Abstract
Background
The relationship between testosterone (T) plasma concentration and cardiovascular risk is unclear, with evidence supporting increased risk in men with both low and high T levels. Few studies have assessed cardiovascular (CV) risk as a function of plasma T levels using objective biomarkers.
Aim
To determine the relationship between T levels and high-sensitivity (hs) CV risk biomarkers.
Methods
10,041 male patients were identified in the database of a commercial clinical laboratory performing biomarker testing. Patients were grouped by total T concentration and associations with the following biomarkers were determined: cardiac troponin I (cTnI), endothelin-1 (ET-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-17A (IL-17A), N-terminal pro-B-type natriuretic peptide (NTproBNP), high-density lipoprotein (HDL), hs c-reactive protein (CRP), hemoglobin A1c (HbA1c), and leptin.
Outcomes
The association of CV risk markers with levels of T in men.
Results
The median (interquartile range) age in the cohort was 58 (48, 68) years, and the median plasma T level was 420 (304, 565) ng/dL; T levels did not vary with patient age. An inverse relationship between plasma T levels and CV risk was observed for 9 of 10 cardiovascular markers: cTnI, ET-1, IL-6, TNF-α, NTproBNP, HDL, hsCRP, HbA1c, and leptin. Even after adjusting for age, BMI, HbA1c, hsCRP, and HDL levels, the CV markers IL-6, ET-1, NTproBNP, and leptin were significantly associated with T <250 ng/dL.
Clinical Implications
Men with low T levels may be at increased risk for increased CVD as seen by elevated CV risk markers.
Strength & Limitations
This study is performed in a group of 10,041 men and is the first study to exam CV risk associated with circulating T levels using a large panel of 10 objective biomarkers. This study is limited by an absence of clinical data indicating if men had preexisting CVD or other CV risk factors.
Conclusion
Men with low plasma T levels exhibit elevations in CV risk markers, consistent with a potential increased risk of cardiovascular disease.
MeSH Keywords: Hypogonadism, biomarkers, cardiovascular diseases, troponin I, endothelin-1, interleukin-6, tumor necrosis factor-alpha, interleukin-17, pro-brain natriuretic peptide, leptin
INTRODUCTION
Men begin to experience a decline in testosterone (T) levels after age 30.1 This decline in plasma T levels can be a harbinger of declining health; hypogonadal men have nearly twice the mortality risk of men with normal T levels.2 Studies have found that hypogonadism is associated with an increased risk of cardiovascular disease (CVD).3, 4 The relationship between T level and CV risk has been a controversial topic in recent years – several studies have shown that testosterone therapy (TTh) is associated with an increase in CV events,5–7 whereas the majority of meta-analyses have demonstrated no association between testosterone and CV events.8–14 While multiple studies have observed that TTh in hypogonadal men decreases the risk of all-cause mortality when compared with untreated hypogonadal men, 2, 15, 16 other studies have found no relationship between testosterone therapy and CVD.17, 18 Still other studies support the conclusion that untreated hypogonadal men can have an increased risk of CV events.3, 19, 20
While most studies investigating the relationship between T levels and CV risk have used CV outcomes such as myocardial infarction and stroke to determine risk, none have linked CV risk with a panel of high-sensitivity biomarkers. High-sensitivity (hs) immunoassays can quantify circulating levels of cardiac troponin I (cTnI), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-17A (IL-17A), and endothelin-1 (ET-1). Small elevations in hs cTnI levels, even in the absence of clinical symptoms, are a sign of underlying cadiomyocyte injury and cardiac disease.21 Studies have shown that asymptomatic men and women with cTnI levels above the 99th percentile of the reference range have a significantly increased risk of CVD, coronary heart disease, and all causes of death.22 Elevated plasma IL-6 levels are observed in men with atherosclerosis and are associated with a 3-fold increased risk of death from CV causes.23 Elevated levels of TNF-α in asymptomatic men are associated with clinical and subclinical CVD, as well as heart failure.24 Another cytokine, IL-17A, mediates immune and inflammatory responses, and high levels in asymptomatic men are associated with worse atherosclerosis and vessel wall plaque instability.23, 25, 26 Finally, elevated levels of ET-1 accelerate development of atherosclerotic disease by inducing smooth muscle cell hyperplasia,27 and is a predictor of increased 10-year mortality in otherwise asymptomatic individuals.28
In addition to these novel biomarkers, traditional CV risk biomarkers including N-terminal pro-brain natriuretic peptide (NTproBNP), high-density lipoprotein (HDL) cholesterol, high-sensitivity C-reactive protein (CRP), leptin, and hemoglobin A1c (HbA1c) can also facilitate detection of subclinical heart disease including myocardial cell damage, vulnerable vessel wall plaque, and vascular inflammation.29–33 Elevated NTproBNP, a peptide released with myocardial stretching, can predict up to 6-fold higher mortality and hospitalization for CV reasons in asymptomatic patients.34 Low HDL levels are a known health risk, and for every 10% reduction in HDL, the risk of CVD increases by 13%.35 Apparently healthy men with elevated hs CRP levels are at 1.5–3-fold increased risk of cardiovascular events.36 Finally, HbA1c also predicts future CVD. Every 1% increase in HbA1c is associated with a 20–30% increase in CV events and all-cause mortality.37 Together, these 10 biomarkers can facilitate determination of CV risk.
