Abstract
Although rodent models of traumatic brain injury (TBI) reliably produce cognitive and motor disturbances, behavioral characterization resulting from left and right hemisphere injuries remains unexplored. Here we examined the functional consequences of targeting the left versus right parietal cortex in lateral fluid percussion injury, on Morris water maze (MWM) spatial memory tasks (fixed platform and reversal) and neurological motor deficits (neurological severity score and rotarod). In the MWM fixed platform task, right lateral injury produced a small delay in acquisition rate compared to left. However, injury to either hemisphere resulted in probe trial deficits. In the MWM reversal task, left-right performance deficits were not evident, though left lateral injury produced mild acquisition and probe trial deficits compared to sham controls. Additionally, left and right injury produced similar neurological motor task deficits, impaired righting times, and lesion volumes. Injury to either hemisphere also produced robust ipsilateral, and modest contralateral, morphological changes in reactive microglia and astrocytes. In conclusion, left and right lateral TBI impaired MWM performance, with mild fixed platform acquisition rate differences, despite similar motor deficits, histological damage, and glial cell reactivity. Thus, while both left and right lateral TBI produce cognitive deficits, laterality in mouse MWM learning and memory merits consideration in the investigation of TBI-induced cognitive consequences.
Keywords: Traumatic brain injury, Lateral fluid percussion injury, Spatial memory, Reversal learning, Behavioral flexibility, Functional asymmetry
1. Introduction
The fluid percussion injury model of traumatic brain injury (TBI) in laboratory animals elicits functional and pathophysiological hallmarks of human TBI, including cognitive dysfunction, intracranial hemorrhage, edema, and progressive gray matter damage [1]. Originally developed as a midline injury in cats [2] and rabbits [3], a lateral fluid percussion injury was used in rats [4], and further adapted for mice [5], now widely used given the utility of transgenic lines. Lateral fluid percussion injury produces a combined focal cortical contusion and diffuse subcortical neuronal injury in rats [6], the extent and location of which is subject to small changes in craniotomy position [7]. It is noteworthy that small alterations in craniotomy position in rats lead to differences in cognitive performance [8]. In mice, left [9,10] and right [5,11] craniotomy placement generally differs across research groups; however, there are no systematic studies investigating craniotomy position on measures of learning and memory or motor effects in mice.
Several lines of evidence suggest existence of left-right hemisphere molecular [12,13], morphological [12,14], and functional differences [15,16] at hippocampal synapses in mice. Left-right morphological differences exist in the mouse hippocampus where left originating CA3 inputs innervate small spines, whereas inputs originating from the right CA3 innervate larger, mushroom-shaped spines [12,14]. The molecular composition of these smaller spines also differ, exhibiting a higher density of GluN2B subunits in post-synaptic spines receiving left CA3 input [12,17]. Morphological differences; size of left infrapyramidal mossy fiber projections, also positively correlate with precision in swimming navigation [18]. Furthermore, long-term potentiation (LTP) reveals hemispheric specialization in which LTP induction of CA3 synaptic inputs to CA1 when input originates from the left, but not the right CA3, using spike timing-dependent LTP [15], or conventional high-frequency stimulation-induced LTP [16]. Similarly, in behaving mice, silencing of left, but not right, CA3 pyramidal neurons resulted in Y-maze task deficits [16], and inversus viscerum mice (bred to express only a right phenotype at CA3-CA1 synapses) exhibited dry maze task deficits [19]. This evidence of structural and behavioral hemispheric differences raises the question of whether left versus right hemisphere TBI will reveal differing patterns of cognitive deficits.
The Morris water maze (MWM) is frequently used to assess TBI-induced spatial learning and memory impairments. Although hippocampal lesions [20] or TBI [10] severely impairs MWM performance, mice with bilateral hippocampal lesions demonstrate improved performance with multiple training trials [20]. Accordingly, MWM performance may employ brain areas in addition to the hippocampus, such as the striatum [21], basal forebrain [22], insular cortex [23], etc.; making this task a useful model to study the functional consequences of a lateralized brain injury in mice. MWM task variations are also used to infer the underlying processes affecting performance. Here, the Fixed Platform task assesses reference memory acquisition, and the reversal task; cognitive flexibility. Moreover, the Cued task, where the location of the hidden platform is made visible, infers sensorimotor and motivational influences. Based on the established left-right molecular and morphological asymmetry in the mouse hippocampus, we examine whether unilateral TBI of the left versus right hemisphere will elicit differential patterns of spatial memory and motor deficits in mice.
