Abstract
Rationale
Low physical activity is highly prevalent among COPD patients and is associated with increased healthcare utilization and mortality and reduced HRQL. The addition of a website to pedometer use is effective at increasing physical activity; however, the timeline of change and impact of environmental factors on efficacy is unknown.
Methods
U.S. Veterans with COPD were randomized (1:1) to receive either (1) a pedometer and website which provided goal-setting, feedback, disease-specific education, and an online community forum or (2) pedometer alone for 3 months. Primary outcome was change in daily step count. Secondary outcomes included 6MWT distance, HRQL, dyspnea, depression, COPD knowledge, exercise self-efficacy, social support, motivation, and confidence to exercise. Generalized linear mixed-effects models evaluated the effect of the pedometer plus website compared to pedometer alone.
Results
Data from 109 subjects (98.5% male, mean age 68.6±8.3 years) were analyzed. At 13 weeks, subjects in the pedometer plus website group had significant increases daily step count from baseline relative to the pedometer alone group (804±356.5 steps per day, p=0.02). The pedometer plus website group had significant improvements in daily step count from baseline beginning in week 3 which were sustained until week 13. In subgroup analyses, the pedometer plus website attenuated declines in daily step count during the transition from summer to fall. No significant differences in secondary outcomes were noted between groups.
Conclusions
A website added to pedometer use improves daily step counts, sustains walking over 3 months, and attenuates declines in physical activity due to season.
MeSH KeyWords: COPD, Physical Activity, Rehabilitation, Randomized Trial, Season
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States [1] and a leading cause of morbidity and mortality worldwide [2]. Low levels of physical activity (PA) in COPD patients are often present early in the disease course [3] and are associated with poor outcomes, including worse health-related quality of life (HRQL) [4], increased risk of acute exacerbations and higher rates of healthcare utilization [5–9], and increased mortality [10–12], independent of lung function. Despite the strong evidence linking low PA with greater morbidity and mortality, effective interventions to promote PA in individuals with COPD remain limited.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines endorse pulmonary rehabilitation as an integral, non-pharmacological component in disease management [13]. However, while supervised pulmonary rehabilitation programs improve exercise capacity and HRQL in persons with COPD, the impact of pulmonary rehabilitation on daily PA is unclear [14, 15]. Data support that a range of types and intensities of PA beyond aerobic exercise, which is emphasized in pulmonary rehabilitation, may be beneficial in COPD [16]. Increased low-intensity PA was significantly associated with a decreased risk of COPD-related hospitalizations, whereas increased high-intensity PA demonstrated no benefit [17].
Intervention programs which combine the use of wearable monitors (i.e. pedometers, accelerometers) with goal-setting can increase daily PA in COPD patients [15]. Many types of goal-setting, including in-person counseling, daily diary use [18, 19], and messaging through smartphone applications[20, 21], have demonstrated short-term efficacy in increasing daily PA among COPD patients. In our previous Taking Healthy Steps (THS) study[22], goal-setting was mediated through the use of a pedometer (Omron HJ-720 ITC, Omron Healthcare, Inc., Bannockburn, IL) plus a website based on the Theory of Self-Regulation which provided individualized step-count goals, iterative step-count feedback, education on disease self-management and motivation, and an online community of social support. Subjects in the intervention group demonstrated increased daily step count and improved HRQL at four months[23].
While many wearable monitor-based PA coaching interventions have demonstrated short-term efficacy in both increasing PA and improving secondary outcomes such as exercise capacity and HRQL, detailed investigations into the physiological, psychosocial, and environmental factors which may influence efficacy remain scarce. In most studies, objective measurements of PA are taken at only 2 time points (baseline and end-of-study) through the use of 7–14 day sampling periods[18–21], precluding the ability to assess exactly when increases in PA occur after initiation of an intervention. Although PA data from intermediate time points from subjects in the intervention arm are available in a subset of studies[19], continuous daily PA monitoring in control subjects is not routinely collected or reported. Lastly, there is emerging evidence linking environmental factors such as temperature and weather to daily PA[24–26]; to date the impact of temperature patterns on PA interventions has not been reported.
In the current study, Every Step Counts (ESC), we extend the use of a pedometer plus the website first described in the THS study in an independent and well-characterized cohort. In the THS study, COPD subjects were identified through ICD-9 codes (without spirometric validation), enrollment was automated, and the primary outcomes (change in daily step count and HRQL) were assessed online[22]. In ESC, daily objective PA monitoring in both groups, as well as in-person assessments of physiological and psychosocial variables, were obtained, providing a unique opportunity to examine (1) the effect of combining the pedometer plus website on daily step count as well as a comprehensive set of secondary physiological and psychosocial variables, (2) the timeline of change in daily step count following initiation of the intervention, (3) the effect of season on daily PA and the response to the intervention, and (4) detailed subject feedback on engagement with the intervention to guide the design and implementation of future interventions.
METHODS (See also Methods section of the online data supplement)
Participants and Trial Registration
Participants were recruited from the general pulmonary clinics at VA Boston. Inclusion and exclusion criteria are listed in Table 1.
Table 1.
Enrollment criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Subjects were at their baseline clinical status, received medical clearance from a healthcare provider to participate, and were not involved in another exercise program. The protocol (#2328) was approved by the VA Boston Healthcare System Committee on Human Research, and informed consent was obtained from each participant. The trial was registered as a randomized clinical trial on ClinicalTrials.gov (registration number NCT01772082).
Randomization Groups
Baseline daily step count was collected in all subjects using an Omron HJ-720 ITC pedometer for 7 days prior to randomization. Subjects received no step-count feedback since an opaque sticker was placed on the pedometer display. Subjects were then randomized (1:1) by a computer algorithm [23] with blocking stratified on season and baseline 6-minute walk test (6MWT) distance (dichotomized by ≥ 1190 feet or < 1190) to either pedometer plus website (intervention) or pedometer alone (control) groups (Figure 1). Randomization assignments were generated with random block sizes which were not disclosed to study staff. Assignments were communicated to study staff through the ESC website, and subjects were notified by telephone of their assignment groups. Due to the nature of the intervention, participant blinding was not possible; however the research assistant conducting assessments at the study conclusion (3 months) was blinded to group assignment.
