Abstract
Alcoholic liver disease remains a major cause of liver-related morbidity and mortality, which ranges from alcoholic steatohepatitis to fibrosis/cirrhosis and hepatocellular carcinoma, and the related mechanisms are understood poorly. In this study, we aimed to investigate the role of miR-34a in alcohol-induced cellular senescence and liver fibrosis. We found that hepatic miR-34a expression was upregulated in ethanol-fed mice and heavy drinkers with steatohepatitis compared with respective controls. Mice treated with miR-34a Vivo-Morpholino developed less severe liver fibrosis than wild-type mice after 5 weeks of ethanol feeding. Further mechanism exploration showed that inhibition of miR-34a increased cellular senescence of hepatic stellate cells (HSCs) in ethanol-fed mice, although it decreased senescence in total liver and hepatocytes, which was verified by the changes of senescence-associated β-galactosidase and gene expression. Furthermore, enhanced cellular senescence was observed in liver tissues from steatohepatitis patients compared with healthy controls. In addition, the expression of transforming growth factor-β1, drosophila mothers against decapentaplegic protein 2 (Smad2), and Smad3 was decreased after inhibition of miR-34a in ethanol-fed mice. Our in vitro experiments showed that silencing of miR-34a partially blocked activation of HSCs by lipopolysaccharide and enhanced senescence of HSCs. Furthermore, inhibition of miR-34a decreased lipopolysaccharide-induced fibrotic gene expression in cultured hepatocytes. In conclusion, our data suggest that miR-34a functions as a profibrotic factor that promotes alcohol-induced liver fibrosis by reducing HSC senescence and increasing the senescence of hepatocytes.
Alcoholic liver disease (ALD) is a predominant cause of liver-related mortality in developed countries. The pathogenesis of ALD in humans is characterized by steatosis, which is the accumulation of fat in hepatocytes.1 Along with the development of ALD, steatosis can progress to steatohepatitis, fibrosis and cirrhosis (8% to 20%), or hepatocellular carcinoma (3% to 10%).2
The entire pathophysiology of ALD still is incompletely understood, although the role of alcohol exposure has been identified, including alcohol exposure–induced hepatocyte apoptosis, necrosis, and necroptosis, as well as inflammatory cytokines released from hepatic macrophages, which are activated as a result of translocation of lipopolysaccharide (LPS) from intestine.3 In addition, miRNAs may be involved in ALD progression. miRNAs are small noncoding RNA molecules with 20 to 22 nucleotides that function in transcriptional and post-transcriptional regulation of gene expression.4 There is accumulating evidence that miRNAs play an important role in the pathogenesis of ALD.5 In recent years, increased miR-34a expression has been reported in alcoholic hepatitis.6 However, the functional role of miR-34a in ethanol-induced liver fibrosis remains unclear.
Liver fibrosis is a common consequence of ALD and other chronic liver insults, which is characterized by accumulation of excess extracellular matrix (ECM) components, including collagen type I, α 1 (Col1α1).7 In a setting of chronic fibrogenic stimuli, myofibroblast-like cells produce large quantities of ECM components. Extensive investigation has shown that hepatic stellate cells (HSCs) are the major source of ECM in liver fibrosis. Previous studies have shown that miR-34a is involved in the development of fibrosis in many organs. For example, miR-34a has been found to promote lung epithelial injury and pulmonary fibrosis.8 miR-34a can contribute to cardiac tissue fibrosis by directly targeting drosophila mothers against decapentaplegic protein 4 (Smad4)9 and promote radiation-induced fibrosis in a murine skin model by inhibition of c-Met production.10 Furthermore, it has been reported that the miR-34a/sirtuin 1 (SIRT1)/p53 signaling pathway is activated in hepatocytes but not in HSCs, which resulted in the apoptosis of hepatocytes and thus activated HSCs, eventually contributing to the progression of carbon tetrachloride–induced rat liver fibrosis.11 Interestingly, miR-34a has been identified to be upregulated in human fibrotic livers compared with normal human liver.12 Based on these observations, we speculate that miR-34a may contribute to the progression of alcoholic liver fibrosis.
Cellular senescence is an irreversible cell-cycle arrest in the G0/G1 phase that occurs when cells experience oncogenic insults or stress signals.13 Senescent cells are characterized by high senescence-associated β-galactosidase (SA-β-gal) activity and a high expression level of markers related to cell-cycle arrest, such as, p53, p16, and p21.14 Although senescence is a well-known mechanism of tumor suppression, emerging evidence has indicated that it may play a role in aging, tissue repair, and even malignancy.15 Cellular senescence of hepatocytes, cholangiocytes, HSCs, and immune cells has been shown in a wide spectrum of chronic liver disorders.16 Senescence of activated HSCs can lead to regression of carbon tetrachloride–induced liver fibrosis.17 Increased senescence in hepatocytes also has been observed in ethanol-induced liver injury and fibrosis.18 In addition, a number of senescence-associated miRNAs has been reported,19 including the members of the miR-34 family, which are downstream effectors of p53-mediated cellular senescence.20 miR-34a has been found to induce senescence in human hepatocellular carcinoma,21 but Cui et al22 found that miR-34a inhibits lung fibrosis by inducing fibroblast senescence. Because miR-34a is a prime putative player that induces senescence, cell-cycle arrest, and apoptosis,23 and the role of miR-34a–regulated cellular senescence is different in lung fibrosis from that in the heart and skin fibrosis, we are interested to explore the effect of miR-34a on cellular senescence and liver fibrosis during alcoholic liver injury.
Given these observations, we hypothesized that miR-34a may promote ethanol-induced liver fibrosis by regulating cellular senescence in HSCs and hepatocytes. In the present study, we mainly explored the effect of inhibition of miR-34a by miR-34a Morpholino on the development of liver fibrosis and cellular senescence in the alcoholic liver injury mouse model. Meanwhile, through in vitro experiments, we examined whether silencing of miR-34a influences the activation and senescence of cultured human HSCs simulated with LPS. Mainly, we assessed the role of miR-34a–regulated cellular senescence in ALD by posing the following four questions: i) is miR-34a and cellular senescence altered in ethanol-exposed mice and ALD human liver tissues, ii) does modulation of miR-34a alter cellular senescence in vitro and in animals with ALD, iii) is there a different mechanism in altered senescence response between HSCs and hepatocytes in ALD, and iv) what are the downstream target genes of miR-34a involved in cellular senescence in ALD?