2. Materials and methods
2.1. Mice
All experiments used adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine), left injury (n = 10), left sham (n = 10), right injury (n = 8), right sham (n = 7), (further described in the supplement), and complied with EC Directive 86/609/EEC, conducted in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
2.2. Craniotomy and induction of lateral fluid percussion injury (FPI)
Under isoflurane anesthesia (2.5%, 250 mL/min) a sagittal scalp incision was made and a 2.7 mm trephine craniectomy performed over the left or right parietotemporal cortex, further description of which is included in the supplement. After a 2 h recovery period, mice were anesthetized with isoflurane (4%, 400 mL/min) and immediately subjected to a moderate lateral fluid percussion injury (1.94 ± 0.1 atm left lateral, 1.92 ± 0.1 atm right lateral injury), described in the supplement.
2.3. Behavioral and cognitive assessment paradigms
2.3.1. Neurological motor impairment evaluation
Mice were evaluated in Rotarod and Neurological Severity Score, 2 days prior to injury, and 1, 2, 3, 7, 14 and 21 days post-injury (see Fig. 1). Single Rotarod (IITC Life Science Rota-Rod, Woodland Hills, CA, with 3 cm diameter rotating drums) trials per day used an accelerating protocol, a description of which appears in supplementary information. A 10-point Neurological Severity Score (NSS) assessed the functional neurological status of mice based on the presence of reflexes and the ability to perform motor and behavioral tasks (see Supplementary Table 1). Further description appears in supplementary information.
Fig. 1. Experimental timeline.
Experimental timeline (relative to injury) of neurological motor battery (indicated by down arrows) and spatial learning and memory tasks of the MWM, for left lateral and right lateral fluid percussion injuries.
2.3.2. Learning and memory assessment
The MWM consisted of a circular, galvanized steel tank (1.8 m in diameter, 0.6 m height) filled with opaque water (maintained at 20 °C ± 2 °C) with a submerged platform (10 cm diameter) and distal and proximal visual cues, further described in the supplement. A description of the Fixed Platform, Reversal, and Cued Tasks also appears in supplementary information (see Fig. 1).
2.4. Histology and lesion volume quantification
Animals were anesthetized, transcardially perfused with paraformaldehyde and brains extracted. Brains were sectioned (50 μm thickness) on a Leica VT1000S vibratome, Nissl stained using cresyl violet acetate, and digitally imaged. Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscope Facility. Further detail and the lesion volume quantification description appears in supplementary information.
2.5. Qualitative assessment of glial cell response to injury
Immunohistochemistry (IHC) was performed in left and right injured and sham animals (n = 3/group) surviving for 3 days post-injury. Mice were perfused as described for histology and 40 μm coronal brain sections cut by vibratome were processed for IHC [24] using antibodies for IBA1 (Wako, Abcam; 1:300), and GFAP (Dako, Encor Biotechnology; 1:20,000). Images were collected on a Zeiss 700 confocal microscope for qualitative assessment of each protein.
2.6. Statistical analysis
Differences were considered significant when P < 0.05, and analyses were conducted using IBM SPSS Statistics 22 (IBM Software, New York, NY). To assess TBI-induced changes to MWM performance, mean latency-to-goal (seconds) in left and right injury was compared to left or right sham, respectively. To compare the effects of right vs. left injury, mixed-factor ANOVA analyses were conducted on MWM latencies normalized as percent-of-sham-control (using the corresponding hemisphere right and left sham group: injured animal score/mean sham score × 100%). This normalization approach increases sensitivity to detect the effects of TBI and reduces the influence of minor sham control differences [25]. Normalized data were also analyzed using a one sample t-test to compare left or right to sham performance (value of 100). Motor differences and MWM acquisition were analyzed using a mixed factor ANOVA, probe trial measures by independent group t-test, and righting times and lesion volume by 2-way ANOVA. Post hoc tests for MWM acquisition (Fixed Platform and Reversal tasks) were conducted using a Bonferroni adjustment, and for NSS and Rotarod used a Tukey HSD adjustment.
