Abstract
M1 macrophages release pro-inflammatory factors during inflammation. They transition to an M2 phenotype and release anti-inflammatory factors to resolve inflammation. An imbalance in the transition from M1 to M2 phenotype in macrophages contributes to the development of persistent inflammation. CD163, a member of the scavenger receptor cysteine-rich family, is an M2 macrophage marker. The functional role of CD163 during the resolution of inflammation is not completely known. We postulate that CD163 contributes to the transition from M1 to M2 phenotype in macrophages. We induced CD163 gene in THP-1 and primary human macrophages using polyethylenimine nanoparticles grafted with a mannose ligand (Man-PEI). This nanoparticle specifically targets cells of monocytic origin via mannose receptors. Cells were challenged with a single or a double stimulation of lipopolysaccharide (LPS). A CD163 or empty plasmid was complexed with Man-PEI nanoparticles for cell transfections. Quantitative RT-PCR, immunocytochemistry, and ELISAs were used for molecular assessments. CD163-overexpressing macrophages displayed reduced levels of tumor necrosis factor-alpha (TNF)-α and monocytes chemoattractant protein (MCP)-1 after a single stimulation with LPS. Following a double stimulation paradigm, CD163-overexpressing macrophages showed an increase of interleukin (IL)-10 and IL-1ra, and a reduction of MCP-1. This anti-inflammatory phenotype was partially blocked by an anti-CD163 antibody (effects on IL-10 and IL-1ra). A decrease in the release of TNF-α, IL-1β, and IL-6 was observed in CD163-overexpressing human primary macrophages. The release of IL-6 was blocked by an anti-CD163 antibody in the CD163-overexpressing group. Our data show that the induction of the CD163 gene in human macrophages under inflammatory conditions produces changes in cytokine secretion in favor of an anti-inflammatory phenotype. Targeting macrophages to induce CD163 using cell-directed nanotechnology is an attractive and practical approach for inflammatory conditions that could lead to persistent pain, i.e. major surgeries, burns, rheumatoid arthritis, etc.
Keywords: CD163, transfection, primary human macrophages, THP-1, lipopolysaccharide, inflammation
1. Introduction
Persistent inflammation can promote the development of chronic diseases, such as rheumatoid arthritis, osteoarthritis, metabolic syndrome-associated disorders (including type 2 diabetes and atherosclerosis), Alzheimer’s disease, asthma, or Crohn’s disease (Libby, 2006, Okin and Medzhitov, 2012, Wong and Lord, 2004). Most of these chronic inflammatory diseases result in persistent pain due to the sensitization of peripheral terminals and a subsequent central sensitization. The research in this field has been mainly focused on reducing pro-inflammatory final effectors (cytokines, prostaglandins, etc.), and, therefore, the current therapies (i.e. glucocorticoids, antibodies against cytokines, COX inhibitors, etc.) are based on the inhibition, specifically or globally, of acute pro-inflammatory mediators. These therapies have been shown to improve the quality of life of patients suffering from chronic inflammatory diseases, but they have many downfalls. For example, they produce multiple side effects, are not effective for all patients, and, most importantly, do not target the underlying causes of the disorder. Even though there have been significant advances in the identification of anti-inflammatory effectors, our understanding of the upstream molecular signaling that drives, promotes, or alters the normal resolution of inflammation is not complete. Therefore, the elucidation of the molecular mechanisms that drive the normal resolution of inflammation will provide the foundation to develop a novel and promising therapeutic approach aiming to restore tissue homeostasis.
Resolution of inflammation is an active and coordinated process preceded by an early cytodestructive phase (Serhan, 2011, Serhan, et al., 2007). Cells of the monocyte/macrophage lineage have been recognized as essential in both phases (Wynn, et al., 2013, Dunster, 2016). Usually, macrophages are divided into 2 categories (M1 and M2), each of which is predominant in the initiation or resolution of inflammation (Martinez and Gordon, 2014). The M1/M2 dichotomy is based on the response of macrophages to an external stimuli (Martinez and Gordon, 2014). For example, the presence of lipopolysaccharide (LPS), interferon-gamma or tumor necrosis factor-alpha (TNF-α) polarize macrophages towards and M1 phenotype. This macrophage phenotype produces pro-inflammatory cytokines (interleukin [IL]-1, IL-6, IL-12, TNF-α, etc.) and oxidative metabolites (nitric oxide and superoxide) that promote host defense and removal of damaged tissue (Gordon, 2003, Gordon and Taylor, 2005, Roszer, 2015). In contrast, macrophages with an M2 phenotype (usually labeled as anti-inflammatory) represent a heterogeneous population of macrophages composed by multiple sub-groups or sub-phenotypes (Martinez and Gordon, 2014). For instance, the M2a macrophage phenotype is characterized by the cellular surface expression of CD163/CD206 (Martinez and Gordon, 2014). Macrophages that adopt an M2b phenotype express markers such as CD86 and class II major histocompatibility complex (MHCII), and macrophages that adopt an M2c phenotype express signaling lymphocyte-activation molecule (SLAM) (Martinez and Gordon, 2014). All these M1/M2 hallmarks do not necessarily exclude each other and usually coexist and adapt depending of the stimulus or the conditions of the system (Davis, et al., 2013). Until date, all functions of these markers and phenotypes are not thoroughly understood. When macrophages are unable to change their phenotype from M1 to M2, inflammation does not resolve, becoming persistent or chronic (Nathan and Ding, 2010). Therefore, therapies that specifically target macrophages could result in the resolution of inflammation or pain. In fact, promoting an M2 phenotype has been shown to decrease pain-related behaviors in murine models of pain (Hasegawa-Moriyama, et al., 2012, Kiguchi, et al., 2015) This study focuses on exploring the functions of CD163 in human macrophages phenotype beyond its use as a phenotype marker. To our knowledge, the functional capabilities of CD163 in the acquisition of an M2 phenotype in macrophages have not been explored.
CD163 was first described as a cysteine–rich scavenger receptor exclusively expressed in M2 macrophages (Zwadlo, et al., 1987). In fact, CD163 binds to the hemoglobin-haptoglobin complex (Hb-Hp) (Kristiansen, et al., 2001), which degradation causes the release of compounds with anti-oxidative and anti-inflammatory effects (i.e. IL-10, free iron, biliverdine, and carbon monoxide) (Otterbein, et al., 2003, Stocker, et al., 1987, Otterbein, et al., 2000). Moreover, the expression of CD163 in circulating human monocytes is associated with the resolution phase of inflammation following major surgeries (Philippidis, et al., 2004). Similarly, CD163-positive macrophages are a hallmark of wound healing in humans (Evans, et al., 2013). Although this evidence shows that CD163 is a definitive marker of an M2 macrophage phenotype associated with the resolution of inflammation, the cause-effect relationship between CD163 and the resolution of inflammation has not yet been explored. Thus, we hypothesize that the specific induction of CD163 gene expression in human macrophages promotes a transition from an M1 to an M2 macrophage phenotype. To test our hypothesis, we used THP-1 and primary human macrophages stimulated with LPS. First, we investigated whether the specific blockade of CD163, using a specific antibody delayed the reduction of pro-inflammatory cytokines or the increase of anti-inflammatory cytokines. Second, we overexpressed the CD163 gene using Man-PEI nanoparticles and measured cytokine secretion as functional outcomes to determine M1 and M2 phenotypes. Third, we confirmed the CD163-dependence of the observed effects by blocking the CD163 receptor using a specific antibody.
One of the translational and clinically relevant features of our study is the utilization of a novel cell-directed gene induction technique using nanotechnology. Specifically, we used polyethyleneimine (PEI) grafted with a mannose receptor ligand (Man-PEI). This feature makes Man-PEI a powerful tool to develop a specific cell (macrophage)-directed gene therapy. Man-PEI preferentially targets monocytes, since these are the only cells that express mannose receptors. In addition to this clear advantage of our approach, Man-PEI has other superior features when compared to other techniques, such as naked DNA, electroporation, lipoplexes, diethylaminoethyl-dextran, or viruses. These alternative techniques produce low transfection efficiencies, short-lasting transfections, cytotoxicity, and immunogenic responses. We have previously demonstrated that Man-PEI efficiently induces different genes in human macrophages under inflammatory conditions without inducing cytotoxicity or significant immunogenicity (Bernal, et al., 2016). Furthermore, this nanoparticle possesses a strong clinical background, since it has been successfully used to induce HIV gene antigens, which activate monocytes from HIV-positive patients, improving clinical outcomes (Lisziewicz, et al., 2012, Rodriguez, et al., 2013).
2. Material and Methods
2.1. THP-1 cell culture conditions, cell differentiation and lipopolysaccharide (LPS) stimulation
Immortalized human acute monocytic leukemia cell line (THP-1) was cultured in Roswell Park Memorial Institute 1640 media (RPMI 1640. Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were plated in 75 cm2 flasks at 8 × 104 cells/cm2 and incubated at 37°C in a 5% CO2 atmosphere. The viability of THP-1 cells was monitored in each experiment using the Trypan blue assay (dye exclusion method). Briefly, the cell suspension was diluted at a 1:1 ratio and incubated at room temperature with a Trypan blue dye (Life Technologies, Grand Island, NY) for one minute. Dead cells were identified as Trypan blue-positive cells. Cells were counted using a hemocytometer (VWR International, Wayne, PA).
THP-1 monocytes were differentiated into macrophages by incubating them with 60 ng/mL phorbol-12-myristate-13-acetate (PMA, Sigma, St. Louis, MO) for 36 hours (37°C, 5 % CO2) (Landry, et al., 2012). Cells were seeded on 24-well plates (250,000 cells/mL per well) for 1 hour and then stimulated with 5 μg/mL of LPS (Escherichia coli O111:B4, Sigma).
