Abstract
Knowledge of the molecular mechanisms of acute pancreatitis is largely based on studies using rodents. To assess similar mechanisms in humans, we performed ex vivo pancreatitis studies in human acini isolated from cadaveric pancreata from organ donors. Because data on these human acinar preparations are sparse, we assessed their functional integrity and cellular and organellar morphology using light, fluorescence, and electron microscopy; and their proteome by liquid chromatography–tandem mass spectrometry. Acinar cell responses to the muscarinic agonist carbachol (CCh) and the bile acid taurolithocholic acid 3-sulfate were also analyzed. Proteomic analysis of acini from donors of diverse ethnicity showed similar profiles of digestive enzymes and proteins involved in translation, secretion, and endolysosomal function. Human acini preferentially expressed the muscarinic acetylcholine receptor M3 and maintained physiological responses to CCh for at least 20 hours. As in rodent acini, human acini exposed to toxic concentrations of CCh and taurolithocholic acid 3-sulfate responded with trypsinogen activation, decreased cell viability, organelle damage manifest by mitochondrial depolarization, disordered autophagy, and pathological endoplasmic reticulum stress. Human acini also secreted inflammatory mediators elevated in acute pancreatitis patients, including IL-6, tumor necrosis factor-α, IL-1β, chemokine (C-C motif) ligands 2 and 3, macrophage inhibitory factor, and chemokines mediating neutrophil and monocyte infiltration. In conclusion, human cadaveric pancreatic acini maintain physiological functions and have similar pathological responses and organellar disorders with pancreatitis-causing treatments as observed in rodent acini.
The physiological functions of the exocrine pancreas are the synthesis, storage, and secretion of digestive enzymes. Disorders in these functions often lead to pancreatitis, an inflammatory disease of the pancreas that can cause significant morbidity and even mortality. Among gastrointestinal diseases, pancreatitis is the most common reason for hospital admissions in the United States.1, 2 Although the clinical description of the disease has been with us for >100 years, therapy has not progressed beyond supportive measures.3, 4 To define molecular targets to develop specific therapies, investigators have largely relied on nonhuman tissues.4, 5, 6
It is widely held that the pathobiologic events in acute pancreatitis are initiated in the pancreatic acinar cell in response to various insults, such as alcohol abuse, cigarette smoking, hyperlipidemia, and gallstones.7 Studies performed using preparations enriched in pancreatic acinar cells from experimental animals have been used to examine both physiological and pathological responses. Using agents that are known to cause pancreatitis in vivo, investigators have demonstrated that complex signaling, trafficking, processing, and organellar and secretory changes that characterize the early phases of the disease also occur in ex vivo preparations of acini from rodents.8, 9, 10, 11 These acini preparations were developed almost 40 years ago,12 and since then virtually all in vitro studies have been performed using rat, mouse, or guinea pig pancreatic acini. The relevance of the responses and mechanisms reported in these systems has had limited validation in human acinar cells. For example, whether human acinar cells have the same complement of functional cell surface receptors, exhibit biphasic secretory responses to agonists, and demonstrate similar disordering of signaling pathways and organellar dysfunction observed in the acinar cells of another species remains largely unknown. A few studies have suggested that some fundamental characteristics of rodent acinar cells are conserved in humans. For example, using isolated human acinar cells or pancreatic fragments from surgical resections, investigators have reported that amylase secretion is retained, although the responsiveness in these preparations appeared to be low.13, 14
Furthermore, preliminary studies suggest that pancreatitis responses are retained with these ex vivo preparations, such as the effects of bile acids and a role of ryanodine receptor in pathologic Ca2+ signaling in the acinar cell.15, 16 The disorders of acinar cell organelles that have emerged as central events in the early phases of the pancreatitis response in rodent acini are not examined in these preparations.17, 18, 19, 20 These disorders play a necessary role in triggering necrosis and inflammation, which are the hallmarks of the disease.
An important limitation for functional studies using pancreatic acini is the need to perform experiments soon after they are isolated. That is, freshly isolated pancreatic acinar cells rapidly change their phenotype when placed in culture. This includes losses of polarity, secretory responsiveness, calcium mobilization in response to stimulation, and other aspects of differentiation. Furthermore, none of the currently available pancreas-derived cell lines, which are driven by neoplastic elements (eg, rat AR42J cell line), appear to fully represent the acinar cell phenotype and function. Finally, until recently, access to functional human pancreatic acini has been limited to the availability of rare surgical specimens from diseased human pancreata.14
Approximately 20 years ago, investigators began isolating human pancreatic islets from cadaveric donor pancreata for transplantation.21, 22 The isolation procedure has been refined and optimized over time to obtain highly functional, pure islet preparations for clinical transplantation.23 The success of the clinical transplantation relies on minimal damage to the exocrine pancreas during pancreas preservation and digestion to minimize the release of endogenous proteases from acinar cells. It also relies on optimal separation of islets from acinar cells to prevent protease damage to islets and to improve viability and function of isolated islet cells.24, 25 These refined procedures also allow attainment of good-quality dispersed acini and other components of the pancreas. On the basis of the preliminary studies of others, we have developed protocols to optimize the preservation of such human dispersed acini, obtained as a by-product of pancreatic islet isolation. To ensure reproducibility of the results obtained with these preparations, we have established a standardized protocol for isolating acini across our institutions. This protocol is accompanied by inclusion criteria based on the assessment of their purity, viability, and functional responses before using them for ex vivo studies.
We find that these human pancreatic acini preparations are highly enriched in acinar cells, contain low amounts of ductal cells and stromal cells, and are depleted of islets and endothelial cells. Proteomic analyses show a similar proteome profile in acini preparations obtained from organ donors of diverse ethnicity. The proteome is enriched in proteins involved in protein production and secretion, as expected for professional secretory cells. The isolated acini are viable, maintain polarity over 20 hours, and demonstrate a robust secretory response to cholinergic stimulation. More important, they also retain key pancreatitis responses to supraphysiologic concentrations of carbachol (CCh), such as suppressed digestive enzyme secretion, intra-acinar activation of trypsinogen, and histological and biochemical evidence of cell injury. Also, as in pancreatic acini from rodents, they develop similar pancreatitis responses to the bile acid, taurolithocholic acid 3-sulfate (TLCS), including the secretion of cytokines/chemokines.26, 27 Similar to those isolated from animals, human acini begin to lose neurohumoral responsiveness and other characteristics of differentiated cells after prolonged culture. Our study shows that human acini obtained from cadaveric pancreata are suitable for studies that require a preparation highly enriched in acinar cells and that aim to elucidate the pathogenic mechanisms of pancreatitis.
Materials and Methods
Isolation of Human Acini
We obtained deidentified human pancreatic tissue devoid of islets of Langerhans, produced as a by-product of isolating islets for clinical transplantation from brain-dead organ donors without morphological or histological evidence of pancreatic disease. Cadaveric donor pancreata acquired after informed consented for use in islet transplants and research were processed at the Beckman Research Institute of City of Hope (Duarte, CA) using a modification of the Ricordi method, as previously described.28 In addition, human acini isolated at the University of Wisconsin Department of Surgery, Islet Research Laboratory (Madison, WI), were used in this study (Figure 1B). Similar cell isolation techniques and experimental approaches were used in all centers involved in this research. In brief, the whole pancreas is received at the institution, preserved in University of Wisconsin solution with variable cold ischemia (<12 hours from harvesting of the organ). Then, after trimming off the fat and other connective tissue surrounding the pancreas, the pancreatic duct is cannulated and the organ is perfused with cold collagenase solution (collagenase NB1 + neutral protease NB; SERVA, Heidelberg, Germany). Then, later, a collagenase-containing enzyme solution (collagenase HA + thermolysin + rhDNase I; VitaCyte LLC, Indianapolis, IN) is used in a Ricordi digestion chamber at 37°C. The chamber is shaken to liberate islets and other pancreatic cells. After pancreas digestion, islets and nonislet cells (acini and other exocrine pancreatic cells) are separated by centrifugation on a continuous Biocoll gradient (Biochrom AG, Berlin, Germany). Once separated, the acini fractions are washed and preserved in cold CMRL 1066 media (ICN Biomedicals, Costa Mesa, CA), containing human serum (hAB; from human male AB plasma), and transferred to our laboratories for studies. On arrival, acini are washed twice in Dulbecco's modified Eagle’s medium (ThermoFisher Scientific, Waltham, MA), containing 0.1% bovine serum albumin and 100 μg/mL soybean trypsin inhibitor (Sigma-Aldrich, St. Louis, MO), and further purified by allowing us to sediment by gravity through Dulbecco’s modified Eagle’s medium containing 2% bovine serum albumin. The resultant cell pellets were resuspended in 199 media (ThermoFisher Scientific), containing 100 μg/mL soybean trypsin inhibitor, and used for experiments. In this study, human acini were kept untreated or stimulated with physiological or toxic concentrations of the cholinergic agonist CCh or the bile acid TLCS; both were purchased from Sigma-Aldrich. In a subset of studies, acini were treated with cholecystokinin (sulfated CCK-8; Research Plus, Barnegat, NJ). All cell incubations were performed in 199 medium, containing 100 μg/mL soybean trypsin inhibitor, in a cell culture incubator at 37°C in an atmosphere of 5% CO2. The study was performed in accordance with regulations and protocols approved by the institutional review boards of the Beckman Research Institute of City of Hope, the University of Wisconsin, and the Cedars-Sinai Medical Center (Los Angeles, CA; institutional review board Pro00032114).
Figure 1.
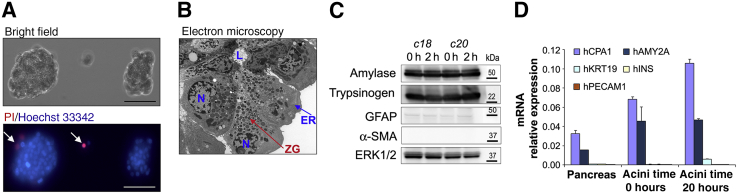
Characterization of isolated human pancreatic acini. A: Untreated acini were placed in a cell culture fluorodish with a glass bottom, cultured for 2 hours, stained with propidium iodide (PI) and Hoechst 33342, and observed under a fluorescence inverted microscope to assess cell morphology and viability. Arrows indicate PI-positive cells. B: Representative electron micrograph of untreated human acini showing typical acinar cell ultrastructure and acinar lumen (L), with nuclei (N) in basal position, endoplasmic reticulum (ER) network surrounding the nucleus, and abundant zymogen granules (ZG). C and D: Untreated human acini were cultured for up to 20 hours. Panels show representative Western blot images (C) and real-time quantitative PCR analysis (D) for markers of acinar, stellate, ductal, endothelial, and β cells. mRNA isolated from pancreatic tissues samples (Pancreas) from organ donors was used for comparison. Data are expressed as means ± SD (D). n = 3 (D). Scale bars = 100 μm (A). Original magnification, ×1500 (B). c, organ donor case (patient coding) used as a source of human acinar preparation; ERK, extracellular signal regulated kinase; GFAP, glial fibrillary acidic protein; hAMY2A, human pancreatic α-amylase; hCPA1, human carboxypeptidase A1; hINS, human insulin; hKRT19, human cytokeratin-19; hPECAM1, human platelet endothelial cell adhesion molecule-1; α-SMA, α-smooth muscle actin; h, hours.
