm.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez Rodriguez JJ, et al. Pancreatitis and eosinophilic gastroenteritis. Int Surg. 1973;58(6):415–9. [PubMed] [Google Scholar]
- 49.Caglar E, Karismaz K, Dobrucali A. A case of eosinophilic gastroenteritis mimicking gastric lymphoma associated with pancreatitis due to duodenal involvement. Turk J Gastroenterol. 2012;23(5):585–9. [PubMed] [Google Scholar]
- 50.Goyal H, et al. Differences in Severity and Outcomes Between Hypertriglyceridemia and Alcohol-Induced Pancreatitis. N Am J Med Sci. 2016;8(2):82–7. doi: 10.4103/1947-2714.177307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braganza JM, Dormandy TL. Micronutrient therapy for chronic pancreatitis: rationale and impact. JOP. 2010;11(2):99–112. [PubMed] [Google Scholar]
- 52.Esposito I, et al. Mast cell distribution and activation in chronic pancreatitis. Hum Pathol. 2001;32(11):1174–83. doi: 10.1053/hupa.2001.28947. [DOI] [PubMed] [Google Scholar]
- 53.Kweon MN, et al. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106(2):199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124(4):639–46. doi: 10.1016/j.jaci.2009.08.035. quiz 647–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knol EF. Requirements for effective IgE cross-linking on mast cells and basophils. Mol Nutr Food Res. 2006;50(7):620–4. doi: 10.1002/mnfr.200500272. [DOI] [PubMed] [Google Scholar]
- 56.Raithel M, et al. Immunoglobulin E production in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2003;15(7):801–7. doi: 10.1097/01.meg.0000059143.68845.af. [DOI] [PubMed] [Google Scholar]
- 57.Raithel M, et al. Stimulation of immunoglobulin E formation in chronic pancreatitis by alcohol drinking and exocrine pancreatic insufficiency. Z Gastroenterol. 2001;39(4):269–76. doi: 10.1055/s-2001-12870. [DOI] [PubMed] [Google Scholar]
- 58.Jyonouchi H. Non-IgE mediated food allergy - update of recent progress in mucosal immunity. Inflamm Allergy Drug Targets. 2012;11(5):382–96. doi: 10.2174/187152812803250971. [DOI] [PubMed] [Google Scholar]
- 59.Caubet JC, et al. Non IgE-mediated Gastrointestinal Food Allergies in children. Pediatr Allergy Immunol. 2016 doi: 10.1111/pai.12659. [DOI] [PubMed] [Google Scholar]
- 60.Tian L, et al. Eosinophilic pancreatitis: Three case reports and literature review. Mol Clin Oncol. 2016;4(4):559–562. doi: 10.3892/mco.2016.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, VA, Das M, Dwivedi PD. Allergenic diversity among plant and animal food proteins. Food Reviews International. 2011;28(3):277–298. [Google Scholar]
- 62.Manohar M, VA, Venkateshaiah SU, Mishra A. Immunological Responses Involved In Promoting Acute and Chronic Pancreatitis. J Clin Immunol Res. 2017;1:1–8. [Google Scholar]
- 63.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–68. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 64.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraneveld AD, et al. The two faces of mast cells in food allergy and allergic asthma: the possible concept of Yin Yang. Biochim Biophys Acta. 2012;1822(1):93–9. doi: 10.1016/j.bbadis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Braganza JM. Mast cell: pivotal player in lethal acute pancreatitis. QJM. 2000;93(7):469–76. doi: 10.1093/qjmed/93.7.469. [DOI] [PubMed] [Google Scholar]
- 67.Hoogerwerf WA, et al. The role of mast cells in the pathogenesis of pain in chronic pancreatitis. BMC Gastroenterol. 2005;5:8. doi: 10.1186/1471-230X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dib M, et al. Role of mast cells in the development of pancreatitis-induced multiple organ dysfunction. Br J Surg. 2002;89(2):172–8. doi: 10.1046/j.0007-1323.2001.01991.x. [DOI] [PubMed] [Google Scholar]
- 69.Masato Yanagawa KU, Ikemune Manami, Ikeura Tsukasa, Shimatani Masaaki, Fukui Toshiro, Takaoka Makoto, Nishio Akiyoshi, Satoi Sohei, Okazaki Kazuichi. The role of basophil via TLR signaling in patients with type 1 autoimmune pancreatitis. Pancreatology. 2016;16(4):S 40. [Google Scholar]
- 70.Stellato C, et al. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Invest. 1997;99(5):926–36. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeshima A, et al. Eosinophilic gastroenteritis presenting with acute pancreatitis. J Med. 1997;28(3–4):265–72. [PubMed] [Google Scholar]
- 72.Manohar M, VAK, Venkateshaiah SU, Mishra A. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2017 Sep 19; doi: 10.1152/ajpgi.00210.2017. Accepted, Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallaert B, et al. Airway neutrophil inflammation in nonasthmatic patients with food allergy. Allergy. 2002;57(5):405–10. doi: 10.1034/j.1398-9995.2002.13527.x. [DOI] [PubMed] [Google Scholar]
- 74.Merza M, et al. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice With Severe Acute Pancreatitis. Gastroenterology. 2015;149(7):1920–1931 e8. doi: 10.1053/j.gastro.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 75.Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19(11):2513–20. doi: 10.1111/jcmm.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munoz-Cano R, et al. Mechanisms of Anaphylaxis Beyond IgE. J Investig Allergol Clin Immunol. 2016;26(2):73–82. doi: 10.18176/jiaci.0046. quiz 2p following 83. [DOI] [PubMed] [Google Scholar]
- 77.Hahn J, et al. Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin Exp Rheumatol. 2016;34(4 Suppl 98):6–8. [PubMed] [Google Scholar]
- 78.Demols A, et al. CD4(+)T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118(3):582–90. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 79.Vonlaufen A, et al. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213(3):239–48. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 80.Yamada T, et al. Role of T cells in development of chronic pancreatitis in male Wistar Bonn/Kobori rats: effects of tacrolimus. Am J Physiol Gastrointest Liver Physiol. 2001;281(6):G1397–404. doi: 10.1152/ajpgi.2001.281.6.G1397. [DOI] [PubMed] [Google Scholar]
- 81.Frossard JL, et al. Cd40 ligand-deficient mice are protected against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2001;121(1):184–94. doi: 10.1053/gast.2001.25483. [DOI] [PubMed] [Google Scholar]
- 82.Palomares O. The role of regulatory T cells in IgE-mediated food allergy. J Investig Allergol Clin Immunol. 2013;23(6):371–82. quiz 2 p preceding 382. [PubMed] [Google Scholar]
- 83.Eigenmann PA, Frossard CP. The T lymphocyte in food-allergy disorders. Curr Opin Allergy Clin Immunol. 2003;3(3):199–203. doi: 10.1097/00130832-200306000-00008. [DOI] [PubMed] [Google Scholar]