Based on our previous work, two stimulation paradigms were utilized: single stimulation (acute inflammation paradigm) and double stimulation (sub-acute stimulation paradigm) (Bernal, et al., 2016). For the single stimulation experiments, THP-1 macrophages were incubated from 24 to 96 hours after a single LPS stimulation (5 μg/mL). For double stimulation experiments, THP-1 macrophages were incubated for 48 hours after a single LPS stimulation (5 μg/mL). Then, supernatants were removed and fresh media was added before the second challenge with LPS (5 μg/mL). The time point for a second stimulation (48 hours) was chosen based on the time point in which gene overexpression is consistent (Bernal, et al., 2016). Subsequently, cells and/or supernatants were collected at 4 and 24 hours after the second LPS stimulation. In both paradigms, cells were transfected with a plasmid that encodes for the CD163 gene (pCD163) or an empty vector plasmid (pEmpty) at the same time as the first LPS stimulation.
2.2. Primary monocytes cell culture and stimulation
Human peripheral blood CD14+ monocytes were purchased from LONZA (Lonza Walkersville, MD). Upon arrival, aliquots were thawed at 37°C and transferred to 50 mL conical tubes. Cells were washed twice with 10 mL of supplemented RPMI (10% FBS, 1% penicillin/streptomycin) and 20 U/mL of DNase I (Sigma Aldrich, St. Louis, MO), followed by centrifugation at 200 × g for 15 min. Cells were then re-suspended in RPMI containing 10% FBS, 1% penicillin/streptomycin, 1% sodium pyruvate, and 100 ng/mL of recombinant human M-CSF (eBioscience, San Diego, CA). M-CSF was used to differentiate monocytes into macrophages. Primary cells were cultured for 5–6 days in a 75-cm2 tissue culture flask at 37°C with 5% of CO2. Macrophages were harvested, and non-adherent cells and media were removed. The cells remaining in the culture flask were detached by adding 12 mL of trypsin for 30 min (37 °C, 5% CO2). Cells were plated at 250,000 cells/mL in 24-well plates, seeded for 1 hour, stimulated with LPS (5 μg/mL, Escherichia coli O111:B4, Sigma), and transfected with a plasmid containing the CD163 gene or an empty vector. Supernatants and cells were harvested at 48 and 96 hours after LPS stimulation and stored at −80°C until used.
2.3. Cell transfection using Man-PEI nanoparticles
Transfection of both THP-1 macrophages and primary human macrophages were performed using a nanoparticle (polyethylenimine, PEI) grafted with a mannose receptor ligand (Man-PEI; Polyplus Transfection, New York, NY). The Man-PEI nanoparticle was complexed with a cDNA plasmid using a pCMV6-XL4 vector, following the manufacturer’s instructions. A nitrogen per DNA phosphate (N/P) ratio of 5 was used, since these conditions induce efficient gene induction without cytotoxicity, as demonstrated elsewhere (Lisziewicz, et al., 2001). We have confirmed these findings in our laboratory with human macrophages under inflammatory conditions using LPS as stimulus (Bernal, et al., 2016). A working mixture of 1 μL of Man-PEI nanoparticle and 50 μL of NaCl (150 mM) was used to prepare the cell transfection. This solution was mixed with either a CD163 plasmid (pCD163, 0.5 μg of plasmid in 50 μL of NaCl) or an empty vector plasmid as a negative control (pEmpty L of NaCl) or an empty vector plasmid as a negative control (pEmpty, 0.5 μg of plasmid in 50 μL of NaCl), and incubated for 30 minutes at room temperature. The plasmids were purchased from Origene (Rockville, MD). Then, 100 μL of the Man-PEI + plasmid preparation were added to primary macrophages or THP-1 macrophages (in 24-well plates, 250,000 cells/well). Lipopolysaccharide (single stimulation, or first stimulation in double stimulation paradigm) was added to cells concomitantly with the Man-PEI + plasmid complexes. For a main control group, the Man-PEI nanoparticle alone (1 μL of Man-PEI in 100 μL of 150 nM NaCl), non-complexed with plasmids, was added to THP-1 macrophages stimulated with LPS. This group was used to normalize the Man-PEI + pCD163 and Man-PEI + pEmpty groups in THP-1 macrophages. Since Man-PEI did not show a significant effect in human THP-1 macrophages (Bernal, et al., 2016) and our access to primary monocytes was limited, all values in the Man-PEI + pCD163 group for primary human macrophages were normalized against the Man-PEI + pEmpty group, which was given a value equal to 1.
2.4. RNA isolation and quantitative real time-PCR
Cells were collected at different time points following single and double LPS stimulation paradigms. For the single stimulation paradigm, collections were performed at 24, 48, 72, and 96 hours. For the double stimulation paradigm, collections were performed at 4 and 24 hours after the second stimulus. Cells were collected using BL+TG buffer and stored at −80°C. The RNA was isolated from THP-1 macrophages and primary macrophages using ReliaprepTM RNA Cell Miniprep System (Promega, Madison, WI), according to the manufacturer’s protocols. Levels of mRNA were determined as described previously (Ndong, et al., 2012). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA from each sample using Script Reverse Transcription Supermix (BioRad, Hercules, CA) under the following conditions: 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. We quantified the expression of TNF-α (59°C), IL-6 (60°C), IL-10 (59°C), CD163 (57°C), and β-actin (57°C) using the SsoAdvanced Universal SYBR Green Supermix (BioRad) under the following conditions: 1 cycle of 98 °C for 30 sec, 45 cycles of 98 °C for 15 sec, followed by 30 sec of the primer-specific annealing temperature. The primers for quantitative PCR are shown in table 1. All samples were run in duplicate using the CFX96 Real-Time PCR system (Bio-Rad, Hercules, CA). The expression of mRNA for our molecules of interest was normalized in each sample using the β-actin expression. To determine the fold change of each gene we used the ddCt method, as previously described (Livak and Schmittgen, 2001).
Table 1.
Primers used for RT-PCR analyses.
| PCR Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-6 | ATGCAATAACCACCCCTGAC | GAGGTGCCCATGCTACATTT |
| IL-10 | CATCGATTTCTTCCCTGTGAA | TCTTGGAGCTTATTAAAGGCATTC |
| TNF-α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| CD163 | GCTACATGGCGGTGGAGACAA | ATGATGAGAGGCAGCAAGATGG |
| β-actin | AGAGCTACGAGCTGCCTGAC | AGCACTGTGTTGGCGTACAG |
2.5. Immunocytochemistry and fluorescence microscopy
The protein expression of CD163 (intracellular and membrane-anchored form) and CD206 (a marker for mannose receptor) was determined by immunocytochemistry and fluorescence microscopy. Briefly, a rounded coverslip (12 mm diameter) was placed into the wells of a 12-well plate. Fibronectin (500 μg/μl, Sigma Aldrich, St. Louis, MO) was added to each well and incubated for 12 hours at 4°C. The fibronectin was removed, and the coverslips were washed with PBS. Then, cells were seeded (250,000 cells/well/mL) and incubated for 1 hour before stimulation with LPS, using the single stimulation paradigm. The following treatments were added concomitantly with LPS: Man-PEI alone, Man-PEI + pCD163, or Man-PEI + pEmpty. Cell cultures under these conditions were incubated for 48, 72, and 96 hours. We based our time points for these experiments on our mRNA results following pCD163 induction (see the results section), in which CD163 mRNA expression was significantly increased at 48 hours and remained elevated until 96 hours of incubation. Following these incubation times, cells were fixed with 4% formaldehyde/PBS solution for 30 minutes at room temperature, then rinsed and permeabilized with 0.25% Triton-X100 in PBS for 5 minutes (room temperature). Non-specific antibody binding was prevented by adding 0.5% fetal bovine serum to the cells for 1 hour at room temperature. Cells were then incubated overnight at 4°C with a mouse antibody against human CD163 (Serotec, Raleigh NC, 1:150) and a rabbit antibody against human CD206 (Abcam, Cambridge MA, 1:250). Then, cells were rinsed and incubated for 1 hour at room temperature with the proper secondary antibodies. The secondary antibodies used were: anti-mouse secondary antibody raised in goat and conjugated to Alexa 555 (Life technologies, Grand Island, NY, 1:1000), and anti-rabbit secondary antibody raised in donkey and conjugated to Alexa 488 (Life technologies, Grand Island, NY, 1:1000). The antibodies and the blocking solution were diluted in PBS that contained 0.25% Triton-X100. Coverslips containing cells were mounted on slides using an anti-fade medium (Vectashield; Vector Laboratories, Burlingame CA, USA) containing 4′, 6-diamidino-2-phenylindole dihydrochloridehydrate (DAPI, Sigma, St. Louis, MO) to allow visualization of cell nuclei (blue fluorescence). Slides were examined using a Leica DMIL microscope (Model: 11521258) and a Leica DFC345 FX Digital Camera (Leica Microsystems Inc., Buffalo Grove, IL). The percent of CD163 or CD206 positive cells was determined by quantifying the percentage of red or green cells respectively, based on the DAPI positive cells in six random fields (40X) per slide. Fluorescence intensities for CD163 or CD206 were quantified in each individual cell analyzed within its local region of interest. The mean background intensity was calculated from areas outside of the cell perimeter. The average intensity per cell was determined after background subtraction using Sigma Scan Pro software (Systat Software Inc., San Jose, CA).