Electron Microscopy
Electron microscopy analysis was performed on cells fixed at 4°C in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 2 hours. After post-fixation in 2% OsO4, cells were dehydrated in ethanol and then in polypropylene. Samples were embedded in epoxy resin. Sections (80 nm thick) were stained with uranyl acetate and examined in a Philips CM 120 electron microscope (Philips, Amsterdam, the Netherlands), and captured images were converted to TIFF files for further analysis.
Immunofluorescence Analysis
Human acini were fixed in suspension with 4% paraformaldehyde (methanol free) for 30 minutes at room temperature and then permeabilized with 0.1% Triton X-100 in phosphate-buffered saline. Non-specific binding was blocked with 5% rabbit or 5% goat serum. Then, cells were stained with primary antibodies against trypsinogen (sc-67388) and translocase of outer membrane 20 (Tom20; sc11415) from Santa Cruz Biotechnology (Dallas, TX); calnexin (ab22595) from Abcam (Cambridge, MA); light chain 3 (LC3) B (2775) from Cell Signaling Technology (Danvers, MA); or Lamp-2 (L-06668) from Sigma-Aldrich. The cells were then labeled with Alexa Fluor 488 (green)–, Alexa Fluor 555 (yellow)–, and Alexa Fluor 633 (far red)–conjugated secondary antibodies (ThermoFisher Scientific), and DAPI as nuclear counterstain. Images were acquired with a Zeiss LSM 710 confocal microscope (Carl Zeiss Microscopy, Thornwood, NY) and analyzed with Volocity 6.3 Image software (Perkin-Elmer, Waltham, MA).
Proteomic Analysis
Proteomic analyses were performed with the assistance of the Cedars-Sinai Mass Spectrometry and Biomarker Discovery Core. For liquid chromatography–tandem mass spectrometry, untreated human acini were centrifuged at 250 × g for 3 minutes; the resulting cell pellets were prepared in parallel by filter-assisted sample preparation using trypsin as the protease for digestion into peptides. Peptides were desalted using C18 spin columns before each run. Finished peptides were analyzed by liquid chromatography–tandem mass spectrometry with a 70-minute chromatogram, using C18 liquid chromatography upstream of an Orbitrap Elite mass spectrometer (ThermoFisher Scientific) at the Cedars-Sinai Medical Center Biomarker Discovery Platform Core. The acquired data sets were analyzed using MaxQuant version 1.3.0.5 (www.maxquant.org). Proteins were identified by searching tandem mass spectrometry spectra against the human UniProt database (September 11, 2012 release). Carbamidomethylation of cysteines was set as a fixed modification, and oxidation of methionines and acetylation of the protein N-terminus were set as variable modifications. Trypsin was chosen as the enzyme specificity, with a maximum of two missed cleavages allowed. The maximum false-discovery rates for reported peptide and protein identifications were subject to a 1% cutoff.
A core proteome of 1044 (approximately 69% of all identified proteins) common protein identifications was produced (Supplemental Table S1). The relative abundance of individual proteins was quantified across the four samples on the basis of label-free quantification values determined using the MAX–label-free quantification algorithm.29 Log10 values of label-free quantification were plotted for comparison of key protein categories as a heat map, with values spanning approximately three orders of magnitude. Using the DAVID 6.7 functional annotation database,30, 31 we interrogated the Gene Ontology (GO) database to classify the enriched proteins by the biological processes they are involved in and the Kyoto Encyclopedia of Genes and Genomes database to determine the significantly enriched metabolic pathways of the human acini.
Amylase Secretion
Amylase secretion was measured using the Phadebas Amylase test (Magle Life Sciences, Cambridge, MA), as described,32, 33 and expressed as the percentage of amylase secreted into the medium to the total amylase (medium plus cells).
Measurement of Calcium Mobilization and Calcium Entry
Human acini were resuspended in a buffer containing 140 mmol/L NaCl, 4.7 mmol/L KCl, 1.13 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L d-glucose, 10 mmol/L HEPES (adjusted to pH 7.2 by NaOH), 10 mmol/L pyruvate, and 1 mmol/L glutamine. Then, they were loaded for 30 minutes at 37°C with 2 μmol/L Fura-2 AM (ThermoFisher Scientific). After being washed, acini were suspended in the same buffer but depleted of CaCl2, and cholinergic-induced fluorescence signals, indicating changes in [Ca2+]i, were measured at 37°C in a Shimadzu RF 1501 spectrofluorometer (Shimadzu, Kyoto, Japan) with excitation at 340 and 380 nm and emission at 510 nm. Changes in [Ca2+]i were measured as the ratio of fluorescence emission at 510 nm/340/380-nm excitation, as previously described.34 In addition, calcium entry after addition of 2 mmol/L CaCl2 to the buffer was measured as area under the curve. A specific inhibitor of Ca2+ influx (CM4620; CalciMedica, La Jolla, CA) that blocks calcium release-activated calcium channels was used to determine the presence of these channels in human pancreatic acini.
Mitochondrial Membrane Potential
Mitochondrial membrane potential was determined using the cationic fluorescent dye tetramethylrhodamine methyl ester; it accumulates and fluoresces as a function of mitochondrial polarity. Acini were resuspended in a buffer containing 140 mmol/L NaCl, 4.7 mmol/L KCl, 1.13 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L d-glucose, 10 mmol/L HEPES (adjusted to pH 7.2 by NaOH), 10 mmol/L pyruvate, and 1 mmol/L glutamine. Then, they were loaded for 20 minutes at 37°C with 1 μmol/L tetramethylrhodamine methyl ester. Tetramethylrhodamine methyl ester fluorescence intensity in response to treatments was measured at 37°C at 543 nm (excitation) and 570 nm (emission) using a Shimadzu RF-1501 Spectrofluorophotometer (Shimadzu, Tokyo, Japan), as described.35 Data are presented as fluorescence arbitrary units.
Trypsinogen Activation
Trypsin activity was measured in homogenates of pancreatic acini using Boc-Gln-Ala-Arg-AMC as a substrate by a fluorogenic assay, as reported.19, 20, 36, 37, 38
PI Uptake
To determine cell viability, live acinar cells were labeled with 2 μg/mL propidium iodide (PI) and 0.5 μg/mL Hoechst 33342 (Sigma-Aldrich) in phosphate-buffered saline. Then, the acini were observed using a Nikon Eclipse TE2000 inverted microscope (Nikon, Tokyo, Japan) to estimate the percentage of visible PI-positive necrotic cells and pyknotic nuclei in apoptotic cells (bright Hoechst staining).
Cell death was quantified in human acinar cells by PI uptake, a measure of cellular necrosis.39 Briefly, cells were treated with reagents for up to 3 hours and then labeled with PI (2 μg/mL medium) for the last 10 minutes of the incubation period, washed with phosphate-buffered saline to remove excess PI, and lysed in radioimmunoprecipitation assay buffer [50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 0.25% deoxycholic acid, 1% Triton X-100, 0.1% SDS, and a mixture of protease and phosphatase inhibitors; Roche Applied Science, Basel, Switzerland]. PI fluorescence was measured by fluorometry at 535/617 nm, and values were normalized to those of total protein concentration in cell lysates.
Western Blot Analysis
After treatments, human acini were harvested and lysed in radioimmunoprecipitation assay buffer [20 mmol/L Tris-HCl (pH 7.4), 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS] supplemented with protease and phosphatase inhibitors (Complete ULTRA and PhosSTOP; Sigma-Aldrich). Equal amounts of proteins were loaded onto Novex 4% to 20% Tris-Glycine Gels (ThermoFisher Scientific), and proteins were electrophoretically transferred onto nitrocellulose membranes using the Trans-Blot TurboTM Transfer Pack and the Trans-Blot TurboTM Transfer System (Bio-Rad, Hercules, CA). Membranes were incubated with primary and secondary antibodies; proteins were detected with chemiluminescence reagents using the PXi6 Touch Imaging System (Syngene, Cambridge, UK). Primary antibodies used were as follows: α-amylase (A8273), α-smooth muscle actin (A5228), and glial fibrillary acidic protein (G3893) from Sigma-Aldrich; trypsinogen (MAB1482) from EMD Millipore (Billerica, MA); high-mobility group protein 1 (3935) from Cell Signaling Technology; CCK-A receptor (CCKAR; G222) from One World Lab (San Diego, CA); and β-actin (ab20272) from Abcam.
RNA Analysis by Real-Time Quantitative PCR
Human acini preparations and human pancreatic tissues were used for these studies. Pancreatic tissues samples were obtained from deceased organ donors or from surgical resections from pancreatic cancer patients after approved institutional review board procedures (Cedars-Sinai Medical Center, institutional review board Pro00034086). Shortly after surgical resection, resected pancreas tissues were examined at the Surgical Pathology Department. Normal tissue samples distant from the tumor margins were collected and preserved in RNAlater (ThermoFisher Scientific) for subsequent RNA analysis or were formalin fixed for histological examination to ensure normal tissue morphology. Similar procedure was followed for tissue sections from cadaveric pancreata. Total RNA was extracted from human acini and RNAlater tissue samples using the RNeasy Plus Mini Kit (catalog number 74034; Qiagen, Germantown, MD), per the manufacturer's instructions. The extracted RNA samples were analyzed using an Agilent 2100 Bioanalyzer system (Agilent Biotechnologies, Palo Alto, CA). Reverse transcription was performed with the iScript Reverse Transcription Supermix (catalog number 170-8840; Bio-Rad) using 1 μg of total RNA, and the synthesized cDNA samples were used as templates for real-time quantitative PCR analysis. All reactions were performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad), and the amplifications were done with the iTaq Universal SYBR Green Supermix (Bio-Rad). The gene-specific oligonucleotide primers used are listed in Table 1. Relative transcript levels were calculated using the comparative 2−ΔΔCt method and normalized to the housekeeping gene, 18S rRNA.
Table 1.
