Abstract
Objectives
The objective of this study was to evaluate the response rate and toxicities of the combination of oral topotecan and carboplatin in patients with untreated extensive stage small cell lung cancer (ES-SCLC). Previous studies have suggested improved outcomes with a topoisomerase I inhibitor in combination with a platinum agent.
Methods
Twenty-six patients with previously untreated, ES-SCLC were evaluable in this phase II trial. All patients received oral topotecan 2.0 mg/m2 per day on days 1 through 5 and carboplatin at an area under curve of 5 on day 5. Treatment was repeated every 21 days up to a total of 6 cycles. All patients received G-CSF.
Results
There were no complete responses and 16 partial responses, for an overall response rate of 62% (95% CI: 41–80). Median time to progression was 6.0 months (95% CI: 4–8), with a median overall survival of 12 months (95% CI: 8–16). This study was closed to accrual early with 26 of a planned 39 patients enrolled because of grade 5 adverse events in 4 (15%) patients (3 neutropenic infections, 1 sudden cardiac death). Eighty-five percent of patients experienced grade 3 or higher hematologic events. The most common severe nonhematologic events included diarrhea, vomiting, dyspnea, hypoxia, and hypotension.
Conclusions
Although this drug regimen has activity as first-line therapy in ES-SCLC, it is associated with excessive hematologic toxicity, which occurred in spite of growth factor support. Despite promising survival estimates, this particular combination and dose level of oral topotecan and carboplatin cannot be recommended.
Keywords: small cell lung cancer, topotecan, filgastrim, phase II
Extensive stage small cell lung cancer is sensitive to chemotherapy, with response rates of 70% to 90%.1–4 Despite a favorable initial response to chemotherapy, the prognosis for these patients remains poor, with a median survival time of 8 to 9 months. Treatment results in the 1990s have plateaued with no major advances in therapy as compared with the 1970s.5,6
Both topotecan and carboplatin have shown activity against small cell lung cancer. Topotecan, a topoisomerase-I inhibitor, resulted in an objective response rate of 39% when used as a single agent in a phase II study of patients with chemonaive extensive stage small cell lung cancer.7 In combination with paclitaxel in previously untreated patients, an overall response rate of 69% and 1-year survival of 50% were observed.8 Oral topotecan is now available and offers the ease of administration with less hematological toxicities. In a trial of oral versus intravenous topotecan in previously treated small cell lung patients, response rates and median survival were comparable, but there was less grade 3 to 4 neutropenia in patients receiving the oral form.9
A Japanese phase III trial has shown superior response rates and median survival (12.9 vs. 9.4 months) for irinotecan plus cisplatin compared with etoposide plus cisplatin (EP) in patients with extensive stage small cell lung cancer.10 This data suggests that a topoisomerase I inhibitor with a platinum agent may be advantageous to the standard etoposide or platinum regimen in extensive stage disease.
Oral topotecan and cisplatin have been evaluated in a phase I trial. The recommended dose of oral topotecan was 2.0 mg/m2 daily for 5 days.11 The main dose limiting toxicities were myelosuppression and diarrhea. There was less myelosuppression with administration of cisplatin on day 5 compared with day 1.
The present study evaluated the combination of oral topotecan on days 1 t o 5 and carboplatin on day 5 as first-line therapy in patients with extensive stage small cell lung cancer. On the basis of earlier studies, carboplatin was felt to be an appropriate substitute for cisplatin, with comparable efficacy but less toxicity in patients with incurable disease.12 It was hypothesized that less myelosuppression would be observed with the oral form of topotecan. However, because of the myelosuppression observed with other topotecan or platinum combinations, G-CSF was included in this study. The primary objectives of this study were to evaluate the confirmed response rate and tolerability with this 2-drug regimen. The secondary objectives were to obtain preliminary estimates of survival and time-to-tumor progression.