2.6. ELISA and Multiplex assays
Cells were cultured as mentioned above, and supernatants from all groups were collected at different time points following single or double LPS stimulation paradigms. Supernatants were collected at 24, 48, 72, and 96 hours for the single stimulation paradigm, while collections were made at 4 and 24 hours for the double stimulation paradigm. Supernatants were collected and stored at −80°C until enzyme-linked immunosorbent assays (ELISA) were performed. Tumor necrosis factor-alpha (TNF-α), interleukin (IL)-10, IL-6 and transforming growth factor-beta (TGF-β) concentrations were measured with commercial sandwich ELISA kits (Human TNF-α/IL /IL-10/IL-6/TGF-βELISA Ready-SET-Go!, eBioscience, San Diego, CA) in stored supernatants. The sensitivity of these ELISA kits was 4 pg/mL for human TNF-α, 8 pg/mL, 8 pg/mL for human TGF-β, and 2 pg/mL for human IL-6 and IL-10. The levels of the soluble form of CD163 (sCD163) and IL-1 receptor antagonist (IL-1ra) were also measured with a commercial ELISA kits (Human CD163/IL-ra ELISA kit, R&D systems, McKinley Place, MN). The sensitivity of the sCD163 ELISA kit was 0.613 ng/mL, while the sensitivity of the IL-1ra kit was 1.83 pg/mL. The ELISA assays were performed, following the manufacturer’s instructions. We also quantified the levels of IL-4, IL-1ra, and IL-1β by Multiplex assays (Human-3 plex HCYTOMAG-60K kit, MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel, Millipore Billerica, MA) using the Luminex 100 IS system (Luminex, Austin, TX) for detection and analysis of the samples. The detection sensitivity for IL-1ra, IL-1β, and IL-4 were 1.8 pg/mL, 2.4 pg/mL, and 2.7 pg/mL, respectively. Levels of IL-4 for all samples are not shown because all values were under the detection limit of the standard curve.
2.7. Westernblot analysis
Confluent THP-1 monocytes were differentiated into macrophages as described before. THP-1 macrophages were counted and plated at a density of 5 × 105 cells/mL. The cells were transfected as previously described using Man-PEI nanoparticles complexed with an empty vector (pEmpty) or a plasmid that encodes de CD163 gene (pCD163). The nanoparticles alone were added to a group of cells as control. Cell lysates were collected 48 hours after the transfection using 200 μl of Rippa buffer (Invitrogen, Carlsbad, CA) containing protease inhibitor (Sigma Aldrich, St. Louis, MO). The samples were sonicated three times for five seconds and then centrifuged at 10,000 g × 10 min at 4°C. The protein rich supernatant was used to assess the expression of CD163 by Westernblot. Samples and standard protein markers were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gels, Bio-Rad, Hercules, CA) and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Non-specific binding was prevented by blocking the membrane with 5% bovine serum albumin in Tris-buffered saline for 1 hour. Then, membranes were incubated overnight with a mouse anti-human CD163 antibody (1:200, clone EDHu-1, Bio-Rad, Hercules, CA). The next day, blots were incubated for 1 h at room temperature with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:3000; KPL, Gaithersburg, MD). The signal was visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher, Waltham, MA) and imaged using the Li-cor C-DiGit scanner (Li-cor, Lincon, NE). Blots were stripped (10 min at room temperature) and re-probed with a mouse anti-Beta-Actin antibody incubated overnight at 4°C (1:3000; Sigma Aldrich, St. Louis, MO). This protein was used as the loading control. The next day, blots were incubated for 1 h at room temperature with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:3000; KPL, Gaithersburg, MD). The signal was visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher, Waltham, MA) and imaged using the Li-cor C-DiGit scanner (Li-cor, Lincon, NE). The density of the bands was measured using Image J software. The ratio of CD163 to the corresponding loading control bands (Beta-Actin) was calculated. Then the relative expression of CD163 was normalized to the pEmpty group, which was assigned a value of 1. Three independent studies were conducted to obtain an n=10 for pCD163 and pEmpty groups. One sample for the nanoparticle group (Man-PEI) was used in each experiment for a total of 3 observations (n=3).
2.8. Functional blockade of CD163
To test whether the normal expression of CD163 in stimulated human macrophages plays a role in the resolution of LPS-induced inflammatory effects, we blocked CD163 with a monoclonal anti-CD163 antibody (Clone RM3/1) that has been shown to inhibit the functional properties of CD163 in monocyte cultures (Philippidis, et al., 2004). We used THP-1 and primary human macrophages challenged with LPS as described above. CD163 was blocked using 20 μg/mL of the anti-CD163 antibody (Clone RM3/1, Santa Cruz Biotechnology, Santa Cruz, CA, sc-33715). For the control group, we used an isotype antibody (Mouse IgG, Santa Cruz Biotechnology, Santa Cruz, sc-3877) 48 hours after the addition of LPS (the time in which CD163 is up-regulated following LPS stimulation). Supernatants were collected 24 hours after the addition of the antibody or isotype control (72 hours after LPS) for further cytokine measurements. This time point after LPS stimulation was selected because pro-inflammatory cytokines are returning to basal levels at this time, and anti-inflammatory cytokines peak following LPS stimulation, as demonstrated in our previous studies (Bernal, et al., 2016).
In another series of experiments, the anti-CD163 antibody was used to block CD163 in THP-1 and primary human cells transfected with the CD163 gene or the empty vector. In the single stimulation paradigm, 20 μg/mL of the anti-CD163 antibody or isotype control was added at 24, 48, and 72 hours after the transfection of THP-1 cells. Supernatants were collected at 96 hours for cytokine ELISA analyses. For the double stimulation paradigm, 20 μg/mL of the anti-CD163 antibody or its isotype control was added concomitantly with the second LPS challenge to THP-1 cells. Supernatants were collected at 4 and 24 hours after the second LPS stimulation for further cytokine measurement, using ELISA analyses. When primary human cells were used, the anti-CD163 antibody (20 μg/mL) or its isotype control was added at 24, 48, and 72 hours after cell transfection. The supernatants were collected at 48 hours and 96 hours after the transfection of primary human macrophages for further cytokine ELISA analyses.
2.9. Statistics
In all studies where THP-1 macrophages were used, the values from pEmpty- and pCD163-transfected cells were normalized against the Man-PEI group. For primary monocytes, all values in the pCD163 group were normalized against the pEmpty-transfected group. In the experiments where the antibody or isotype control was used, all values were normalized against the empty vector group. Statistical analysis was performed using GraphPad Prism 6.01 (GraphPad Software Inc., La Jolla, CA). Unpaired t-test and one-way ANOVA followed by Bonferroni’s post hoc test was used as appropriate. A p<0.05 was considered significant. Data is presented as mean ± standard deviation (SD).
3. Results
3.1. Blockade of CD163 following LPS stimulation
First, anti-inflammatory cytokines were studied in THP-1 macrophages (IL-10 and TGF-β, Fig. 1A and 1B) during the resolution stage of LPS stimulation. We observed that the blockade of CD163 using an anti-CD163 antibody resulted in a reduction of TGF-β (1948.71 ± 713.32 pg/mL) when compared to LPS alone (3079.50 ± 533.03 pg/mL, Fig. 1B). No changes were observed in the levels IL-10 (Fig. 1A). The isotype control antibody did not produce any significant effect when compared to LPS alone group or the anti-CD163 group. Then, pro-inflammatory cytokines were studied in LPS stimulated THP-1 macrophages (TNF-α, MCP-1, IL-1β and IL-6). We observed that the blockade of CD163 resulted in an increase of TNF-α (5769.51 ± 2719.68 pg/mL) when compared to LPS alone (2858.45 ± 1304.04 pg/mL) or the isotype group (3270.79 ± 1352.58 pg/mL) (Fig. 1D); no changes were observed in IL-1, MCP-1 or IL-6 among groups (Fig. 1C, 1E and 1F, respectively).
Figure 1. Changes of cytokine concentration in THP-1 macrophages challenged with a single LPS stimulation and a CD163 antibody.
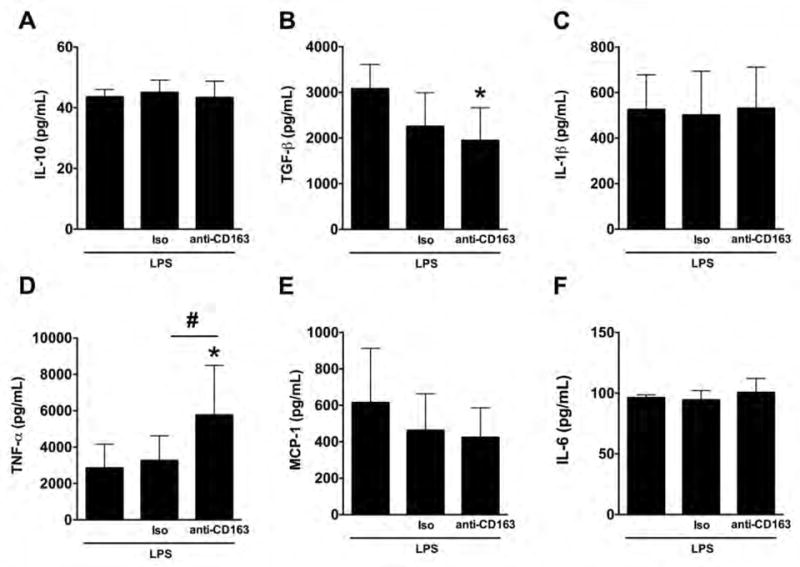
Quantification for IL-10 (A), TGF-β (B), IL-1β (C), TNF-α (D), MCP-1 (E), and IL-6 (F) protein concentration in THP-1 macrophages with a single LPS stimulation and incubated with the anti-CD163 antibody (RM3/1) or its isotype control (Iso) for 24 hours (72 hours after the addition of LPS). N = 6–10 per group. *p<0.05, vs. LPS, #p<0.05, vs. isotype control by student’s t-test.
The enhanced pro-inflammatory phenotype found in LPS-stimulated THP-1 macrophages during the resolution of the inflammatory stage following CD163 blockade was replicated in human primary macrophages. When anti-inflammatory cytokines were studied in human primary macrophages, we observed that the blockade of CD163 did not change the concentration of IL-10 (Fig. 2A) or TGF-β (Fig. 2B) compared to any control group. However, when pro-inflammatory cytokines were studied in human primary macrophages, we observed that the blockade of CD163 resulted in an increase in TNF-α (1393.94 ± 343.67 pg/mL) compared to the isotype group (1033.17 ± 229.83 pg/mL), and reached a statistical difference of P=0.05 when compared to the LPS alone group (1080.55 ± 298.93 Fig. 2D); no changes were observed in IL-1β, MCP-1 or IL-6 among groups (Fig. 2C, 2E and 2F, respectively).
