Abstract
Introduction
Relations between heart failure and clinically manifested stroke are well known, but the associations between heart and brain early abnormalities are not totally clear.
Aims
We explore relations of subclinical brain abnormalities with early cardiac dysfunction in a large healthy middle-aged biracial cohort.
Methods
The CARDIA study enrolled 5,115 young adults aged 18–30 years at baseline (1985–1986). We assessed 719 Caucasian and African-American participants of the CARDIA study, with echocardiograms and brain MRI at follow-up year-25 (2010–2011). Echocardiography assessed aortic root diameter; LVEF; circumferential, longitudinal, and radial deformation. Cerebral MRI DTI, and, on a subset, ASL perfusion sequences were used to assess white matter fractional anisotropy and grey matter cerebral blood flow (CBF). Linear regression explored relations between cardiac parameters and cerebral measures, adjusting for anthropometrics, risk factors, and brain constitutional variation.
Results
Mean age 50±4 years, SBP 118±15mmHg; 60% white, and 48% men. Mean CBF was 46±9mL/100g/min and white matter fractional anisotropy was 0.31±0.02. Worse circumferential deformation and larger aortic root related to worse white matter fractional anisotropy. Worse radial systolic deformation was related to worse CBF in multivariable models. LVEF did not relate to early brain abnormalities.
Conclusions
In spite of no apparent effect of LV ejection fraction, early subclinical cardiac dysfunction and brain abnormalities are present and associated in middle-aged generally healthy individuals.
Keywords: Myocardial dysfunction, cardiac strain, cerebrovascular disorders
Introduction
Relations between chronic heart failure and clinically manifested stroke are well known.[1] However, this is unlikely to reflect the underlying, more prevalent cerebral and cardiac sub-clinical disease that precedes cognitive impairment related to aging.[2] In fact, relations between heart and brain are complex and not totally clear.[3] Developing knowledge in early, less severe stages of cardiac dysfunction might provide useful insights on the development of cerebral abnormalities.
Using advanced imaging techniques and measures, these subclinical relationships can now be studied in large community based cohorts. Speckle tracking echocardiography (STE)-derived cardiac tissue deformation parameters may be more sensitive to incipient myocardial alterations compared to left ventricular (LV) chamber dysfunction assessed by traditional echocardiographic measures.[4] Similarly, brain tissue volume, microstructural changes in the parenchymal, vessel structure, and cerebral perfusion can now be measured in large population studies using advanced brain MRI techniques.[5, 6] However, there are few studies that simultaneously utilize cardiovascular and neuro-imaging; those that do examine older populations and have not used STE techniques.[7]
We hypothesized that early functional parameters of the brain would be associated with measures of early cardiac dysfunction in a large cohort of middle-aged Caucasians and African-Americans. We explore the relations of incipient brain alterations assessed by advanced MRI techniques with standard parameters of aortic root and LV ejection fraction, as well as novel LV early dysfunction parameters using STE-derived strain rate.
Methods
Participant selection
The CARDIA Study, which has been described previously [8], is a longitudinal study of the development and determinants of cardiovascular disease in 5,115 young adults aged 18–30 years at baseline (1985–1986). The sample was recruited from four US cities (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California) to be approximately balanced within center by sex, age (18–24 years and 25–30 years), race (white, black), and education (≤ high school, > high school).[8] In 2010 – 2011 the 25 year (Y25) follow-up exam (72% participation) was conducted. All participants provide written informed consent at each examination, and institutional review boards from each field center and the coordinating center approves the study annually.
The BRAIN sub-study, conducted as a part of the Y25 follow-up exam, is designed to characterize the brain morphology, pathology, physiology and function using state-of-the art MR technology.[5] The sample for the CARDIA-BRAIN sub-study was selected randomly within four strata of ethnicity/race (black, white) and sex from three of the CARDIA field centers: Birmingham, AL, Minneapolis, MN, and Oakland, CA. The only exclusion criteria at the time of sample selection was presence of severe claustrophobia, pacemaker, defibrillator, neuro-stimulator, ferro-magnetic or unknown aneurysm clip, 3T MR incompatible metal implant of any kind or potentially-dangerous foreign metal objects in the body. Female participants of childbearing age who did not test negative on a pregnancy test prior to the scheduled MRI exam were also excluded. Consent for participation in the BRAIN sub-study was administered separately from the main study and in addition to the main study IRB approval; the protocol was reviewed and approved by the IRBs governing participating sites.