MATERIALS AND METHODS
Patient Eligibility
Eligibility criteria consisted of the following: histologic or cytologic confirmation of small cell lung cancer; extensive stage disease with no prior chemotherapy; age ≥18 years; measurable disease; Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better. In addition, all patients must have had the following laboratory parameters within 14 days of registration: absolute neutrophil count ≥1.5 × 103/µL; platelet count ≥100 × 103/µL; aspartate aminotransferase ≤5× the institutional upper limit of normal (ULN); serum alkaline phosphatase ≤5 × ULN; total bilirubin ≤1.5 × ULN or direct bilirubin within normal limits; serum creatinine ≤1.5 × ULN or calculated creatinine clearance of ≥50 mL/min using the Cockcroft-Gault formula.
Contraindications to enrollment included untreated CNS metastases, clinically significant infection, hypersensitivity to Escherichia coli derived proteins, uncontrolled angina pectoris, congestive heart failure within 3 months, uncontrolled cardiac arrhythmias, major surgery within 3 weeks, concurrent radiation therapy, earlier thoracic radiation except for superior vena cava syndrome to a maximum of 3 fractions, prior malignancy unless there had been no evidence of disease for 3 years, and pregnant or nursing women.
Dosing and Response
Patients received topotecan 2.0 mg/m2 per day orally days 1 to 5 and carboplatin at an area under the curve (AUC) of 5 (calculated with the Calvert formula) on day 5. Treatment was repeated every 21 days. All patients received G-CSF 5 µg/kg subcutaneously for the first 10 days of each cycle or until the ANC was >10,000/µL after the nadir if this occurred sooner. Treatment was continued for a maximum of 6 cycles. The National Cancer Institute’s Common Toxicity Criteria, version 2.0, was used to assess adverse events after each chemotherapy cycle.
Tumor response was followed with either a chest x-ray or CT scan of the chest after each even-numbered treatment cycle. The RECIST criteria were used for the purpose of assessing response (available at: http://www.nci.nih.gov/bip/RECIST.htm). Confirmatory repeat scans at least 4 weeks after a response were required. Chemotherapy was continued for up to 6 cycles for patients with stable disease or a partial or complete response. Chemotherapy was discontinued for patients with progressive disease. This trial was approved at all treatment centers’ institutional review board. Written informed consent was obtained according to federal and institutional guidelines.
Statistical Analyses
A single-stage phase II study with an interim analysis based on a Simon design was conducted to assess the efficacy and toxicity of this regimen. The primary endpoint was confirmed tumor response rate. A treatment success was defined as either a complete response or partial response (PR) observed on 2 consecutive evaluations at least 4 weeks apart.
This trial was designed to test the null hypothesis that the true treatment success rate was at most 0.50 against the alternative that it was at least 0.70. On the basis of the Simon design, a sample size of 39 patients provided 90% power to test the above hypothesis with a 2-sided alpha of 0.10. This regimen was considered promising if at least 24 of the 39 evaluable patients had a confirmed response. A planned interim analysis was to be conducted after the first 23 evaluable patients were followed for at least 3 cycles. Accrual was to continue if 12 or more confirmed responses were observed in the first 23 evaluable patients. A regimen with fewer than 12 confirmed responses was to permanently close to accrual due to lack of efficacy. Accrual was not to be suspended for the interim analysis; however, additional patients enrolled during this time were not to be included in the interim analysis evaluation. Because of the toxicity observed in this trial, accrual was halted early and thus the decision rules outlined above for both the final and interim analyses were not evaluated.
A preliminary pilot was also performed to determine whether G-CSF should be included in the regimen. The first 6 patients were treated with G-CSF and were evaluated after their first cycle of treatment. If either a grade 5 event occurred or 1 or more of the 6 patients experienced febrile neutropenia or 2 or more patients experienced absolute neutrophil count (ANC) <500 for >5 days, the remaining patients were to be treated with G-CSF. Otherwise, a second cohort of 6 patients was to be treated and evaluated without G-CSF.