Figure 1.
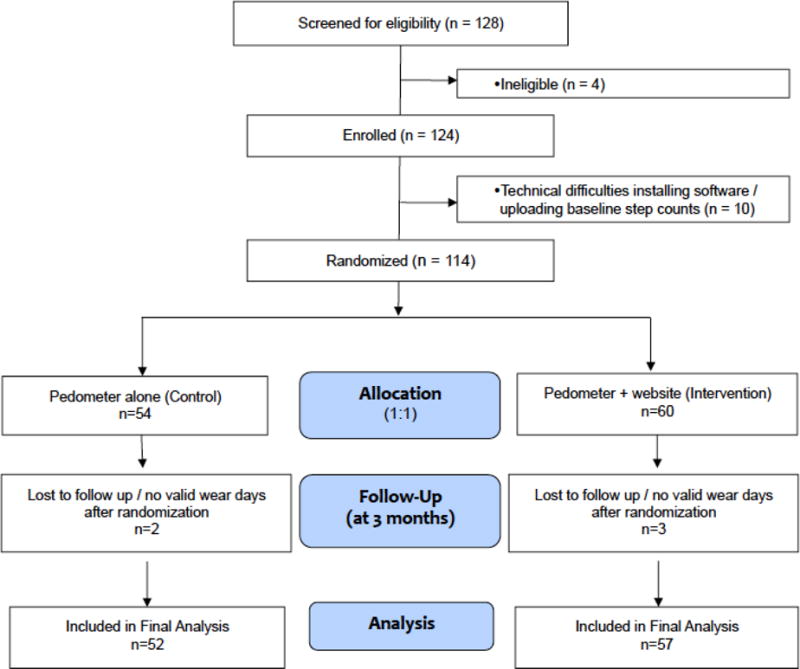
CONSORT diagram
Subjects in the pedometer plus website group were instructed to wear the pedometer daily during all waking hours, to upload step counts weekly, and were given access to a website which provided the four key components of individualized goal-setting, iterative step-count feedback for self-monitoring, educational and motivational content to enhance disease self-management and self-efficacy, and an online community forum for social support. Subjects received step-count goals every week based on their current recorded step count or previously set goal. The goals were the minimum value of three possible numbers: 1) the previous goal + 400 steps, 2) the average of the most recently uploaded seven days of step counts + 400 steps, or 3) 10,000 steps.
Subjects randomized to the pedometer alone group were given a pedometer and written materials about exercise at study entry, but were not assigned step-count goals. They were instructed to wear the pedometer daily while awake and to upload step counts at least monthly via the website; the website had no content except a display of the study week. Both groups uploaded step-count data to the same study server via the website.
Outcome Assessments
Daily step count, the primary outcome, was assessed objectively using the Omron pedometer. To identify days when the device was not worn by the subject, a wear day was defined by wear time ≥ 8 hours and ≥ 100 steps [22, 23]. Baseline daily step count was the average of all wear days assessed during the 7 days prior to randomization [27, 28]. Following randomization, daily step counts were averaged each week if the subject had ≥ 3 wear days for that week[29]. The difference between average daily step count and baseline step count was determined for weeks 1 through 13. The definition of a wear day and calculation of baseline and follow-up daily step counts were similarly applied to both groups.
In-person evaluation of secondary outcomes. including 6MWT distance, exercise adherence, HRQL, dyspnea, depression, COPD knowledge, exercise self-efficacy, social support, and motivation and confidence to exercise daily, were performed at baseline and end of study (3 months). Spirometry[30] was performed using an Eaglet spirometer (nSpire Health, Inc.), a 6MWT[31] was conducted according to ATS guidelines without a practice walk, and standardized questionnaires were administered, including the St. George’s Respiratory Questionnaire (SGRQ) [32], modified Medical Research Council [33] (MMRC), Beck Depression Inventory-II [34], Bristol COPD Knowledge Questionnaire [35], Exercise Self-Regulatory Efficacy Scale [36], and Medical Outcome Study Social Support survey [37]. For additional details please see the online supplement. Season was ascertained for each week of the study in which subject participated, including the time of enrollment (week 1), based upon whether ≥ 4 days that week fell into the calendar month groupings as follows: winter (December, January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). The number of wear days and the hours of wear time during wear days were examined as surrogates of exercise adherence in both groups, and the number of website logins was a measure of adherence to website use in the pedometer plus website group. At the end of the study, participants in both groups completed a feedback survey about use of the pedometer and website.
Significant adverse events and pulmonary events were tracked during the study. Significant adverse events were defined as deaths and/or hospitalizations for any cause and were reportable to the Internal Review Board. Pulmonary events were self-reported at monthly telephone interviews during the study and were confirmed by medical record review whenever possible. Pulmonary events included confirmed diagnoses of pneumonia and acute exacerbations of COPD, defined as worsening symptoms which resulted in an unplanned visit to a healthcare provider or hospitalization associated with a new prescription for antibiotics and/or systemic steroids.
Statistics
Power Calculation
Based on pilot data from a cohort of subjects with moderate COPD [38], to have adequate power (β=80%) to detect a difference of at least 1000 steps per day[39] at an α=0.05, an estimated enrollment of 100 subjects (50 in each arm) was required.
Analysis
Univariate comparisons were made using a Chi-square or Fisher’s exact test for discrete data and either a Student’s t-test or Wilcoxon rank-sum test for continuous data. To evaluate the effect of the intervention and the impact of season on change in daily step count, generalized linear mixed effects models (PROC MIXED, SAS v9.4, Cary, NC) for repeated measures employing a first order auto-regressive covariance matrix were constructed with change in daily step count from baseline as the dependent variable and randomization group, FEV1 % predicted (included a priori), and season of study week as the independent variables. Secondary subgroup analyses of subjects enrolled in each season were conducted using an identical model. A p-value <0.05 was considered statistically significant. Additional details are available in the online supplement.