3. Results
3.1. Left or right hemisphere injury produces neurological motor deficits
NSS Measure
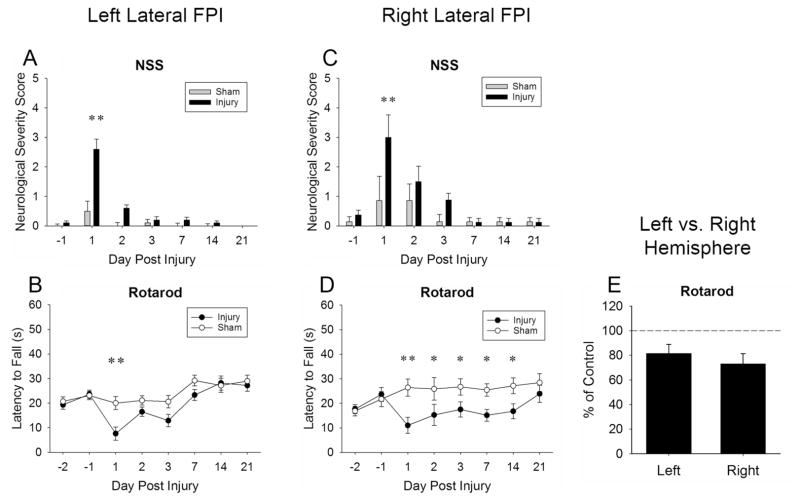
NSS scores increased for left and right lateral injury on post-injury day one. A significant interaction for both left (Fig. 2A), F(6, 108) = 11.93, P < 0.001; and right lateral (Fig. 2C) NSS, F(6, 78) = 2.39, P < 0.05, revealed an increased NSS in both left and right lateral injured animals on post-injury day 1 (P < 0.01) compared to day −1, not present in both left and right lateral sham animals.
Fig. 2. Left and right hemisphere injury produced neurological motor deficits.
NSS scores increased for left (Panel A) and right (Panel C) lateral injury on post-injury day one. Injury also produced impaired rotarod performance in both left (Panel B) and right (Panel D) lateral hemispheres, with no significant difference in performance between hemispheres (Panel E, represents main effect of injury marginal means). Values represent means ± SEM; Panels A and C **P < 0.01 vs. injury day −1. Panels B and D *P < 0.05, **P < 0.01 vs. sham.
Rotarod measure
Both left and right lateral Injury produced impaired rotarod performance, with no left-right performance differences. A significant interaction for both left lateral (Fig. 2B), F(7, 126) = 2.47, P < 0.05; and right lateral (Fig. 2D) latency to fall, F(7, 91) = 2.54, P < 0.05, revealed a significant decrease in latency to fall compared to sham in left lateral injury on post-injury day 1 (P < 0.01), and in right lateral injured animals on post-injury days 1 (P < 0.01), 2 (P < 0.05), 3 (P < 0.05), day 7 (P < 0.05), and 14 (P < 0.05). Direct comparison of left and right cohorts on percent of sham control revealed no significant interaction (Fig. 2E) P = 0.10, and no main effect of hemisphere P = 0.47 in latency to fall.
3.2. Left or right hemisphere injury produces memory deficits in the MWM probe trial, with mild left-right hemisphere differences in fixed platform acquisition rates
Fixed Platform Task
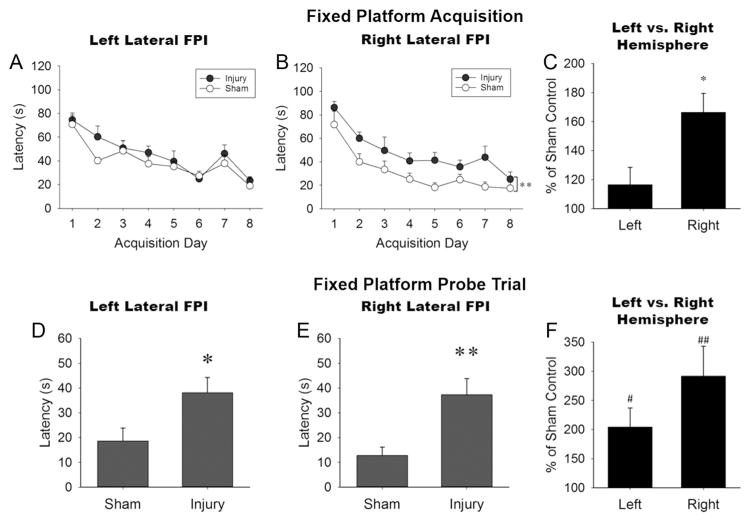
The right lateral injury group showed a small but significant delay in the acquisition of reference memory compared to sham, and worse performance than left injured animals. Injury to either hemisphere elicited impairment in the expression of reference memory. The acquisition of reference memory showed no significant interaction for left lateral (Fig. 3A) P = 0.75; or right lateral (Fig. 3B) P = 0.88 injuries. A significant main effect of injury was found for the right lateral cohort, F(1, 13) = 10.05, P < 0.01, but not left (P = 0.15). Right lateral injured animals demonstrated longer latencies to the platform than sham animals. A comparison of normalized left-right acquisition performance revealed a significant main effect of hemisphere, F(1, 16) = 7.87, P < 0.05, indicating greater acquisition deficits in the right lateral cohort compared to the left (Fig. 3C), but the Injury X Day interaction failed to achieve statistical significance (P = 0.07). The fixed platform probe trial revealed significantly longer latencies to the platform location in injured mice compared to sham animals for both left (Fig. 3D), t(18) = 2.41, P < 0.05, and right lateral injuries (Fig. 3E), t(13) = 3.17, P < 0.01. Analyses of normalized probe trial measurements confirmed injury-induced increases in mean latency for both left, t(9) = 3.16, P < 0.05, and right, t(7) = 3.74, P < 0.01, cohorts relative to sham levels (one sample t-test). The normalized latencies did not reveal differences between hemispheres (P = 0.16, independent samples t-test). The number of platform crossings, percentage of time spent in the target quadrant, and representative swim path traces during the probe trial are shown in Supplementary Figure 1.