The results for the primary endpoint of confirmed tumor response rate is computed as the number of confirmed complete response or PR divided by the total number of evaluable patients. Exact binomial confidence intervals for the true treatment success rate were constructed. The distribution of TTP and overall survival time is estimated using the method of Kaplan-Meier.13 All patients were included in the analysis except patients who never received study treatment.
RESULTS
Demographics
Twenty-seven patients were enrolled from November 2001 to December 2002. One patient did not receive any treatment because of patient refusal and was excluded from all analyses. The remaining 26 patients received at least 1 cycle of chemotherapy. Patient characteristics are shown in Table 1. The median age was 63 years, and 62% were female. Eighty-five percent of the patients had an ECOG performance score of <2. Almost half (46%) had 2 or more sites of metastatic disease.
TABLE 1.
Baseline Patient Characteristics
| Variable | A (N = 26) |
|---|---|
| Age, median (min, max) | 63.0 (44.0, 78.0) |
| Gender, No. (%) | |
| Female | 16 (62%) |
| Male | 10 (38%) |
| Race, No. (%) | |
| White | 26 (100%) |
| Brain metastases, No. (%) | |
| Yes | 1 (4%) |
| No | 25 (96%) |
| Number metastases sites, No. (%) | |
| 0,1 | 14 (54%) |
| ≥2 | 12 (46%) |
| Prior palliative RT, No. (%) | |
| Yes | 1 (4%) |
| No | 25 (96%) |
| Performance score, No. (%) | |
| 0–1 | 22 (85%) |
| 2 | 4 (15%) |
| Whole brain RT, No. (%) | |
| Yes | 1 (4%) |
| No | 25 (96%) |
Drug Administration
Ten patients (38%) received all 6 cycles of chemotherapy. Of the 16 patients who did not complete 6 cycles, treatment was discontinued because of disease progression in 5 patients and adverse reactions in 5 patients. Four patients died while on study, 1 patient went off treatment for other medical problems, and 1 pursued alternative treatment options. Refer Table 2 for the complete distribution of treatment cycles received.
TABLE 2.
Follow-up of the 26 Evaluable Patients
| Variable | A (N = 26) |
|---|---|
| Number cycles received, No. (%) | |
| 1 | 3 (12%) |
| 2 | 2 (8%) |
| 3 | 2 (8%) |
| 4 | 6 (23%) |
| 5 | 3 (12%) |
| 6 | 10 (38%) |
| Progression status, No. (%) | |
| Progression-free | 4 (15%) |
| Progression | 22 (85%) |
| Follow-up status, No. (%) | |
| Alive | 2 (8%) |
| Dead | 24 (92%) |
| Reason end treatment, No. (%) | |
| Completed study per protocol | 10 (38%) |
| Refused further treatment | 1 (4%) |
| Adverse reactions | 4 (15%) |
| Disease progression | 5 (19%) |
| Alternate treatment | 1 (4%) |
| Other medical problems | 1 (4%) |
| Died on study | 4 (15%) |
| Confirmed response | |
| PR | 16 (62%) |
| Stable | 6 (23%) |
| No post baseline assessment | 4 (15%) |
Thirteen patients (50%) had one or more dose reduction(s) for topotecan, and 4 patients (15%) had one or more dose reduction(s) for carboplatin. Fifty-four percent of patients had one or more dose reduction(s) for either agent. Seventy-three percent of patients had one or more treatment delays.
Adverse Events
All 26 patients were evaluable for the adverse event endpoint and all adverse events are reported regardless of attribution to study treatment. One of the first 6 patients enrolled did experience a febrile neutropenic event, and so all subsequent patients were treated with G-CSF. The most common adverse events were hematologic, with 85% of patients reporting at least one grade 3 or higher hematologic event. Forty-two percent of patients developed grade 3 (n = 3) or 4 (n = 7) neutropenia (with 1 patient with neutropenic fever but an unrecorded neutrophil level), and 77% of patients had grade 3 (n = 15) or grade 4 (n = 5) thrombocytopenia. Six patients (23%) required platelet or red blood cell transfusions during their course of treatment.