Echocardiography Protocol
The CARDIA examination year 25 echocardiography protocol for image acquisition and interpretation, as well as quality control procedures, have been reported previously.[9] In summary, Artida™ cardiac ultrasound machines (Toshiba Medical Systems; Otawara, Japan) were used to acquire standard and speckle tracking echocardiography (STE) images. When assessing standard echocardiography-derived paramerets, M-mode was used to measure the aortic root and two-dimensional 2-chambers and 4-chambers views were used to assess ejection fraction (LVEF), following recommendations from the American Society of Echocardiography (ASE).[10, 11]
STE images were analyzed in a 16-segment basis for LV mid-wall layer, using Advanced Cardiology Package Wall Motion 2D Tracking™ (Toshiba Medical Systems; Otawara, Japan). The peak of the averages was used to compute 4-chamber longitudinal strain rates from long axis views; as well as circumferential and radial strain rates from mid-ventricular short-axis views (Figure 1).[12] A given STE image set (4-chamber, 2-chamber, or short-axis) was excluded if more than 3 segments were improperly tracked. Longitudinal and circumferential strain rate measures have by convention negative values; radial strain rate parameters have positive values. We chose strain rate as it is thought to be more load-independent than strain alone. Note that strain rate values may vary according to vendors’ specifications. Reference values and reproducibility profile in the CARDIA study has been previously published.[9, 13]
Figure 1.
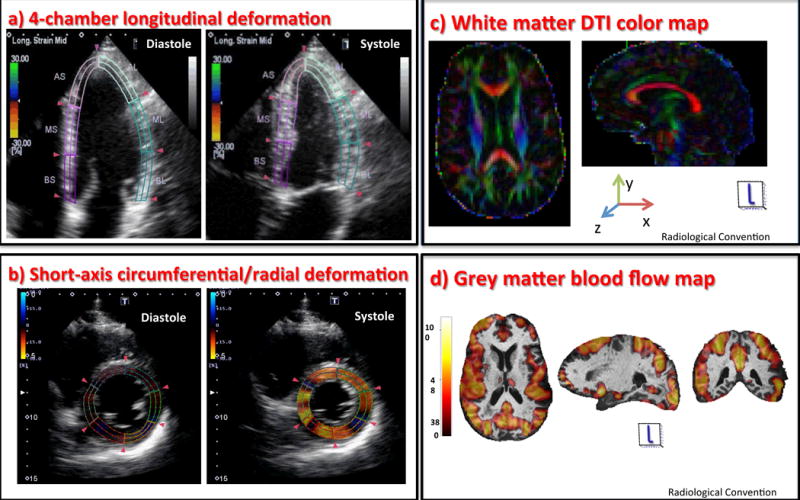
Assessment of cardiac deformation by speckle tracking echocardiography: Left ventricular images from 4-chamber (a) and short-axis (b) were analyzed in a 16-segment basis. Illustration of brain magnetic resonance imaging processing protocol: Brain white matter Diffusion Tensor Imaging (c) – The DTI map is displayed in colors showing the direction of the white matter tracts; grey matter cerebral blood flow map (d) – obtained from ASL sequences overlaid on T1-weighted images.
DTI – Diffusion Tensor Imaging; ASL – Arterial Spin Labeling.
Brain Magnetic Resonance Protocol
All scans were performed using 3 Tesla Siemens Tim Trio‐VB 15 platform (Siemens AG, Healthcare Sector, Erlangen, Germany) [sites in Oakland and Minneapolis] or 3 Tesla Philips 3T Achieva/2.6.3.6 platform [site in Birmingham] with an 8-channel head coil. Brain MRI imaging analyses were automated and performed at the CARDIA Brain MRI Reading Center (University of Pennsylvania, Philadelphia, PA).[5] Image analysis used fully automated computer programs to generate various image maps, each corresponding to a target MRI variable to be reported. All supratentorial brain tissue was automatically segmented[14, 15] into 109 anatomic regions of interest (ROI) in each hemisphere and then collapsed into three anatomic summary ROI’s for this analysis: total brain, total gray matter and total white matter.