Figure 2. Changes of cytokine concentration in primary human macrophages challenged with a single LPS stimulation and a CD163 antibody.
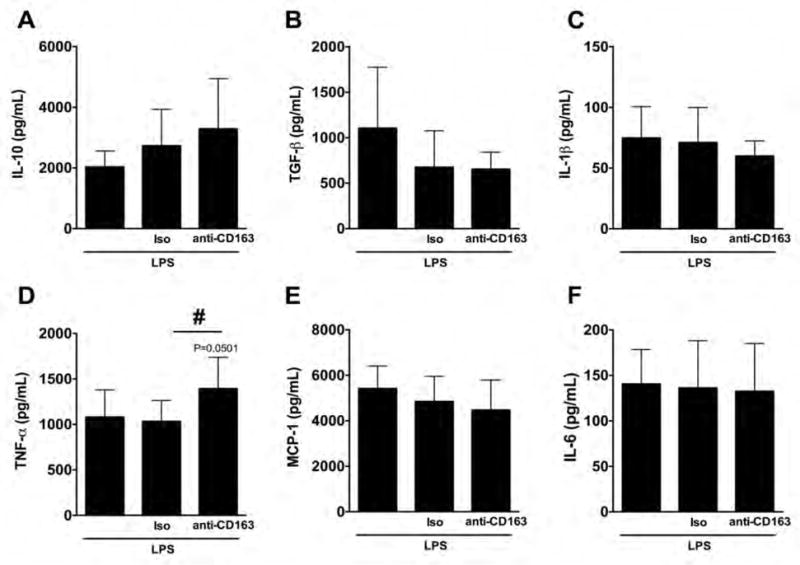
Quantification for IL-10 (A), TGF-β (B), IL-1β (C), TNF-α (D), MCP-1 (E), and IL-6 (F) protein concentration in primary human macrophages with a single LPS stimulation and incubated with the anti-CD163 antibody (RM3/1) or its isotype control (Iso) for 24 hours (72 hours after the addition of LPS). N = 6–10 per group. #p<0.05, vs. isotype control by student’s t-test.
3.2. CD163 overexpression in THP-1 macrophages following LPS stimulation
The transfection of LPS-stimulated THP-1 macrophages (single stimulation) with pCD163 using Man-PEI nanoparticles resulted in a significant increase in CD163 mRNA expression at 48, 72, and 96 hours after transfection compared to pEmpty or Man-PEI group (Fig. 3). The changes observed at the 24-hour incubation time point in the pCD163 group reached a statistical difference of p=0.056 when compared to the pEmpty group.
Figure 3. CD163 mRNA induction in THP-1 macrophages challenged with a single LPS stimulation.
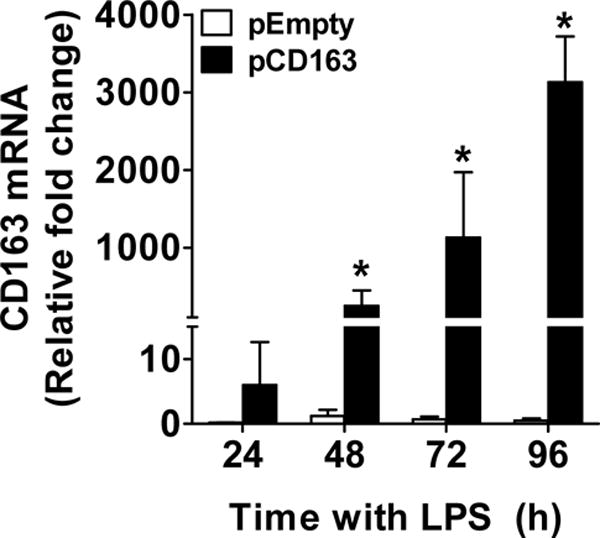
Quantification for CD163 mRNA induction using Man-PEI complexed with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) in LPS-stimulated THP-1 macrophages at 24, 48, 72 and 96 hours after transfection. The expression of CD163 mRNA was normalized to the respective levels of β-actin expression in each group, and then calculated as fold change against the expression of the control group (Man-PEI) at each time point. N = 5–6 per group. *p<0.05 vs. pEmpty by student’s t-test.
We observed that the transfection of THP-1 cells using pCD163 resulted in an overexpression of CD163 at the protein level (fluorescence intensity) at the 48-hour time point compared to the pEmpty or Man-PEI group (Fig. 4A and 4B). We did not observe any significant changes at 72 or 96 hours after transfection. Similarly, when the percent of CD163-positive cells was determined, we found that the percentage of CD163-positive cells in the pCD163 group (75.0±6.3%) was significantly higher than in the pEmpty group (53.0±2.6%) 48 hours after transfection (P<0.05). There were no differences between groups in the number of CD163-positive cells at 72 hours (pCD163 = 46.6±1.8% vs. pEmpty = 39.4±8.3%) and 96 hours (pCD163 = 38.5±7.9% vs. pEmpty = 38.8±2.7%) after transfection. The increase of CD163 protein at the 48-hour time point in the pCD163 group when compared to the pEmpty group was confirmed by Westernblot analysis (Fig. 5).
Figure 4. CD163 protein induction in THP-1 macrophages challenged with a single LPS stimulation.
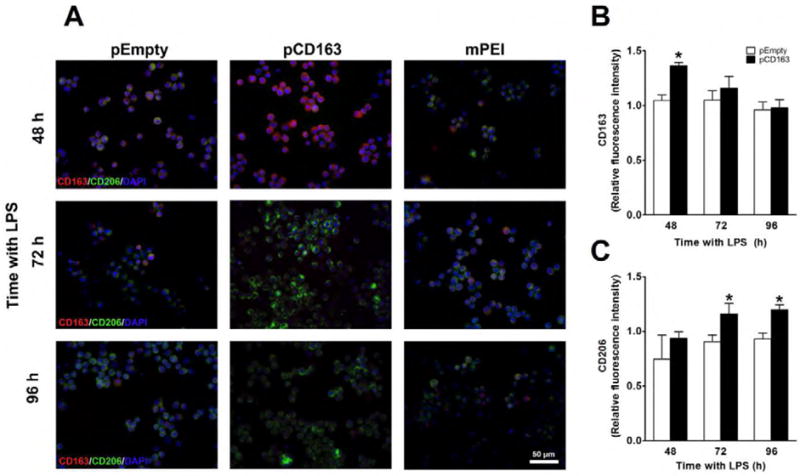
Microscopic images of nuclear staining using DAPI (blue), CD163 protein (red) and mannose receptor (CD206, green) in LPS-stimulated THP-1 macrophages transfected with Man-PEI complexed with an empty vector (pEmpty), a plasmid encoding for CD163 gene (pCD163) or the Man-PEI nanoparticle alone (A). Quantification of the relative average fluorescence intensity of CD163 (B) or mannose receptor (CD206, C) in LPS-stimulated THP-1 macrophages from 48 to 96 hours after transfection. The quantification of the relative average fluorescence intensity was normalized to the respective levels in the Man-PEI group, which was assigned a value equal to 1. N = 3 per group. *p< 0.05 vs. pEmpty by student’s t-test.
Figure 5. CD163 protein induction in THP-1 macrophages challenged with a single LPS stimulation.
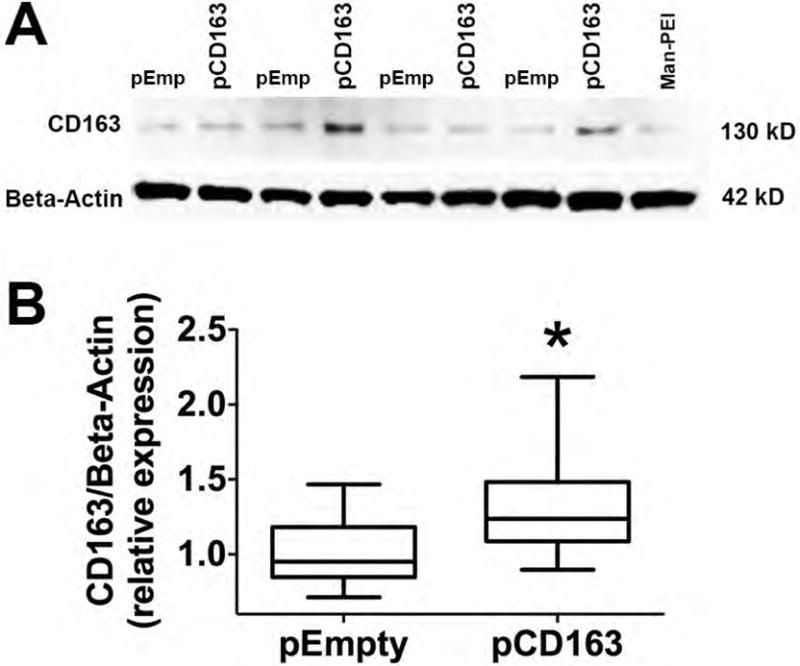
Representative Westernblot images of CD163 and Beta-Actin proteins in LPS-stimulated THP-1 macrophages transfected with Man-PEI complexed with an empty vector (pEmp or pEmpty), a plasmid encoding for CD163 gene (pCD163) or the Man-PEI nanoparticle alone (A). Quantification of the relative expression (band density) of CD163 in LPS-stimulated THP-1 macrophages from 48 hours after transfection (B). The quantification of the relative density was normalized to the respective levels in the pEmpty group, which was assigned a value equal to 1. N = 10 for pCD163 or pEmpty group. *p< 0.05 vs. pEmpty by student’s t-test.