There were four grade 5 adverse events (15%), leading to early termination of the study. These included 3 treatment-related deaths because of neutropenic infection and 1 sudden cardiac death. It was uncertain whether the sudden cardiac death was treatment related. All patients with grade 5 toxicity had received G-CSF. No correlation could be identified between creatinine clearance and risk of grade 5 toxicity.
Similarly, no correlation existed between grade 5 events and pre-enrollment performance status (PS). Four of the 26 had a PS of 2, with the rest having a PS of 0 or 1. There was a single grade 5 event in the PS 2 patients (25%), compared with 3 events in the PS 0 to 1 patients (14%). The difference is not statistically significant (P = 0.51).
The most frequently reported nonhematologic events of any grade were fatigue (96%), nausea (62%), alopecia (46%), diarrhea (46%), vomiting (42%), dyspnea (27%), anorexia (19%), and hypotension (19%). Fifty-four percent of patients experienced at least one grade 3 or higher nonhematologic event, with 23% of patients experiencing at least one grade 4 or higher nonhematologic event. Table 3 outlines the specific severe (grade: ≥3) hematologic and nonhematologic events that were reported in at least 5% of the study population.
TABLE 3.
Severe (grade ≥3) Hematologic and Nonhematologic Events Occurring in 5% or More of Patients (n = 26 Patients)
| Grade | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 3 | 4 | 5 | Total | |||||
|
|
|
|
|
|||||
| Adverse Event | N | % | N | % | N | % | N | % |
| Thrombocytopenia | 15 | 57.7 | 5 | 19.2 | — | — | 20 | 76.9 |
| Neutropenia | 3 | 11.5 | 7 | 26.9 | — | — | 11* | 42.3 |
| Leukopenia | 2 | 7.7 | 5 | 19.2 | — | — | 7 | 26.9 |
| Anemia | 6 | 23.1 | 1 | 3.8 | — | — | 7 | 26.9 |
| Transfusion-pRBc | 4 | 15.4 | — | — | — | — | 4 | 15.4 |
| Dyspnea | 3 | 11.5 | — | — | — | — | 3 | 11.5 |
| Hypotension | 1 | 3.8 | 2 | 7.7 | — | — | 3 | 11.5 |
| Hypoxia | 3 | 11.5 | — | — | — | — | 3 | 11.5 |
| Vomiting | 2 | 7.7 | 1 | 3.8 | — | — | 3 | 11.5 |
| Diarrhea—no colostomy | 3 | 11.5 | — | — | — | — | 3 | 11.5 |
| Infection–ANC | — | — | — | — | 3 | 11.5 | 3 | 11.5 |
| Nausea | 2 | 7.7 | — | — | — | — | 2 | 7.7 |
| Neuromotor | 2 | 7.7 | — | — | — | — | 2 | 7.7 |
| Edema | — | — | 2 | 7.7 | — | — | 2 | 7.7 |
| Transfusion–PLT | 2 | 7.7 | — | — | — | — | 2 | 7.7 |
| Febrile neutropenia | 2 | 7.7 | — | — | — | — | 2 | 7.7 |
Includes 1 patient with undefined neutropenia who were nonetheless recorded as having neutropenic fever which by definition requires an ANC <1000.
All adverse events included regardless of perceived relationship to study treatment.
Response Data and Survival
All 26 patients were evaluable for response. There were no complete responses. Sixteen patients achieved a PR, for an overall response rate of 62% (95% CI: 41%–80%). One of the responders did not have the lesions followed on CT, as outlined per protocol, but response was confirmed with chest x-ray at cycle number 4. In addition, 6 patients (23%) had stable disease.
There were 2 patients alive at the time of data analysis with follow-up times of 37 and 42 months: 1 of these patients progressed at cycle 4 after an initial PR and the second progressed over 1 year after completing 5 cycles of the study regimen and achieving a PR. Twenty-two patients (85%) had documented disease progression. Table 2 outlines the follow-up details of the 26 evaluable patients.