Fig. 3. Both left and right lateral injury produced MWM Fixed Platform task deficits.
During Fixed Platform acquisition (Panels A, B, C) only the right lateral injury (Panel B) showed a small but significant delay in the acquisition of reference memory compared to sham; and compared to left injured animals (Panel C, represents main effect of injury marginal means). During the Fixed Platform Probe trial (Panels D, E, F), both left and right lateral injured mice demonstrated latency impairments relative to sham (Panels D, E, F), but with no left-right differences (Panel F). Values represent mean ± SEM; *P < 0.05, **P < 0.01 vs. sham; #P < 0.05, ##P < 0.01 vs. 100 (sham performance).
Reversal Task
No performance difference was found between the hemipheres in reversal task learning; however, left lateral injury elicited modest impairments relative to sham in reversal acquisition and expression. There was no significant interaction for either left lateral (Fig. 4A), P = 0.92; or right lateral (Fig. 4B), P = 0.39; injury in reversal acquisition learning. However, a main effect of injury was found for the left lateral injury cohort, F(1, 18) = 7.93, P < 0.05, but not the right lateral injury cohort, F(1, 13) = 1.89, P = 0.19. Specifically, left injured animals demonstrated longer latencies to the platform than sham animals. Direct comparison of left and right injury revealed no significant interaction (Fig. 4C) P = 0.39, and no significant main effect of hemisphere P < 0.28 on percent of sham control. The reversal probe trial revealed significantly longer latencies to the new platform location in left lateral injured mice compared to sham animals (Fig. 4D), t(18) = 2.75, P < 0.05, but not right lateral injured mice (Fig. 4E), P = 0.37. Direct comparison of left vs. right injury using the normalized reversal probe trial latencies, showed no performance differences (P = 0.17, independent samples t-test), though the left, t(9) = 2.90, P < 0.05, but not right (P = 0.22), injury group differed from sham levels (one sample t-test) (Fig. 4F). The number of platform crossings, percentage of time spent in the target quadrant, and representative swim path traces during the probe trial are shown in Supplementary Figure 2.
Fig. 4. The MWM Reversal Task did not reveal any left-right differential vulnerability to cognitive flexibility.
Both reversal task acquisition (Panels A, B, C) and reversal probe trial (Panels D, E, F) produced no left-right performance differences in reversal task learning (Panels C [main effect of injury marginal means] and F). However left lateral injury demonstrated modest latency impairments relative to sham in both reversal acquisition (Panel A), and reversal probe trial (Panel D, F). Values represent mean ± SEM; *P < 0.05 vs. sham; #P < 0.05 vs. 100 (sham performance).