We then confirmed that the induction of CD163 overexpression resulted in the expression of an M2a phenotype macrophage surface marker, CD206. The transfection of LPS-stimulated THP-1 macrophages (single stimulation) with pCD163 resulted in a significant increase in CD206 expression at the protein level (fluorescence intensity) at 72- and 96-hour incubation time points when compared to the pEmpty or Man-PEI group (Fig. 4A and 4C). No changes in CD206 expression were observed at 48 hours after transfection. The percent of CD206 positive cells remained similar in both pCD163 and pEmpty groups at 48 hours (pCD163 = 40.9±3.2% vs. pEmpty = 39.9±1.5%), 72 hours (pCD163 = 91.4±4.3% vs. pEmpty = 89.9±4.2%), and 96 hours (pCD163 = 77.2±4.1% vs. pEmpty = 78.3±4.3%).
We also measured the levels of the soluble form of CD163 (sCD163) after CD163 overexpression. The levels of sCD163 increased in both pCD163 and pEmpty groups at 72 and 96 hours following a single stimulation of LPS, consistent with previous observations (Hintz, et al., 2002, Weaver, et al., 2006), and no differences between groups were observed (Supplementary fig. 1). These data suggest that the levels of cellular CD163 do not necessarily determine the levels of sCD163. We speculate that the overexpression of cellular CD163 does not influence the cleavage mechanisms that promote the shedding of sCD163.
3.3. Cytokine profile of CD163-overexpressing THP-1 macrophages
When anti-inflammatory cytokines were studied in LPS-stimulated CD163-overexpressing THP-1 macrophages, we did not find any change in IL-10 (Fig. 6A), IL-1ra (Fig. 6B), or TGF-β (Fig. 6C) when compared to the pEmpty group at any time point tested. However, CD163-overexpressing macrophages produced lower concentrations of the pro-inflammatory cytokines TNF-α (pCD163 = 0.63 ± 0.10, pEmpty = 0.98 ± 0.10 Fig. 6D) and MCP-1 (pCD163 = 0.97 ± 0.10, pEmpty = 1.19 ± 0.05, Fig. 6E) when compared to the pEmpty group at 96 hours (no changes were found at 24, 48, or 72 hours). No differences in IL-1β (Fig. 6F) and IL-6 (Fig. 6G) were found at any time point between pCD163 or pEmpty groups. We did not detect IL-4 in our system. The addition of the RM3/1 antibody against CD163 (membrane-bound form) or its respective isotype control (48 hours after transfection) did not block effects on TNF-α or MCP-1 observed in CD163-overexpressing cells at 96 hours (data not shown). The effects of CD163 overexpression on the cytokine concentration in THP-1 cells in the single LPS stimulation paradigm are summarized in the table 2.
Figure 6. Changes in cytokine expression in CD163-overexpressing THP-1 macrophages challenged with a single LPS stimulation.
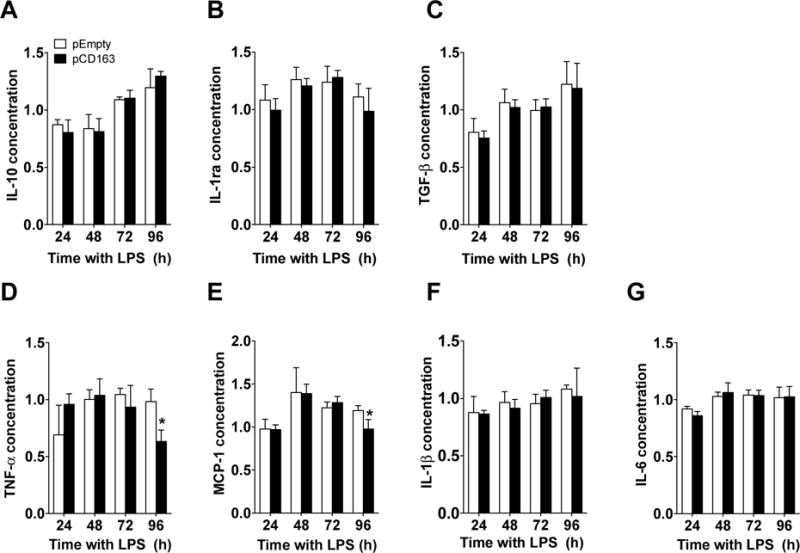
Quantification for IL-10 (A), IL-1ra (B), TGF-β (C), (C), TNF-α (D), MCP-1 (E), IL-1β (F) and IL-6 (G) protein concentration in THP-1 macrophages with a single LPS stimulation and transfected with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) at 24, 48, 72 and 96 hours after transfection. The concentration of each cytokine/chemokine was normalized to the control group (Man-PEI), which was assigned a value equal to 1. N = 4–9 per group. *p<0.05, vs. pEmpty by student’s t-test.
Table 2.
Effects of CD163 and its antibody reversal effects in macrophage cytokines
| Cytokines -THP-1, LPSx1 |
Effect of pCD163 | Reversal effect of anti-CD163 |
|---|---|---|
| TNF-α | ↓ | (−) |
| MCP-1 | ↓ | (−) |
| Cytokines -THP-1, LPSx2 |
Effect of pCD163 | Reversal effect of anti-CD163 |
|---|---|---|
| IL-10 | ↑ | (+) |
| IL-1ra | ↑ | (+) |
| MCP-1 | ↓ | (−) |
| Cytokines -Primary Macrophages |
Effect of pCD163 | Reversal effect of anti-CD163 |
|---|---|---|
| IL-10 | ↓ | (−) |
| TNF-α | ↓ | (−) |
| IL-1β | ↓ | (−) |
| IL-6 | ↓ | (+) |
Note: LPSx1 = LPS single stimulation paradigm, LPSx2 = LPS double stimulation paradigm, ↑ = Significant increase expression, ↓ = Significant decrease expression, (−) = No significant reversal effect, (+) = Significant positive reversal effect. The effect of pCD163 is in relation to pEmpty. The reversal effect of anti-CD163 antibody is in relation to pCD163.
Additionally, we measured the mRNA expression of two major pro-inflammatory cytokines (TNF-α, IL-6) and one anti-inflammatory cytokine (IL-10) after the overexpression of CD163 under the abovementioned conditions. We did not observe differences in the mRNA levels of TNF-α between pCD163 and pEmpty groups at any time point (Supplementary fig. 2A). We observed that IL-6 mRNA was elevated at 24 hours in CD163-overexpressing macrophages when compared to the pEmpty group, and no changes were observed at other time points (Supplementary fig. 2B). An increase in IL-10 mRNA at 72 hours (no changes in other time points) was found in CD163-overexpressing macrophages when compared to the pEmpty group (Supplementary fig. 2C).
3.4. Cytokine profile of CD163-overexpressing macrophages in a model of sub-acute inflammation
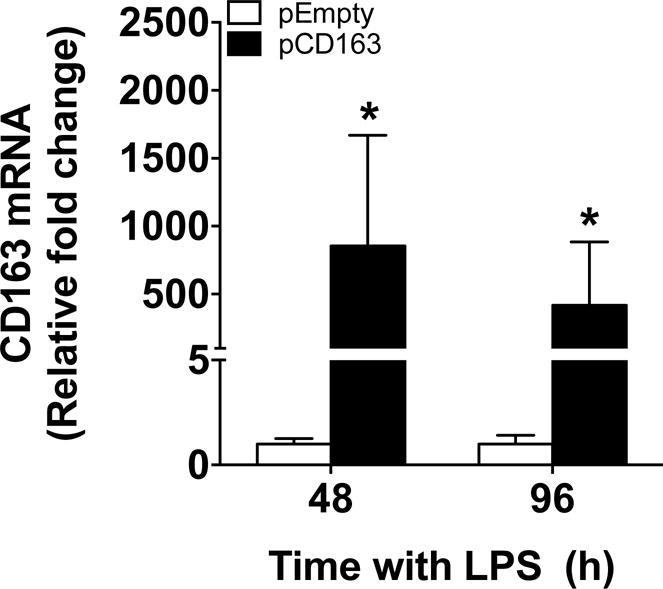
We confirmed that CD163 mRNA was significantly increased in THP-1 macrophages transfected with pCD163 in a double LPS stimulation paradigm when compared to the pEmpty group at 4 and 24 hours after the second stimulus (+4 and +24, respectively; Fig. 7).
Figure 7. CD163 mRNA induction in THP-1 macrophages challenged with a double LPS stimulation.
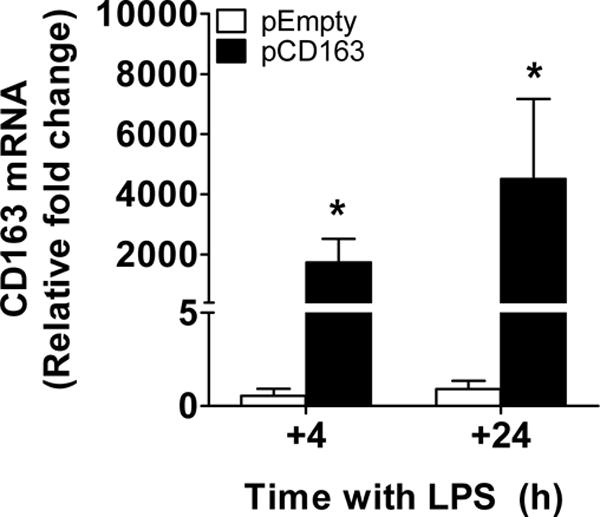
Quantification for CD163 mRNA using Man-PEI complexed with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) in THP-1 macrophages with a double LPS stimulation at 4 and 24 hours after the second stimulus (+4 and +24 respectively). The expression of CD163 mRNA was normalized to the respective levels of β-actin expression in each group, and then calculated as fold change against the expression of the control group (Man-PEI) at each time point, which was assigned a value equal to 1. N = 4–6 per group. *p<0.05 against the pEmpty transfected group by student’s t-test.