Several studies have found associations between the above CV biomarkers and hypogonadism. Dhindsa et al. found an increase in TNF-α, as well as CRP and leptin, in hypogonadal men.38 Elevated IL-6 and CRP levels have also been reported in men with low T levels.39 Finally in a study of over 3,000 men, Colangelo et al. observed that men with low T levels are at increased risk for diabetes mellitus and elevated HbA1c levels when compared to eugonadal men.40 While several studies have examined the relationship between T levels and CV risk using individual biomarkers, in this study we present our findings examining the association between T levels and CV risk using a large panel of 10 objective biomarkers, all of which have previously been linked to CV health.
METHODS
Study Design and Subject Identification
In collaboration with Singulex Clinical Laboratories (SCL, a CLIA-certified and CAP-accredited laboratory), we accessed de-identified patient data from the SCL database between January 2013 and September 2014. Laboratory tests found in this database are marketed for patients with or at risk for CVD, as determined by his or her community-based physician. All male patients >18 years of age with data regarding circulating levels of Total T, hs cTnI, hs IL-6, hs TNF-α, and hs IL-17A were included for analysis, a total of 10,041 subjects. Biomarker results for Leptin, NTproBNP, HDL, hsCRP, ET-1 and HbA1c, were included when available (92%, 95%, 88%, 93%, 28%, and 75%, respectively). For each patient, all samples for all 10 biomarkers were drawn on a single day; 47% of tests were ordered by primary care providers, 22% of tests were ordered by cardiologists, 17% of tests were ordered by internists, 7% of tests were ordered by osteopathic providers, and 7% were ordered by other specialists.
Testing Methods
Immunoassays for hs cTnI, hs IL-6, hs TNF-α, hs IL-17A, and hs ET-1 were used to quantify plasma concentrations. Leptin was measured using a plate-based sandwich immunoassay and a standard plate spectrophotometer. Total T, NTproBNP, HDL, hsCRP, and blood HbA1c were measured on the Roche cobas® 6000 analyzer. The total T reportable range was 2.5–7500 ng/dL, functional sensitivity 12 ng/dL, standardized via ID-GC/MS, repeatability CV range from 1.5% to 10.2%, and day-to-day CV range from 2.4% to 18.5% based on samples at 1450 ng/dL and 4.5 ng/dL respectively.
Reference Limits
The hs cTnI, hs IL-6, hs TNF-α, hs IL-17A, hs ET-1, and leptin assays were Lab Developed Tests with reference limits (RLs) determined as the 99th percentile from a reference population of apparently healthy subjects without CVD (95th percentile for leptin). The RL for T in the SCL, derived from internal standardization testing, is 250 ng/dL. The RLs for NTproBNP, HDL, hsCRP, and HbA1c were based upon the manufacturer-determined standards for the instrument.
Statistical Analysis
To investigate the co-variable effect on the relationship between T levels and biomarkers, patients were partitioned into groups based on age and body mass index (BMI). Median T levels were determined for these age and BMI groups, and a p-value for trend was determined using an analysis of variance (ANOVA) linear contrast model. Age and BMI levels were compared between men with T levels < 250 ng/dL and men with levels ≥ 250 ng/dL using the Wilcoxon Rank Sum test.