List of Primer Sequences for Real-Time Quantitative PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| 18S rRNA | 5′-AGTCCCTGCCCTTTGTACACA-3′ | 5′-CGATCCGAGGGCCTCACTA-3′ |
| hAMY2A | 5′-CAATGATGCTACTCAGGTCA-3′ | 5′-GTAATCCTTCTCCAGTGCAA-3′ |
| hCCKAR | 5′-CGTCCTTCCAAAGAGTGGCA-3′ | 5′-GCATCCGCTTGTTCCGAATC-3′ |
| hCCKBR | 5′-GCCAGACCTGGTCCGTACT-3′ | 5′-GTCACTGTCGCCGTCAAAG-3′ |
| hCHRM1 | 5′-GGAGACAGAGAACCGAGCAC-3′ | 5′-CCTGGAGGAGTCTCTGGTGA-3′ |
| hCHRM2 | 5′-CTTTGGGACCTGTGGTGTGT-3′ | 5′-ATTTTTGTGGTCCGCTTGAC-3′ |
| hCHRM3 | 5′-AGATGGACCAAGACCACAGC-3′ | 5′-GTGGAGTTGAGGATGGTGCT-3′ |
| hCPA1 | 5′-CAAGTTTGCCAATTCCGAAG-3′ | 5′-CTGATCCAGCTCATCCTGGT-3′ |
| hDDIT3 | 5′-GCACCTCCCAGAGCCCTCACT-3′ | 5′-GTCTACTCCAAGCCTTCCCCC-3′ |
| hINS | 5′-GCCATCAAGCAGATCACT-3′ | 5′-TAGAGAGCTTCCACCAGG-3′ |
| hKRT19 | 5′-AGCCGGACTGAAGAATTGAA-3′ | 5′-TCTTCCAAGGCAGCTTTCAT-3′ |
| hPECAM1 | 5′-TGAGGGTGAAGGTGATAGCC-3′ | 5′-GGGTTTGCCCTCTTTTTCTC-3′ |
| hSLC10A6 | 5′-GCAAAGGTGCAGGACAATTT-3′ | 5′-CATAGGCCAGTGGGAAACTC-3′ |
| hSQSTM1 | 5′-CAGAGAAGCCCATGGACAG-3′ | 5′-AGCTGCCTTGTACCCACATC-3′ |
| hXBP1s | 5′-TGAGTCCGCAGCAGGT-3′ | 5′-ATCTGAAGAGTCAATACCGC-3′ |
Cytokine Secretion
Human acini were left untreated or stimulated for 16 hours with CCh or TLCS, and cells and conditioned media were subsequently harvested. Media were assayed using a membrane-based human cytokine array kit (ARY005B; Proteome Profiler Antibody Arrays Human Cytokine Array; R&D Systems, Minneapolis, MN), following manufacturer's instructions. The cytokine array kit consists of a membrane-based sandwich immunoassay that can detect a total of 36 cytokines/chemokines spotted in duplicate onto a membrane. Briefly, concentrated conditioned media samples were mixed with a cocktail of biotinylated detection antibodies and then incubated with the array membrane, which was spotted in duplicate with capture antibodies to specific target proteins. Captured proteins were detected by enhanced chemiluminescence, and signals were captured on a PXi6 Touch Imaging System (Syngene). The optical density of each cytokine spot was measured using GeneTools 4.3.1 analysis software (SynGene), and analyzed relative to the reference spots. Data are presented as arbitrary units of the averaged optical density values.
Statistical Analysis
All experiments were performed in triplicate, unless otherwise stated. Data are presented as means ± SD. Data were subjected to analysis of variance, followed by the Tukey post hoc test, and two-tailed t-test for comparison between two groups. P < 0.05 was considered significant.
Results
Characteristics of the Donors and the Human Pancreatic Acinar Preparations
This study was performed using human acini preparations obtained from 21 cadaveric donor pancreata. The characteristics of the organ donors are listed in Table 2. As indicated, organ donors included both men (71%) and women (29%), ranging in age from 22 to 61 years, and of diverse ethnicity (Hispanics, 48%; whites, 28%; blacks, 14%; Asians, 5%; and Pacific Islanders, 5%). The predominant cause of death was catastrophic injuries, including stroke (48%), head trauma (33%), and anoxia (19%). The main inclusion criteria at the islet transplant centers for accepting and processing the pancreas for islet isolation were as follows: i) <12 hours of cold ischemia time (from harvesting the organ to starting the isolation procedure); ii) pancreata preserved in approved preservation solution (University of Wisconsin solution); iii) donor age ranging from 15 to 65 years; and iv) cause of death not affecting the integrity of the pancreas. Exclusion criteria included the following: i) history or biochemical evidence of diabetes mellitus (glycated hemoglobin, >6.1%); ii) non–heart-beating cardiac donors; iii) evidence of histological pancreas damage; and iv) evidence of diseases, including malignancies, sepsis, viral infections or positive serology, inflammatory diseases, and clotting disorders, high-risk sexual behavior, acute alcohol intoxication, or other factors unacceptable to the surgical team.
Table 2.
Donor Characteristics
| Characteristics | Description |
|---|---|
| Ethnicity | Hispanics (n = 10), whites (n = 6), blacks (n = 3), Asians (n = 1), and Pacific Islanders (n = 1) |
| Sex | Male (n = 15) and female (n = 6) |
| Age, years | Means ± SD, 40.4 ± 13.8; median, 40.05; range, 22–61 |
| Body mass index, kg/m2 | Means ± SD, 30.0 ± 6.2; median, 30.8; range, 20.6–42.1 |
| HbA1c, % | <6 (n = 17), ≥6 (n = 1), and NA (n = 3) |
| Serological analysis∗ | All negative results |
| Cause of death | Stroke (n = 10), head trauma (n = 7), and anoxia (n = 4) |
| Alcohol abuse | One donor (5 years of use) |
| Smoking | NA |
| Non-clinical drug use | None |
| Prescription medication | Hypertension drugs (n = 1) |
Numbers in parentheses indicate the number of donors of a total of 21.
HbA1c, glycated hemoglobin; NA, not available.
Performed for cytomegalovirus, hepatitis B virus, and hepatitis C virus.
At our research laboratories, we have restricted the use of human acini preparations to those from organ donors with no history of drug abuse or heavy drinking, and only to those received at our research laboratories in cold media within 2 to 8 hours after pancreas processing. Furthermore, only those preparations displaying high viability of acinar cells (>95%), as determined by PI uptake analysis, and absence of histological evidence of plasma membrane blebbing were used (Figure 1). In our experience, most of the preparations received shortly after processing of the pancreas (2 to 8 hours) were of acceptable quality using the criteria indicated above. Besides the morphological and viability analyses, we also measured basal and CCh-induced amylase secretion to determine secretory function in most of the preparations.
Light and electron microscopy as well as biochemical analyses indicate that cell preparations obtained after islet depletion consist mainly of acinar cells. However, negligible amounts of ductal and quiescent stellate cells can also be found (not shown), as is the case with rodent acini.40 Figure 1 summarizes representative data on the morphology and purity of the human acinar preparations we used. Figure 1A shows a bright-field examination of two acini from a preparation received 8 hours after pancreas processing and kept in culture for 2 hours. Note the lack of plasma membrane blebbing and the high cell viability (Figure 1A). Electron microscopy shows that the human acini display ultrastructural features similar to those published in studies using rodent acini,41 with acinar cells organized in a polarized manner around the lumen, an extensive endoplasmic reticulum (ER) network, and abundant zymogen granules in the middle and apical areas (Figure 1B). Immunoblot analysis (Figure 1C) confirmed abundant expression of digestive enzymes in pancreatic acini (α-amylase and trypsinogen). In addition, we observed low levels of the glial and stellate cell marker glial fibrillary acidic protein and no α-smooth muscle actin, a marker of pericytes and activated pancreatic stellate cells. To further substantiate the enrichment of our acinar cell preparations, we measured mRNA expression levels of the human acinar cell markers carboxypeptidase A1 and pancreatic α-amylase, the β cell marker insulin, the endothelial cell marker platelet endothelial cell adhesion molecule, and the ductal cell marker keratin 19. As illustrated in Figure 1D, compared with the high expression levels of the acinar cell markers, there was minimal expression of ductal, endothelial, and β cell markers.
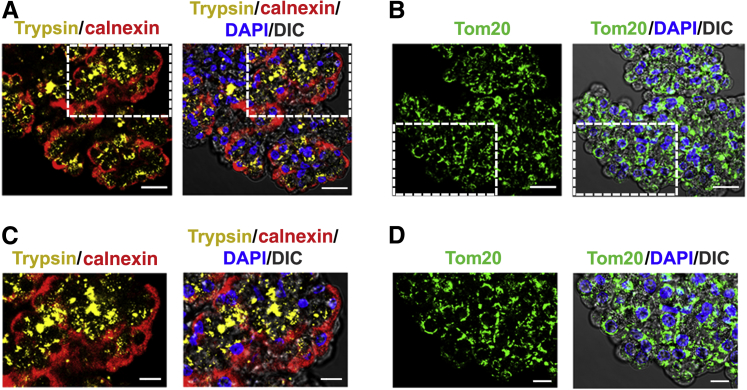
The immunofluorescence studies illustrated in Figure 2 were performed to assess relative locations in acinar cells of key organelles: zymogen granules using trypsinogen as marker; the ER network, identified by the ER transmembrane protein calnexin; and the mitochondria network using translocase of outer membrane 20. The figure shows calnexin staining located preferentially in the basolateral area of the cells in the acinus and translocase of outer membrane 20 mainly in the perinuclear region. In contrast, trypsinogen is located close to the apical area of the acinar cells in packets representing zymogen granules, as shown in Figure 1B.
Figure 2.
The distribution of intracellular organelles retains its polarity in isolated human acini. A and B: Untreated freshly isolated human acini were formaldehyde fixed and immunostained with antibodies against trypsinogen (Trypsin; zymogen granules; yellow stain), calnexin (endoplasmic reticulum; red stain), and translocase of outer membrane 20 (Tom20; mitochondria; green stain). The signals were detected with Alexa Fluor 555–, Alexa Fluor 633–, and Alexa Fluor 488–conjugated secondary antibodies, respectively. Nuclei were stained with DAPI (blue). Images were analyzed under a confocal microscope. C and D: Higher magnifications of the boxed areas in A and B, respectively. Scale bars: 10 μm (A and B); 20 μm (C and D). DIC, differential interference contrast.
Human Acinar Proteome
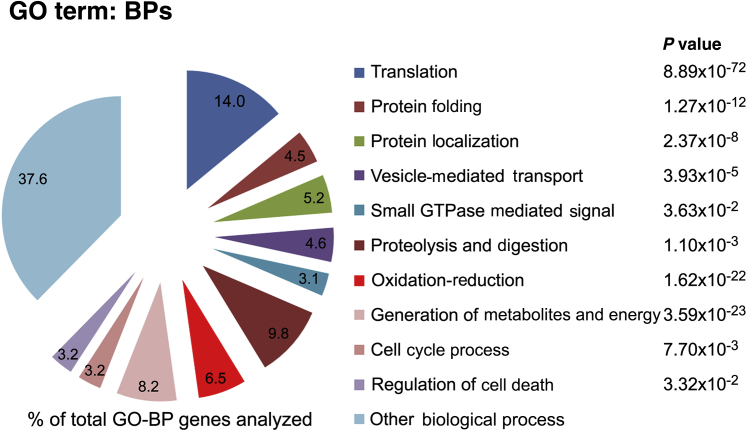
Proteomic studies on functional human acinar cells from normal pancreas have been limited by the scarcity of available samples. To better characterize the human acinar preparations, we performed quantitative proteomic profiling using untreated cells obtained from cadaveric pancreata from four organ donors. The selected donors were of relatively similar age but of different ethnicity and sex (Table 3). Proteins were analyzed by label-free liquid chromatography–tandem mass spectrometry using short-run (70-minute) chromatograms that identify mainly the highly abundant proteins. Among the 1509 confidently identified proteins, 1044 (approximately 69% of all identified proteins) were common across the four samples analyzed (Supplemental Table S1). We used the DAVID functional annotation database to retrieve GO information. A P value threshold of < 0.05 and a false-discovery rate of <1% were used to confidently predict enriched GO terms among the identified common core proteome. As expected for a professional secretory cell, the GO analysis revealed that most proteins identified fall in biological processes, such as translation, protein folding, proteolysis and digestion, protein location and vesicle-mediated transport, generation of metabolites and energy, and oxidation-reduction (Figure 3). These cellular functions constitute >50% of the annotation hits in the GO biological processes category.