When anti-inflammatory cytokines/chemokines were measured under these conditions, we observed that CD163-overexpressing THP-1 macrophages displayed higher concentrations of IL-10 (at +4 hours; pCD163 = 2.52 ± 1.20, pEmpty = 1.28 ± 0.34; Fig. 8A) and IL-1ra (at +24 hours; pCD163 = 2.41 ± 0.21, pEmpty = 1.95 ± 0.23; Fig. 8B) when compared to the pEmpty group. There were no changes in the concentration of TGF-β between the pCD163 and pEmpty groups at the tested time points (Fig. 8C). The effects on IL-10 and IL-1ra observed in the pCD163 group were replicated in independent experiments, and the RM3/1 anti-CD163 antibody blocked these effects (Fig. 9A and 9B). The isotype control antibody did not produce any significant change when compared to the pCD163 group, and it was significantly different to pCD163+anti-CD163 antibody group for IL-10 but not for IL-1ra. A weak unspecific effect of this isotype antibody has been previously observed (Luo, et al., 2014, Moura, et al., 2012).
Figure 8. Changes of cytokine expression in CD163-overexpressing THP-1 macrophages challenged with a double LPS stimulation.
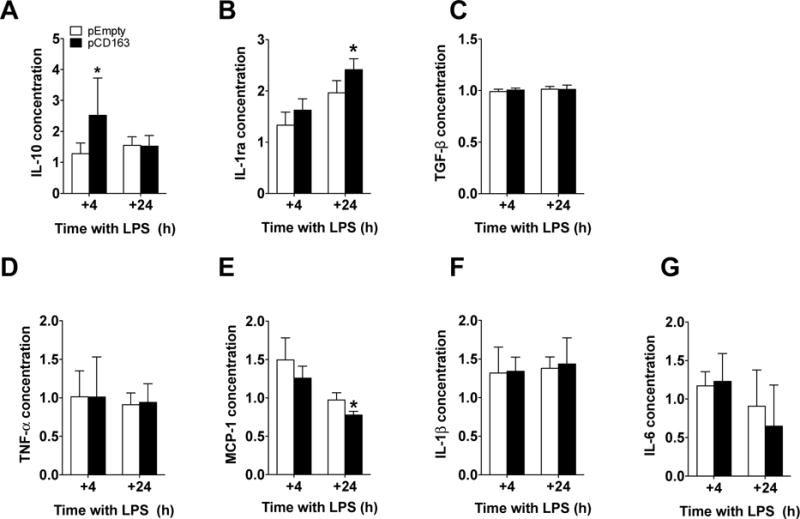
Quantification for IL-10 (A), IL-1ra (B), TGF-β (C), TNF-α (D), MCP-1 (E), IL-1β (F) and IL-6 (G) protein concentration in THP-1 macrophages with a double LPS stimulation and transfected with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) at 4 and 24 hours after the second stimulus (+4 and +24 respectively). The concentration of each molecule was normalized to the control group (Man-PEI), which was assigned a value equal to 1. N = 5–13 per group. *p<0.05, vs. pEmpty by student’s t-test.
Figure 9. Changes of cytokine expression in CD163-overexpressing THP-1 macrophages challenged with a double LPS stimulation and a CD163 antibody.
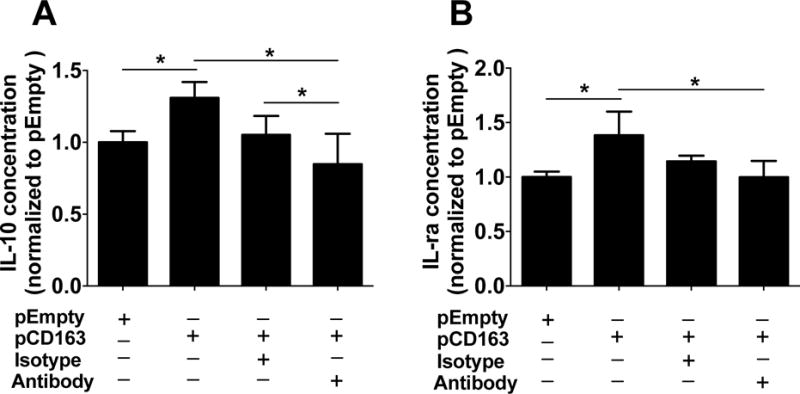
Quantification for IL-10 (A) and IL-1ra (B) protein concentration in THP-1 macrophages transfected with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) and incubated with either anti-CD163 antibody (RM3/1) or its isotype control antibody at 4 (for IL-10) and 24 (for IL-1ra) hours after a second LPS stimulus. The protein concentration of each group was normalized to the control group (pEmpty), which was assigned a value equal to 1. N= 5–12 per group. *p<0.05 using student’s t-test.
When pro-inflammatory cytokines were measured under these conditions, we observed that CD163-overexpressing THP-1 macrophages displayed lower concentrations of MCP-1 (at +24 hours; pCD163 = 0.77 ± 0.04, pEmpty = 0.97 ± 0.09; Fig. 8E) when compared to the pEmpty group. There were no changes in the concentration of TNF-α (Fig. 8D), IL-1β (Fig. 8F), or IL-6 (Fig. 8G) between the pCD163 and pEmpty groups at the tested time points. The addition of the RM3/1 anti-CD163 antibody did not block the effects on MCP-1 observed in CD163-overexpressing cells (data not shown). The effects of CD163 overexpression on the cytokine concentration in THP-1 cells in the double LPS stimulation paradigm are summarized in the table 2.
When major cytokines were evaluated at the mRNA level, we did not observe differences in the amount of TNF-α (Supplementary fig. 3A), IL-6 (Supplementary fig. 3B), or IL-10 (Supplementary fig. 3C) between the pCD163 and pEmpty groups.
3.5. CD163 overexpression and cytokine profile in primary human macrophages following LPS stimulation
Using the same transfection approach and Man-PEI nanoparticles in a single LPS stimulation paradigm, we observed an overexpression of CD163 mRNA in primary human macrophages transfected with pCD163 when compared with the pEmpty vector at both 48 and 96 hours (Fig. 10). The concentration of the soluble form of CD163 did not differ between the CD163 and pEmpty groups at any time point in human primary macrophages (Supplementary fig. 4).
Figure 10. CD163 mRNA induction in primary human macrophages challenged with a single LPS stimulation.
Quantification for CD163 mRNA induction using Man-PEI complexed with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) in primary human macrophages with a single stimulation of LPS at 48 and 96 hours after transfection. The expression of CD163 mRNA was normalized to the respective levels of β-actin expression in each group, and then calculated as fold change against the expression of the pEmpty group at each time point, which was assigned a value equal to 1. N = 6–9 per group. *p<0.05 against the pEmpty group by student’s t-test.
We also determined the cytokine production profile of CD163-overexpressing cells as a functional outcome. When anti-inflammatory cytokines were measured under these conditions, we observed that CD163-overexpressing human primary macrophages displayed a slight, yet significant, reduction in IL-10 (at 48 hours; pCD163 = 0.90 ± 0.09, pEmpty = 1 ± 0.06; Fig. 11A), but no changes were observed in IL-1ra or TGF-β when compared to the pEmpty group (Fig. 11B and 11C).
Figure 11. Changes of cytokine expression in CD163-overexpressing primary human macrophages challenged with a single LPS stimulation.
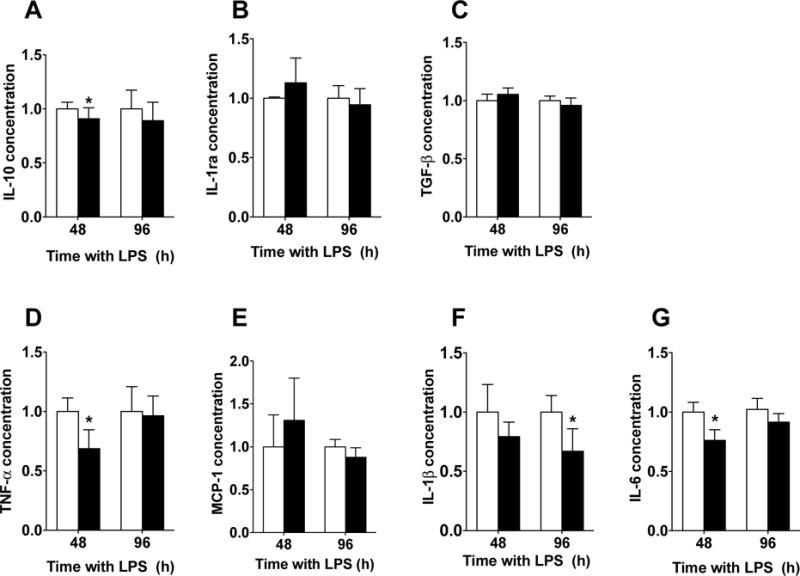
Quantification for IL-10 (A), IL-1ra (B), TGF-β (C), TNF-α (D), MCP-1 (E), IL-1β (F) and IL-6 (G) protein concentration in primary human macrophages with a single LPS stimulation and transfected with either a plasmid encoding for CD163 gene (pCD163) or the empty vector (pEmpty) at 48 and 96 hours after transfection. The concentration of each molecule was normalized to the control group (pEmpty), which was assigned a value equal to 1. N= 5–8 per group. *p<0.05 vs. pEmpty by student’s t-test.
When pro-inflammatory cytokines were measured under these conditions, we observed that CD163-overexpressing human primary macrophages displayed lower concentrations of TNF-α (at 48 hours; pCD163 = 0.68 ± 0.15, pEmpty = 1 ± 0.11; Fig. 11D), IL-1β (at 96 hours; pCD163 = 0.67 ± 0.19, pEmpty = 1 ± 0.14; Fig. 11F), and IL-6 (at 48 hours; pCD163 = 0.76 ± 0.08, pEmpty = 1 ± 0.08; Fig. 11G) when compared to the pEmpty group. There were no changes in the concentration of MCP-1 (Fig. 11E) between the pCD163 and pEmpty groups at the tested time points. The effects of CD163 overexpression on the cytokine release in primary human cells are summarized in the table 2.