Materials and Methods
Materials
Reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise indicated. The rabbit polyclonal antibodies against α-smooth muscle actin (α-SMA), desmin, fibronectin 1 (Fn1), p16 (used for Western blot assay), and plasminogen activator inhibitor-1 (PAI-1) were purchased from Abcam (Cambridge, MA). The antibodies against hepatocyte nuclear factor 4-α and p16 were purchased from Santa Cruz Biotechnology (Dallas, TX). The SA-β-gal staining and activity assay kits were purchased from Millipore Sigma (Billerica, MA). The inhibitor for miR-34a and the control inhibitor were purchased from Thermo Fisher Scientific (Waltham, MA). The miRNA Isolation Kit for RNA purification was purchased from Thermo Fisher Scientific (Rockford, IL), and all selected primers were purchased from Qiagen (Valencia, CA). The iScript Complementary DNA Synthesis Kit (170-8891) and iTaq Universal SYBR Green Supermix (172-5124) were purchased from Bio-Rad (Hercules, CA).
Animal Models
All animal experiments were performed in accordance with protocols approved by the Baylor Scott & White Health Institutional Animal Care and Use Committee. Male C3H/HeOu/J mice (weight, 25 to 30 g) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a temperature-controlled environment (22°C) with 12:12-hour light-dark cycles. The mice (age, 10 wk; n = 15) were divided randomly into 3 groups: wild-type mice, ethanol-fed mice (Lieber-DeCarli liquid diet ethanol, ethanol feeding for 5 weeks, Rodent Liquid Diet Lieber-DeCarli '82; Bio-Serv, Flemington, NJ), and ethanol-fed mice (for 5 weeks) treated with miR-34a Morpholino (CAACAACCAGCTAAGACACTGCCAA; Gene Tools, LLC, Philomath, OR) by two tail vein injections (one on day 3 and one on day 7, 30 mg/kg body weight) for 5 weeks. The inhibitors of miR-34a are small, chemically synthetic, single-stranded, nucleic acids designed to specifically bind to and inhibit endogenous miR-34a molecules. For chronic intragastric ethanol administration, mice were implanted aseptically with gastrostomy catheters as described.24, 25 A dose of liquid ethanol (5%) or control solution was infused for 5 weeks.24 After 5 weeks of treatment, mice were weighed and anesthetized. Livers then were excised, weighed, and portions were fixed in formalin, frozen in Optimal Cutting Temperature medium (Sakura Finetek USA, Torrance, CA), snap-frozen in liquid nitrogen, and stored in −80°C for further use.
Human Healthy Control and Steatohepatitis Samples
Healthy human liver (n = 3) and liver samples of steatohepatitis patients with heavy alcohol consumption (n = 3) were purchased from Xeno Tech (Kansas, KS). The samples were used for RNA extraction, frozen section slides, and protein extraction. The patients' characteristics are listed in Table 1.
Table 1.
Characteristics of Healthy Controls and Steatohepatitis Patients
| Groups | Samples ID | Product name | Diagnosis | Sample | Sex | Age, years | Alcohol use | Alcohol frequency | Origin |
|---|---|---|---|---|---|---|---|---|---|
| Control | H1255 | HHPL.NT | Normal | Liver | Female | 56 | No | NA | Xeno Tech |
| H1293 | HHPL.NT | Normal | Liver | Female | 52 | No | NA | Xeno Tech | |
| H1296 | HHPL.NT | Normal | Liver | Male | 46 | No | NA | Xeno Tech | |
| Steatohepatitis | H0959 | HHPL.HST | Steatohepatitis | Liver | Male | 48 | Yes | Heavy | Xeno Tech |
| H1063 | HHPL.HST | Steatohepatitis | Liver | Male | 43 | Yes | Heavy | Xeno Tech | |
| H1259 | HHPL.HST | Steatohepatitis | Liver | Male | 64 | Yes | Heavy | Xeno Tech |
The human liver samples listed were purchased from Xeno Tech (Kansas City, KS).
NA, not applicable.
Isolation of Mouse HSCs and Hepatocytes, and Transient Transfection in Cultured Human HSC Lines and Human Hepatocytes
Mouse HSCs and hepatocytes were isolated by laser capture microdissection as described previously26 (using desmin as a marker of HSCs and hepatocyte nuclear factor 4-α as a marker of hepatocytes). The RNA from laser capture microdissection–isolated HSCs and hepatocytes were extracted with the Arcturus PicoPure RNA isolation kit (Thermo Fisher Scientific, Grand Island, NY) according to the instructions provided by the vendor. Expression of cyclin-dependent kinase inhibitor 2A (p16), cyclin-dependent kinase inhibitor 1A (p21), and transforming growth factor-β1 (TGF-β1) was measured in these cells by quantitative PCR. The in vitro studies were performed in human HSC (HHSC) lines and human hepatocytes (both Sciencell, Carlsbad, CA). HHSCs and human hepatocytes were seeded onto six-well plates the day before transfection. The cells were transfected with inhibitors of miR-34a or negative control miRNAs using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific). After culture with the transfection mix for 24 hours, the cells were cultured in normal medium or medium containing 20 ng/mL LPS for 24 hours. Cells then were harvested and the expression of fibrosis and senescent markers was evaluated by quantitative PCR or immunofluorescence. All studies were performed in quadruplicate unless otherwise specified.
Liver Histologic Analysis and Measurement of Serum Chemistry
Liver histology was evaluated in liver sections (4- to 5-μm thick) from mice and human samples by staining with hematoxylin and eosin. Hepatic fibrosis was assessed by Sirius Red staining in liver sections (4- to 5-μm thick) (10 different fields were analyzed from three different samples obtained from three different animals). Images were obtained by a Leica scanner (Buffalo Grove, IL). Collagen deposition in liver sections with Sirius Red staining was quantified using Image Pro-Premier software version 9.0 (Media Cybernetics, Rockville, MD). The serum levels of alanine aminotransferase were measured by a Dimension RxL Max Integrated Chemistry System (Dade Behring, Deerfield, IL) located at Baylor Scott & White Health. The expression of p16 was detected in human liver sections by immunohistochemistry.
Total RNA Extraction and Quantitative PCR Analysis
Total RNA was extracted from the liver samples as well as cultured HHSCs and human hepatocytes using a miRNA isolation Kit. The expression of fibrosis markers such as α-SMA, Fn1, and Col1α1, and senescence markers such as p16, PAI-1, and monocyte chemoattractant protein-1/CC-chemokine ligand 2 (MCP1/CCL2) were measured by quantitative PCR.27 Primers were designed for the sequences described on https://www.ncbi.nlm.nih.gov/nuccore per the listed accession numbers. The mouse PCR primers for Col1α1 (PPM03845F), α-SMA (PPM04483A), p16 (PPM02906F), p21 (PPM02901B), CCL2 (PPM03151G), PAI-1 (PPM03093C), SIRT1 (PPM05054A), TGF-β1 (PPM02991B), drosophila mothers against decapentaplegic protein 2 (Smad2) (PPM04430C), drosophila mothers against decapentaplegic protein 3 (Smad3) (PPM04461C), and glyceraldehyde-3-phosphate dehydrogenase (PPM02946E) were purchased from Qiagen. The human PCR primers for α-SMA (PPH01300B), Col1a1 (PPH01299F), CCL2 (PPH00192F), Fn1 (PPH00143B), p16 (PPH00207C), TGF-β1 (PPH00508A), PAI-1 (PPH00215F), and glyceraldehyde-3-phosphate dehydrogenase (PPH00150F) were purchased from Qiagen. The genes related to cellular senescence (p16, p21, CCL2, and PAI-1) were analyzed using Ingenuity Pathway Analysis (IPA) software (Qiagen, Redwood City, CA) for the functionally relevant pathway. IPA is a web-based functional analysis software that helps researchers to model, analyze, and understand the complex biological and chemical systems in life science research.28 By using IPA, we can understand if there is a functional relationship between miR-34a and the earlier-described senescent genes.