To examine the association between T and CV risk, patients were grouped into 100 ng/dL increments of plasma T levels. The fraction of the patient population that exceeded the RLs for all CV biomarkers was determined for each T group. The Jonckheere-Terpstra (JT) test was used to determine the p-value for ordered percentile trends.
For logistic regression models, T levels were dichotomized at 250 ng/dL to define men with low T levels, while blood biomarkers were dichotomized at their respective reference limits. Maximum likelihood estimation point was used to determine the odds ratio of increased CV biomarker risk for T levels < 250 ng/dL with 95% Wald confidence intervals. Models were controlled initially for age and BMI only. We also constructed CV risk models controlled for age, BMI, HbA1c, hsCRP, and HDL. Only patients with all 5 covariates were used in logistic regression models, a total sample size of 4,265 for cTnI, IL-6, TNF-α, and IL-17A (ET-1, leptin, and NTproBNP were modeled with 1,359, 4,220, and 4,257 patients respectively). BMI was only available for 53% of the patient population.
Distribution of all variables was assessed by the Kolmogorov-Smirnov Test for normality. All variables were found to be statistically different than a Gaussian (normal) distribution, and thus all data are represented as median and interquartile range. Statistical significance was assessed at the p=0.05 level. All analyses were performed using SAS v9.3.
RESULTS
Patient Characteristics
The patient population age, BMI, hsCRP, HDL, HbA1c, cTnI, IL-6, IL-17A, TNF-α, ET-1, leptin, and NTproBNP levels are presented in Table 1. Median age was 58 years (range 18–97 years). Interestingly, men with T < 250 ng/dL were not significantly older than eugonadal men (p = 0.2104). The median (IQR) BMI was 28 (25, 32). Men with T < 250 ng/dL had significantly higher BMIs when compared with eugonadal men (median BMI 31 vs. 27, respectively, p < 0.0001). Additionally, men with T levels < 250 ng/dL had elevated hsCRP, HDL, HbA1c, cTnI, IL-6, IL-17A, TNF-α, leptin, and NTproBNP levels when compared with men with T levels ≥ 250 ng/dL (p < 0.01 for all). The overall median (IQR) T concentration was 421 (305, 565) ng/dL and 1,518 patients (15%) had T levels < 250 ng/dL (Figure 1a). Figure 1b and Figure 1c display the median T concentrations for patient subsets by age (grouped by every 10 years) and by BMI (normal, overweight, or obese per the NIH definition). T levels were similar across age groups (p=0.71) and decreased linearly with increasing BMI (p<0.0001).