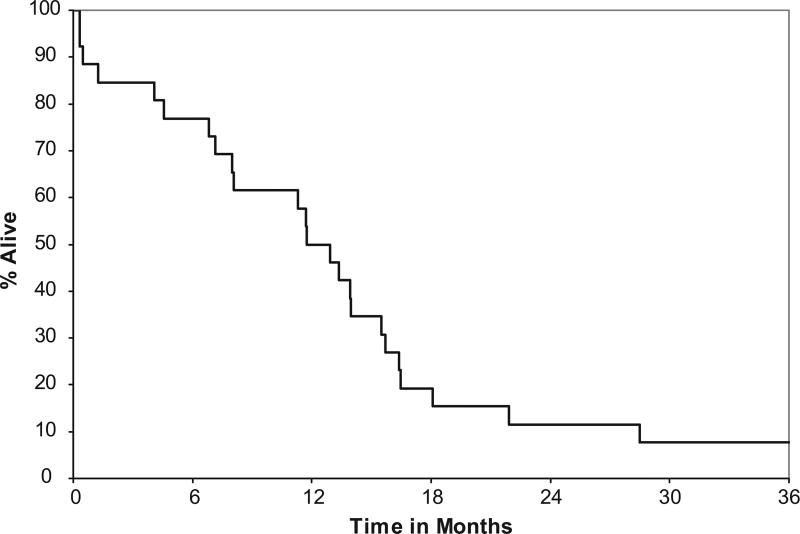
The median time to progression was 6 months (95% CI: 4–8), with a 1 year progression-free survival of 9% (95% CI: 2–34). Median overall survival was 12 months (95% CI: 8–16), with a 1-year survival estimate of 50% (95% CI: 34–73) and 2 year survival estimate of 12% (95% CI: 4–33) (Fig. 1).
FIGURE 1.
Overall survival.
DISCUSSION
Both topotecan and carboplatin have established roles in the treatment of small cell lung cancer. Data from Japan suggested a superior response rate and median survival with the combination of a platinum agent and a topoisomerase I inhibitor in untreated patients with extensive stage disease.10 This data has spawned other platinum or topoisomerase I inhibitor combination trials.
The overall response rate for this study was of 62% for this study (all PRs), which is comparable to that observed in other reports. The median overall of 12 months is marginally superior to the expected median survival of 8 to 9 months, and approaches the findings observed with the combination of irinotecan and cisplatin in the Japanese phase III trial J 9511. Two subsequent trials published in 2006 comparing oral topotecan or cisplatin to EP14 and irinotecan or cisplatin to EP15 reported median survivals of approximately 9 months, which were non superior to EP. Similarly, the results of S0124, designed to confirm J9511, were reported in abstract form at ASCO in 2008 and failed to show a survival advantage for irinotecan or cisplatin though less toxicity was observed.16
The survival data observed in this study are tempered by the unacceptable toxicity. Myelosuppression has been demonstrated to be the main dose-limiting toxicity of chemotherapy combinations with topotecan. A phase II trial of alternating etoposide or cisplatin and topotecan or paclitaxel resulted in an overall response rate of 77% and median survival of 10.5 months, but 70% of patients experienced grade 4 neutropenia.17 In a comparison study with intravenous topotecan, the oral form was associated with less myelosuppression, suggesting that oral topotecan may be a promising agent for combination regimens. However, in this study, 85% of patients experienced grade 3 or higher hematological events. Despite G-CSF, approximately one-third of patients had grade 3 or 4 neutropenia, with 3 deaths because of neutropenic infection. Therefore, it appears that in spite of the use of oral topotecan, the combination of this drug with carboplatin is associated with excessive myelosuppression.