Table 3.
Proteomic Analysis: Characteristics of the Sample Donors
| Case ID | Sex | Ethnicity | Age, y | BMI, kg/m2 | Cause of death |
|---|---|---|---|---|---|
| c7 | Male | Hispanic | 61 | 42.1 | Stroke |
| c8 | Female | Pacific Islander | 54 | 33.7 | Stroke |
| c16 | Male | Hispanic | 49 | 40.0 | Anoxia |
| c19 | Male | White | 47 | 31.7 | Stroke |
BMI, body mass index; c, organ donor case (patient coding) used as a source of human acinar preparation; ID, identification.
Figure 3.
Gene Ontology (GO) analysis of the human pancreatic acini proteome. Protein extracts from untreated, freshly isolated, human acini from four organ donors (Table 3 provides organ donor information) were analyzed by high-precision liquid chromatography–tandem mass spectrometry in an Orbitrap Elite analyzer; data were quantified using MaxQuant version 1.3.0.5. Of the total proteins identified, 70% were common to all samples and used for further analysis. GO information was retrieved from the DAVID annotation tool. Diagram shows percentage of total genes analyzed corresponding to the indicated GO biological processes (BPs). A P value threshold of < 0.05 was used to confidently predict enriched GO terms among the proteins.
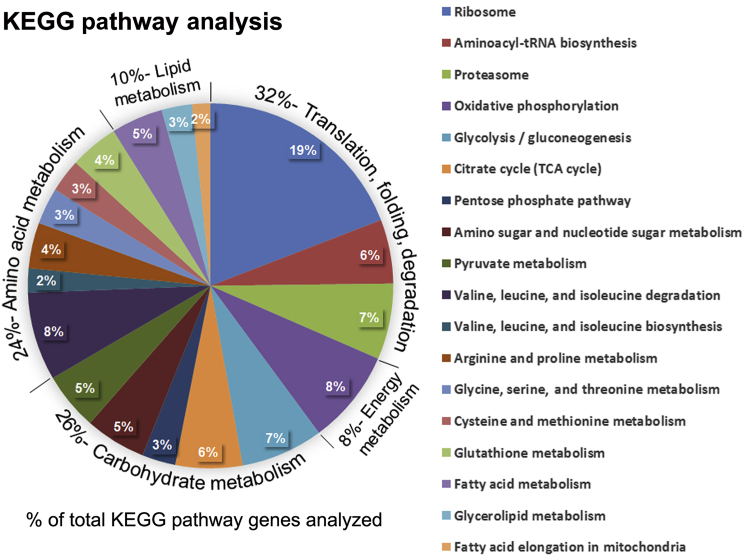
We also interrogated the DAVID database to determine the more prominent Kyoto Encyclopedia of Genes and Genomes terms. Figure 4 indicates the percentage of total identified genes that fall in each Kyoto Encyclopedia of Genes and Genomes metabolic pathway or molecular cellular process (Figure 4). As shown in the figure, 32% of the hits were classified into cellular processes related to protein translation, folding, and degradation, and 8% into those involved in energy metabolism (oxidative phosphorylation). Among the category of metabolism, 26% of the annotation hits are involved in carbohydrate metabolism, 24% in amino acid metabolism, and the remaining 10% in lipid metabolism. The main subfamilies within these categories are indicated in the legend of Figure 4. Overall, the GO terms and Kyoto Encyclopedia of Genes and Genomes classifications of the identified proteome in the human acinar preparations indicate that, as expected, most proteins are involved in metabolic and cellular processes required to support the uniquely high rate of digestive enzyme production and the secretory phenotype of the pancreatic acinar cell.
Figure 4.
Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of the human acinar proteome. The data sets originating from the human acinar proteome indicated in Figure 3 were used to interrogate the DAVID analysis tool for the significantly enriched KEGG pathways. The diagram shows the percentage of total enriched genes that fall in each metabolic pathway or molecular cellular process. TCA, tricarboxylic acid.
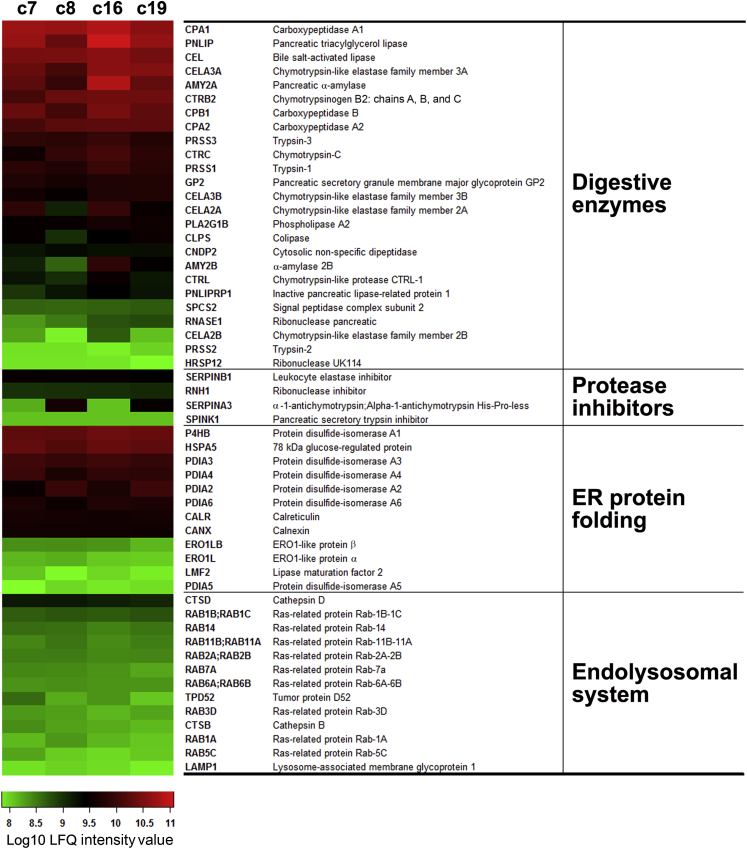
As stated, our cell preparations were obtained from organ donors of both sexes and with diverse ethnicity, genetic background, and cause of death (Table 3). To assess donor-related sample variability, we analyzed the relative abundance of selected proteins identified in the four preparations (Figure 5). A total of 54 proteins were selected on the basis of their high abundance (eg, digestive enzymes) and because they are involved in cellular processes of functional importance for the acinar cell (ie, protease inhibition, ER protein folding, and endolysosomal function). The heat map in Figure 5 shows relative amounts of the indicated proteins determined by label-free quantification. As shown in the heat map, the relative abundance of most of the selected proteins was strikingly similar between the four samples. These proteomic data support the validity of using human acini from cadaveric donor pancreata as experimental platforms to investigate the physiology and pathophysiology of the exocrine parenchyma.
Figure 5.
The relative abundance of selected proteins in the human pancreatic acinar proteome is similar across donor tissue sources. The human acinar proteomic data sets originating from the four organ donors (c7, c8, c16, and c19 [c indicates organ donor case (patient coding) used as a source of human acinar preparation]) indicated in Figure 3 and Table 3 were analyzed to determine the interdonor variability. The heat map shows relative amounts of the indicated selected proteins determined by label-free quantification (LFQ). Selected proteins include all identified digestive enzymes, protease inhibitors, abundant protein biosynthetic components (endoplasmic reticulum chaperones and foldases), and proteins in the endolysosomal system.
Physiological Responses of the Human Pancreatic Acini
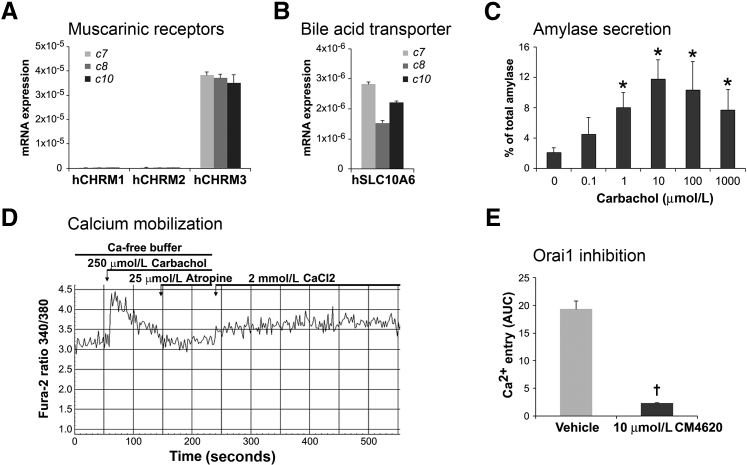
Experimental studies using rodent pancreatic acini showed that the muscarinic acetylcholine receptor M3 (gene CHRM3) is the principal receptor for mediating physiological secretory responses of these cells to acetylcholine and acetylcholine agonists, including CCh.42, 43 Consistent with these data, we found robust expression of CHRM3 but not of CHRM1 and CHRM2 in human pancreatic acini (Figure 6A). Figure 6B shows that human acini also express the organic anion transporter, solute carrier family 10 member 6. This transporter has been shown in rodent acinar cells to mediate the transport of sulfated bile acids, including TLCS, into the cell from its apical surface, causing pancreatitis.44, 45, 46 Apical entry of bile acids is thought to occur during gallstone pancreatitis in humans.47
Figure 6.
Human acini express the human muscarinic acetylcholine receptor M3 (hCHRM3) and exhibit a biphasic secretory response to cholinergic stimulation. A and B: mRNA extracts from human acini isolated from three organ donor pancreata (c7, c8, and c10 [c indicates organ donor case (patient coding) used as a source of human acinar preparation]) were analyzed by real-time quantitative PCR to determine the expression of the indicated muscarinic receptors (A) and the bile acid transporter solute carrier family 10 member 6 (SLC10A6; B). All three human acinar preparations analyzed preferentially express the muscarinic receptor CHRM3 and the SLC10A6 transporter. C: Human acini were stimulated for 30 minutes with the cholinergic agonist carbachol at the indicated concentrations, and amylase release was measured using the Phadebas test. As shown, human acinar cells respond to carbachol stimulation by releasing amylase in a typical biphasic pattern also reported for rodent acini. D and E: Characterization of carbachol-induced calcium response in human acini. Acini were loaded with Fura-2 AM and then stimulated with 250 μmol/L carbachol in the absence of free Ca2+ in the extracellular buffer. As shown, carbachol causes a rapid, transit elevation of cytosolic Ca2+ in Fura-loaded cells. Atropine was then added to terminate the Ca2+-releasing signal. Adding Ca2+ to the extracellular media (2 mmol/L CaCl2) leads to Ca2+ entry, and this effect is blocked by preincubation with CM4620, a specific inhibitor of the store-operated Ca2+ channel, Orai (E). Data are expressed as means ± SD (C and E). n = 3 independent experiments (C and E). ∗P < 0.05 versus basal (no carbachol); †P < 0.05 versus vehicle (dimethyl sulfoxide). AUC, area under the curve.