Axial DTI (Diffusion Tensor Imaging) sequences (Figure 1) were performed with 30 diffusion directions (b-value of 1000 s/mm2 plus three images with b-value = 0 s/mm2); repetition time (TR) of 7400 ms; echo time (TE) of 82 ms. Sixty Four slices with an 112 × 112 matrix, were acquired with a voxel-size of 2.19×2.19 and 2.2 mm spacing to assess white matter (WM) fractional anisotropy (FA).[16] FA assessed WM microstructure integrity by evaluating the axonal water diffusion and was used to detect early changes in cerebral WM. FA can virtually range from 0 (indicating isotropic diffusion) to 1 (indicating that the diffusion is confined to that direction alone), values closer to 1 indicate higher WM tissue integrity.
Axial Psuedo-Continus Arterial Spin Labeling (PCASL) technique assessed a measure of brain perfusion as volume of flow per unit brain mass per unit time; mL/100g/min. Here we present the measured PCASL for assessing gray matter cerebral blood flow (CBF), as it is more reliably obtained than measures in the white matter (Figure 1).[17]
Clinical Covariates
We included several demographic and cardiovascular risk factors that may contribute independently to cardiac function and brain outcomes. Total education (the sum of all years of formal education), smoking status (never, former, or current), and use of anti-hypertensive (HTN) medication were assessed by questionnaire. Heart rate (HR) was computed by counting beats for 30s and multiplying by 2. Systolic blood pressure (SBP) was the average of the last two measurements (total of three), both acquired after 5 minutes of rest. Body-mass index (BMI) was computed dividing the participant weight (Kg) by the square of their height (m2). Diabetes in examination year 25 was defined by the presence of one of the following ADA criteria: self-reported history of hypoglycemic medication use, fasting glucose ≥ 126 mg/dL, glucose tolerance test ≥ 200 mg/dL, or HbA1c ≥ 6.5%. Additional description on clinical covariates has been published for the CARDIA study.[18]
Statistical analysis
Linear regression models were used to explore relations between echocardiography-derived cardiac parameters (independent variables) and MRI-derived cerebral measures (dependent variables). Early LV systolic function echocardiography parameters were: longitudinal systolic strain rate (Ell Srs), circumferential systolic strain rate (Ecc Srs), and radial systolic strain rate (Err Srs). Standard LV ejection fraction and aortic root were also assessed. Brain MRI parameters evaluated as dependent variables were white matter fractional anisotropy and grey matter cerebral blood flow.
We show multivariable models adjusted for age, total brain volume, sex, race, total education, SBP, HTN medication, diabetes, HR, smoking status, and BMI. Covariates were selected based on previous work in CARDIA.[5] We additionally corrected for head size individual variation using total intracranial volume in the analyses of white matter fractional anisotropy, and using total grey matter tissue volume in the analyses of grey matter CBF.
Results
Of 3474 participants that had echocardiograms in the CARDIA year-25, 719 also underwent brain MRI examination. The CARDIA participants in this study were on average overweight, middle-aged men and women, with slight predominance of Caucasians (Table 1). Brain MRI and echocardiography parameters are shown in Table 2.
Table 1.
Participant clinical characteristics (n = 693)
| Parameter | Mean (SD) |
|---|---|
| Age (years) | 50.3 (3.5) |
| SBP (mmHg) | 118.2 (14.7) |
| BMI (Kg/m2) | 28.9 (5.8) |
| HR (beats/30s) | 32.6 (5.2) |
| Total education (years) | 14.9 (2.6) |
| Proportion (%) | |
| Caucasian | 59.9 |
| Men | 47.8 |
| Current smokers | 16.2 |
| Former smoker | 23.7 |
| Using anti-HTN medication | 22.2 |
| Diabetes | 9.7 |
SBP – systolic blood pressure, BMI – body-mass index; HR – heart rate; HTN – hypertensive.
Table 2.
Brain magnetic resonance imaging and echocardiography variables description
| Parameter | n | Mean | SD/IQR | |
|---|---|---|---|---|
| Brain MRI | GM CBF, mL/100g/min | 544 | 56.0 | 11.4 |
| WM Fractional anysotropy | 701 | 0.31 | 0.02 | |
|
| ||||
| Echocardiography | LVEF, % | 675 | 61.8 | 7.0 |
| Ell Srs, s−1 | 627 | −0.7 | 0.1 | |
| Ecc Srs, s−1 | 664 | −0.7 | 0.2 | |
| Err Srs, s−1 | 664 | 1.8 | 0.7 | |
| Ao root/height, mm/m | 712 | 1.8 | 0.2 | |
GM – grey matter; WM – white matter; Ell Srs – longitudinal peak systolic strain rate; Ecc Srs – circumferential peak systolic strain rate; Err Srs – radial peak systolic strain rate; LVEF – left ventricular ejection fraction; Ao – aorta.