Other studies have also demonstrated the increased toxicity with platinum or topotecan combinations. In a 4-arm Cancer and Leukemia group study in extensive stage small cell lung cancer, there were 3 deaths (25%) in the cisplatin or topotecan arm.18 Similarly, in a trial evaluating the combination of paclitaxel, carboplatin, and topotecan in extensive and limited stage disease, 8% of patients had treatment-related death from sepsis.19 More specifically, of 12 patients with an ECOG performance status of 2, the treatment-related mortality was 42%. The aforementioned 2006 trial by Hanna et al reported a treatment-related death rate of 5.8%. In that study, the rate of grade 5 toxicity was significantly related to a PS >1 (22.5% vs. 3.6%).15 Similar difficulties have been observed in platinum or topotecan studies in patients with ovarian cancer and other solid tumors.20,21
The trial by Eckardt et al14 using oral topotecan or cisplatin showed difficulty with excessive hematologic toxicity similar to our study. After initially starting with a regimen of topotecan 2.0 mg/m2 and cisplatin 60 mg/m2, 7 grade 5 toxicities were seen among the first 40 patients to receive topotecan 2.0 mg/m2, and the study was subsequently amended to a starting topotecan dose of 1.7 mg/m2.
In contrast, 3 trials have demonstrated manageable hematologic toxicity with topotecan and carboplatin or cisplatin when used in lower doses than this study. Vecchione, et al reported a Phase II trial of topotecan and carboplatin in first-line therapy for advanced ovarian cancer in which they used a dose of 1.5 mg/m2 i.v. from d1 to d3 and carboplatin AUC = 5 i.v. on d3 of a 21 day cycle. Eighty percent of patients in that trial experienced a grade 3 or 4 hematologic toxicity, and 45% required a dose reduction of the topotecan. Nonetheless, 79% of patients completed the planned 6 cycles of therapy.22 Similarly, Koensgen et al conducted a Phase I or II trial in second line therapy for platinum sensitive recurrent ovarian cancer using topotecan and carboplatin, established an MTD of topotecan 0.75 mg/m2 i.v. from d1 to d3 and carboplatin AUC = 5 i.v. d3 of a 21-day cycle. Only 5 of 26 patients developed any grade 4 hematologic toxicity and 58% developed grade 3 or 4 leukopenia.23 The superior tolerability of both of these regimens is likely reflective of the lower dose of topotecan rather than a difference of i.v. versus oral administration.
More recently, Sorensen et al published a trial using topotecan 0.75 mg/m2 i.v. from d1 to d3 and cisplatin 50 mg/m2 i.v. on d3 on a 21-day cycle in extensive stage SCLC. Grade 3 and 4 hematologic toxicities of anemia, neutropenia, and thrombocytopenia were reported in 9.5%, 66.7%, and 21.4% of patients, respectively.24 Although direct conversion between cisplatin and carboplatin is not possible, the dose of cisplatin in the Sorensen et al trial is in a general sense lower than the carboplatin dose used in our trial and likely accounts for the tolerability of their regimen.
What accounts for the increased toxicity of platinum and topotecan combinations? The main mechanism of action of the platinum compounds is through DNA strand breakage resulting from platinum-associated intra- and interstrand DNA crosslinks. However, more recent studies have shown that platinum agents also decrease topoisomerase I activity in cancer cell lines.25 This downregulation of topoisomerase I by platinum agents is compounded by camptothecins in vitro, resulting in a synergistic cytotoxic effect.26 This may account for the improved response rates seen with camptothecin or platinum combinations, but also may exacerbate the known myelotoxicity associated with topotecan.
The possibility that decreased renal clearance of the chemotherapeutic agents accounted for the increased toxicity of this combination was considered. However, no correlation could be noted between creatinine clearance and risk of grade 5 toxicity.
In summary, the oral topotecan and carboplatin combination does have activity as first-line therapy for extensive stage small cell lung cancer. Median survival in this study was somewhat better than that seen with other combinations, but the small patient number limits the conclusions that can be drawn from this. Despite its apparent clinical utility, the toxicities observed in this study imply that this combination at the doses employed should not be used. In addition, the incidence of severe myelosuppression did not seem to be abated by the use of oral topotecan or by the administration of G-CSF. On the basis of this and earlier data, there may be a synergistic toxicity on cancer cell and hematopoietic cell toxicity with this combination. However, given the results of this study and the previously reported results by Eckardt et al and Natale et al, significant caution regarding appropriate dosing is clearly warranted. A Phase III trial based on the Sorensen regimen is currently being conducted by the Danish Oncological Lung Cancer Group (Clinical trials gov identifier: NCT00812266).