Table 1.
Characteristics of the Patient Population.
| N (% < 250 ng/mL) | All Patients | T >=250 ng/dL | T <250 ng/dL | p-value | |
|---|---|---|---|---|---|
| Age (years) | 10,041 (15.1%) | 58 (48,68) | 58 (48,68) | 58 (48,69) | 0.2104 |
| BMI | 5,346 (14.9%) | 28 (25,32) | 27 (25,31) | 31 (27,36) | <.0001 |
| hsCRP (μU/mL) | 9,303 (14.9%) | 1.3 (0.9,3.0) | 1.2 (0.9,2.7) | 2.5 (1.1,5.6) | <.0001 |
| HDL (mg/dL) | 8,844 (14.8%) | 47 (39,58) | 48 (40,58) | 43 (35,52) | <.0001 |
| HbA1C (%) | 7,494 (15.0%) | 5.6 (5.4,5.9) | 5.6 (5.3,5.9) | 5.8 (5.5,6.4) | <.0001 |
| cTnI (pg/mL) | 10,041 (15.1%) | 1.2 (0.7,2.2) | 1.2 (0.7,2.2) | 1.3 (0.8,2.5) | <.0001 |
| IL-6 (pg/mL) | 10,041 (15.1%) | 1.4 (0.9,2.4) | 1.3 (0.8,2.2) | 2.0 (1.2,3.4) | <.0001 |
| IL-17A (pg/mL) | 10,041 (15.1%) | 0.4 (0.2,0.6) | 0.4 (0.2,0.6) | 0.4 (0.2,0.7) | <.0001 |
| TNF-α (pg/mL) | 10,041 (15.1%) | 2.5 (2.0,3.2) | 2.5 (1.9,3.2) | 2.7 (2.1,3.6) | <.0001 |
| ET-1 (pg/mL) | 2,836 (13.0%) | 2.5 (2.1,3.1) | 2.5 (2.1,3.0) | 2.5 (2.0,3.2) | 0.0584 |
| Leptin (ng/mL) | 9,216 (15.2%) | 7.1 (3.7,13.4) | 6.5 (3.4,11.8) | 13.1 (6.9,24.0) | <.0001 |
| NTproBNP (pg/mL) | 9,507 (15.1%) | 40 (18,97) | 39 (18,93) | 43 (18,124) | 0.0038 |
Data are represented as median value + interquartile range. P-values determined by Wilcoxon Rank Sum. cTnI, cardiac troponin I; ET-1, endothelin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IL-17A, interleukin-17A; NTproBNP, N-terminal pro-B-type natriuretic peptide; HDL, high-density lipoprotein, hsCRP, hs c-reactive protein; HbA1c, hemoglobin A1c.
Figure 1.
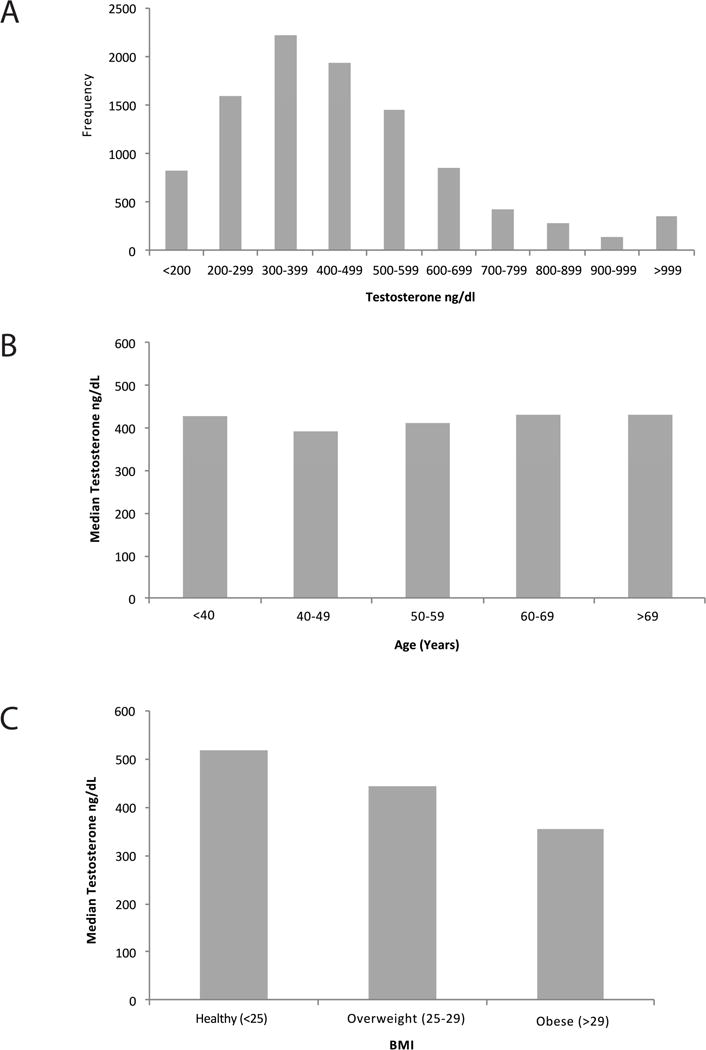
Plasma testosterone levels within the cohort. A) Distribution of T levels of men included in the study. T < 250 ng/dL was used to define hypogonadism. B) T concentrations are similar across age groups, p-value for trend = 0.5601. C) T concentrations decrease linearly with worsening BMI, p-value for trend < 0.0001.