In multivariable models (Table 3), lower white matter fractional anisotropy was associated with parameters of early LV dysfunction as measured by reduced circumferential systolic deformation (less negative Ecc Srs) and to an enlarged aortic root.
Table 3.
Multivariable linear regression for white matter (WM) and grey matter (GM) brain parameters assessed by magnetic resonance imaging
| Parameters | WM Fractional Anisotropy
|
GM cerebral Perfusion (mL/100g/min)
|
||||
|---|---|---|---|---|---|---|
| n | coeff | p | n | coeff | p | |
| LVEF, % | 633 | −0.0002 | 0.061 | 498 | 0.05 | 0.475 |
| Ell Srs, s−1 | 590 | −0.0005 | 0.945 | 461 | −8.00 | 0.075 |
| Ecc Srs, s−1 | 623 | −0.0110 | 0.042 | 488 | −0.31 | 0.923 |
| Err Srs, s−1 | 623 | 0.0001 | 0.937 | 488 | 1.83 | 0.021 |
| Ao root/height, mm/m | 671 | −0.0078 | 0.038 | 517 | 3.06 | 0.170 |
Ell Srs – longitudinal peak systolic strain rate; Ecc Srs – circumferential peak systolic strain rate; Err Srs – radial peak systolic strain rate; LVEF – left ventricular ejection fraction; Ao – aortic. Models adjusted for age, total brain volume, sex, race, total education, systolic blood pressure, use of medication for hypertension, diabetes, heart rate, smoking status, and BMI.
Early LV dysfunction assessed by decreased radial deformation (less positive Err Srs) showed association to reduction in grey matter cerebral blood flow, as well as a trend toward decreased longitudinal deformation (less negative Ell Srs).
Discussion
In a large biracial cohort of healthy mid-aged adults, we present a broad investigation of subclinical cerebrovascular/cardiovascular diseases associations derived from a comprehensive assessment of cardiac mechanics and remodeling with white matter and grey matter cerebral abnormalities assessed by advanced brain imaging techniques. Our exploratory analyses show that incipient brain imaging alterations are associated with early LV dysfunction, but not with LV ejection fraction. The ability to detect these associations before clinical manifestations of cardiac or cerebral disease suggests at some extent cardiac dysfunction and brain abnormalities evolve simultaneously.
Cerebral hypoperfusion on a consequence of low cardiac output is a major determinant of brain abnormalities in advanced HF, although other factors are thought to be involved in this process.[19] This is underscored by the fact that not all patients with advanced HF who undergo heart transplantation recover cerebral perfusion to values associated with a normal cardiac output.[20] However, it is still unclear how cerebral hypoperfusion relates to chronic heart failure, particularly in early stages, as compensatory mechanisms are activated. In fact, cerebral hypoperfusion appears to be more related to acute HF when compared to chronic patients.[21]
Echocardiography techniques have shown an important role in the assessment of patients after stroke.[22] However, its ability to stratify stroke risk in subclinical phases is still unclear. Speckle tracking echocardiography is an accurate method to evaluate subclinical cardiac disease and has shown ability to predict CV events in diverse clinical settings.[23, 24] Recently, MRI-derived brain abnormalities have been associated to subclinical decreases in longitudinal strain.[25] The effects of an exposition to cardiovascular risk factors in middle-aged adults on heart and brain subclinical abnormalities is still unclear, but may be important in global risk prediction.[26] A comprehensive assessment of cardiac mechanics beyond longitudinal deformation may provide important insights in how subclinical abnormalities in the brain and in the heart relate.
Several pathways proposed in studies of patients with HF might help explain our findings. In clinical HF patients, chronic exposure to underperfusion and tissue hypoxemia may lead to axon and myelin alterations assessed by water diffusion MRI techniques.[6] FA reflects early abnormalities of white matter microstructure and is associated in our study with reduced LV circumferential strain rate. Importantly, other mechanisms, beyond hypoxia and hypoperfusion, may be related to FA changes in early subclinical cerebral abnormalities, such as demyelination and/or loss of tissue organization.