Acknowledgments
Supported by Public Health Service grants CA-25224, CA-37404, CA-35431, CA-35113, CA-35195, CA-35103, CA-63849, CA-35269, CA-52352, CA-37417, CA-35272, and CA-63848.
The study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic.
Footnotes
Additional participating institutions: Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, MD, PhD); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, MD); Meritcare Hospital CCOP, Fargo ND 58122 (Preston D. Steen, MD); Ochsner CCOP, New Orleans, LA 70121 (Carl G. Kardinal, MD); Rapid City Regional Oncology Group, Rapid City, SD 57709 (Larry P. Ebbert, MD); Centracare Clinic, St. Cloud, MN 56301 (Harold E. Windschitl, MD); and Michigan Cancer Consortium, Ann Arbor, MI 48106 (Philip Stella, MD).
References
- 1.Choi NC, Carey RW, Kaufman SD, et al. Small cell carcinoma of the lung: a progress report of 15 years’ experience. Cancer. 1987;59:6–14. doi: 10.1002/1097-0142(19870101)59:1<6::aid-cncr2820590106>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Maksymiuk AW, Jett JR, Earle JD, et al. Sequencing and schedule effects of cisplatin plus etoposide in small cell lung cancer: results of a North Central Cancer Treatment Group randomized clinical trial. J Clin Oncol. 1994;12:70–76. doi: 10.1200/JCO.1994.12.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Morstyn G, Ihde DC, Lichter AS, et al. Small cell lung cancer 1973–1983: early progress and recent obstacles. Int J Radiat Oncol Biol Phys. 1984;10:515–539. doi: 10.1016/0360-3016(84)90032-4. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen M, Lassen U, Palshof T, et al. Topotecan and cisplatin in combination with concurrent twice-daily chemoradiation in limited disease small cell lung cancer-a Danish Oncological Lung Cancer Group (DOLG) phase II trial. Lung Cancer. 2008;60:252–258. doi: 10.1016/j.lungcan.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Murray N, Turrisi AT., III A review of first-line treatment for small cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 6.Chute JP, Venzon DJ, Hankins L, et al. Outcome of patients with small cell lung cancer during 20 years of clinical research at the US National Cancer Institute. Mayo Clin Proc. 1997;72:901–912. doi: 10.1016/S0025-6196(11)63359-4. [DOI] [PubMed] [Google Scholar]
- 7.Schiller JH, Kim K, Hutson P, et al. Phase II study of topotecan in patients with extensive-stage small cell carcinoma of the lung: an Eastern Cooperative Oncology Group Trial. J Clin Oncol. 1996;14:2345–2352. doi: 10.1200/JCO.1996.14.8.2345. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam S, Belani CP, Day R, et al. Phase II study of topotecan and paclitaxel for patients with previously untreated extensive stage small cell lung cancer. Ann Oncol. 2004;15:247–251. doi: 10.1093/annonc/mdh061. [DOI] [PubMed] [Google Scholar]
- 9.von Pawel J, Gatzemeier U, Pujol JL, et al. Phase ii comparator study of oral versus intravenous topotecan in patients with chemosensitive small cell lung cancer. J Clin Oncol. 2001;19:1743–1749. doi: 10.1200/JCO.2001.19.6.1743. [DOI] [PubMed] [Google Scholar]
- 10.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge MJ, Loos WJ, Gelderblom H, et al. Phase I pharmacologic study of oral topotecan and intravenous cisplatin: sequence-dependent hematologic side effects. J Clin Oncol. 2000;18:2104–2115. doi: 10.1200/JCO.2000.18.10.2104. [DOI] [PubMed] [Google Scholar]