Immunofluorescence and Western Blot Assay
The expression of α-SMA in liver section (6- to 8-μm thick) and the expression of PAI-1 in cultured HHSCs were evaluated by immunofluorescence as described.27 For the detection of PAI-1 expression, the cells were seeded on glass coverslips. Before staining, the cells were washed with 1× phosphate-buffered saline. Then, immunofluorescent staining for PAI-1, similar to staining in liver sections, was performed. After staining, images were obtained using the Leica AF 6000 Modular Systems. The protein of liver samples was extracted with lysis buffer and quantified by the bicinchoninic acid method (Pierce Biotechnology, Inc., Rockford, IL). Then, the protein expression of α-SMA, Fn1, and p16 was evaluated by immunoblotting as described.26 Protein expression was visualized and quantified using the LI-COR Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE).
SA-β-Gal Staining and Activity Assay
Cellular senescence was measured in frozen liver sections from mice (n = 4) and human samples (n = 3; 10-μm thick) by staining for SA-β-gal using a commercially available kit according to the instructions provided by the vendor. The pictures from one representative slide are shown for SA-β-gal staining. Images were obtained by a Leica scanner. The SA-β-gal–positive areas were measured in at least three microscope fields from each liver section using the Image Pro-Analyzer software. SA-β-gal activity in HHSCs was detected by a commercial kit (mentioned earlier) according to provided instructions.
Statistical Analysis
All data are expressed as means ± SEM. The differences between groups were analyzed by an unpaired t-test when two groups were compared or by one-way analysis of variance when more than two groups were compared using SPSS 22.0 software (IBM, Armonk, NY). A P value < 0.05 was used to indicate statistically significant differences.
Results
Expression of miR-34a Is Upregulated along with Enhanced Cellular Senescence in the Livers from Steatohepatitis Patients with Heavy Alcohol Consumption
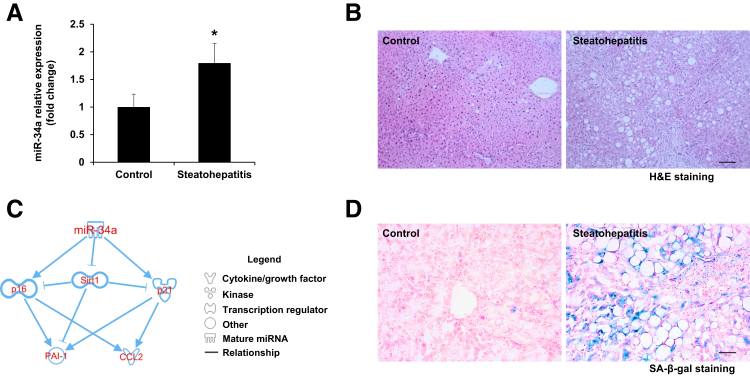
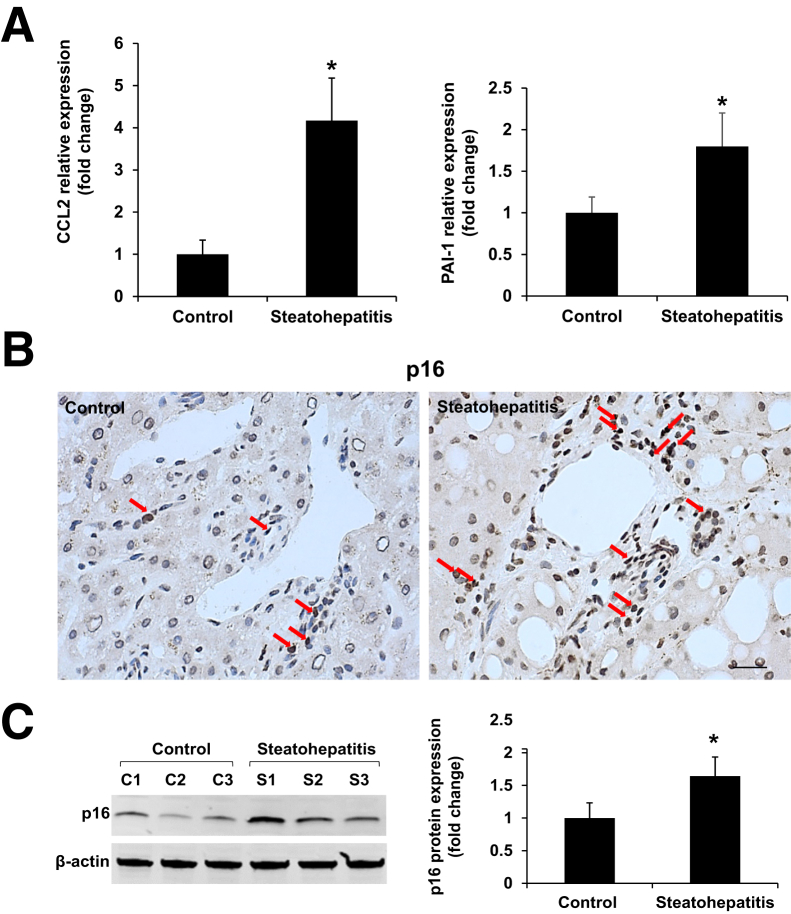
miR-34a plays a critical role in both liver physiology and the pathogenesis of alcoholic liver diseases. Quantitative PCR revealed that expression of miR-34a was increased in the livers of steatohepatitis patients with heavy alcohol consumption compared with healthy controls (Figure 1A). Typical pathologic changes were observed in liver sections from steatohepatitis patients compared with healthy controls (Figure 1B). IPA was performed to ascertain the cellular context of the differentially expressed signaling mechanisms related to miR-34a–mediated liver injury. IPA analysis indicated that the cellular senescence pathway was the most altered signaling through p16, p21, CCL2, PAI-1, and SIRT1-related pathologic mechanisms (Figure 1C). SA-β-gal staining showed the increased cellular senescence in livers from patients with steatohepatitis compared with healthy controls (Figure 1D). As well, the messenger RNA (mRNA) expression of senescence markers (PAI-1 and CCL2) was increased in steatohepatitis patients compared with healthy controls (Figure 2A). Increased protein expression of the senescence marker p16 in livers from steatohepatitis patients was verified by immunohistochemistry and Western blot assay when compared with healthy controls (Figure 2, B and C).