RESULTS
Between April 2012 and August 2015, 114 participants were enrolled and randomized (Figure 1). Enrollment was concluded after achieving target enrollment. Data from 109 Veterans (52 control, 57 intervention) who had 1) baseline step-count data, i.e. ≥ 5 wear days at baseline[27], 2) follow-up step-count data, i.e. ≥ 1 week with step-count data after randomization, and 3) completed the baseline and end-of-study in-person visits were analyzed on an intention-to-treat basis. Nine participants in the pedometer alone group and two participants in the pedometer plus website group met the inclusion criterion of COPD based solely on the finding of emphysema on CT scan; all others had a FEV1/FVC ratio < 0.70. Although there were significantly more subjects in the pedometer alone group whose COPD was diagnosed on the basis of chest CT, there were no significant differences in age, BMI, pack-years, SGRQ Total Score (SGRQ-TS), MMRC dyspnea score, or baseline daily step count in subjects diagnosed on the basis of CT compared to subjects diagnosed using spirometry data. Subjects in the pedometer plus website group had higher rates of inhaled long-acting muscarinic antagonist use compared to the pedometer alone group, p=0.01. There were no other differences in baseline characteristics by randomization group (Table 2).
Table 2.
Baseline subject characteristics
| Characteristic | Total N=109 |
Pedometer + Website (Intervention) N=57 |
Pedometer Alone (Control) N=52 |
Between Group P-value |
|---|---|---|---|---|
|
| ||||
| Sex (Male) | 95 (98.2%) | 56 (98.3%) | 51 (98.1%) | 1.0 |
|
| ||||
| Age | 68.6±8.3 | 68.4±8.7 | 68.8±7.9 | 0.52 |
|
| ||||
| White race | 100 (91.7%) | 53 (93%) | 47 (90.4%) | 0.73 |
| BMI (kg/m2) | 29.3±5.6 | 29.4±5.76 | 28.7±5.56 | 0.55 |
|
| ||||
| Pack-years | 61.6±41.4 | 61.9±34 | 61.1±48.0 | 0.24 |
|
| ||||
| Current smoker | 40 (36.7%) | 22 (36.1%) | 18 (34.6%) | 0.7 |
|
| ||||
| Current oxygen use | 26 (23.9%) | 15 (26.3%) | 11 (21.2%) | 0.65 |
|
| ||||
| Medication use | ||||
| LAMA | 59 (54.1%) | 38 (66.7%) | 21 (40.4%) | 0.01 |
| LABA | 53 (48.6%) | 33 (57.9%) | 23 (44.2%) | 0.18 |
| Inhaled corticosteroid | 59 (54.1%) | 33 (57.9%) | 26 (50%) | 0.45 |
|
| ||||
| Comorbidities | ||||
| Coronary Artery Disease | 23 (21.1%) | 13 (25.0%) | 10 (17.5%) | 0.36 |
| Hypertension | 63 (57.8%) | 36 (63.2%) | 27 (51.9%) | 0.25 |
| Congestive Heart Failure | 8 (7.3%) | 4 (7.7%) | 4 (7.0%) | 1.00 |
| Arthritis | 39 (35.8%) | 17 (29.8%) | 22 (42.3%) | 0.23 |
| Diabetes | 28 (25.6%) | 16 (28.1%) | 12 (23.1%) | 0.66 |
| Depression | 41 (37.6%) | 25 (43.9%) | 16 (30.8%) | 0.17 |
| Psychiatric Illness | 30 (27.5%) | 19 (33.3%) | 11 (21.2%) | 0.20 |
| Back Pain | 44 (40.4%) | 21 (36.8%) | 23 (44.2%) | 0.44 |
|
| ||||
| Baseline daily step count | 3444.8±2438.6 | 3148.6±2469.0 | 3769.5±2386.3 | 0.19 |
|
| ||||
| FEV1 (L) | 1.87±0.62 | 1.80±0.56 | 1.93±0.68 | 0.28 |
|
| ||||
| FEV1 % predicted | 62.6±21.6 | 60.2±21.2 | 65.2±21.9 | 0.22 |
|
| ||||
| 6-MWT distance (m) | 388.1±81.3 | 382.5±89.4 | 394.2±71.8 | 0.46 |
|
| ||||
| SGRQ-TS | 33.5±16.4 | 34.9±16.8 | 31.9±15.9 | 0.54 |
|
| ||||
| MMRC dyspnea score | 1.00 | |||
| 0-2 | 65 (59.6%) | 34 (59.7%) | 31 (59.6%) | |
| 3-4 | 44 (40.3%) | 23 (40.4%) | 21 (40.4%) | |
|
| ||||
| Beck’s Depression Inventory | 9.0 ±9.3 | 9.2 ±9.5 | 8.8 ±9.0 | 0.84 |
|
| ||||
| MOS Social Support | 3.6 ±1.1 | 3.5 ±1.1 | 3.7 ±1.2 | 0.23 |
|
| ||||
| Bristol COPD Knowledge | 41.4 ±16.8 | 44.1 ±14.6 | 38.5 ±18.6 | 0.08 |
|
| ||||
| Exercise Self-Efficacy | 63.6 ±22.1 | 60.9 ±22.8 | 66.5 ±21.2 | 0.19 |
|
| ||||
| Motivation to exercise daily* | 5.1 ±3.0 | 4.8 ±2.8 | 5.4 ±3.1 | 0.34 |
|
| ||||
| Confidence can exercise daily± | 7.4 ±2.4 | 7.0 ±6.3 | 7.9 ±7.3 | 0.05 |
|
| ||||
| Season of enrollment | 0.29 | |||
| Winter (Dec, Jan, Feb) | 12 (11.0%) | 4 (7.0%) | 8 (15.4%) | |
| Spring (Mar, Apr, May) | 34 (31.2%) | 18 (31.6%) | 16 (30.8%) | |
| Summer (Jun, Jul, Aug) | 28 (25.7%) | 13 (22.8%) | 15 (28.9%) | |
| Fall (Sep, Oct, Nov) | 35 (32.1%) | 22 (38.6%) | 13 (25.0%) | |
BMI = body mass index, LAMA = long-acting muscarinic antagonist, LABA = long-acting beta-agonist, 6-MWT = 6-minute walk test, SGRQ-TS = St. George’s Respiratory Questionnaire Total Score, MMRC = Modified Medical Research Council, MOS = Medical Outcomes Study
In the Bristol’s COPD Knowledge, Exercise Self-Regulatory Efficacy, and Medical Outcomes Study Social Support questionnaires, higher scores represent greater knowledge, efficacy, and social support, respectively.