In our cohort of healthy participants, brain white matter microstructural integrity showed consistent associations with aortic root dimensions. Increases in the aortic root diameter have shown strong association with brain structural abnormalities and cognitive impairment.[27] Aortic root enlargement appears to be related to alterations of vascular elasticity in different populations.[28] Aortic root enlargement has shown to relate with high blood pressure, and low pulse pressure.[29] MRI-derived aortic stiffness and pulse wave velocity have shown to relate with early brain abnormalities.[30–32] Moreover, dilation of the aorta associates with atherosclerosis along the vessel, which is related to brain abnormalities.[33] Although our study is limited in its assessment of vascular elasticity, the findings suggest that vascular properties, as measured by aortic root dimensions, may relate to brain white matter abnormalities independent of traditional risk factors.
In clinical HF patients, a lower cerebral perfusion is associated with disease severity and long-term prognosis.[34] Cortical areas may be particularly affected by reduced brain perfusion in clinical HF, possibly leading to cognitive impairment.[35, 36] Our results, in a healthy biracial cohort of middle-aged men and women, show that LV early dysfunction was still associated to an impaired grey matter perfusion after adjustment to anthropometrics and CV risk factors. Common factors may simultaneously increase the risk for cardiac dysfunction and reduced cerebral perfusion in early stages.
Interestingly, lower white matter fractional anisotropy was associated with reduced circumferential strain whereas decreased grey matter cerebral blood flow was associated with reduced radial strain. We hypothesize based on previous studies that compensative mechanisms may have been activated to maintain the cardiac output stable. In early subclinical phases of LV systolic dysfunction, radial deformation may be maintained secondary to an increased LV torsion that restores LVEF in the face of reduced circumferential deformation.[37] Different areas in the brain may be affected in each phase of subclinical cardiac dysfunction. These compensatory mechanisms may also explain why LVEF did not relate to subclinical brain abnormalities in our study. Although causal inference is not possible in this cross-sectional exploratory investigation, deleterious cerebral hypoperfusion appears to be prevented at some extent by cardiac compensating mechanisms, when subclinical heart dysfunction is present.
This study is an exploratory investigation on early stages heart and brain concomitant abnormalities and has several limitations. Also, our findings need to be replicated in different populations for external validity.
In a large biracial cohort of healthy middle-aged men and women, we demonstrate that, in spite of no apparent effect of LV ejection fraction, parameters of early subclinical cardiac dysfunction are independently related to cerebral white matter microstructural abnormalities and grey matter perfusion, as assessed by advanced brain MRI techniques. Our findings indicate that early cardiac and cerebrovascular alterations are present and associated in middle-aged otherwise healthy individuals.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Sources of Funding: This work was supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham [HHSN268201300025C & HHSN268201300026C], Northwestern University [HHSN268201300027C], University of Minnesota [HHSN268201300028C], Kaiser Foundation Research Institute [HHSN268201300029C], and Johns Hopkins University School of Medicine [HHSN268200900041C]. CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging. Dr Armstrong was funded by Universidade Federal do Vale do São Francisco [Petrolina, Brazil] and by the Johns Hopkins University [Baltimore, Maryland]. There are no relations with industries to disclose in this study.
Footnotes
Disclosures: This manuscript has no relevant conflict of interest. This manuscript has been reviewed by CARDIA for scientific content.
References
- 1.Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. doi: 10.1161/STROKEAHA.111.628479. [DOI] [PubMed] [Google Scholar]
- 2.Warsch JR, Wright CB. The aging mind: vascular health in normal cognitive aging. J Am Geriatr Soc. 2010;58(Suppl 2):S319–324. doi: 10.1111/j.1532-5415.2010.02983.x. [DOI] [PubMed] [Google Scholar]
- 3.Pozuelo L. Fine-tuning a heart-brain connection: anxiety in atrial fibrillation. Circ Heart Fail. 2012;5:307–308. doi: 10.1161/CIRCHEARTFAILURE.112.967778. [DOI] [PubMed] [Google Scholar]
- 4.Morris DA, Boldt LH, Eichstadt H, et al. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5:610–620. doi: 10.1161/CIRCHEARTFAILURE.112.966564. [DOI] [PubMed] [Google Scholar]