Figure 1.
The expression of miR-34a Morpholino (miR-34a) and cellular senescence are increased in livers from heavy alcohol consumers with steatohepatitis. A: Expression of miR-34a was upregulated in the livers from steatohepatitis patients with heavy alcohol consumption compared with healthy controls. B: Histologic changes were detected by hematoxylin and eosin (H&E) staining. C: Ingenuity Pathway Analysis software showed that several genes implicated in cellular senescence are regulated by miR-34a, including p16, p21, CCL2, and plasminogen activator inhibitor-1 (PAI-1). D: Increased cellular senescence in heavy alcohol consumers with steatohepatitis was verified by senescence-associated β-galactosidase (SA-β-gal) staining compared with healthy controls. n = 3 (A). *P < 0.05 versus healthy controls. Scale bars: 100 μm (B); 50 μm (D). Original magnification, ×10 (B); ×20 (D). Sirt1, sirtuin 1.
Figure 2.
The mRNA and protein levels of senescence markers are increased in the livers of heavy alcohol consumers with steatohepatitis compared with healthy controls. A: The hepatic mRNA expression of senescence-related genes [CCL2 and plasminogen activator inhibitor-1 (PAI-1)] was increased in steatohepatitis patients compared with healthy controls. B and C: Increased expression of p16 in livers from steatohepatitis patients was observed by immunohistochemistry and Western blot assays compared with healthy controls (red arrows show direct p16-positive staining). n = 3 (A and C). *P < 0.05 versus healthy controls. Scale bar = 25 μm (B). Original magnification, ×40 (B). C, healthy control; S, steatohepatitis.
Ethanol Feeding Increases miR-34a Expression and miR-34a Morpholino Treatment Decreases Liver Damage in Ethanol-Fed Mice
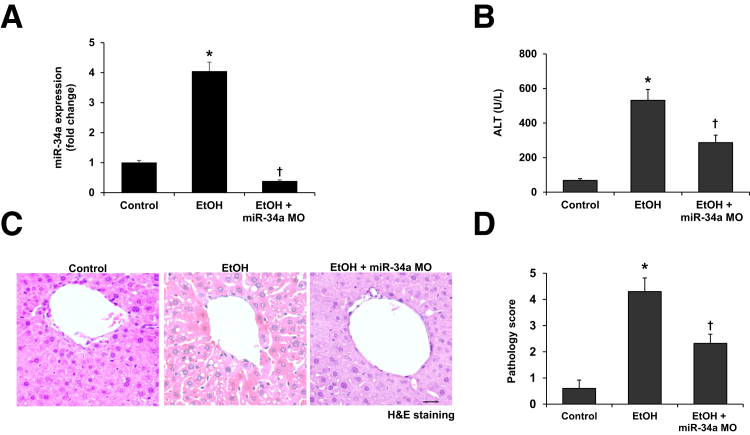
Ethanol feeding significantly increased the hepatic expression of miR-34a compared with wild-type control mice. Furthermore, miR-34a expression was decreased in ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice (Figure 3A). Treatment with miR-34a Morpholino to ethanol-fed mice decreased alanine aminotransferase serum levels compared with ethanol-fed mice (Figure 3B). In ethanol-fed mice, liver cell swelling, irregular nucleus size, shrinkage in varying degrees, and fatty degeneration of a large number of liver cells were all observed (Figure 3C). These pathologic changes and pathologic scores were reduced in ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice (Figure 3, C and D).
Figure 3.
Ethanol feeding induces upregulation of miR-34a in the liver and results in alcoholic liver injury. A: Expression of miR-34a was detected by real-time PCR. B: Inhibition of miR-34a decreased alanine aminotransferase (ALT) serum levels in ethanol-fed mice. C and D: Histologic changes were measured by hematoxylin and eosin (H&E) staining and scored. n = 4 (A–D). *P < 0.05 versus wild-type control mice; †P < 0.05 versus EtOH-fed mice. Scale bar = 25 μm (C). Original magnification, ×40 (C). EtOH, ethanol; miR-34a MO, miR-34a Morpholino.
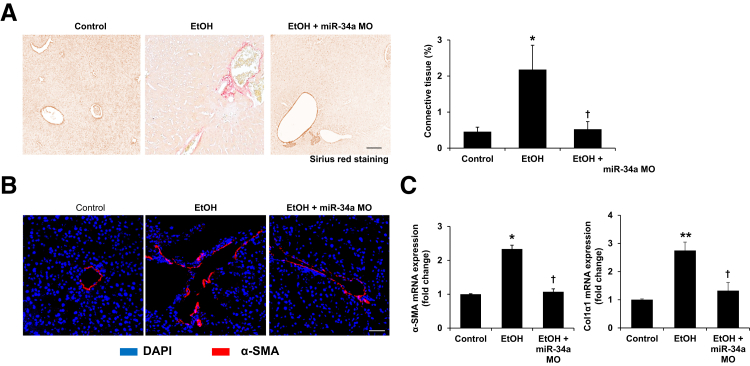
miR-34a Morpholino Treatment Decreases Ethanol-Induced Liver Fibrosis
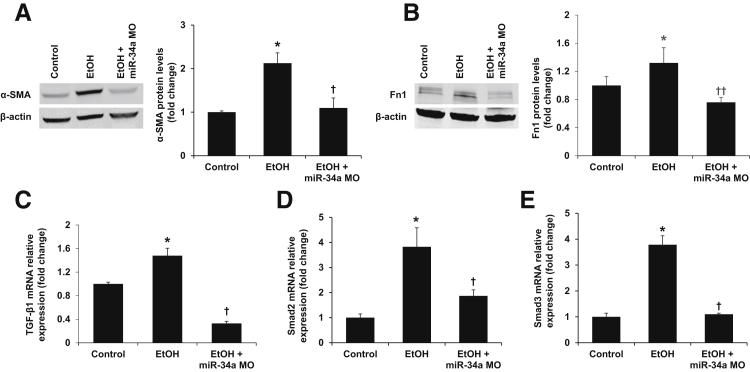
Sirius red staining showed that liver fibrosis was increased in ethanol-fed mice compared with control mice, but was reduced significantly in ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice (Figure 4A). By immunofluorescence, increased expression of α-SMA, a marker of HSC activation, was observed in ethanol-fed mice compared with control mice (Figure 4B). In mice fed with ethanol and treated simultaneously with miR-34a Morpholino, there was reduced expression of α-SMA compared with ethanol-fed mice (Figure 4B). Furthermore, the mRNA expression of fibrosis markers α-SMA and Col1α1, as well as protein expression of α-SMA and Fn1, was decreased in total liver samples from miR-34a Morpholino-treated mice with ethanol feeding compared with ethanol-fed mice (Figures 4C and 5, A and B). There also was decreased expression of TGF-β1, Smad2, and Smad3 in total liver samples from ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice (Figure 5, C–E). In addition, the increased expression of TGF-β1 was observed in isolated HSCs and hepatocytes from ethanol-fed mice compared with control mice, which was reduced by miR-34a Morpholino treatment (Figure 6, D and E). These findings suggest that TGF-β1/Smad signaling is involved in miR-34a modulation of alcoholic liver fibrosis.