These cytokine effects in CD163-overexpressing primary human macrophages were replicated and challenged with the addition of the RM3/1 anti-CD163 antibody. We observed that the anti-CD163 antibody blocked the effects on IL-6 when compared to the pCD163 group (Supplementary fig. 5A). The isotype antibody control group was not significantly different from either the pCD163 group or the anti-CD163 group. The anti-CD163 antibody or its isotype antibody did not reverse the effects on TNF-α, IL-1β, or IL-10 in human primary macrophages (Supplementary fig. 5B, 6C, and 5D, respectively).
4. Discussion
The major findings of this study are: 1) The blockade of CD163 in human macrophages at the resolution phase of LPS-induced inflammatory response resulted in an exacerbated pro-inflammatory cellular phenotype; and 2) The overexpression of CD163 in human macrophages resulted in the expression of M2 markers and an a clear reduction of the inflammatory phenotype (cytokine profile) produced by LPS in all the studied conditions.
The classic effects of CD163 have been associated with the scavenging activity of the hemoglobin-haptoglobin (Hb-Hp) complexes. The degradation of the Hb-Hp complex through the CD163 receptor produces metabolites with anti-inflammatory properties (Philippidis, et al., 2004, Etzerodt and Moestrup, 2013). However, CD163 could serve as a receptor for other molecules and drive different responses. For example, under certain conditions, CD163 acts as an adhesion receptor for erythroblasts, promoting the growth and survival of these cells (Fabriek, et al., 2007). Additionally, CD163 has been described as a contributor to THP-1 cell immune response under infections, serving as a bacterial (Fabriek, et al., 2009) or viral receptor (Guo, et al., 2014). Similarly, the crosslink of CD163 with some antibodies triggers pro-inflammatory immune responses (Ritter, et al., 2001). On the other hand, CD163 seems to bind to dust mites (Dai, et al., 2016) and cockroach allergens (Mushaben, 2015), which protects the host from inflammatory reactions. Together, these data show that the conditions or stimuli present in a system can determine the immunomodulatory actions of CD163, which are not limited to the Hb-Hp complex interaction. In fact, using CD163 gene overexpression techniques, it has been demonstrated that CD163 not only interacts with the Hb-Hp complexes, but also with T-lymphocytes and viruses as a receptor (Guo, et al., 2014, Nielsen, et al., 2013, Madsen, et al., 2004, Frings, et al., 2002).
The current study uncovers a new function for CD163, which promotes changes in the phenotype in human macrophages under LPS inflammatory conditions in the absence of factors that have been described to interact with CD163, namely, Hb-Hp complexes, erythroblasts, bacteria, allergens, viruses, and other types of cells. Macrophages express multiple markers following LPS stimulation, which probably contribute to the acquisition of a clear pro-inflammatory phenotype. In our hands, the overexpression of CD163 favored the induction of some markers of an M2a phenotype that encompassed a cytokine profile associated to the resolution of inflammation in both THP-1 and primary macrophages under different LPS stimulation paradigms.
Our approach demonstrates that the mechanism by which CD163 promotes this cellular phenotype is in part due to the extracellular engagement of a ligand present in the inflammatory milieu. This is demonstrated by the use of the RM3/1 antibody, which blocks an extracellular domain of CD163 and, therefore, its potential interaction with its ligand. In all circumstances (non-overexpressed and overexpressed CD163 cells), this antibody induced a pro-inflammatory cytokine profile in our settings. Our studies did not identify the responsible ligand that interacts with CD163 following LPS stimulation, but a likely candidate is the tumor necrosis factor-like weak inducer of apoptosis (TWEAK) (Bover, et al., 2007, Moreno, et al., 2009). This molecule, which is produced by macrophages upon LPS stimulation (Chacon, et al., 2006), induces apoptosis in macrophages by binding to the Fn14 receptor, via the nuclear factor-κB pathway (Brown, et al., 2003). Interestingly, CD163 acts as a decoy receptor for TWEAK, which blocks its apoptotic effects (Bover, et al., 2007). TWEAK induces the production of pro-inflammatory factors, such as IL-6, MCP-1, IL-8, and MMP-9 (Kim, et al., 2004). Even though the direct interaction of CD163 and TWEAK does not occur in all conditions (Fick, et al., 2012), there is consistent evidence showing that TWEAK binds to CD163, and this interaction blocks other functions of TWEAK, i.e. its myogenic effects (Akahori, et al., 2015). Therefore, we speculate that in our system an interaction between CD163 and TWEAK could be promoting an anti-inflammatory macrophage phenotype. However, further studies are needed to characterize the CD163/TWEAK interaction under our experimental settings.
In contrast to our findings, it has been shown that CD163-positive macrophages under a double LPS stimulation paradigm display a higher intracellular content of TNF-α in comparison to their CD163-negative counterparts (Alves-Januzzi, et al., 2015). Here, we show that CD163 overexpression resulted in a reduction of released TNF-α. A potential explanation of these findings is that CD163 could reduce the release of TNF-α, which would result in an accumulation of this cytokine in the intracellular space. This hypothesis is interesting, since it opens the possibility of several potential mechanisms for CD163 immunomodulatory effects, namely the reduction of the de novo production of pro-inflammatory factors, and the reduction of the release of these molecules.
Our data (i.e. regarding IL-10) are in accordance with previous findings, in which the presence of CD163-positive macrophages was associated with the resolution of inflammation and higher production of IL-10 (Philippidis, et al., 2004, Etzerodt and Moestrup, 2013). The use of an empty vector as a control group demonstrates that the observed effects are CD163 specific. Our additional control group, using only the nanoparticle, also rules out the possibility that the observed anti-inflammatory effect is related to the mannose ligand, since it has been shown that mannose receptors could trigger anti-inflammatory responses (Chieppa, et al., 2003). Additionally, we have previously demonstrated that this nanoparticle produces a minimal and transient immunogenic effects and no cytotoxicity under our current conditions (Bernal, et al., 2016), which also rules out the possibility of a masking effect induced by the polyethyleneimine nanoparticle or the mannose ligand interaction.
We further confirmed the specificity of our observations through the functional blockade of a specific extracellular domain of CD163 (scavenger receptor cysteine rich [SRCR] domain nine) on IL-10, IL-1ra, and IL-6 with a specific antibody, RM3/1. These findings are in line with previous studies that show the blocking effect of the RM3/1 antibody on IL-10 release in human macrophages (Philippidis, et al., 2004, Landis, et al., 2013). Interestingly, in our experiments, RM3/1 antibody affected TNF-α in non-transfected macrophages after LPS stimulation, but it did not affect this cytokine in CD163-overexpressing macrophages. Thus, the binding of molecules to the different CD163 SRCR extracellular domains could trigger differential effects in different conditions. In fact, it has been shown that the crosslinking of CD163 with antibodies also triggers some pro-inflammatory responses by the activation of its different extracellular domains and via alternative intracellular pathways that involve casein kinase II or protein kinase C (Ritter, et al., 2001). For instance, targeting the third SRCR domain using the EDhu-1 antibody increases inositol production, IL-6, and granulocyte-monocyte colony stimulating factor (Van den Heuvel, et al., 1999). Similarly, the binding of bacteria to the second SRCR domain induces the release of TNF-α (Fabriek, et al., 2009). This evidence suggests that pro-inflammatory cytokines could be modulated through different CD163 SRCR domains in different conditions.
Our findings could set the foundation for a novel therapeutic approach for conditions with the risk of developing chronic inflammation and chronic pain, such as major surgeries or large burns. Inducing an efficient transition from M1 to M2 phenotype in macrophages by inducing CD163 as a gene therapy could directly restore a homeostatic state by targeting the cause of the problem. The induction of M2 macrophages has been shown to reduce pain-related behaviors in mice models of postoperative or neuropathic pain using a PPAR-γ agonist (Hasegawa-Moriyama, et al., 2012) or IL-4 (Kiguchi, et al., 2015).
The clinical relevance of our approach lies in the fact that in our studies we use human primary macrophages (Bosshart and Heinzelmann, 2016, Heil, et al., 2002), demonstrating that our target, CD163, retains its anti-inflammatory functions in clinically relevant human cells under potent (LPS) inflammatory conditions. Even though our approach show differential responses of THP-1 and primary human macrophages, as shown elsewhere, cells (Bosshart and Heinzelmann, 2016, Heil, et al., 2002) CD163 consistently induced an anti-inflammatory phenotype if both types of cells. We believe that inducing CD163 could not only be beneficial for promoting resolution of inflammation and treating related pain conditions, but also protect from bacterial infections. CD163 can bind to bacteria through the interaction of fibronectin peptides, which amplifies the phagocytosis of S. aureus and improves the killing efficiency of this pathogen (Kneidl, et al., 2012). This feature is particularly relevant for conditions with open injuries or tissue damage, such as major surgeries or burns.
Another clinically relevant feature of our study is the use of a clinically proven gene delivery method, Man-PEI nanoparticles (Lisziewicz, et al., 2012, Rodriguez, et al., 2013). This polyethylenimine nanoparticle is an endotoxin-free cationic polymer that forms stable complexes with nucleic acids, protecting DNA from degradation (Grzelinski, et al., 2006, Urban-Klein, et al., 2005). Although PEI does not have cell specificity, the modified nanoparticle, Man-PEI preferentially targets cells that express mannose receptors (Diebold, et al., 1999). Thus, Man-PEI is ideal to preferentially target macrophages. Moreover, mannose receptors are involved in endocytosis and antigen delivery processes, which increase the uptake of the Man-PEI complexes and enhance the specificity to target cells (Diebold, et al., 1999, Martinez-Pomares, 2012). Our previous studies further confirm the safety and efficiency of these nanoparticles for gene induction in macrophages under inflammatory conditions (Bernal, et al., 2016).