“Motivation to exercise” was assessed by subject self-report to the question “Overall, how motivated are you to exercise each day?” using a scale from 1-10 (1=not motivated, 10=extremely motivated).
“Confidence can exercise daily” was assessed by subject self report to the question “Overall, how confident are you that you can exercise each day?” using a scale from 1-10 (1=not at all confident, 10=extremely confident).
In generalized linear mixed effects models for repeated measures, randomization group was a significant predictor of change in daily step count (p = 0.03). Figure 2 illustrates the change in daily step count from baseline for weeks 1 through week 13, by treatment group. At week 13, subjects in the pedometer plus website group had an average change from baseline that was 804 steps per day (SE ±356.5) greater than the change observed in the pedometer alone group (between-group difference p = 0.02). Subjects in the pedometer plus website group demonstrated a significant (p<0.05) increase in daily step count from baseline (within-group change in step count) starting in week 3, which was sustained until the end of the study (week 13 – Figure 2 and Supplementary Table 1). Subjects in the pedometer alone group had daily step counts that did not differ significantly from baseline for the duration of the study.
Figure 2. Change from baseline daily step count by randomization group.
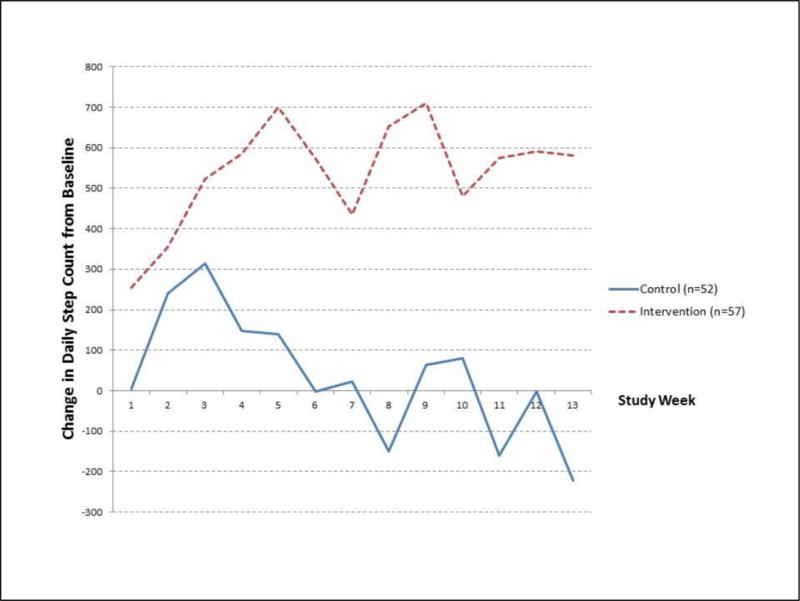
Control group = Pedometer alone, Intervention group = Pedometer plus website
Values plotted on the y-axis are from least square means solutions for change in daily step count from a generalized linear mixed-effects model for repeated measures, adjusting for FEV1 % predicted and season of intervention week. At week 13, subjects in the pedometer + website group walked an average change in step count from baseline of 804 steps more than subjects in the pedometer-only group (p = 0.02).
These findings were unchanged when we excluded COPD subjects (n = 11) diagnosed by CT scan alone and had a FEV1/FVC ratio > 0.7. Findings were similar in models which included adjustment for baseline daily step count as well as in models where FEV1 % predicted was removed as a covariate. When we included adjustment for dichotomized baseline 6MWT distance (dichotomized ≥1190 feet or <1190 feet), the between-group difference at week 13 was attenuated (612.7 ± 339.9 steps/day higher in the pedometer plus website group, p = 0.07).
There were no significant differences by randomization group in the secondary outcomes (Table 3) or in absolute daily step counts at 3 months. Subjects in the pedometer plus website group had an average absolute daily step count of 3,589 (±2423) at 3 months while subjects in the pedometer alone group averaged 3,664 (±2507) steps per day (p-value 0.95). There were no significant between-group differences with respect to change in 6MWT distance, SGRQ-TS, MMRC dyspnea score, Beck’s depression score, Bristol COPD knowledge score, exercise regulatory self-efficacy, social support, and motivation or confidence to exercise at 3 months. However, significant within-group changes were observed. In the pedometer plus website group, motivation to exercise daily increased (1.1 ± 2.4, one sample Student’s t-test p = 5 × 10−3). In the pedometer alone group, the Bristol’s COPD knowledge score increased (5.8 ± 12.6, p =1.6 × 10−3), dyspnea increased (MMRC, 0.3 ± 1.1, p-value=0.03), and exercise self-regulatory efficacy decreased (−5.9 ± 21.1, p =0.05).
Table 3.