Figure 4.
Inhibition of miR-34a protects mice from alcoholic liver fibrosis. A: Sirius red staining showed enhanced collagen deposition in ethanol-fed mice compared with control mice, but reduced collagen deposition in ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice. B: There was lower immunoreactivity for α-smooth muscle actin (α-SMA) in liver sections from ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice. C: There was increased mRNA expression of α-SMA and collagen type I, α 1 (Col1α1) in total liver of ethanol-fed mice compared with control mice, although these two gene expressions were reduced in ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice. n = 3 (A–C). *P < 0.05, **P < 0.01 versus control mice; †P < 0.05 versus EtOH-fed mice. Scale bars: 50 μm (A and B). Original magnification, ×20 (B). EtOH, ethanol; miR-34a MO, miR-34a Morpholino.
Figure 5.
Inhibition of miR-34a reduces the expression of α-smooth muscle actin (α-SMA) and fibronectin as well as the activation of the transforming growth factor-β1 (TGF-β1)/Smad2/3 signaling pathway. A and B: Immunoblot for α-SMA and fibronectin was performed. The expression of these proteins in the liver was decreased after miR-34a Morpholino treatment in ethanol-fed mice. C–E: Quantitative PCR assay for TGF-β1, Smad2, and Smad3. The hepatic mRNA expression of TGF-β1, Smad2, and Smad3 was decreased after miR-34a Morpholino treatment in ethanol-fed mice. n = 3 (A–E) *P < 0.05 versus control mice; †P < 0.05, ††P < 0.01 versus EtOH-fed mice. EtOH, ethanol; Fn1, fibronectin-1; miR-34a MO, miR-34a Morpholino.
Figure 6.
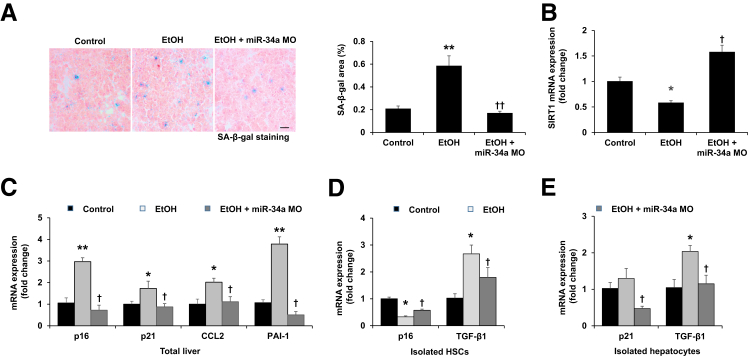
Inhibition of miR-34a results in decreased cellular senescence in total liver and hepatocytes but increased senescence in HSCs. A: There was enhanced cellular senescence shown by senescence-associated β-galactosidase (SA-β-gal) staining in liver sections from ethanol-fed mice compared with control mice. Cellular senescence was reduced in the livers from ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice. B: The hepatic mRNA expression of sirtuin 1 (SIRT1) was decreased in ethanol-fed mice, although it increased after miR-34a Morpholino treatment. C: There was increased mRNA expression of senescence-related genes [p16, p21, CCL2, and plasminogen activator inhibitor-1 (PAI-1)] in total liver from ethanol-fed mice compared with control mice and decreased expression of these senescence-related genes in total liver from ethanol-fed mice treated with miR-34a Morpholino compared with ethanol-fed mice. D: The expression of p16 was decreased and the expression of transforming growth factor-β1 (TGF-β1) was increased in HSCs isolated from ethanol-fed mice compared with control mice, changes partly were reversed in ethanol-fed mice treated with miR-34a Morpholino. E: The expression of p21 and TGF-β1 was increased in hepatocytes isolated from ethanol-fed mice compared with wild-type mice, changes were reversed in ethanol-fed mice treated with miR-34a Morpholino. n = 4 (A); n = 3 (B–E). *P < 0.05, **P < 0.01 versus control mice; †P < 0.05, ††P < 0.01 versus EtOH-fed mice. Scale bar = 25 μm (A). Original magnification, ×40 (A). EtOH, ethanol; miR-34a MO, miR-34a Morpholino.
miR-34a Morpholino Treatment Decreases Ethanol-Induced Cellular Senescence in Total Liver and Isolated Hepatocytes, but Increases Senescence in Isolated HSCs from Ethanol-Fed Mice
To further evaluate the underlying mechanisms of miR-34a modulation of liver fibrosis during ALD, we measured the changes in cellular senescence in total liver, isolated HSCs, and hepatocytes. We observed enhanced senescence (reflected by SA-β-gal staining) in livers of ethanol-fed mice compared with control mice (Figure 6A). However, administration of miR-34a Morpholino to ethanol-fed mice reduced cellular senescence in total liver compared with ethanol-fed mice (Figure 6A). As shown by quantitative PCR, ethanol-fed mice had increased expression of the senescence-related genes (p16, p21, CCL2, and PAI-1) and decreased SIRT1 mRNA expression in total liver compared with control mice, which was reversed by treatment with miR-34a Morpholino (Figure 6, B and C). Interestingly, the expression of the senescence gene p16 was decreased in HSCs isolated from ethanol-fed mice compared with control mice, which was reversed owing in part to miR-34a Morpholino treatment (Figure 6D), suggesting that senescence of HSCs was increased after miR-34a Morpholino treatment in ethanol-fed mice. Conversely, miR-34a Morpholino treatment decreased ethanol-induced expression of the senescence gene p21 in hepatocytes (Figure 6E).