Cardiovascular Risk and Testosterone Levels
Cardiovascular risk was defined the as percent of the patient population with an elevated plasma concentration that exceeded the biomarker 99%-tile reference limit. When stratified by T levels, a significant ordered relationship between low T levels and each CV biomarker was observed, with the exception of IL-17A. The proportion of men with elevated hs cTnI, hs IL-6, hs TNF-α, hs ET-1, leptin, NTproBNP, HDL, hsCRP, and HbA1c increased as T concentrations decreased (p<0.05 for all markers) (Table 2). Interestingly, while patients with low T levels were at an increased CV risk according to all biomarkers, hs cTnI, HDL, hsCRP, and HbA1c exhibited a U-shape risk distribution, suggesting additional CV risk at very high T levels (Figure 2).
Table 2.
Fraction of patients with biomarker levels above the reference limit.
| High Sensitivity Biomarkers (% Patients > 99%-tile RL) | Standard Biomarkers (% Patients Exceeded RL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) Age | IL-6 | cTnI | TNF-α | IL-17A | ET-1 | NTproBNP | Leptin | HDL | hsCRP | HbA1c | ||
| All Patients | 58 (15) | 4.8 | 6.7 | 9.6 | 1.5 | 6.8 | 15.7 | 8.8 | 12.7 | 24.4 | 24.0 | |
| Patients Grouped by Plasma Testosterone Level (ng/dL) | <200 | 60 (15) | 10.9 | 9.7 | 14.5 | 2.5 | 15.6 | 23.8 | 27.3 | 23.0 | 47.4 | 41.0 |
| 200–299 | 57 (14) | 6.2 | 6.7 | 11.8 | 1.8 | 7.2 | 15.4 | 16.2 | 20.0 | 34.6 | 37.9 | |
| 300–399 | 57 (15) | 4.8 | 6.4 | 9.0 | 1.5 | 6.5 | 15.6 | 9.4 | 13.5 | 26.8 | 27.4 | |
| 400–499 | 58 (14) | 3.6 | 6.6 | 7.7 | 1.2 | 5.4 | 15.8 | 5.7 | 8.7 | 20.1 | 19.0 | |
| 500–599 | 58 (15) | 4.2 | 6.2 | 8.9 | 1.5 | 7.5 | 14.2 | 3.5 | 8.1 | 16.5 | 17.3 | |
| 600–699 | 58 (15) | 2.2 | 5.9 | 8.1 | 1.2 | 5.1 | 13.4 | 1.8 | 7.9 | 14.4 | 15.0 | |
| 700–799 | 58 (15) | 4.0 | 4.9 | 10.0 | 1.6 | 5.9 | 14.9 | 2.6 | 8.4 | 15.5 | 10.4 | |
| 800–899 | 57 (15) | 1.1 | 5.4 | 7.9 | 1.8 | 3.7 | 12.1 | 1.5 | 8.7 | 11.1 | 10.6 | |
| 900–999 | 58 (15) | 4.3 | 8.5 | 7.8 | 1.4 | 6.0 | 12.2 | 2.2 | 6.8 | 13.1 | 13.2 | |
| >999 | 58 (14) | 3.1 | 7.8 | 10.0 | 1.7 | 5.2 | 13.7 | 1.7 | 14.7 | 19.1 | 15.0 | |
| P-value for trend | 0.5601 | <0.0001 | 0.0261 | <0.0001 | 0.1229 | 0.0013 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Reference Limit | >7.2 pg/mL | 5.8 or 6.1 pg/mL* | >4.2 pg/mL | >3.3 pg/mL | >3.7 pg/mL | >124 or >449 pg/mL* | >25.2 ng/mL | <35 mg/dL | >0.9 μU/mL | 5.9% | ||
Figure 2.