Change from baseline of secondary outcomes, by randomization group.
| Pedometer Alone (Control) |
Pedometer + Website (Intervention) |
P−value | |
|---|---|---|---|
| Δ 6-MWT distance (m) | 2.6 (46.7) | −0.9 (55.8) | 0.72 |
| Δ SGRQ-TS | −0.67 (12.7) | −0.9 (9.9) | 0.92 |
| Δ MMRC | 0.3 (1.1) | 0.1 (1) | 0.27 |
| ΔBeck’s Depression Inventory | −0.3 (5.3) | −0.6 (3.6) | 0.71 |
| ΔBristol’s COPD Knowledge | 5.8 (12.6) | 1.1 (11.9) | 0.05 |
| ΔExercise Self-Regulatory Efficacy | −5.8 (21.1) | −2.0 (21.5) | 0.36 |
| ΔMedical Outcomes Study Social Support | −0.2 (0.7) | 0.0 (0.7) | 0.17 |
| ΔMotivation to exercise daily | 0.5 (2.7) | 1.1 (2.6) | 0.27 |
| ΔConfidence to exercise daily | −0.6 (2.2) | −0.1 (2.2) | 0.22 |
Negative values indicate a decrease from baseline.
6-MWT = 6-minute walk test, SGRQ-TS= St. George’s Respiratory Questionnaire Total Score, MMRC = Modified Medical Research Council.
Season of the study week was a significant predictor of change in daily step count in the primary model (p= 0.02). Because our study was conducted over the course of 3 months, most subjects experienced a change of season during the study. Subgroup analysis of subjects enrolled during the summer (n = 28) showed a significant overall difference in change in daily step count by randomization group (p=0.01). At week 13, subjects in the pedometer plus website group (n = 13) had an average change in daily step count from baseline that was 1983 steps higher than subjects in the pedometer alone group (n = 15) (between-group p=0.0076). During weeks 11–13, concurrent with the transition from summer to fall, subjects in the pedometer alone group had a significant decline in daily step count from baseline (from −1057 steps in week 11 to −1750 steps in week 13, within-group p-values from 0.05 to 0.0017) whereas subjects in the pedometer plus website group demonstrated sustained to slightly improved daily step counts from baseline (Figure 3). When we examined the data using calendar month (rather than intervention week) as the abscissa, the decline in step count in the pedometer alone group coincides with decreasing average daily temperatures recorded during the study period (Supplementary Figure E1). There were no significant differences in the number of pulmonary events in the fall/winter (defined as events occurring after September 1st) by randomization group among subjects enrolled in the summer (see Supplementary Results).
Figure 3. Change in daily step count by randomization group among subjects enrolled in summer.
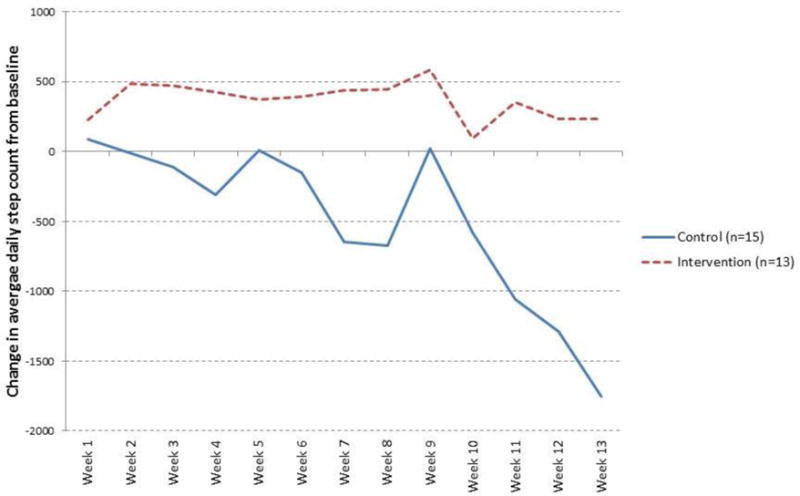
Control group = pedometer alone, Intervention group = pedometer plus website
Values plotted on the y-axis are from least square means solutions for change in daily step count from a generalized linear mixed-effects model for repeated measures, adjusting for FEV1 % predicted and season of intervention week. Subjects in the pedometer-alone group demonstrated a significant decline in daily step count from baseline during the last three weeks of the study period. Of the 28 subjects enrolled during summer, 23 (82%) ended the study (at week 13) in fall or winter when temperatures in the northeastern United States are cooler.
For subjects enrolled in other seasons (winter, spring, fall), no statistically significant between-group differences in change in step count were identified in the primary model (see Supplementary Table 2) or in subgroup analyses (using separate models). When we constructed a model examining the interaction between randomization group and season of enrollment (in all subjects simultaneously), between-group differences were again noted only among subjects enrolled during the summer, with the pedometer plus website group demonstrating an average 860.6 ± 416.8 (p = 0.04) more steps per day over the entire study. In this model, subjects in both the pedometer plus website and pedometer alone groups enrolled in spring had significant within-group improvements from baseline, consistent with known improvements in daily PA with increasing temperatures characteristic of the transition from spring to summer[24, 25]. Although participants enrolled in spring in the intervention group had higher mean improvements, because both groups improved, the between-group differences in spring were not statistically significant.
We examined the impact of comorbid medical conditions on daily physical activity levels. Although there were no significant differences in the frequency of self-reported comorbidities by randomization group, within-group differences in both baseline and change in daily step count by self-reported comorbid conditions were observed. In the pedometer alone group, subjects who reported a history of arthritis had a significantly lower baseline daily step count relative to pedometer-alone subjects without arthritis (2819.6 ± 1844.7 versus 4466.1 ± 2522.7, p=0.01). Interestingly, subjects in the pedometer alone group with arthritis had significantly higher changes in step count at 3 months relative to pedometer-alone subjects without arthritis (934.3 ± 1498.7 versus −756.4 ± 2177.7, p = 0.02). There were no differences in baseline daily step count by self-reported comorbidities in the pedometer plus website group, however, subjects in the intervention group with depression had significantly less improvement in their daily step count at 3 months (−276.6 ± 973.4 versus 1000.2 ± 2123.8, p = 0.01).
Compliance with pedometer use, a surrogate for exercise adherence, was high, with an overall percentage of pedometer wear days of 86 % for the cohort over the study period (87% in the pedometer alone group and 85.8 % in the pedometer plus website group). Although there was a decrease in percent of wear days by month 3, there were no differences between randomization groups (Table 4). There were no significant differences in average wear time by randomization group, 16.1± 6.3 hours/day in the pedometer alone group versus 15.2 ± 2.4 hours/day in the pedometer plus website group, (p = 0.84).