Silencing of miR-34a Decreases the Expression of Fibrosis Genes in Cultured HHSCs and Hepatocytes and Increases the Expression of Senescence Markers in HHSCs
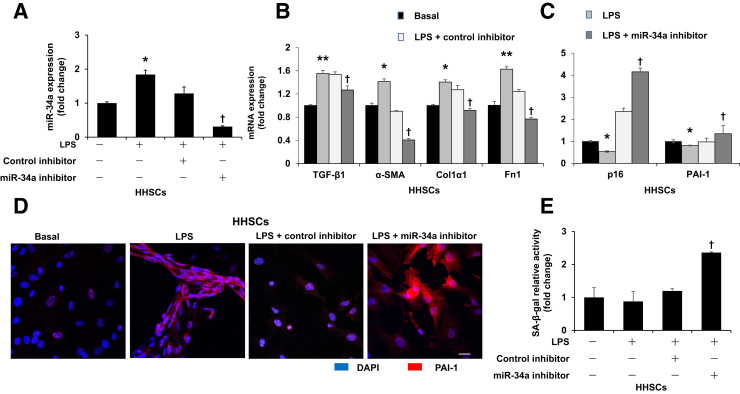
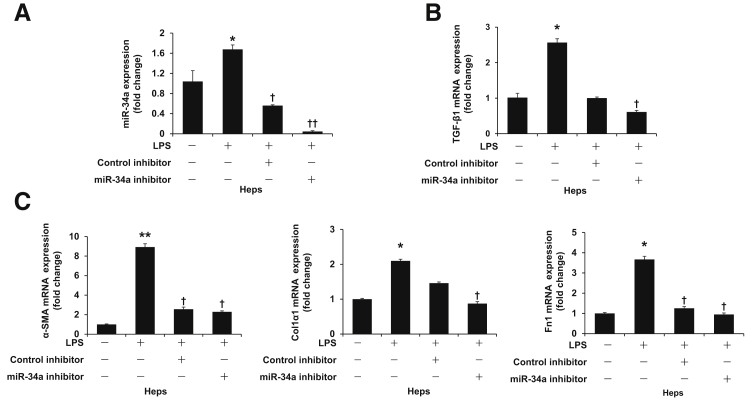
By quantitative PCR, we showed that LPS increased the expression of TGF-β1, α-SMA, Col1α1, and Fn1 in HHSCs compared with the basal group; however, the expression of these genes was decreased after silencing of miR-34a (Figure 7, A and B), which suggests that silencing of miR-34a inhibited activation of HSCs. Similarly, silencing of miR-34a decreased LPS-induced fibrosis gene expression in cultured human hepatocytes (Figure 8). At the same time, LPS decreased mRNA expression of the senescence-related gene p16 in HHSCs compared with the basal group, which was reversed by incubation with miR-34a inhibitor (Figure 7C). Silencing of miR-34a increased mRNA and protein expression of the senescence-related gene PAI-1 in HHSCs treated with LPS (Figure 7, C and D). Moreover, silencing of miR-34a increased SA-β-gal activity in cultured HHSCs stimulated with LPS (Figure 7E).
Figure 7.
Silencing of miR-34a decreases lipopolysaccharide (LPS)-induced fibrotic gene expression and increases senescence gene expression in cultured human HSCs (HHSCs) simulated with LPS. A: Silencing of miR-34a was confirmed by quantitative PCR in HHSCs. B: LPS induced expression of fibrosis genes [transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), collagen type I, α 1 (Col1α1), and fibronectin 1 (Fn1)] in HHSCs, which was blocked by inhibition of miR-34a. C: LPS induced decreased mRNA expression of senescence genes (p16 and PAI-1) in cultured HHSCs, which was reversed after miR-34a inhibitor treatment. D: Representative immunofluorescence picture of plasminogen activator inhibitor-1 (PAI-1) in HHSCs. E: Inhibition of miR-34a increased senescence-associated β-galactosidase (SA-β-gal) activity in HHSCs stimulated by LPS. n = 4 (A–E). *P < 0.05, **P < 0.01 versus basal; †P < 0.05 versus HHSCs stimulated with LPS. Scale bar = 25 μm (D). Original magnification, ×40 (D).
Figure 8.
Silencing of miR-34a decreases lipopolysaccharide (LPS)-induced fibrosis gene expression in cultured human hepatocytes simulated by LPS. A: Silencing of miR-34a was confirmed by quantitative PCR in cultured human hepatocytes. B: LPS increased the expression of transforming growth factor-β1 (TGF-β1) in cultured human hepatocytes, this increase was blocked by inhibition of miR-34a. C: LPS induced increased mRNA expression of fibrosis genes [α-smooth muscle actin (α-SMA), collagen type I, α 1 (Col1α1), and fibronectin 1 (Fn1)] in cultured hepatocytes, which was reversed after miR-34a inhibitor treatment. n = 4 (A and C). *P < 0.05, **P < 0.01 versus basal; †P < 0.05, ††P < 0.01 versus hepatocytes stimulated with LPS. Heps, hepatocytes.
Discussion
Long-term alcohol consumption and abuse results in liver damage, fibrosis/cirrhosis, and, ultimately, liver failure.1 The development of alcoholic liver fibrosis/cirrhosis is associated with the activation/proliferation of HSCs, secretion of proinflammatory cytokines, and increased deposition of ECM proteins.29 Several studies have shown that expression of liver miRNAs is altered in ethanol-fed mice,5, 30 but the functions of these miRNAs in the pathogenesis of ALD, especially in alcoholic liver fibrosis, remains largely unclear. In our study, the findings elucidated that the expression of miR-34a was increased in mice liver during alcoholic liver injury as well as in the livers of heavy drinkers with steatohepatitis compared with healthy controls. After inhibition of miR-34a by Vivo Morpholino treatment, cellular senescence increased in HSCs and decreased in total liver and hepatocytes, resulting in decreased liver fibrosis in ethanol-fed mice. In in vitro experiments, silencing of miR-34a similarly increased the senescence of cultured human HSCs stimulated by LPS. Therefore, our data suggest that the profibrotic effect of miR-34a is related to decreased cellular senescence in HSCs and increased senescence in total liver and hepatocytes during ALD.