A key response of pancreatic acinar cells is digestive enzyme secretion, stimulated by neurohumoral agents. Although there is more than one distinct class of cell surface receptor on the acinar cell identified in rodents and other species, physiological studies in humans suggest that cholinergic ligands, acting through muscarinic acetylcholine receptors, are the most important for digestive enzyme secretion.48 As shown in Figure 6C, similar to rodent acinar cells,32 CCh-stimulated amylase release in isolated human acini followed the characteristic biphasic dose-response curve. Amylase release during a 30-minute incubation period increased progressively with increasing concentrations of CCh to a maximal response at 10 μmol/L. Greater supramaximal concentrations of CCh caused less amylase secretion; this pattern is often described as inhibition of secretion.
Coupled with exocytosis responses, the human pancreatic acini preparations also displayed robust Ca2+ mobilization after cholinergic stimulation. As shown in Figure 6D, CCh at maximal concentrations (250 μmol/L) induced a rapid and transitory elevation of cytosolic Ca2+ in Fura-loaded cells, followed by Ca2+ entry into the cytoplasm on addition of calcium to the extracellular space. Studies using murine and human acinar cells demonstrated that Ca2+ influx in these cells is regulated by calcium release-activated calcium channels localized in the plasma membrane.16, 49 We found that a specific inhibitor of Orai1 (CM4620), the main component of calcium release-activated calcium channels, effectively blocked CCh-induced Ca2+ entry (Figure 6E), demonstrating the presence of functional calcium release-activated calcium channels in our cell preparations. Together, our data indicate that human pancreatic acini isolated from cadaveric pancreata display cholinergic-induced secretory and Ca2+ signaling responses similar to those of freshly isolated rodent acini.
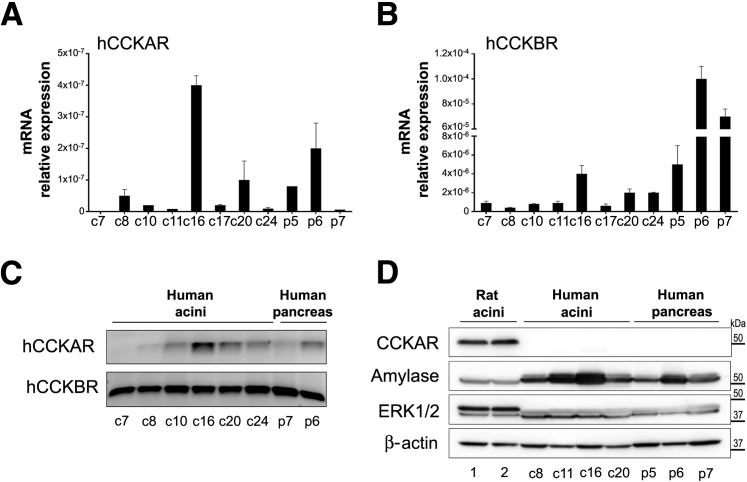
Besides responses to CCh and TLCS, we also investigated whether our human acini preparations display functional responses to the hormone CCK. CCK peptides have actions in many systems, including the gut and the nervous system.50 CCK effects are mediated via two receptors: CCKAR, which binds sulfated (but not nonsulfated) CCK peptides with high affinity; and the CCK-B receptor, which has preferential binding for gastrin but also binds both sulfated and nonsulfated CCK peptides with low affinity. In vivo studies demonstrated that CCK stimulates pancreatic enzyme secretion in humans via cholinergic pathways; in rodents, these secretory responses are also mediated via CCK receptors in acinar cells.48 In this respect, freshly isolated dispersed rodent acini display robust physiological and pathologic responses to CCK in culture; attainment of similar responses in human acini has been challenging.51 Studies by Ji et al52 found secretory responses after stimulation with physiological concentrations of CCh and other secretagogues in human acini obtained from surgically resected pancreas tissues; minimal or no responses were found after CCK treatment. However, Murphy et al14 reported functional responses to physiological concentrations of CCK in human pancreatic acini obtained from surgically resected tissues, and similar results were obtained using ex vivo human pancreatic slices.53 The apparent discrepancy between these studies may be attributable to the challenges in obtaining healthy pancreatic acini and the extremely low expression levels of CCKAR in human pancreatic tissues54 and human dispersed acini.51, 52
Initial pilot studies using six of our human acini preparations indicated that CCK-8 stimulated amylase secretion by 3.5-fold only at supraphysiologic concentrations (10 nmol/L), whereas physiological (1 to 10 pmol/L) or maximal (100 pmol/L) concentrations were ineffective (data not shown). Similarly, CCK-8 (1 to 10 nmol/L) did not elicit changes in cytosolic Ca2+ levels in Fura-loaded cells (data not shown). These studies indicated that, similar to rodent acini, our human acini preparations display robust functional responses to cholinergic stimulation but only minimal responses to high concentrations of CCK-8 (10 nmol/L), which are toxic in rodent acini. Because the pilot studies were discouraging, we did not perform further studies using CCK-8.
To determine CCKAR and CCK-B receptor expression, we performed real-time quantitative PCR using RNA extracted from eight batches of human acini, cadaveric pancreas tissue from one organ donor, and surgically resected pancreas tissues from two cancer patients (normal portions distant from tumor margins). As shown in Figure 7, A–C, all human acini preparations and the pancreatic tissues analyzed expressed low RNA levels of CCKAR, but relatively higher levels of CCK-B receptor. We next analyzed protein levels of CCKAR by Western blotting using those human acini batches with higher CCKAR RNA expression, and compared them with rat acini preparations. For these studies, we selected an antibody produced against a synthesized human CCKAR peptide. The immunoblots showed robust bands consistent with the expected molecular weight for CCKAR in the rat acini but no immunoreactivity in the human acini or the human pancreas tissue preparations (Figure 7D). Taken together, our data suggest that our human acini preparations express low or negligible levels of the CCKA receptor and lack functional responses to CCK-8. However, these acini preparations efficiently respond to physiological and clinical relevant concentrations of other stimuli, including the cholinergic agonist CCh and the bile acid TLCS.
Figure 7.
Human acini express low levels of the cholecystokinin-A receptor (CCKAR). A and B: mRNA extracts from untreated human acini isolated from eight organ donor pancreata (batches c7, c8, c10, c11, c16, c17, c20, and c24 [c indicates organ donor case (patient coding) used as a source of human acinar preparation]), cadaveric pancreas tissues from an organ donor (p5; normal parenchyma), and surgically resected pancreas tissues from two cancer patients (p6 and p7; normal parenchyma distant from tumor margins) were analyzed by real-time quantitative PCR (qPCR) to determine the expression of hCCKAR (A) and human CCK-B receptor (CCKBR; B). Expression levels are relative to those of 18S. C: qPCR products from studies illustrated in A and B. cDNA (50 ng) was used per reaction, and reactions were run for 40 cycles. D: Panels show immunoblot images of protein levels of CCKAR and amylase in human acini and pancreatic tissue specimens. Extracellular signal regulated kinase (ERK) 1/2 and β-actin were used as loading controls.
Ex Vivo Pancreatitis Responses in Human Pancreatic Acini
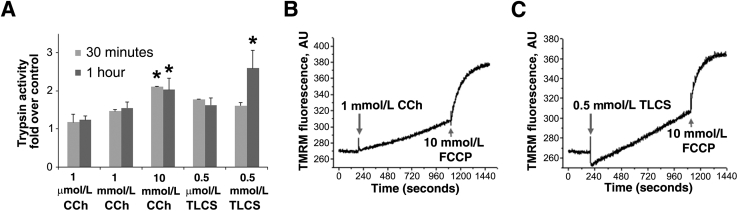
To investigate whether human acini isolated from cadaveric pancreata reproduce pancreatitis responses observed in rodent acini, we incubated human acini with toxic concentrations of CCh (1 to 10 mmol/L)55 and the bile acid TLCS (0.5 mmol/L).44, 56 One of the responses central to the mechanism of pancreatitis is an inappropriate intra-acinar activation of several of the proenzymes, particularly trypsinogen.32, 55, 57 In addition, mitochondria dysfunction has been shown to mediate the development of pancreatitis. Recent studies revealed findings of mitochondrial depolarization and decreased ATP production during experimental pancreatitis; the depolarization is attributable to sustained opening of the mitochondrial permeability transition pore.10, 35, 58, 59, 60, 61, 62 We found that both CCh (1 and 10 mmol/L) and TLCS (0.5 mmol/L) caused trypsinogen activation to trypsin in human acini (Figure 8A). In addition, as shown in Figure 8, B and C, CCh or TLCS at similar concentrations induced mitochondrial depolarization. These data indicate that our human acinar preparations are appropriate for studying the pathogenic mechanisms of pancreatitis.
Figure 8.
Carbachol (CCh) or taurolithocholic acid 3-sulfate (TLCS) at a high concentration induces trypsinogen activation and mitochondrial depolarization in human acini. A: Human acini were incubated for the indicated times in the absence (control) or presence of CCh or TLCS. Trypsin activity was measured in cell homogenates by enzymatic assay using a specific fluorogenic substrate. Graph indicates trypsin activity in stimulated cells relative to control values (set at 1.0). B and C: Mitochondrial membrane potential was measured in acini loaded with the fluorescent probe tetramethylrhodamine methyl ester (TMRM) and treated with CCh (B) or TLCS (C). p-Trifluoromethoxy-phenylhydrazone (FCCP) was used to completely dissipate the mitochondrial membrane potential. Data are expressed as means ± SD (A). n = 3 independent experiments (A). ∗P < 0.05 versus control. AU, arbitrary units.
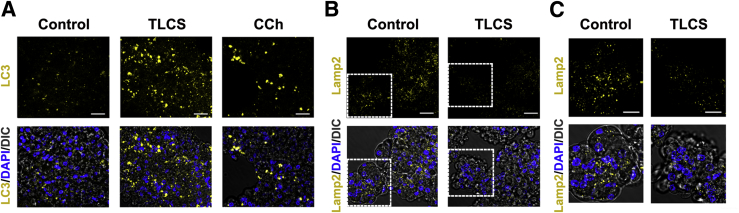
Disorders of autophagy function have recently been described as a central pancreatitis mechanism using rodent models.17, 20, 63, 64, 65, 66, 67, 68 These studies show that there is inhibition of autophagic flux in pancreatitis, resulting from impairment of lysosomal and/or autophagic pathways. These disorders result in inappropriate activation of zymogens as well as other pathologic responses of pancreatitis. As an example, levels of lysosome-associated membrane proteins decrease in rodent models of pancreatitis, and genetic ablation of lysosome-associated membrane protein-2 results in pancreatitis.17 Accumulation of the membrane form of the microtubule-associated protein LC3 in intracellular vacuoles is widely used to monitor autophagy. The results in Figure 9 show that both TLCS and supraphysiologic concentrations of CCh caused accumulation of LC3 puncta in human acini, and this effect was associated with decreases in lysosome-associated membrane protein-2 protein levels. These results fully mirror findings in rodent acini and support a role for acinar cell autophagic/lysosomal dysfunction in human pancreatitis.
Figure 9.