Cued Task and Swim Speed
The injury-induced MWM performance impairments were likely not due to sensory-motor or motivational impairments measured injury to either hemisphere did not affect performance in the Cued Task (Supplementary Figure 3), or swim speed (Supplementary Figures 4 and 5).
3.3. Injury severity and histological outcome in left and right hemisphere injury groups
The injury procedure produced a mortality rate of 20.8% for the left lateral cohort, and 22.7% for the right lateral cohort, which resulted in their exclusion from all other measures.
Righting Time
Neither the interaction between hemisphere and injury (Fig. 5A, D), P = 0.84, nor the main effect of hemisphere, P = 0.79, achieved significance; however, a main effect of injury, F(1, 31) = 44.58, P < 0.001, showed longer righting times in injured animals than sham controls.
Fig. 5. Left and right hemisphere injury produced impaired righting time and histological outcome.
Both left and right lateral injury produced significantly greater righting times (Panels A, D) and lesion volume %’s (Panels B, E) compared to sham animals. No left-right differences were found in righting time or lesion volume %. Representative coronal sections of left (Panel C) and right (Panel F) lateral injury-induced lesion are shown. Values represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham.
Lesion Volume Percentage
Neither the injury by hemisphere interaction (Fig. 5B, E), P = 0.53, nor main effect of hemisphere, P = 0.86, achieved significance; however, there was a main effect of injury, F(1, 12) = 23.08, P < 0.001, in which injured mice showed significant lesion volumes relative to shams.
3.4. Hippocampal glial cell response to left and right hemisphere injury
Hippocampal IHC of microglial (IBA1) and astrocytes (GFAP) showed robust glial response to TBI. IBA1 imaging in the dentate gyrus revealed prominent shift from ramified microglial phenotype in sham brains (Fig. 6A), to a reactive state with larger cell bodies and multiple lobular processes, a phenotype seen ipsilateral to both left and right hemisphere injury. Similarly, GFAP imaging revealed a reactive astrocyte response ipsilateral to both left and right hemisphere injury compared to sham (Fig. 6B), with extensive hypertrophy of cellular processes. Contralateral left and right injured hippocampus also showed both microglial (Fig. 6A) and astrocyte (Fig. 6B) reactivity, but at a much reduced level.
Fig. 6. Hippocampal glial cell response to left and right hemisphere injury.
Microglial (IBA1 +; green) morphology (Panel A) showed non-reactive, ramified state within the dentate gyrus of sham controls (see insets for cell detail). A robust reactive phenotype was observed in the dentate gyrus ipsilateral to both left and right hemisphere injury, where microglia exhibited enlarged, rounded cell bodies and lobular processes (arrows; inset). Astrocyte (GFAP +; red) morphology (Panel B) showed thin process bearing stellate cells within the dentate gyrus of sham controls (see insets for cell details). Reactive hypertrophic phenotype, with enlarged cell bodies and increased, thick cell processes (arrows; inset) was seen in the dentate gyrus ipsilateral to both left and right hemisphere injury. Cell nuclei co-stained with DAPI. IPSI = ipsilateral to injury; CON = contralateral to injury; H = hilus; GCL = granule cell layer; ML = molecular layer; Bar = 30 μm large images, 20 μm insets.
4. Discussion
The present study demonstrated that left or right hemisphere TBI produces cognitive deficits in the MWM paradigm. Injury to either hemisphere impaired the expression of reference memory, as assessed in the probe trial, while right, but not left, lateral injury delayed acquisition in the fixed platform. In contrast, cognitive flexibility deficits measured by reversal acquisition and the reversal probe trial did not differ between left and right hemisphere, though modest left hemisphere impairments were seen relative to sham.
The left and right lateral injury deficits seen in the Fixed Platform probe trial are consistent with existing left [27] and right [30] lateral injury reference memory expression deficits. These results support findings that both hippocampal hemispheres contribute to spatial learning [30]. MWM Fixed Platform acquisition deficits [5,11] have been shown in right hemisphere injury, though contrary to the present findings, others have noted acquisition deficits also from left lateral injuries [9,10]. Comparison of left-right hemisphere injury across studies is difficult due to methodological differences, such as post-injury training timing, injury magnitude and model, and varying numbers of training days or trials per day. Accordingly, one unique contribution of the present work was the identical experimental conditions under which left and right hemisphere injury was studied.