- 5.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10:e0122138. doi: 10.1371/journal.pone.0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar R, Woo MA, Macey PM, et al. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011;307:106–113. doi: 10.1016/j.jns.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luijckx GJ, van den Berg MP. Heart failure and the brain, a wake-up call. Eur J Heart Fail. 2011;13:597–598. doi: 10.1093/eurjhf/hfr055. [DOI] [PubMed] [Google Scholar]
- 8.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong AC, Ricketts EP, Cox C, et al. Quality Control and Reproducibility in M-Mode, Two-Dimensional, and Speckle Tracking Echocardiography Acquisition and Analysis: The CARDIA Study, Year 25 Examination Experience. Echocardiography. 2015;32:1233–1240. doi: 10.1111/echo.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Moreira HT, Nwabuo CC, Armstrong AC, et al. Reference Ranges and Regional Patterns of Left Ventricular Strain and Strain Rate Using Two-Dimensional Speckle-Tracking Echocardiography in a Healthy Middle-Aged Black and White Population: The CARDIA Study. J Am Soc Echocardiogr. 2017;30:647–658 e642. doi: 10.1016/j.echo.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldszal AF, Davatzikos C, Pham DL, et al. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 16.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Pallas F, Larson DF. Cerebral blood flow in the diabetic patient. Perfusion. 1996;11:363–370. doi: 10.1177/026765919601100502. [DOI] [PubMed] [Google Scholar]
- 20.Gruhn N, Larsen FS, Boesgaard S, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 21.Tavazzi L, Senni M, Metra M, et al. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473–481. doi: 10.1161/CIRCHEARTFAILURE.112.000161. [DOI] [PubMed] [Google Scholar]
- 22.Pallesen LP, Ragaller M, Kepplinger J, et al. Diagnostic Impact of Transesophageal Echocardiography in Patients with Acute Cerebral Ischemia. Echocardiography. 2016;33:555–561. doi: 10.1111/echo.13131. [DOI] [PubMed] [Google Scholar]
- 23.Collier P, Phelan D, Klein A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Haberka M, Liszka J, Kozyra A, et al. Two-dimensional speckle tracking echocardiography prognostic parameters in patients after acute myocardial infarction. Echocardiography. 2015;32:454–460. doi: 10.1111/echo.12666. [DOI] [PubMed] [Google Scholar]
- 25.Russo C, Jin Z, Homma S, et al. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128:1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamberg F, Parhofer KG, Lochner E, et al. Diabetes mellitus: long-term prognostic value of whole-body MR imaging for the occurrence of cardiac and cerebrovascular events. Radiology. 2013;269:730–737. doi: 10.1148/radiol.13130371. [DOI] [PubMed] [Google Scholar]
- 27.Paul RH, Gunstad J, Poppas A, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovasc Dis. 2005;20:129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong WY, Wong WH, Chiu CS, et al. Aortic root dilation and aortic elastic properties in children after repair of tetralogy of Fallot. Am J Cardiol. 2006;97:905–909. doi: 10.1016/j.amjcard.2005.09.141. [DOI] [PubMed] [Google Scholar]
- 29.van Rossum AC, Bedaux WL, Hofman MB. Morphologic and functional evaluation of coronary artery bypass conduits. J Magn Reson Imaging. 1999;10:734–740. doi: 10.1002/(sici)1522-2586(199911)10:5<734::aid-jmri18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.van Elderen SG, Brandts A, van der Grond J, et al. Cerebral perfusion and aortic stiffness are independent predictors of white matter brain atrophy in type 1 diabetic patients assessed with magnetic resonance imaging. Diabetes Care. 2011;34:459–463. doi: 10.2337/dc10-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King KS, Chen KX, Hulsey KM, et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267:709–717. doi: 10.1148/radiol.13121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandts A, van Elderen SG, Westenberg JJ, et al. Association of aortic arch pulse wave velocity with left ventricular mass and lacunar brain infarcts in hypertensive patients: assessment with MR imaging. Radiology. 2009;253:681–688. doi: 10.1148/radiol.2533082264. [DOI] [PubMed] [Google Scholar]
- 33.Arangalage D, Ederhy S, Dufour L, et al. Relationship between cognitive impairment and echocardiographic parameters: a review. J Am Soc Echocardiogr. 2015;28:264–274. doi: 10.1016/j.echo.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Kim JS, Yun SC, et al. Association of cerebral blood flow with the development of cardiac death or urgent heart transplantation in patients with systolic heart failure. Eur Heart J. 2012;33:354–362. doi: 10.1093/eurheartj/ehr345. [DOI] [PubMed] [Google Scholar]
- 35.Almeida OP, Garrido GJ, Beer C, et al. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012;33:1769–1776. doi: 10.1093/eurheartj/ehr467. [DOI] [PubMed] [Google Scholar]
- 36.Alves TC, Rays J, Fraguas R, Jr, et al. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. J Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- 37.Yoneyama K, Gjesdal O, Choi EY, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126:2481–2490. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]