Autophagic puncta accumulate, and the lysosomal membrane protein lysosome-associated membrane protein (LAMP)-2 decreases in ex vivo pancreatitis using human acini. A and B: Immunofluorescence analysis of light chain 3 (LC3; A) and LAMP-2 (B) in human acini incubated for 3 hours without (Control) or with 0.5 mmol/L taurolithocholic acid 3-sulfate (TLCS) or 1 mmol/L carbachol (CCh). Nuclei were stained with DAPI (blue). C: Higher magnification of the boxed areas in B. Scale bars: 20 μm (A and B); 10 μm (C). DIC, differential interference contrast.
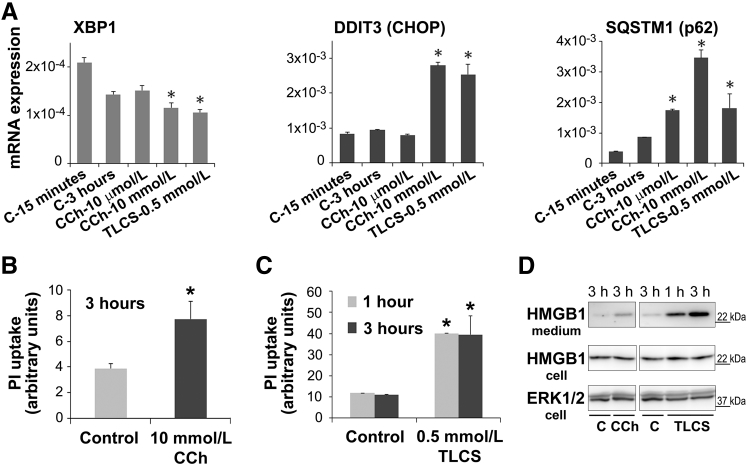
The participation of ER stress and the unfolded protein response in the pathogenic mechanisms of pancreatitis is a matter of current active investigation.69, 70, 71, 72, 73 The unfolded protein response regulates ER function, protein processing, and secretion. An emerging concept from studies in rodent pancreas is that there are both protective and pathologic ER stress/unfolded protein response signaling responses in the exocrine pancreas.72, 74, 75 These studies suggest that an increase in the cell death–associated ER stress transcriptional factor, C/EBP homologous protein, and a decrease in the transcription factor, spliced X box protein-1 (XBP), are causative in the mechanism of pancreatitis. Moreover, studies using Xbp1-deficient mice have demonstrated that XBP1 is required for the secretory phenotype of the acinar cell76 and, in particular, regulates amylase secretion in isolated murine acini.71 We found that, compared with human acini cultured for only 15 minutes, XBP1 levels decreased by 30% in cells cultured for 3 hours (Figure 10A) and by >50% in cells cultured for >20 hours (data not shown). This effect was associated with a reduction in CCh-induced secretory capacity (data not shown), a phenomenon that also occurs in rodent pancreatic acini after prolonged culture. Compared with control cells, 3-hour treatment with supramaximal concentrations of CCh or TLCS induced a significant further decrease in XBP1 expression and concomitant up-regulation of C/EBP homologous protein and sequestosome-1 (p62), a mediator of disordered autophagy and a putative C/EBP homologous protein gene target (Figure 10A). Taken together, the data indicate that, in human acini, CCh and TLCS at concentrations that cause pancreatitis responses induce disordered autophagy, ER stress, and unfolded protein response dysregulation.
Figure 10.
Dysregulation of endoplasmic reticulum (ER) stress pathways in ex vivo pancreatitis is associated with acinar cell death. RNA was extracted from untreated human acini after 15 minutes of incubation (C-15 minutes) or from acini incubated for 3 hours in the absence (C-3 hours) or presence of carbachol (CCh) or taurolithocholic acid 3-sulfate (TLCS) at the indicated concentrations. A: The ER stress regulators spliced X box protein-1 (XBP1) and DNA damage inducible transcript 3 (DDIT3; CHOP) and the cellular stress marker sequestosome 1 (SQSTM1; p62) were measured by real-time quantitative PCR. B and C: Human acini were treated with toxic concentrations of CCh or TLCS for the indicated times. Cell death was assessed by propidium iodide (PI) uptake. D: Cells were kept untreated [control (C)] or treated for the indicated times with 10 mmol/L CCh or 0.5 mmol/L TLCS, and then conditioned media were collected. Panels show representative immunoblot images of protein levels of the high-mobility group protein 1 (HMGB1) in conditioned media and cell homogenates. Extracellular signal regulated kinase (ERK) 1/2 was used as loading control. Data are expressed as means ± SD (A) or means ± SEM (B and C). n = 3 independent experiments (A); n = 4 independent experiments (B and C). ∗P < 0.05 versus control. h, hours.
We next measured whether CCh- and TLCS-induced pancreatitis responses were associated with acinar cell death. Human acini were treated for 1 and 3 hours with 10 mmol/L CCh or 0.5 mmol/L TLCS (Figure 10, B and C). After 1 hour of treatment, and as determined by PI uptake, CCh had little effect on cell necrosis (data not shown), but TLCS significantly increased cell death (Figure 10C). After 3 hours of treatment, both CCh (Figure 10B) and TLCS (Figure 10C) significantly increased PI uptake by twofold and fourfold, respectively. Consistent with these findings, TLCS and CCh also induced significant release into the media of high-mobility group protein 1, an inflammatory mediator released by necrotic cells (Figure 10D).
Secretome Profile of Cytokines and Chemokines in Human Acini
Acute pancreatitis in humans and experimental animals is characterized by local and systemic inflammation, with a marked increase of inflammatory mediators (eg, cytokines, chemokines, and proteases) in pancreas, lungs, and blood. Experimental evidence indicates that, during the early stages of acute pancreatitis, injured pancreatic acinar cells release cytokines and chemokines that trigger recruitment of inflammatory cells into the pancreas and promote systemic inflammation.9, 77, 78 Studies using freshly isolated rodent pancreatic acinar cells have identified some of the inflammatory mediators produced by these cells in response to injury: tumor necrosis factor-α, IL-6, IL-10, chemokine (C-C motif) ligand (CCL) 2/monocyte chemoattractant protein 1, and intercellular adhesion molecule 1, among others.77, 78, 79 However, a comprehensive profiling of the cytokine/chemokine secretome in rodent and human acinar cells is lacking.
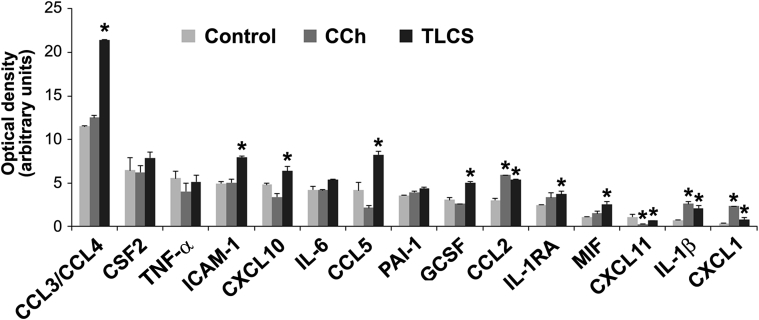
Using our preparations of human pancreatic acini, we measured the release of cytokines and chemokines by a membrane-based human cytokine/chemokine array (36 cytokine/chemokines; catalog number ARY005B; R&D Systems). On arrival to our laboratories, human acini were kept untreated or stimulated for 16 hours with toxic concentrations of CCh (1 mmol/L) or TLCS (0.5 mmol/L). After the incubation period, conditioned media were collected and assayed. As illustrated in Figure 10A, we detected 15 cytokines/chemokines (of 32 tested) in the conditioned media. Figure 10B lists factors tested but not detected.
We found that human acini in culture released inflammatory mediators, including the cytokines IL-6, tumor necrosis factor-α, colony-stimulating factor 2 (granulocyte-macrophage colony-stimulating factor), plasminogen activator inhibitor-1, and IL-1 receptor antagonist, and the chemokine CXCL10. TLCS treatment further stimulated the release of IL-1β (3.0-fold), macrophage inhibitory factor (2.3-fold), CXCL1 (2.1-fold), CCL5/regulated on activation normal T cell expressed and secreted (2.0-fold), CCL3/CCL4 (2.0-fold), CCL2/monocyte chemoattractant protein 1 (1.8-fold), granulocyte colony-stimulating factor (1.6-fold), and intercellular adhesion molecule 1 (1.6-fold). CCh, at the concentration tested, was less effective than TLCS but significantly increased the release of CXCL1 (6.0-fold), IL-1β (3.5-fold), and CCL2/monocyte chemoattractant protein 1 (2.0-fold). Several of the detected factors were previously identified in ex vivo and in vivo models of acute pancreatitis and in acute pancreatitis patients.80 They were found to modulate inflammatory responses and neutrophil and monocyte infiltration into the pancreatic tissues.
Discussion
The results of this study demonstrate that pancreatic acini isolated from cadaveric human pancreas are suitable to investigate mechanisms of acute pancreatitis related to organellar disorders. The data indicate that many of the organellar responses to supraphysiologic cholinergic stimulation and exposure to pancreatitis causing bile acids are identical to those observed in rodent pancreatic tissues. Furthermore, the findings presented herein justify the use of rodent tissues for the investigation of many of the pathobiologic responses of acute pancreatitis aimed to elucidate disease mechanisms and identify potential targets for therapy. Moreover, the results suggest that novel findings made in pancreatic tissue from experimental animals can be appropriately validated in human pancreatic tissue. Thus, the present study both initiates validation of key mechanistic steps and responses using pancreatic acini from humans and justifies using pancreatic acini from animals for the bulk of the necessary mechanistic work associated with elucidation of the pathogenesis of pancreatic disorders.
Our data indicate that human acini isolated from cadaveric human pancreatic tissues (that exhibit no overt pancreas pathology) are homogeneous with respect to purity and cell identity. Using microscopy as well as protein and mRNA (real-time quantitative PCR) analyses for markers of acinar, ductal, endothelial, β, and mesenchymal cells, we confirmed that acinar cells represent the vast majority of the cell population in the dispersed acini. Novel proteomic data presented herein reveal that, as expected for professional secretory cells, the human acinar preparations are enriched in proteins involved in protein transcription, biosynthesis, and secretion, as well as cellular processes for generation of energy. Moreover, the identified proteome shows low variability across different donors in relation to the relative abundance of digestive enzymes and regulators of ER and endolysosomal functions. Deeper mass spectral coverage should be of value in identifying important differences between donors with regard to their sex, ethnicity, and other factors.
Human acini express major cell surface receptors previously identified in rodent acinar cells, including the muscarinic acetylcholine receptor M3 and the bile acid transporter solute carrier family 10 member 6, and respond to cholinergic stimuli in a similar manner to several well-documented responses in rodent acini.42, 43, 44, 45, 46 We demonstrated that human pancreatic acini used 2 to 8 hours after pancreas processing maintain functional secretory responses to cholinergic stimulation. However, as described for freshly isolated rodent acini, the decline in secretory capacity associated with long-term culture constitutes one of the main challenges to working with human acini isolated from cadaveric donor pancreata. The mechanisms underlying the loss of secretory capacity of primary acinar cells in culture are associated with dysregulation of endolysosomal pathways18 and a decrease in levels of the ER stress–associated transcription factor XBP1 (Figure 10A). In addition, a decline in mitochondria function, resulting in an ATP decrease, likely contributes to the loss of secretory capacity in isolated acinar cells.