Taken together, our data shows that under potent inflammatory conditions, the induction of the CD163 gene using Man-PEI nanoparticles resulted in changes in macrophage cytokine secretion, which are in favor of an anti-inflammatory phenotype. These findings suggest that targeting macrophages via CD163 could be beneficial to promote an efficient resolution of inflammation and a subsequent reduction in the risk of developing chronic inflammation, delayed tissue repair, and/or persistent pain. Although the exact mechanism by which CD163 produces these effects is still unknown, our data provide new insights into the functions of this molecule. Ongoing studies are focused on elucidating the upstream ligands and downstream molecular mechanisms of the overexpression of CD163, and the use of this approach in in vivo models of chronic pain.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Rita Allen Foundation and American Pain Society- Pain Scholar Award (EAR-S), NIH-NIGMS R15GM109333 (EAR-S), Pharmacy Research Summer Internship (PAAV, LB, RG, DWF and CMV) for funding, and CAPES Foundation - Ministry of Education of Brazil, PDSE: BEX 10794/14-0 for funding DWF’s scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interest
The authors declare no potential conflicts of interest.
References
- Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012;22:R733. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Lord JM. Factors underlying chronic inflammation in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2004;52:379. [PubMed] [Google Scholar]
- Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunster JL. The macrophage and its role in inflammation and tissue repair: mathematical and systems biology approaches. Wiley Interdiscip Rev Syst Biol Med. 2016;8:87. doi: 10.1002/wsbm.1320. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Moriyama M, Ohnou T, Godai K, Kurimoto T, Nakama M, Kanmura Y. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochem Biophys Res Commun. 2012;426:76. doi: 10.1016/j.bbrc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Kobayashi Y, Saika F, Sakaguchi H, Maeda T, Kishioka S. Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain. 2015;156:684. doi: 10.1097/j.pain.0000000000000097. [DOI] [PubMed] [Google Scholar]
- Zwadlo G, Voegeli R, Schulze Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- Evans BJ, Haskard DO, Sempowksi G, Landis RC. Evolution of the Macrophage CD163 Phenotype and Cytokine Profiles in a Human Model of Resolving Inflammation. Int J Inflam. 2013;2013:780502. doi: 10.1155/2013/780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal L, Alvarado-Vazquez A, Ferreira DW, Paige CA, Ulecia-Moron C, Hill B, Caesar M, Romero-Sandoval EA. Evaluation of a nanotechnology-based approach to induce gene-expression in human THP-1 macrophages under inflammatory conditions. Immunobiology. 2016 doi: 10.1016/j.imbio.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J, Bakare N, Calarota SA, Banhegyi D, Szlavik J, Ujhelyi E, Toke ER, Molnar L, Lisziewicz Z, Autran B, Lori F. Single DermaVir immunization: dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS One. 2012;7:e35416. doi: 10.1371/journal.pone.0035416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez B, Asmuth DM, Matining RM, Spritzler J, Jacobson JM, Mailliard RB, Li XD, Martinez AI, Tenorio AR, Lori F, Lisziewicz J, Yesmin S, Rinaldo CR, Pollard RB. Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J Acquir Immune Defic Syndr. 2013;64:351. doi: 10.1097/QAI.0b013e3182a99590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry RP, Jacobs VL, Romero-Sandoval EA, DeLeo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J, Gabrilovich DI, Varga G, Xu J, Greenberg PD, Arya SK, Bosch M, Behr JP, Lori F. Induction of potent human immunodeficiency virus type 1-specific T-cell-restricted immunity by genetically modified dendritic cells. J Virol. 2001;75:7621. doi: 10.1128/JVI.75.16.7621-7628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndong C, Landry RP, Deleo JA, Romero-Sandoval EA. Mitogen activated protein kinase phosphatase-1 prevents the development of tactile sensitivity in a rodent model of neuropathic pain. Mol Pain. 2012;8:34. doi: 10.1186/1744-8069-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72:711. [PubMed] [Google Scholar]
- Weaver LK, Hintz-Goldstein KA, Pioli PA, Wardwell K, Qureshi N, Vogel SN, Guyre PM. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006;80:26. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- Luo J, Li C, Xu T, Liu W, Ba X, Wang X, Zeng X. PI3K is involved in beta1 integrin clustering by PSGL-1 and promotes beta1 integrin-mediated Jurkat cell adhesion to fibronectin. Mol Cell Biochem. 2014;385:287. doi: 10.1007/s11010-013-1837-x. [DOI] [PubMed] [Google Scholar]
- Moura DF, de Mattos KA, Amadeu TP, Andrade PR, Sales JS, Schmitz V, Nery JA, Pinheiro RO, Sarno EN. CD163 favors Mycobacterium leprae survival and persistence by promoting anti-inflammatory pathways in lepromatous macrophages. Eur J Immunol. 2012;42:2925. doi: 10.1002/eji.201142198. [DOI] [PubMed] [Google Scholar]
- Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabriek BO, Polfliet MM, Vloet RP, van der Schors RC, Ligtenberg AJ, Weaver LK, Geest C, Matsuno K, Moestrup SK, Dijkstra CD, van den Berg TK. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109:5223. doi: 10.1182/blood-2006-08-036467. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- Guo L, Niu J, Yu H, Gu W, Li R, Luo X, Huang M, Tian Z, Feng L, Wang Y. Modulation of CD163 expression by metalloprotease ADAM17 regulates porcine reproductive and respiratory syndrome virus entry. J Virol. 2014;88:10448. doi: 10.1128/JVI.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M, Buechler C, Kapinsky M, Schmitz G. Interaction of CD163 with the regulatory subunit of casein kinase II (CKII) and dependence of CD163 signaling on CKII and protein kinase C. Eur J Immunol. 2001;31:999. doi: 10.1002/1521-4141(200104)31:4<999::aid-immu999>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Dai C, Yao X, Gordon EM, Barochia A, Cuento RA, Kaler M, Meyer KS, Keeran KJ, Nugent GZ, Jeffries KR, Qu X, Yu ZX, Aponte A, Gucek M, Dagur PK, McCoy JP, Levine SJ. A CCL24-dependent pathway augments eosinophilic airway inflammation in house dust mite-challenged Cd163(−/−) mice. Mucosal Immunol. 2016;9:702. doi: 10.1038/mi.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushaben DC, Yao Xianglan, Nugent Gayle, Keeran Karen, Yu Zu Xi, Aponte Angel, Gucek Marjan, Levine Stewart J. The Macrophage Scavenger Receptor CD163 Binds the German Cockroach Protein, Vitellogenin, and Attenuates Allergic Sensitization and Airway Hyperreactivity in a Murine Model of Experimental Cockroach-Induced Asthma. Am J Respir Crit Care Med. 2015;191:5661. [Google Scholar]
- Nielsen MJ, Andersen CB, Moestrup SK. CD163 binding to haptoglobin-hemoglobin complexes involves a dual-point electrostatic receptor-ligand pairing. J Biol Chem. 2013;288:18834. doi: 10.1074/jbc.M113.471060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M, Moller HJ, Nielsen MJ, Jacobsen C, Graversen JH, van den Berg T, Moestrup SK. Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J Biol Chem. 2004;279:51561. doi: 10.1074/jbc.M409629200. [DOI] [PubMed] [Google Scholar]
- Frings W, Dreier J, Sorg C. Only the soluble form of the scavenger receptor CD163 acts inhibitory on phorbol ester-activated T-lymphocytes, whereas membrane-bound protein has no effect. FEBS Lett. 2002;526:93. doi: 10.1016/s0014-5793(02)03142-3. [DOI] [PubMed] [Google Scholar]
- Bover LC, Cardo-Vila M, Kuniyasu A, Sun J, Rangel R, Takeya M, Aggarwal BB, Arap W, Pasqualini R. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J Immunol. 2007;178:8183. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Munoz-Garcia B, Martin-Ventura JL, Madrigal-Matute J, Orbe J, Paramo JA, Ortega L, Egido J, Blanco-Colio LM. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Chacon MR, Richart C, Gomez JM, Megia A, Vilarrasa N, Fernandez-Real JM, Garcia-Espana A, Miranda M, Masdevall C, Ricard W, Caubet E, Soler J, Vendrell J. Expression of TWEAK and its receptor Fn14 in human subcutaneous adipose tissue. Relationship with other inflammatory cytokines in obesity. Cytokine. 2006;33:129. doi: 10.1016/j.cyto.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA. The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J. 2003;371:395. doi: 10.1042/BJ20021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- Fick A, Lang I, Schafer V, Seher A, Trebing J, Weisenberger D, Wajant H. Studies of binding of tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) to fibroblast growth factor inducible 14 (Fn14) J Biol Chem. 2012;287:484. doi: 10.1074/jbc.M111.287656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahori H, Karmali V, Polavarapu R, Lyle AN, Weiss D, Shin E, Husain A, Naqvi N, Van Dam R, Habib A, Choi CU, King AL, Pachura K, Taylor WR, Lefer DJ, Finn AV. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat Commun. 2015;6:7792. doi: 10.1038/ncomms8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Januzzi AB, Brunialti MK, Salomao R. CD163 and CD206 expression does not correlate with tolerance and cytokine production in LPS-tolerant human monocytes. Cytometry B Clin Cytom. 2015 doi: 10.1002/cyto.b.21321. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- Landis RC, Philippidis P, Domin J, Boyle JJ, Haskard DO. Haptoglobin Genotype-Dependent Anti-Inflammatory Signaling in CD163(+) Macrophages. Int J Inflam. 2013;2013:980327. doi: 10.1155/2013/980327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, Dopp EA, Droste A, Van Gaalen FA, Sorg C, Hogger P, Beelen RH. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- Bosshart H, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4:438. doi: 10.21037/atm.2016.08.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil TL, Volkmann KR, Wataha JC, Lockwood PE. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J Oral Rehabil. 2002;29:401. doi: 10.1046/j.1365-2842.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- Kneidl J, Loffler B, Erat MC, Kalinka J, Peters G, Roth J, Barczyk K. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol. 2012;14:914. doi: 10.1111/j.1462-5822.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, Czubayko F, Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kursa M, Wagner E, Cotten M, Zenke M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J Biol Chem. 1999;274:19087. doi: 10.1074/jbc.274.27.19087. [DOI] [PubMed] [Google Scholar]
- Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