- 12.Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small cell lung cancer: a Hellenic Co-operative Oncology Group study. Ann Oncol. 1994;5:601–607. doi: 10.1093/oxfordjournals.annonc.a058931. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E, Meier P. Nonparametric estimation of incomplete estimations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Eckardt JR, von Pawel J, Papai Z, et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small cell lung cancer. J Clin Oncol. 2006;24:2044–2051. doi: 10.1200/JCO.2005.03.3332. [DOI] [PubMed] [Google Scholar]
- 15.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 16.Natale RB, Lara PN, Chansky K, et al. S0124: a randomized phase III trial comparing irinotecan/cisplatin (IP) with etoposide/cisplatin (EP) in patients (pts) with previously untreated extensive stage small cell lung cancer (E-SCLC) [abstract 7512] J Clin Oncol. 2008;26(suppl 15):400s. [Google Scholar]
- 17.Jett JR, Hatfield AK, Hillman S, et al. Alternating chemotherapy with etoposide plus cisplatin and topotecan plus paclitaxel in patients with untreated, extensive-stage small cell lung carcinoma: a phase II trial of the North Central Cancer Treatment Group. Cancer. 2003;97:2498–2503. doi: 10.1002/cncr.11377. [DOI] [PubMed] [Google Scholar]
- 18.Lyss AP, Herndon JE, II, Lynch TJ, Jr, et al. Novel doublets in extensive-stage small cell lung cancer: a randomized phase II study of topotecan plus cisplatin or paclitaxel (CALGB 9430) Clin Lung Cancer. 2002;3:205–210. doi: 10.3816/clc.2002.n.004. discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 19.Hainsworth JD, Morrissey LH, Scullin DC, Jr, et al. Paclitaxel, carboplatin, and topotecan in the treatment of patients with small cell lung cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer. 2002;94:2426–2433. doi: 10.1002/cncr.10508. [DOI] [PubMed] [Google Scholar]
- 20.Hoskins P, Eisenhauer E, Vergote I, et al. Phase II feasibility study of sequential couplets of Cisplatin/Topotecan followed by paclitaxel/cisplatin as primary treatment for advanced epithelial ovarian cancer: a National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol. 2000;18:4038–4044. doi: 10.1200/JCO.2000.18.24.4038. [DOI] [PubMed] [Google Scholar]
- 21.Pentheroudakis G, Briasoulis E, Karavassilis V, et al. Phase I trial of intravenous cisplatin-topotecan chemotherapy for three consecutive days in patients with advanced solid tumors: parallel topotecan escalation in two fixed platinum dosing schemes. Chemotherapy. 2005;51:154–161. doi: 10.1159/000085624. [DOI] [PubMed] [Google Scholar]
- 22.Vecchione F, Fruscio R, Dell’Anna T, et al. A phase II clinical trial of topotecan and carboplatin in patients with newly diagnosed advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:367–372. doi: 10.1111/j.1525-1438.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 23.Koensgen D, Stengel D, Belau A, et al. Topotecan and carboplatin in patients with platinum-sensitive recurrent ovarian cancer. Results of a multicenter NOGGO: phase I/II study. Cancer Chemother Pharmacol. 2008;62:393–400. doi: 10.1007/s00280-007-0617-2. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen M, Lassen U, Jensen PB, et al. Phase II study of a 3-day schedule with topotecan and cisplatin in patients with previously untreated small cell lung cancer and extensive disease. J Thorac Oncol. 2008;3:902–906. doi: 10.1097/JTO.0b013e31817e0f58. [DOI] [PubMed] [Google Scholar]
- 25.Aoe K, Kiura K, Ueoka H, et al. Cisplatin down-regulates topoisomerase I activity in lung cancer cell lines. Anticancer Res. 2004;24:3893–3897. [PubMed] [Google Scholar]
- 26.van Waardenburg RC, de Jong LA, van Eijndhoven MA, et al. Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin. J Biol Chem. 2004;279:54502–54509. doi: 10.1074/jbc.M410103200. [DOI] [PubMed] [Google Scholar]