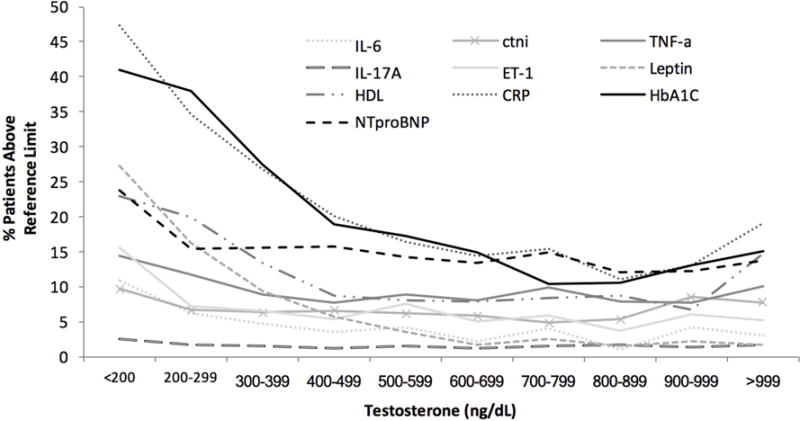
Fraction of patients at increased cardiovascular risk.
Men with Low Testosterone Have Increased Likelihood of CV Biomarker Elevations
The likelihood of CV risk in patients with T < 250 ng/dL was determined using adjusted logistic regression models. Models were first adjusted for two covariates, age and BMI, both of which have been consistently associated with hypogonadism. Models were then adjusted for three biomarkers traditionally associated with hypogonadism, HbA1c, HDL, and hsCRP.38–40 Our adjusted logistic regression models demonstrated that T levels <250 ng/dL predict increased CV risk based on an increased likelihood of elevated cTnI, IL-6, TNF-α, IL-17A, ET-1, leptin, and NTproBNP after adjusting for age and BMI (Figure 3a). When further adjusted for elevated HbA1c, HDL, and hs CRP levels, T levels <250 ng/dL still predicted an increased likelihood of elevated of IL-6, ET-1, leptin, and NTproBNP (Figure 3b).
Figure 3.
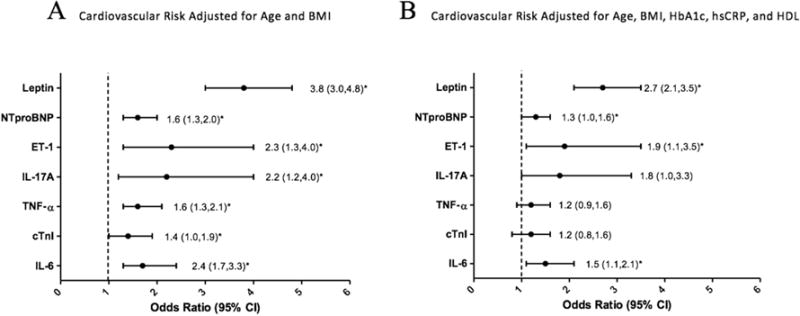
Relationship between low testosterone and increased cardiovascular risk. A) Cardiovascular risk adjusted for age and BMI. B) Cardiovascular risk adjusted for age, BMI, HbA1c, hsCRP, and HDL.
CV Biomarkers and Testosterone Levels ≥ 1000 ng/dL
All biomarkers demonstrate a clear relationship between increased CV risk and low T levels. However, cTnI, HDL, hs CRP, and HbA1c appeared to also follow a U-shaped risk pattern (Table 2). To examine the relationship between CV biomarkers and high T levels, patients with T ≥ 1000 ng/dL were compared with those with T levels between 250–999 ng/dL. Patients < 250ng/dL were not included in this analysis. Among the 4 biomarkers that had a U-shaped risk pattern, only hs cTnI concentrations were significantly higher in the T ≥ 1000 ng/dL group versus the T 250–999 ng/dL group. Median (IQR) cTnI concentration was 1.6 (1.0, 2.6) vs. 1.2 (0.7, 2.2) pg/mL, respectively (p < 0.0001). Despite the elevated hs cTnI concentrations in patients with T ≥ 1000 ng/mL, the proportion of patients with cTnI concentrations exceeding the cTnI reference limit was not significantly higher in the T ≥ 1000 ng/dL group vs. the T 250–999 ng/dL group. These data support a significant association between supraphysiologic plasma T levels and elevations in cTnI.