Albeit that normalization of the reversal dependent measures did not reveal significant differences between left and right hemisphere injury, the observation that left lateral injury produced modest MWM performance impairments relative to sham control mice raises the possibility of potential hemisphere differences. For example, increasing injury magnitude may reveal whether either hemisphere is differentially more vulnerable to disrupted cognitive flexibility. Although few rodent TBI studies have employed the MWM reversal task, a left lateral controlled cortical impact injury study reported deficits in a similar task in mice [31]. Furthermore, superior MWM reversal task accuracy correlated with larger left mossy fiber projections [32], also true in Collins High-lateralized mice known to exhibit larger left mossy fiber projections [18]. As such, the MWM reversal tasks ability to assess changes in cognitive flexibility may yet prove useful in the study of TBI.
Although the present study did not address underlying molecular mechanisms of left and right hemisphere TBI behavioral deficits, the observations reported are consistent with the El-Gaby et al. (2014) theoretical model, which accounts for differential use of distinct left-right hippocampal synapse populations during learning and memory in mice [33]. Specifically, pre-configured CA3-CA1 synapses attributed to stable cell assemblies of the right hippocampus function to facilitate rapid incorporation of spatial information [34]. Left hippocampal CA3-CA1 synapses; however, recruit new place cells to instruct the formation of de novo cell assemblies [35] facilitating representation of salient new locations [36]. Consequently, the fixed platform acquisition impairments of right injured mice may reflect a heavier reliance on plastic but slower left CA3-CA1 hippocampal synapses. Whereas left lateral injury reversal task impairments could be explained by heavier reliance on right CA3-CA1 hippocampal assemblies still pre-assigned to the previous Fixed Platform location. An adaptive advantage of such anatomical dissociation could be an efficient division of labor between left and right hemisphere hippocampi.
Neurological motor behavior was also impaired by both left and right lateral injury, though neither the NSS nor Rotarod performance revealed differential left-right hemisphere motor vulnerability to TBI. Despite acute post-injury motor impairments, assessments of sensorimotor and motivational performance in the MWM cued task and MWM swim speeds revealed no impairment for either left or right lateral injuries. Accordingly, left-right hemisphere injuries, which produced cognitive impairments, did not impact MWM sensorimotor performance or impair learning of MWM procedural components.
Left and right hemisphere injury elicited equivalent injury severity as measured by post-injury righting time. In addition, neurodegeneration in response to injury measured by cortical lesion volume did not differ between left and right hemisphere injury. Qualitative assessment of the glial response to left and right hemisphere injury demonstrated robust activation of hippocampal microglia and astrocytes ipsilateral to the injury, reflecting a significant level of traumatic insult. Contralateral hippocampal structures from the injury site also elicited modest glial reactivity, consistent with previous reports [37]. These data indicate that left and right hemisphere injury generate a similar cellular response to trauma pathology, and suggest that variance in left-right behavioral outcome is not correlated with overt differences in glial reactivity.
5. Conclusions
Left and right lateral TBI both produced MWM performance deficits in mice, with modest left-right differences. The investigation of left versus right lateral injuries contributes to the understanding of the mouse TBI model. Consequently, laterality in mouse MWM learning and memory impairments may be worthy of consideration for future study design and interpretation in the investigation of TBI-induced cognitive consequences.
Supplementary Material
HIGHLIGHTS.
Left or right lateral TBI impaired MWM memory performance.
Right lateral TBI produced modest MWM acquisition deficits compared to left.
Both Left and right TBI produced similar neurological motor task deficits.
Both left and right TBI produced impaired righting times and lesion volumes.
Both left and right lateral TBI produced robust ipsilateral glial cell reactivity.
Acknowledgments
Funding
National Institutes of Health [T32DA007027, 1F31NS095628-01A1 (LDS), DA032933 (AHL)]. Surgical, histological, and IHC evaluation was supported by National Institutes of Health [NS057758 (TMR) and NS056247 (LLP)]. Microscopy was funded in part by NIH-NIND Center Core (5 P30 NS047463), and by the NIH-NCI Cancer Center Support (P30 CA016059). This research was all supported by VCU School of Pharmacy start-up funds.
We would like to express our gratitude to Ms. Krista Mitchnik, whose thought provoking comments were a source of inspiration, and Mr. Dolan Edinboro who assisted with the histological staining.