Table 4.
Percent wear days by randomization group
| Study Month | Pedometer Alone (Control) |
Pedometer + Website (Intervention) |
P-value |
|---|---|---|---|
| 1 | 93.1% | 90.1% | 0.31 |
| 2 | 87.4% | 85.3% | 0.61 |
| 3 | 80.3% | 83.6% | 0.52 |
A similar trend was noted for adherence to website use as evidenced by the website logins in the pedometer plus website group; average monthly logins remained > 4 per month which suggests good adherence to the requested weekly logins (data not shown). While 74% of our subjects rated their ability to use the internet as “basic” or “moderate”, two-thirds of subjects reported using the internet on a daily basis at baseline. There were no differences in the self-reported ability or frequency of internet use by randomization group.
In both groups, the most commonly reported technical problems included the pedometer “popping off” the waistband (72%) and difficulty uploading the step-count data to the website on the first attempt that required assistance from study staff (27%); these difficulties were minor and did not impact the collection of data during the study. There were no differences in technical difficulties with the pedometer or website by randomization group (Supplementary Figure E2). Overall, 94% of subjects enrolled in both groups stated the website was easy to understand while 85% reported it was easy to find time to log in. Overall satisfaction with the program was high in both randomization groups, with 95% of subjects reporting that they would recommend the program to another patient with COPD, and 93% reporting that they planned to continue to walk for exercise after the study ended.
Among all randomized subjects (n = 114), 17 individuals (8 in pedometer-alone, 9 in pedometer + website) reported 24 serious adverse events (10 in pedometer-alone, 14 in pedometer + website) during the study. Events included abdominal pain, anxiety, mental health crisis, headache, congestion, ear pain, rash, skin abscess, kidney problems, a broken toe, and a car accident; none of the events were study-related. There were no differences in incidence rates of serious adverse events by randomization group (2 tailed mid-P exact value = 0.54). When we included covariate adjustment for whether a subject experienced a significant adverse event in our primary model, the between-group difference in change in daily step count remained significant at week 13 (830.5 ± 347 steps higher in the pedometer plus website group, p = 0.02).
Twenty-four pulmonary events (9 in pedometer alone, 15 in pedometer + website) were reported by 21 individuals (8 pedometer alone, 13 pedometer + website subjects) during the study period. There were no differences in the incidence rates of pulmonary events by randomization group (2 tailed mid-P exact = 0.29). When we included covariate adjustment for whether a subject experienced a pulmonary event during the study into our primary model, the between-group difference in change in daily step count at week 13 remained significant (864.3 ± 355.1 higher in the pedometer plus website group, p=0.02). When we included covariate adjustment for whether a subject experienced either a significant adverse event or a pulmonary event, the between-group difference in change in step count at week 13 remained significant (883.3 ±348.4 higher in the pedometer plus website group, p=0.01).
DISCUSSION
Low PA is highly prevalent among patients with COPD and has been definitively associated with reduced HRQL, and increased risk for acute exacerbations and death[5]. Importantly, sedentary behavior and low PA represent potentially modifiable risk factors in COPD patients. Recently, a number of interventions which combine the use of wearable monitors in conjunction with goal-setting have been shown to increase daily PA in COPD patients[18–20]. Our results show that the combination of a pedometer and a website based on the Theory of Self-Regulation improves and maintains daily step count over 3 months among ambulatory COPD patients compared to a pedometer alone. The 4 main components of the intervention–goal-setting, feedback, education and motivation, and social support—appears together to have been efficacious in promoting and sustaining walking. These results in a cohort of patients with COPD referred to a pulmonary clinic not only confirm our previous findings in a cohort of individuals with COPD identified using an administrative database[22, 23], but also extends our understanding of the mechanisms of benefit.
Our step-count data, which were collected daily over 3 months, provides exceptional granularity compared to published studies which assess PA at only a limited number of discrete time points. Significant increases in daily step count were observed approximately 3 weeks after initiating our pedometer plus website program. These data are consistent with results reported in Mendoza et al., where the greatest improvement in step count in the intervention arm (which consisted of goal-setting through the use of daily diaries and in-person counseling) was observed during the first month of the intervention[19]. We speculate that 3-4 weeks may be required to effect initial changes in behavior. Therefore, targeted efforts and counseling to keep patients engaged at the initiation of a program to promote PA are warranted.
The availability of daily step counts for the entirety of the study and in both arms of our cohort also allowed us to examine the impact of changes in season and temperature on daily PA. The efficacy of the pedometer plus website intervention to attenuate the decline associated with the onset of cold temperatures suggests a mechanism which may contribute to the overall efficacy of our intervention. Observational studies have consistently reported declines in daily PA associated with decreasing temperatures in COPD patients[24–26]; the decline in daily step count observed in the pedometer alone group during the transition from warm to cold weather is consistent with these reports. The converse was also observed – increases in daily PA in both groups were observed during the transition from colder to warmer temperatures. Thus, timing the initiation of a PA intervention (for example, >3 weeks prior to the transition to colder weather in temperate climates) may be particularly efficacious in COPD populations. To our knowledge, this is the first study which examines the effect of temperature and season in a PA intervention trial.
The lack of changes in secondary outcomes, such as 6MWT distance and HRQL, in our study warrants discussion. Changes in exercise capacity in PA-focused intervention trials are inconsistent, with some trials demonstrating concurrent improvements[19, 20], while other studies do not[21]. Some of the heterogeneity in response in exercise capacity may be due to the fact that daily PA and exercise capacity are distinct, independent entities with multivariable determinants. Similarly, differences in PA-intervention protocols may also contribute to variability in improvement between studies. It is possible that our intervention yields isolated gains in daily PA; unfortunately, because 6MWT distance was not assessed in the THS study, we are not able to provide evidence to confirm this hypothesis. Interestingly, the lack of improvement in HRQL in our cohort differs from the results reported in the THS study[23]. Differences in study populations between ESC and THS, such as increased baseline variability in daily step counts as well as a sicker and more sedentary cohort in ESC, may also contribute to these findings.