Stimuli that induce pancreatitis in rodents (ie, high doses of TLCS or CCh) similarly elicit early pancreatitis responses in human acinar cells, including inhibition of digestive enzyme secretion, intracellular trypsinogen activation, mitochondrial depolarization, and acinar cell death. Similar to characteristics previously described for rodent experimental models, human pancreatitis ex vivo exhibits early organellar dysfunction, manifested by impaired autophagy and dysregulated ER stress responses. We found that CCh and TLCS cause ER stress and autophagy dysfunction, as evidenced by up-regulation of C/EBP homologous protein and accumulation of the autophagy markers LC3 (membrane form) and p62. These effects are linked to pathological cellular responses, including increased trypsin activity and necrosis, as indicated by release of high-mobility group protein 1, an inflammatory mediator in injured tissues.
Another novel result in this study is cytokine/chemokine secretion profiling in human acini (Figure 11). We found that human acini secrete inflammatory mediators, including IL-6, tumor necrosis factor-α, and plasminogen activator inhibitor-1, and that pancreatitis inputs further stimulate the release of IL-1β, macrophage inhibitory factor, and CCL2/monocyte chemoattractant protein 1. Moreover, on treatment with TLCS, these cells release several chemokines that are involved in neutrophil and monocyte infiltration. The results support the concept that injured acinar cells are a key effector in initiating inflammatory responses during the development of acute pancreatitis.
Figure 11.
Select cytokine/chemokine secretion is up-regulated in ex vivo pancreatitis using human acini. Cells were kept untreated (Control) or treated for 16 hours with 1 mmol/L carbachol (CCh) or 0.5 mmol/L taurolithocholic acid 3-sulfate (TLCS). Cytokines and chemokines released into the media were measured by a membrane-based human cytokine/chemokine array (36 cytokines/chemokines; catalog number ARY005B; R&D Systems). Optical density (arbitrary units) for all 15 detected cytokines/chemokines is shown. The following cytokines/chemokines were tested, but not detected, in conditioned media: IL-1α, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12, IL-13, IL-16, IL-17A, IL-17E, IL-21, IL-32a, IL-18, IL-27, interferon-γ, C5/C5A, chemokine (C-C motif) ligand (CCL) 1, CXCL12, CD40 ligand, and TREM-1. Data are expressed as means ± SD. n = 2 independent experiments. ∗P < 0.05 versus control. CSF, colony-stimulating factor; GCSF, granulocyte CSF; ICAM, intercellular adhesion molecule 1; IL-1RA, IL-1 receptor antagonist; MIF, macrophage inhibitory factor; PAI, plasminogen activator inhibitor; TNF-α, tumor necrosis factor-α; TREM, triggering receptor expressed on myeloid cells 1.
In conclusion, human acini isolated from cadaveric pancreatic tissues maintain physiological functions ex vivo and have similar organellar disorders with pancreatitis-causing treatments as have been observed in rodent acini. Future studies should examine, in more detail, the mechanisms mediating the pancreatitis responses described herein. In addition, when available, studies should include the use of human pancreatic acini isolated from donor pancreata with a history of long-term smoking and/or heavy drinking. Comparison of these preparations with those from healthy-lifestyle donors can reveal signatures and functional responses different from those obtained from donor pancreata without pancreas pathology. They can aid in understanding the molecular basis of increased risk for pancreatitis in smokers and alcohol abusers.
Acknowledgments
We thank Drs. Kevin Ferrari, Mohamed Ei-Sayed, and Fouad R. Kandeel (Southern California Islet Center at City of Hope, Duarte, CA; and Integrated Islet Distribution Program) for providing human pancreatic acini, Dr. Ashok K. Saluja for guidance on working with human acini, and Drs. Michael Dunn and Kenneth Stauderman for providing the Orai1 inhibitor CM4620.
Footnotes
Supported by NIH grants P01DK098108 (A.S.G.) and R01 AA019954 (A.L.) and the Department of Veterans Affairs I01BX001484 (S.J.P.).
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2017.08.017.
Supplemental Data
References
- 1.Yadav D., Lowenfels A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery A.F., Dellon E.S., Lund J., Crockett S.D., McGowan C.E., Bulsiewicz W.J., Gangarosa L.M., Thiny M.T., Stizenberg K., Morgan D.R., Ringel Y., Kim H.P., Dibonaventura M.D., Carroll C.F., Allen J.K., Cook S.F., Sandler R.S., Kappelman M.D., Shaheen N.J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143 doi: 10.1053/j.gastro.2012.08.002. 1179–1187.e1–1187.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afghani E., Pandol S.J., Shimosegawa T., Sutton R., Wu B.U., Vege S.S., Gorelick F., Hirota M., Windsor J., Lo S.K., Freeman M.L., Lerch M.M., Tsuji Y., Melmed G.Y., Wassef W., Mayerle J. Acute pancreatitis-progress and challenges: a report on an International Symposium. Pancreas. 2015;44:1195–1210. doi: 10.1097/MPA.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandol S.J., Saluja A.K., Imrie C.W., Banks P.A. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Lerch M.M., Gorelick F.S. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Gukovsky I., Li N., Todoric J., Gukovskaya A., Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209.e4. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah R.P., Garg P., Saluja A.K. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol. 2012;28:507–515. doi: 10.1097/MOG.0b013e3283567f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen O.H., Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Gu H., Werner J., Bergmann F., Whitcomb D.C., Buchler M.W., Fortunato F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013;4:e816. doi: 10.1038/cddis.2013.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criddle D.N., Murphy J., Fistetto G., Barrow S., Tepikin A.V., Neoptolemos J.P., Sutton R., Petersen O.H. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Voronina S., Collier D., Chvanov M., Middlehurst B., Beckett A.J., Prior I.A., Criddle D.N., Begg M., Mikoshiba K., Sutton R., Tepikin A.V. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J. 2015;465:405–412. doi: 10.1042/BJ20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams J.A., Korc M., Dormer R.L. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- 13.Bläuer M., Sand J., Nordback I., Laukkarinen J. A novel 2-step culture model for long-term in vitro maintenance of human pancreatic acinar cells. Pancreas. 2014;43:762–767. doi: 10.1097/MPA.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 14.Murphy J.A., Criddle D.N., Sherwood M., Chvanov M., Mukherjee R., McLaughlin E., Booth D., Gerasimenko J.V., Raraty M.G., Ghaneh P., Neoptolemos J.P., Gerasimenko O.V., Tepikin A.V., Green G.M., Reeve J.R., Jr., Petersen O.H., Sutton R. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Lewarchik C.M., Orabi A.I., Jin S., Wang D., Muili K.A., Shah A.U., Eisses J.F., Malik A., Bottino R., Jayaraman T., Husain S.Z. The ryanodine receptor is expressed in human pancreatic acinar cells and contributes to acinar cell injury. Am J Physiol Gastrointest Liver Physiol. 2014;307:G574–G581. doi: 10.1152/ajpgi.00143.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen L., Voronina S., Javed M.A., Awais M., Szatmary P., Latawiec D., Chvanov M., Collier D., Huang W., Barrett J., Begg M., Stauderman K., Roos J., Grigoryev S., Ramos S., Rogers E., Whitten J., Velicelebi G., Dunn M., Tepikin A.V., Criddle D.N., Sutton R. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology. 2015;149:481–492.e7. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mareninova O.A., Sendler M., Malla S.R., Yakubov I., French S.W., Tokhtaeva E., Vagin O., Oorschot V., Lüllmann-Rauch R., Blanz J., Dawson D., Klumperman J., Lerch M.M., Mayerle J., Gukovsky I., Gukovskaya A.S. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol. 2015;1:678–694. doi: 10.1016/j.jcmgh.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messenger S.W., Thomas D.D., Falkowski M.A., Byrne J.A., Gorelick F.S., Groblewski G.E. Tumor protein D52 controls trafficking of an apical endolysosomal secretory pathway in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2013;305:G439–G452. doi: 10.1152/ajpgi.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messenger S.W., Thomas D.D., Cooley M.M., Jones E.K., Falkowski M.A., August B.K., Fernandez L.A., Gorelick F.S., Groblewski G.E. Early to late endosome trafficking controls secretion and zymogen activation in rodent and human pancreatic acinar cells. Cell Mol Gastroenterol Hepatol. 2015;1:695–709. doi: 10.1016/j.jcmgh.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mareninova O.A., Hermann K., French S.W., O'Konski M.S., Pandol S.J., Webster P., Erickson A.H., Katunuma N., Gorelick F.S., Gukovsky I., Gukovskaya A.S. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L., Kneteman N.M., Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 22.Halberstadt C., Williams D., Gores P. Isolation of human cadaveric pancreatic islets for clinical transplantation. Methods Mol Biol. 2013;1001:227–259. doi: 10.1007/978-1-62703-363-3_20. [DOI] [PubMed] [Google Scholar]
- 23.Hawthorne W.J., Williams L., Chew Y.V. Clinical islet isolation. Adv Exp Med Biol. 2016;938:89–122. doi: 10.1007/978-3-319-39824-2_7. [DOI] [PubMed] [Google Scholar]
- 24.Loganathan G., Dawra R.K., Pugazhenthi S., Wiseman A.C., Sanders M.A., Saluja A.K., Sutherland D.E., Hering B.J., Balamurugan A.N. Culture of impure human islet fractions in the presence of alpha-1 antitrypsin prevents insulin cleavage and improves islet recovery. Transplant Proc. 2010;42:2055–2057. doi: 10.1016/j.transproceed.2010.05.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loganathan G., Graham M.L., Radosevich D.M., Soltani S.M., Tiwari M., Anazawa T., Papas K.K., Sutherland D.E., Hering B.J., Balamurugan A.N. Factors affecting transplant outcomes in diabetic nude mice receiving human, porcine, and nonhuman primate islets: analysis of 335 transplantations. Transplantation. 2013;95:1439–1447. doi: 10.1097/TP.0b013e318293b7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orabi A.I., Muili K.A., Javed T.A., Jin S., Jayaraman T., Lund F.E., Husain S.Z. Cluster of differentiation 38 (CD38) mediates bile acid-induced acinar cell injury and pancreatitis through cyclic ADP-ribose and intracellular calcium release. J Biol Chem. 2013;288:27128–27137. doi: 10.1074/jbc.M113.494534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth D.M., Murphy J.A., Mukherjee R., Awais M., Neoptolemos J.P., Gerasimenko O.V., Tepikin A.V., Petersen O.H., Sutton R., Criddle D.N. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116–2125. doi: 10.1053/j.gastro.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 28.Qi M., McFadden B., Valiente L., Omori K., Bilbao S., Juan J., Rawson J., Oancea A.R., Scott S., Nair I., Ferreri K., Mullen Y., Dafoe D., Ei-Shahawy M., Kandeel F., Al-Abdullah I.H. Human pancreatic islets isolated from donors with elevated HbA1c levels: islet yield and graft efficacy. Cell Transplant. 2015;24:1879–1886. doi: 10.3727/096368914X683548. [DOI] [PubMed] [Google Scholar]