Abbreviations
- FPI
fluid percussion injury
- IHC
immunohistochemistry
- LTP
long term potentiation
- MWM
Morris water maze
- NSS
neurological severity score
- TBI
traumatic brain injury
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2017.05.032.
Footnotes
Conflict of interest
No competing financial interests exist.
Contributors disclosure
All authors approved the final article. Conception and study design; LDS, AHL, TMR, LLP, data acquisition; LDS, TLS, AJM, NNL, data analysis/interpretation; LDS, AHL, TMR, LLP, manuscript composition; LDS, AHL, TMR, LLP.
References
- 1.Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- 2.Hayes RL, Stalhammar D, Povlishock JT, Allen AM, Galinat BJ, Becker DP, Stonnington DH. A new model of condussive brain injury in the cat produced by extradural fluid volume loading: II. Physiological and neuorpathological observations. Brain Inj. 1987;1:93–112. doi: 10.3109/02699058709034449. [DOI] [PubMed] [Google Scholar]
- 3.Hartl R, Medary M, Ruge M, Arfors KE, Ghajar J. Blood brain barrier breakdown occurs early after traumatic brain injury and is not related to white blood cell adherence. Acta Neurochir Suppl. 1997;70:240–242. doi: 10.1007/978-3-7091-6837-0_74. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, LFA Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 5.Carbonell WS, Maris DO, McCall T, Grady MS. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma. 1998;15:217–229. doi: 10.1089/neu.1998.15.217. http://dx.doi.org/10.1089/neu.1998.15.217. [DOI] [PubMed] [Google Scholar]
- 6.Hicks R, Soares H, Smith D, McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. http://dx.doi.org/10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- 7.Vink R, Mullins PG, Temple MD, Bao W, Faden AI. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J Neurotrauma. 2001;18:839–847. doi: 10.1089/089771501316919201. http://dx.doi.org/10.1089/089771501316919201. [DOI] [PubMed] [Google Scholar]
- 8.Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19:303–316. doi: 10.1089/089771502753594873. http://dx.doi.org/10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- 9.Tchantchou F, Zhang Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma. 2013;30:565–579. doi: 10.1089/neu.2012.2647. http://dx.doi.org/10.1089/neu.2012.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. http://dx.doi.org/10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- 11.Spain A, Daumas S, Lifshitz J, Rhodes J, Andrews PJD, Horsburgh K, Fowler JH. Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J Neurotrauma. 2010;27:1429–1438. doi: 10.1089/neu.2010.1288. http://dx.doi.org/10.1089/neu.2010.1288. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA recetpro epsilon 2 subunits in hippocampal circuitry. Science. 2003;80(300):900–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara Y, Hirase H. Size and receptor density of glutamatergic synapses: a viewpoint from left-right asymmetry of CA3-CA1 connections. Front Neuroanat. 2009;3:10. doi: 10.3389/neuro.05.010.2009. http://dx.doi.org/10.3389/neuro.05.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:19498–19503. doi: 10.1073/pnas.0807461105. http://dx.doi.org/10.1073/pnas.0807461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohl MM, Shipton OA, Deacon RM, Rawlins JNP, Deisseroth K, Paulsen O. Hemisphere-specific optogenetic stimulation reveals left-right asymmetry of hippocampal plasticity. Nat Neurosci. 2011;14:1413–1415. doi: 10.1038/nn.2915. http://dx.doi.org/10.1038/nn1211-1617e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shipton O, El-Gaby M, Apergis-Schoute J, Karl D, Bannerman DM, Paulsen O, Kohl MM. Left-right dissociation of hippocampal memory processes in mice. Proc Natl Acad Sci U S A. 2014;111:15238–15243. doi: 10.1073/pnas.1405648111. http://dx.doi.org/10.1073/pnas.1405648111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Kawakami R, Shinohara Y, Fukaya M, Sakimura K, Mishina M, Watanabe M, Ito I, Shigemoto R. Target-cell-specific left-right asymmetry of NMDA receptor content in schaffer collateral synapses in epsilon1/NR2A knock-out mice. J Neurosci. 2005;25:9213–9226. doi: 10.1523/JNEUROSCI.2134-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.2134-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernasconi-Guastalla S, Wolfer DP, Lipp HP. Hippocampal mossy fibers and swimming navigation in mice: correlations with size and left-right asymmetries. Hippocampus. 1994;4:53–63. doi: 10.1002/hipo.450040107. http://dx.doi.org/10.1002/hipo.450040107. [DOI] [PubMed] [Google Scholar]