Cellular senescence, a stable cell-cycle arrest blocking further proliferation, is involved in multiple pathophysiological processes.17, 31 Ethanol-induced oxidative stress, which is considered a key player leading to cellular senescence because alcohol consumption increases the production of reactive oxygen species and reduces cellular antioxidant levels,32 causes double-strand DNA breaks.33, 34 A previous study showed that hepatocyte senescence, characterized as increased expression of p21 in liver sections of patients with ALD, is linked to the development of fibrosis during alcohol-related liver disease.35 Similarly, in our study, we observed that chronic ethanol feeding increased miR-34a expression, SA-β-gal activity, and senescence gene expression (p16, p21, CCL2, and PAI-1) in total liver as well as p21 expression in hepatocytes, suggesting that upregulation of miR-34a leads to a cellular senescence increase in total liver and hepatocytes and contributes to the progression of liver fibrosis. Interestingly, we observed enhanced cellular senescence in the livers of steatohepatitis patients with heavy alcohol consumption along with upregulation of hepatic miR-34a. The majority of senescence-associated miRNAs are involved in the regulation of p53/p21/pRb or p16/pRb pathways.14 miR-34 is a direct transcriptional target of tumor suppressor p53, upregulation of miR-34a may inhibit SIRT1, thus activating p53, and enhance p53-mediated cell-cycle arrest and apoptosis.36 In our study, we identified that the expression of SIRT1 was decreased in total liver of ethanol-fed mice along with increased expression of miR-34a, which may explain why ethanol-induced expression of miR-34a contributed to the cellular senescence increase in total liver. In support of our findings, other studies showed that enhanced expression of miR-34a contributes to the suppression of hepatocyte proliferation during the late phase of liver regeneration after partial hepatectomy,37 and the prosenescence effect of miR-34a by targeting SIRT1 also has been observed in mesenchymal stem cells and vascular smooth muscle cells.38, 39 Furthermore, activation of miR-34a/SIRT1/p53 signaling has been proved to promote liver fibrosis by inducing apoptosis in hepatocytes.11
During ALD, damaged or senescent hepatocytes can induce activation of HSCs, resulting in liver fibrosis.40 Activation of HSCs is the key step in the development of liver fibrosis.41 In response to alcohol-induced injury, HSCs are activated and α-SMA and Col1a1 are upregulated.42 However, senescence of activated HSCs has been found to limit fibrosis during carbon tetrachloride–induced liver injury.17, 43 In this study, we observed higher expression of miR-34a in alcoholic liver injury and decreased senescence marker (p16) expression in HSCs, although the cellular senescence in total liver and hepatocytes was increased, which contributes to the development of alcohol-induced liver fibrosis. After inhibition of miR-34a, the expression of senescence genes increased in HSCs, but decreased in total liver and hepatocytes from ethanol-fed mice. Along with enhanced HSC senescence resulting from inhibition of miR-34a, cytokines and ECM production was decreased as a result, leading to alleviation of liver fibrosis in ethanol-fed mice. In addition, it is well known that LPS/endotoxin plays an important role in ALD. First, enhanced translocation of Gram-negative, bacteria-derived, endotoxin/LPS itself from the gut into the portal circulation leads to activation of Kupffer cells through the LPS/Toll-like receptor 4 pathways to produce reactive oxygen and nitrogen species, tumor necrosis factor α, and other proinflammatory mediators, providing pivotal noxious effects on other liver cells and particularly on hepatocytes.44 Second, a previous study reported that activation of LPS/Toll-like receptor 4 signaling in HSCs promoted liver fibrogenesis.45 Third, LPS/endotoxin increased activation of NF-κB by high ethanol treatment in rat hepatocytes.46 Fourth, it recently was reported that LPS stimulation in vitro mimics conditions of binge alcohol drinking in vivo.47 Therefore, in view of the vital role of LPS in ALD, we used LPS to stimulate HSCs and hepatocytes in vitro and observed the effects of miR-34a on the expression of fibrosis and senescence markers. We observed that knockdown of miR-34a decreased LPS-induced fibrosis marker expression in human HSCs and hepatocytes and increased senescence gene expression in human HSCs stimulated by LPS, which confirms that inhibition of miR-34a contributes to decreased activation and increased senescence of HSCs. Supporting our work, other studies have reported that miR-34a promotes HSC activation by targeting peroxisome proliferator-activated receptor γ48 or acyl-CoA synthetase long-chain family member 1.49 However, there are few studies related to the effect of miR-34a on senescence of HSCs and our current study provides a better understanding of these effects. Collectively, our findings suggested that miR-34a contributes to the progression of liver fibrosis during ALD. Conversely, there is a study showing that miR-34a can inhibit lung fibrosis by inducing senescence in lung fibroblast.22 Taken together, whether cellular senescence is regulated by miR-34a, inducing either profibrosis or antifibrosis, essentially depends on which cell population is influenced primarily by this cellular process. In future studies, we need to explore the effect of miR-34 further in cellular senescence on specific liver cells in miR-34a knockout mice.
TGF-β1 is an important cytokine expressed after liver injury and is the most important cytokine stimulating fibrogenesis in HSCs.42 In our study, we observed decreased expression of TGF-β1 and downstream molecules Smad2 and Smad3 in ethanol-fed mice with miR-34a Morpholino treatment. Therefore, it may be possible that inhibition of miR-34a decreased hepatocyte damage and senescence, which reduced activation of TGF-β1/Smad2/3 signaling induced by ethanol feeding, resulting in decreased activation of HSCs. Decreased activation of HSCs together with increased senescence of activated HSCs contributes to regression of alcoholic liver fibrosis in ethanol-fed mice with miR-34a Morpholino.
In summary, the present study identifies that miR-34a reduces senescence of HSC and increases cellular senescence in hepatocytes and total liver during alcoholic liver fibrosis, which may present a new therapeutic target for alcohol-induced liver fibrosis. Further detailed studies on the specific genes that may be the targets in HSCs by miR-34a need to be performed to fully understand the mechanism of miR-34a involved in the senescence and liver fibrosis.
Acknowledgments
We thank Dr. Hidekazu Tsukamoto and the tissue sharing program of the animal core of Southern California Research Center for Alcoholic Liver and Pancreatic Disease and Cirrhosis for their assistance with the animal studies in this project.
Footnotes
Supported by Veterans Affairs Merit Award 1I01BX001724 (F.M.), the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White (G.A.), a Veterans Affairs Research Career Scientist Award (G.A.), Veterans Affairs Merit award 5I01BX000574 (G.A.), VA Merit Award 5I01BX002192 (S.G.), NIH grants DK058411, DK076898, DK107310, DK062975 (G.A., F.M., and S.G.), and RO1DK108959 (H.F.), Veterans Affairs Merit Award 1I01BX003031 from the US Department of Veteran's Affairs, Biomedical Laboratory Research and Development Service, and the Southern California Research Center for ALPD and Cirrhosis grant P50AA011999.
Disclosures: None declared.
Contributor Information
Gianfranco Alpini, Email: galpini@medicine.tamhsc.edu.
Fanyin Meng, Email: fmeng@medicine.tamhsc.edu.