Although our website specifically provided motivation and education to promote disease knowledge, confidence, and exercise self-efficacy, we did not see significant between-group differences in improvements in these domains when directly assessed with questionnaires. Efficacy to increase the primary endpoint of change in daily step count without measurable differences in self-reported questionnaire items may also reflect a limitation of the questionnaire tools used. Alternatively, a period longer than 3 months may be needed for changes in these domains to occur.
Our study provides unique insight into patient perceptions and attitudes along with protocol-specific feedback which will help in the design of future pedometer- and web-based interventions to promote PA. Both objective data and subject self-report demonstrate high acceptance and compliance with our pedometer and website, supporting the feasibility of employing similar programs in rural areas where access to in-person counseling and conventional pulmonary rehabilitation programs is limited. Mechanical modifications to prevent pedometer detachment and simplifying step-count data upload represent easily implemented changes which can improve future PA interventions.
The strengths of our study include the randomized design, highly granular, objective PA data, and rich demographic, physiological and psychosocial data. Potential limitations include the paucity of women and that our study was conducted at a single center, both of which limit the generalizability of our findings. Nevertheless, the single center in one geographic region can be viewed as a strength as it allows for uniformity in variables that are difficult to assess that may impact PA such as weather, the built environment of sidewalks and green space, and the general culture of how PA (such as biking to work) is integrated in daily routines. Due to the voluntary nature of the study, it is possible that self-selection for highly motivated or more computer literate populations occurred. This would not be expected to contribute to between-group differences observed in our study given our randomized study design. Due to the nature of the intervention, participant blinding was not possible. However, the impact of the lack of blinding was mitigated by 1) research staff performing the end-of-study visits were blinded to subject group assignment and 2) the use of objectively-assessed endpoints. Finally, a “placebo effect” arising from awareness of PA monitoring may have impacted participant behavior during the study, particularly in the control arm. Wearable monitors have been shown to positively impact daily PA levels in both the general and COPD population [19, 20, 40], even in the absence of a structured goal-setting program. This would, however, be expected to cause a bias towards the null and arguably strengthens the significance of the difference between groups due to the addition of the website to pedometer use in our study.
We acknowledge that the short duration of our study provides helpful but limited information. Observational studies have consistently described a natural history of decline in PA among COPD patients[41], although the rate and pattern of decline following an intervention may vary by patient type and severity[18]. Although gains in daily PA in THS appeared to wane over time [42], our cohort is distinct and extended follow up, including monitoring of acute exacerbations following the intervention period, is currently ongoing.
In conclusion, a website added to a pedometer improves step counts and sustains walking over 3 months, possibly by attenuating declines in PA due to season changes. These novel data contribute significant insights to exercise counseling by healthcare providers and the development of future interventions to promote PA among COPD patients. Overall, our results provide additional evidence to support the use of structured interventions which include goal-setting in combination with monitoring devices to promote PA in COPD. Future studies to identify subpopulations who benefit the most, as well as assessing the long-term efficacy of our protocol, are warranted.
Supplementary Material
Acknowledgments
We thank the Veterans for their participation in this research study.
Funding Sources
Department of Veterans Affairs, Rehabilitation Research and Development Service [Career Development Award 2, F6847W (Moy); CDA2 IK2RX002165 (Wan); Merit O1150-R (Moy)].
Role of the Funding Source(s)
The funding body had no role in the design, collection, analysis or interpretation of the data, in writing the manuscript or in the decision to submit the manuscript for publication.
Abbreviations
- 6MWT
Six minute walk test
- COPD
Chronic obstructive pulmonary disease
- ESC
Every Step Counts
- HRQL
Health-related quality of life
- MMRC
modified Medical Research Council
- MOS
Medical Outcomes Study
- PA
Physical activity
- THS
Taking Healthy Steps
Footnotes
Author Contributions
MM, CR, DG, RK, and EG were involved in the conception and design of all stages of the study. AK, DH, MT were involved in study data collection. EW and AK conducted study analyses and DG provided statistical support. All authors read and approved the final manuscript. MM and EW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest Statement
Dr. Moy received an honorarium for consulting from Astra Zeneca. All other authors declare no relevant conflicts of interest.
Online Data Supplement
This article has an online data supplement.