- 19.Goto K, Kurashima R, Gokan H, Inoue N, Ito I, Watanabe S. Left-right asymmetry defect in the hippocampal circuitry impairs spatial learning and working memory in IV mice. PLoS ONE. 2010;5:1–7. doi: 10.1371/journal.pone.0015468. http://dx.doi.org/10.1371/journal.pone.0015468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlai RT, McNamara A, Williams S, Phillips HS. Hippocampal dysfunction and behavioral deficit in the water maze in mice: an unresolved issue? Brain Res Bull. 2002;57:3–9. doi: 10.1016/s0361-9230(01)00630-x. http://dx.doi.org/10.1016/S0361-9230(01)00630-X. [DOI] [PubMed] [Google Scholar]
- 21.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 22.Waite JJ, Chen AD, Wardlow ML, Thal LJ. Behavioral, biochemical consequences of combined lesions of the medial septum/diagonal band, nucleus basalis in the rat when ibotenic acid, quisqualic acid, and AMPA are used. Exp Neurol. 1994;130:214–229. doi: 10.1006/exnr.1994.1200. [DOI] [PubMed] [Google Scholar]
- 23.Gutiérrez H, Hernández-Echeagaray E, Ramírez-Amaya V, Bermúdez-Rattoni F. Blockade of N-methyl-D-aspartate receptors in the insular cortex disrupts taste aversion and spatial memory formation. Neuroscience. 1999;89:751–758. doi: 10.1016/s0306-4522(98)00360-1. http://dx.doi.org/10.1016/S0306-4522(98)00360-1. [DOI] [PubMed] [Google Scholar]
- 24.Reeves TM, Trimmer PA, Colley BS, Phillips LL. Targeting Kv1. 3 channels to reduce white matter pathology after traumatic brain injury. Exp Neurol. 2016;283:188–203. doi: 10.1016/j.expneurol.2016.06.011. http://dx.doi.org/10.1016/j.expneurol.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townend J. Practical Statistics for Environmental and Biological Scientists. John Wiley & Sons Ltd; Chichester, West Sussex, England: 2013. [Google Scholar]
- 27.Chen Y, Shohami E, Constantini S, Weinstock M. Rivastigmine, a brain-selective acetylcholinesterase inhibitor, ameliorates cognitive and motor deficits induced by closed-head injury in the mouse. J Neurotrauma. 1998;15:231–237. doi: 10.1089/neu.1998.15.231. http://dx.doi.org/10.1089/neu.1998.15.231. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara Y, Hosoya A, Yamasaki N, Ahmed H, Hattori S, Eguchi M, Yamaguchi S, Miyakawa T, Hirase H, Shigemoto R. Right-hemispheric dominance of spatial memory in split-brain mice. Hippocampus. 2012;22:117–121. doi: 10.1002/hipo.20886. http://dx.doi.org/10.1002/hipo.20886. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Loane DJ, Murray MG, Stoica Ba, Faden AI. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma. 2012;29:2475–2489. doi: 10.1089/neu.2012.2511. http://dx.doi.org/10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schöpke R, Wolfer DP, Lipp HP, Leisinger-Trigona MC. Swimming navigation and structural variations of the infrapyramidal mossy fibers in the hippocampus of the mouse. Hippocampus. 1991;1:315–328. doi: 10.1002/hipo.450010322. http://dx.doi.org/10.1002/hipo.450010322. [DOI] [PubMed] [Google Scholar]
- 33.El-Gaby M, Shipton OA, Paulsen O. Synaptic plasticity and memory: new insights from hippocampal left-right asymmetries. Neuroscietist. 2014;21:490–502. doi: 10.1177/1073858414550658. http://dx.doi.org/10.1177/1073858414550658. [DOI] [PubMed] [Google Scholar]
- 34.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. http://dx.doi.org/10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 35.Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. 21/5/1635 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leutgeb JK, Leutgeb S, Moser M, Moser EI, Moser I. Pattern gyms in the dentate separation and CA3 of the hippocampus. Science. 2007;80(315):961–966. doi: 10.1126/science.1135801. http://dx.doi.org/10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 37.Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, Brody DL. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2012;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. http://dx.doi.org/10.1097/NEN.0b013e31821f891f.Repetitive. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.