References
- 1.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altamirano J., Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H., Yu M., Zhao J., Martin B.N., Roychowdhury S., McMullen M.R., Wang E., Fox P.L., Yamasaki S., Nagy L.E., Li X. IRAKM-Mincle axis links cell death to inflammation: pathophysiological implications for chronic alcoholic liver disease. Hepatology. 2016;64:1978–1993. doi: 10.1002/hep.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K., Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 5.Dolganiuc A., Petrasek J., Kodys K., Catalano D., Mandrekar P., Velayudham A., Szabo G. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H., French B.A., Li J., Tillman B., French S.W. Altered regulation of miR-34a and miR-483-3p in alcoholic hepatitis and DDC fed mice. Exp Mol Pathol. 2015;99:552–557. doi: 10.1016/j.yexmp.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty S.K., Tiwari N., Marudamuthu A.S., Puthusseri B., Bhandary Y.P., Fu J., Levin J., Idell S., Shetty S. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol. 2017;187:1016–1034. doi: 10.1016/j.ajpath.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., Qi Y., Du J.Q., Zhang D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets. 2014;18:1355–1365. doi: 10.1517/14728222.2014.961424. [DOI] [PubMed] [Google Scholar]
- 10.Simone B.A., Ly D., Savage J.E., Hewitt S.M., Dan T.D., Ylaya K., Shankavaram U., Lim M., Jin L., Camphausen K., Mitchell J.B., Simone N.L. microRNA alterations driving acute and late stages of radiation-induced fibrosis in a murine skin model. Int J Radiat Oncol Biol Phys. 2014;90:44–52. doi: 10.1016/j.ijrobp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian X.F., Ji F.J., Zang H.L., Cao H. Activation of the miR-34a/SIRT1/p53 signaling pathway contributes to the progress of liver fibrosis via inducing apoptosis in hepatocytes but not in HSCs. PLoS One. 2016;11:e0158657. doi: 10.1371/journal.pone.0158657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda Y., Nakajima M., Tsuneyama K., Takamiya M., Aoki Y., Fukami T., Yokoi T. Retinoid X receptor alpha in human liver is regulated by miR-34a. Biochem Pharmacol. 2014;90:179–187. doi: 10.1016/j.bcp.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridman A.L., Tainsky M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodier F., Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravinthan A.D., Alexander G.J. Senescence in chronic liver disease: is the future in aging? J Hepatol. 2016;65:825–834. doi: 10.1016/j.jhep.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez T., Li Y.M., Yin S., Xu M.J., Feng D., Zhou Z., Zang M., Mukhopadhyay P., Varga Z.V., Pacher P., Gao B., Wang H. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66:601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries L.W. MicroRNAs as mediators of the ageing process. Genes (Basel) 2014;5:656–670. doi: 10.3390/genes5030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rokavec M., Li H., Jiang L., Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 21.Xu X., Chen W., Miao R., Zhou Y., Wang Z., Zhang L., Wan Y., Dong Y., Qu K., Liu C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui H., Ge J., Xie N., Banerjee S., Zhou Y., Antony V.B., Thannickal V.J., Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–178. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 24.Tsukamoto H., Mkrtchyan H., Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 2008;447:33–48. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- 25.Xiong S., She H., Zhang A.S., Wang J., Mkrtchyan H., Dynnyk A., Gordeuk V.R., French S.W., Enns C.A., Tsukamoto H. Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G512–G521. doi: 10.1152/ajpgi.90327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., Onori P., Marzioni M., Alvaro D., Franchitto A., Gaudio E., Glaser S., Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser S., Meng F., Han Y., Onori P., Chow B.K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J.M., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv H., Liu L., Zhang Y., Song T., Lu J., Chen X. Ingenuity pathways analysis of urine metabonomics phenotypes toxicity of gentamicin in multiple organs. Mol Biosyst. 2010;6:2056–2067. doi: 10.1039/c0mb00064g. [DOI] [PubMed] [Google Scholar]
- 29.Friedman S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 30.McDaniel K., Herrera L., Zhou T., Francis H., Han Y., Levine P., Lin E., Glaser S., Alpini G., Meng F. The functional role of microRNAs in alcoholic liver injury. J Cell Mol Med. 2014;18:197–207. doi: 10.1111/jcmm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M.C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J., Keyes W.M. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Dey A., Cederbaum A.I. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Porath I., Weinberg R.A. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q., Fischer A., Reagan J.D., Yan L.J., Ames B.N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aravinthan A., Pietrosi G., Hoare M., Jupp J., Marshall A., Verrill C., Davies S., Bateman A., Sheron N., Allison M., Alexander G.J. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One. 2013;8:e72904. doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X., He L., Hannon G.J. The guardian's little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–11101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 37.Chen H., Sun Y., Dong R., Yang S., Pan C., Xiang D., Miao M., Jiao B. Mir-34a is upregulated during liver regeneration in rats and is associated with the suppression of hepatocyte proliferation. PLoS One. 2011;6:e20238. doi: 10.1371/journal.pone.0020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badi I., Burba I., Ruggeri C., Zeni F., Bertolotti M., Scopece A., Pompilio G., Raucci A. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J Gerontol A Biol Sci Med Sci. 2015;70:1304–1311. doi: 10.1093/gerona/glu180. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F., Cui J., Liu X., Lv B., Liu X., Xie Z., Yu B. Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res Ther. 2015;6:195. doi: 10.1186/s13287-015-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Gea V., Friedman S.L. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 41.Friedman S.L., Roll F.J., Boyles J., Bissell D.M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X., Han X., Yin L., Xu L., Qi Y., Xu Y., Sun H., Lin Y., Liu K., Peng J. Potent effects of dioscin against liver fibrosis. Sci Rep. 2015;5:9713. doi: 10.1038/srep09713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge X., Leung T.M., Arriazu E., Lu Y., Urtasun R., Christensen B., Fiel M.I., Mochida S., Sorensen E.S., Nieto N. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-alpha and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59:1600–1616. doi: 10.1002/hep.26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seki E., De Minicis S., Osterreicher C.H., Kluwe J., Osawa Y., Brenner D.A., Schwabe R.F. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto H., Sato Y., Azumi J., Kato J., Niitsu Y., Tamaki K. Role of endotoxin in NF-kappaB activation by ethanol in rat hepatocytes. Alcohol Clin Exp Res. 2002;26:6S–10S. doi: 10.1097/01.ALC.0000026827.79925.C9. [DOI] [PubMed] [Google Scholar]
- 47.Bala S., Marcos M., Gattu A., Catalano D., Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Chen Y., Wu S., He J., Lou L., Ye W., Wang J. microRNA-34a and microRNA-34c promote the activation of human hepatic stellate cells by targeting peroxisome proliferator-activated receptor gamma. Mol Med Rep. 2015;11:1017–1024. doi: 10.3892/mmr.2014.2846. [DOI] [PubMed] [Google Scholar]
- 49.Yan G., Li B., Xin X., Xu M., Ji G., Yu H. MicroRNA-34a promotes hepatic stellate cell activation via targeting ACSL1. Med Sci Monit. 2015;21:3008–3015. doi: 10.12659/MSM.894000. [DOI] [PMC free article] [PubMed] [Google Scholar]