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 2.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 3.Van Remoortel H, Hornikx M, Demeyer H, Langer D, Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68(10):962–3. doi: 10.1136/thoraxjnl-2013-203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arne M, Janson C, Janson S, Boman G, Lindqvist U, Berne C, Emtner M. Physical activity and quality of life in subjects with chronic disease: chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scand J Prim Health Care. 2009;27(3):141–7. doi: 10.1080/02813430902808643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimeno-Santos E, Frei A, Steurer-Stey C, de Batlle J, Rabinovich RA, Raste Y, Hopkinson NS, Polkey MI, van Remoortel H, Troosters T, Kulich K, Karlsson N, Puhan MA, Garcia-Aymerich J, PR consortium Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–9. doi: 10.1136/thoraxjnl-2013-204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962–9. doi: 10.1016/j.rmed.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One. 2013;8(4):e60400. doi: 10.1371/journal.pone.0060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen HQ, Chu L, Liu IL Amy, Lee JS, Suh D, Korotzer B, Yuen G, Desai S, Coleman KJ, Xiang AH, Gould MK. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):695–705. doi: 10.1513/AnnalsATS.201401-017OC. [DOI] [PubMed] [Google Scholar]
- 9.Zanoria SJ, ZuWallack R. Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10(4):207–13. doi: 10.1177/1479972313505880. [DOI] [PubMed] [Google Scholar]
- 10.Durheim MT, Smith PJ, Babyak MA, Mabe SK, Martinu T, Welty-Wolf KE, Emery CF, Palmer SM, Blumenthal JA. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group. Ann Am Thorac Soc. 2015;12(3):349–56. doi: 10.1513/AnnalsATS.201408-365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moy ML, Gould MK, Liu IA, Lee JS, Nguyen HQ. Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res. 2016;2(1) doi: 10.1183/23120541.00062-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waschki B, Kirsten A, Holz O, Muller KC, Meyer T, Watz H, Magnussen H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–42. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 13.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Varela MV Lopez, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 14.Cindy Ng LW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9(1):17–26. doi: 10.1177/1479972311430335. [DOI] [PubMed] [Google Scholar]
- 15.Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81. doi: 10.1183/13993003.01744-2015. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–8. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, de Batlle J, Ramon MA, Rodriguez E, Farrero E, Benet M, Guerra S, Sauleda J, Ferrer A, Ferrer J, Barbera JA, Rodriguez-Roisin R, Gea J, Agusti A, Anto JM, Garcia-Aymerich J, P.-C.S. Group Benefits of physical activity on COPD hospitalisation depend on intensity. Eur Respir J. 2015;46(5):1281–9. doi: 10.1183/13993003.01699-2014. [DOI] [PubMed] [Google Scholar]
- 18.Altenburg WA, ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med. 2015;109(1):112–21. doi: 10.1016/j.rmed.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza L, Horta P, Espinoza J, Aguilera M, Balmaceda N, Castro A, Ruiz M, Diaz O, Hopkinson NS. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. 2015;45(2):347–54. doi: 10.1183/09031936.00084514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, Loeckx M, Buttery SC, Rubio N, Van der Molen T, Hopkinson NS, Vogiatzis I, Puhan MA, Garcia-Aymerich J, Polkey MI, Troosters T, P.s.g. Mr Papp, P.c. the Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017 doi: 10.1136/thoraxjnl-2016-209026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorrink SN, Kort HS, Troosters T, Zanen P, Lammers JJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J. 2016;48(4):1019–1029. doi: 10.1183/13993003.00083-2016. [DOI] [PubMed] [Google Scholar]
- 22.Martinez CH, Moy ML, Nguyen HQ, Cohen M, Kadri R, Roman P, Holleman RG, Kim HM, Goodrich DE, Giardino ND, Richardson CR. Taking Healthy Steps: rationale, design and baseline characteristics of a randomized trial of a pedometer-based Internet-mediated walking program in veterans with chronic obstructive pulmonary disease. BMC Pulm Med. 2014;14:12. doi: 10.1186/1471-2466-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moy ML, Collins RJ, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, Nguyen HQ, Cohen MD, Goodrich DE, Giardino ND, Richardson CR. An Internet-Mediated Pedometer-Based Program Improves Health-Related Quality-of-Life Domains and Daily Step Counts in COPD: A Randomized Controlled Trial. Chest. 2015;148(1):128–37. doi: 10.1378/chest.14-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alahmari AD, Mackay AJ, Patel AR, Kowlessar BS, Singh R, Brill SE, Allinson JP, Wedzicha JA, Donaldson GC. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir Res. 2015;16:71. doi: 10.1186/s12931-015-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balish SM, Dechman G, Hernandez P, Spence JC, Rhodes RE, McGannon K, Blanchard C. The Relationship Between Weather and Objectively Measured Physical Activity Among Individuals With COPD. J Cardiopulm Rehabil Prev. 2017 doi: 10.1097/HCR.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 26.Furlanetto KC, Demeyer H, Sant’anna T, Hernandes NA, Camillo CA, Pons IS, Gosselink R, Troosters T, Pitta F. Physical Activity of Patients with COPD from Regions with Different Climatic Variations. COPD. 2017;14(3):276–283. doi: 10.1080/15412555.2017.1303039. [DOI] [PubMed] [Google Scholar]
- 27.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danilack VA, Weston NA, Richardson CR, Mori DL, Moy ML. Reasons persons with COPD do not walk and relationship with daily step count. COPD. 2014;11(3):290–9. doi: 10.3109/15412555.2013.841670. [DOI] [PubMed] [Google Scholar]
- 29.The objective monitoring of physical activity : contributions of accelerometry to epidemiology, exercise science and rehabilitation. Springer Berlin Heidelberg; New York, NY: 2016. [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 32.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 33.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 34.Phan T, Carter O, Adams C, Waterer G, Chung LP, Hawkins M, Rudd C, Ziman M, Strobel N. Discriminant validity of the Hospital Anxiety and Depression Scale, Beck Depression Inventory (II) and Beck Anxiety Inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13(3):220–8. doi: 10.1177/1479972316634604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White R, Walker P, Roberts S, Kalisky S, White P. Bristol COPD Knowledge Questionnaire (BCKQ): testing what we teach patients about COPD. Chron Respir Dis. 2006;3(3):123–31. doi: 10.1191/1479972306cd117oa. [DOI] [PubMed] [Google Scholar]
- 36.Davis AH, Figueredo AJ, Fahy BF, Rawiworrakul T. Reliability and validity of the Exercise Self-Regulatory Efficacy Scale for individuals with chronic obstructive pulmonary disease. Heart Lung. 2007;36(3):205–16. doi: 10.1016/j.hrtlng.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 38.Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR. A pilot study of an Internet walking program and pedometer in COPD. Respir Med. 2012;106(9):1342–50. doi: 10.1016/j.rmed.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, Janssens W, Troosters T. The Minimal Important Difference in Physical Activity in Patients with COPD. PLoS One. 2016;11(4):e0154587. doi: 10.1371/journal.pone.0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 41.Waschki B, Kirsten AM, Holz O, Mueller KC, Schaper M, Sack AL, Meyer T, Rabe KF, Magnussen H, Watz H. Disease Progression and Changes in Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192(3):295–306. doi: 10.1164/rccm.201501-0081OC. [DOI] [PubMed] [Google Scholar]
- 42.Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Kim HM, Nguyen HQ, Cohen MD, Goodrich DE, Giardino ND, Richardson CR. Long-Term Effects of an Internet-Mediated Pedometer-Based Walking Program for Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. J Med Internet Res. 2016;18(8):e215. doi: 10.2196/jmir.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