- 29.Cox J., Hein M.Y., Luber C.A., Paron I., Nagaraj N., Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 31.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thrower E.C., Wang J., Cheriyan S., Lugea A., Kolodecik T.R., Yuan J., Reeve J.R., Gorelick F.S., Pandol S.J. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas. 2009;38:930–935. doi: 10.1097/MPA.0b013e3181b8476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandol S.J., Schoeffield M.S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985;260:10081–10086. [PubMed] [Google Scholar]
- 34.Fischer L., Gukovskaya A.S., Penninger J.M., Mareninova O.A., Friess H., Gukovsky I., Pandol S.J. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G875–G886. doi: 10.1152/ajpgi.00558.2005. [DOI] [PubMed] [Google Scholar]
- 35.Shalbueva N., Mareninova O.A., Gerloff A., Yuan J., Waldron R.T., Pandol S.J., Gukovskaya A.S. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology. 2013;144:437–446.e6. doi: 10.1053/j.gastro.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gukovskaya A.S., Vaquero E., Zaninovic V., Gorelick F.S., Lusis A.J., Brennan M.L., Holland S., Pandol S.J. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 37.Thrower E.C., Osgood S., Shugrue C.A., Kolodecik T.R., Chaudhuri A.M., Reeve J.R., Pandol S.J., Gorelick F.S. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1344–G1353. doi: 10.1152/ajpgi.00020.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shugrue C.A., Alexandre M., Diaz de Villalvilla A., Kolodecik T.R., Young L.H., Gorelick F.S., Thrower E.C. Cerulein hyperstimulation decreases AMP-activated protein kinase levels at the site of maximal zymogen activation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G723–G732. doi: 10.1152/ajpgi.00082.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H.Y., Waldron R.T., Gong R., Ramanujan V.K., Pandol S.J., Lugea A. The unfolded protein response plays a predominant homeostatic role in response to mitochondrial stress in pancreatic stellate cells. PLoS One. 2016;11:e0148999. doi: 10.1371/journal.pone.0148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lugea A., Gukovsky I., Gukovskaya A.S., Pandol S.J. Nonoxidative ethanol metabolites alter extracellular matrix protein content in rat pancreas. Gastroenterology. 2003;125:1845–1859. doi: 10.1053/j.gastro.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 41.O'Konski M.S., Pandol S.J. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. 1990;86:1649–1657. doi: 10.1172/JCI114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid S.W., Modlin I.M., Tang L.H., Stoch A., Rhee S., Nathanson M.H., Scheele G.A., Gorelick F.S. Telenzepine-sensitive muscarinic receptors on rat pancreatic acinar cells. Am J Physiol. 1998;274:G734–G741. doi: 10.1152/ajpgi.1998.274.4.G734. [DOI] [PubMed] [Google Scholar]
- 43.Soudah H.C., Lu Y., Hasler W.L., Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol. 1992;263:G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- 44.Kim J.Y., Kim K.H., Lee J.A., Namkung W., Sun A.Q., Ananthanarayanan M., Suchy F.J., Shin D.M., Muallem S., Lee M.G. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 2002;122:1941–1953. doi: 10.1053/gast.2002.33617. [DOI] [PubMed] [Google Scholar]
- 45.Geyer J., Döring B., Meerkamp K., Ugele B., Bakhiya N., Fernandes C.F., Godoy J.R., Glatt H., Petzinger E. Cloning and functional characterization of human sodium-dependent organic anion transporter (SLC10A6) J Biol Chem. 2007;282:19728–19741. doi: 10.1074/jbc.M702663200. [DOI] [PubMed] [Google Scholar]
- 46.Döring B., Lütteke T., Geyer J., Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–168. doi: 10.1016/B978-0-12-394316-3.00004-1. [DOI] [PubMed] [Google Scholar]
- 47.Acosta J.M., Ledesma C.L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- 48.Owyang C., Logsdon C.D. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Gerasimenko J.V., Gryshchenko O., Ferdek P.E., Stapleton E., Hebert T.O., Bychkova S., Peng S., Begg M., Gerasimenko O.V., Petersen O.H. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci U S A. 2013;110:13186–13191. doi: 10.1073/pnas.1300910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rehfeld J.F. Cholecystokinin: from local gut hormone to ubiquitous messenger. Front Endocrinol (Lausanne) 2017;8:1–8. doi: 10.3389/fendo.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saluja A., Logsdon C., Garg P. Direct versus indirect action of cholecystokinin on human pancreatic acinar cells: is it time for a judgment after a century of trial? Gastroenterology. 2008;135:357–360. doi: 10.1053/j.gastro.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 52.Ji B., Bi Y., Simeone D., Mortensen R.M., Logsdon C.D. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- 53.Liang T., Dolai S., Xie L., Winter E., Orabi A.I., Karimian N., Cosen-Binker L.I., Huang Y.C., Thorn P., Cattral M.S., Gaisano H.Y. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem. 2017;292:5957–5969. doi: 10.1074/jbc.M117.777433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagerberg L., Hallstrom B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., Asplund A., Sjostedt E., Lundberg E., Szigyarto C.A., Skogs M., Takanen J.O., Berling H., Tegel H., Mulder J., Nilsson P., Schwenk J.M., Lindskog C., Danielsson F., Mardinoglu A., Sivertsson A., von Feilitzen K., Forsberg M., Zwahlen M., Olsson I., Navani S., Huss M., Nielsen J., Ponten F., Uhlen M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lugea A., Gong J., Nguyen J., Nieto J., French S.W., Pandol S.J. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res. 2010;34:1768–1781. doi: 10.1111/j.1530-0277.2010.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muili K.A., Wang D., Orabi A.I., Sarwar S., Luo Y., Javed T.A., Eisses J.F., Mahmood S.M., Jin S., Singh V.P., Ananthanaravanan M., Perides G., Williams J.A., Molkentin J.D., Husain S.Z. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2013;288:570–580. doi: 10.1074/jbc.M112.428896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grady T., Mah'Moud M., Otani T., Rhee S., Lerch M.M., Gorelick F.S. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol. 1998;275:G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 58.Voronina S.G., Barrow S.L., Gerasimenko O.V., Petersen O.H., Tepikin A.V. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem. 2004;279:27327–27338. doi: 10.1074/jbc.M311698200. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee R., Criddle D.N., Gukovskaya A., Gukvoskaya A., Pandol S., Petersen O.H., Sutton R. Mitochondrial injury in pancreatitis. Cell Calcium. 2008;44:14–23. doi: 10.1016/j.ceca.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odinokova I.V., Sung K.F., Mareninova O.A., Hermann K., Gukovsky I., Gukovskaya A.S. Mitochondrial mechanisms of death responses in pancreatitis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S25–S30. doi: 10.1111/j.1440-1746.2007.05271.x. [DOI] [PubMed] [Google Scholar]
- 61.Sung K.F., Odinokova I.V., Mareninova O.A., Rakonczay Z., Hegyi P., Pandol S.J., Gukovsky I., Gukovskaya A.S. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp Cell Res. 2009;315:1975–1989. doi: 10.1016/j.yexcr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee R., Mareninova O.A., Odinokova I.V., Huang W., Murphy J., Chvanov M., Javed M.A., Wen L., Booth D.M., Cane M.C., Awais M., Gavillet B., Pruss R.M., Schaller S., Molkentin J.D., Tepikin A.V., Petersen O.H., Pandol S.J., Gukovsky I., Criddle D.N., Gukovskaya A.S., Sutton R., Unit N.P.B.R. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut. 2016;65:1333–1346. doi: 10.1136/gutjnl-2014-308553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto D., Ohmuraya M., Hirota M., Yamamoto A., Suyama K., Ida S., Okumura Y., Takahashi E., Kido H., Araki K., Baba H., Mizushima N., Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaccaro M.I. Autophagy and pancreas disease. Pancreatology. 2008;8:425–429. doi: 10.1159/000151480. [DOI] [PubMed] [Google Scholar]
- 65.Vaccaro M.I. Zymophagy: selective autophagy of secretory granules. Int J Cell Biol. 2012;2012:396705. doi: 10.1155/2012/396705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gukovskaya A.S., Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fortunato F., Deng X., Gates L.K., McClain C.J., Bimmler D., Graf R., Whitcomb D.C. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis? Am J Physiol Gastrointest Liver Physiol. 2006;290:G232–G241. doi: 10.1152/ajpgi.00040.2005. [DOI] [PubMed] [Google Scholar]
- 68.Fortunato F., Bürgers H., Bergmann F., Rieger P., Büchler M.W., Kroemer G., Werner J. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137:350–360.e5. doi: 10.1053/j.gastro.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Kubisch C.H., Sans M.D., Arumugam T., Ernst S.A., Williams J.A., Logsdon C.D. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 70.Suyama K., Ohmuraya M., Hirota M., Ozaki N., Ida S., Endo M., Araki K., Gotoh T., Baba H., Yamamura K. C/EBP homologous protein is crucial for the acceleration of experimental pancreatitis. Biochem Biophys Res Commun. 2008;367:176–182. doi: 10.1016/j.bbrc.2007.12.132. [DOI] [PubMed] [Google Scholar]
- 71.Lugea A., Tischler D., Nguyen J., Gong J., Gukovsky I., French S.W., Gorelick F.S., Pandol S.J. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pandol S.J., Gorelick F.S., Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function: a hypothesis. Front Physiol. 2011;2:8. doi: 10.3389/fphys.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sah R.P., Garg S.K., Dixit A.K., Dudeja V., Dawra R.K., Saluja A.K. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J Biol Chem. 2014;289:27551–27561. doi: 10.1074/jbc.M113.528174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waldron R.T., Pandol S., Lugea A., Groblewski G. Endoplasmic reticulum stress and the unfolded protein response in exocrine pancreas physiology and pancreatitis. In: Williams J.A., editor. Pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base; Mountain View, CA: 2015. pp. 88–96. [Google Scholar]
- 75.Lugea A., Waldron R.T., Pandol S.J. Pancreatic adaptive responses in alcohol abuse: role of the unfolded protein response. Pancreatology. 2015;15:S1–S5. doi: 10.1016/j.pan.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee A.H., Chu G.C., Iwakoshi N.N., Glimcher L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blinman T.A., Gukovsky I., Mouria M., Zaninovic V., Livingston E., Pandol S.J., Gukovskaya A.S. Activation of pancreatic acinar cells on isolation from tissue: cytokine upregulation via p38 MAP kinase. Am J Physiol Cell Physiol. 2000;279:C1993–C2003. doi: 10.1152/ajpcell.2000.279.6.C1993. [DOI] [PubMed] [Google Scholar]
- 78.Gukovskaya A.S., Gukovsky I., Zaninovic V., Song M., Sandoval D., Gukovsky S., Pandol S.J. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha: role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grady T., Liang P., Ernst S.A., Logsdon C.D. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966–1975. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 80.Szatmary P., Gukovsky I. The role of cytokines and inflammation in the genesis of experimental pancreatitis. In: Williams J.A., editor. Pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base; Mountain View, CA: 2016. pp. 42–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.