DISCUSSION
In this study, we observe a significant association between elevated CV risk biomarkers and low plasma T levels. As T levels rose outside the hypogonadal range in the patient cohort, the proportion of men with elevated CV risk marker levels decreased. Elevated CV biomarker levels were associated with plasma T levels < 250 ng/dL even after adjustment for age and BMI, further supporting a relationship between low plasma T levels and increased CV risk. These results are consistent with published work supporting an increased CV risk among hypogonadal men.3, 19 Our study, however, adds valuable new information, since we report CV risk as a function of plasma T levels using a large panel of both high-sensitivity and standard biomarkers. These results suggest that men with T levels ≥ 1000 ng/dL are at increased CV risk; a higher proportion of men in this group had elevated cTnI, HDL, hsCRP, and HbA1c than men with T levels of 250–999 ng/dL. Biomarker elevations in the subgroup of men with T ≥ 1000 ng/dL are not as dramatic as those observed in men with T <250 ng/dL; this apparent bimodal CV risk may be due to physiologic effects of both hypogonadism and supraphysiologic T levels, though additional study is needed to more definitively define this relationship.
While prior studies have examined single or limited sets of biomarkers, the panel of 10 biomarkers used in this study integrates a spectrum of inflammatory and CV pathologies. Dhindsa et al. observed both increased inflammation and insulin resistance as evidenced by increased levels of CRP, TNF-α, and leptin in 44 hypogonadal men.38 Gianatti et al. found increased levels of NT-proBNP and hs cTnI in 88 hypogonadal men, supporting an increased risk of coronary artery disease and heart failure.41 Other studies have utilized biomarkers to demonstrate dyslipidemia or an increased risk of developing diabetes.40 Interestingly, other studies have reported a correlation between hypogonadal men and elevated CRP levels, but also report that, as CRP levels increase, erectile function decreases.42 Our study of more than 10,000 men supports previous findings that hypogonadal men are at risk for developing CV disease; moreover, this panel of biomarkers implies that hypogonadal men are specifically at risk for ischemia, heart failure, atherosclerosis, inflammation, and dyslipidemia.43
The molecular mechanisms linking T and CV risk have yet to be precisely defined. Several studies suggest that androgens directly inhibit the development of atherosclerotic plaques through reducing lipid lesions, preventing foam cell formation, preventing endothelial injury, modulating the coagulation pathway, and inhibiting inflammation.38, 44 These studies have found that T and estrogen work synergistically to prevent endothelial injury. Thus, when plasma T levels are too high or too low, the T to estrogen ration is altered, contributing to atherosclerotic plaque formation.44 An alternative hypothesis is that low T may be provoked by an overall worse health status and thus low T could be an important early warning sign of future CVD.45
Strengths of our study include the large sample size and the inaugural use of a large panel of both high-sensitivity and traditional CV risk biomarkers to examine the relationship between CV risk and plasma T levels. Limitations included the absence of clinical data on most patients (including accurate records of the number of men with preexisting CVD or CV risk factors), the lack of a control group of healthy men, unknown timing of blood draws, and variable availability of results for all 10 biomarkers. Due to the retrospective design of this study, reverse causality is a possibility. Additionally, unknown T therapy status at time of laboratory draws is a limitation of the study, as several randomized controlled trials demonstrated that TTh increases T levels and also improves several of the biomarkers examined in this panel; however, we were unable to assess TTh status. The patient population consisted of men referred to the Singulex Clinical Laboratory, patients who already had CVD, or were at risk for CVD, therefore these findings can only be extended to this population. However, the study patient population had BMI levels similar to the average American male and most patients were not at risk for low HDL or high HbA1c levels. Data were not available for free T, sex hormone binding globulin, estradiol, follicle-stimulating hormone, luteinizing hormone, and triglyceride levels during the study period.
Information from these biomarkers could lend further clarity about the observed association between the CV biomarker panel and low plasma T, however, in general, total T is satisfactory for screening potential male hypogonadism and is still useful to monitor T treatment. This association between T level, CVD, and diabetes implies that an initial presentation with symptoms of low T may be an important entry point into the healthcare system, prompting screening for CVD and diabetes. Clinicians should be aware of the potential adverse CV risks associated with low T levels in men and should further evaluate these men using validated screening calculators, such as the Framingham Coronary Heart Disease Risk Score, or refer men to a cardiologist for a more extensive workup.46
CONCLUSION
The present study supports the conclusion that there is an association between plasma T levels <250 ng/dL elevated CV risk compared to men with normal T levels. To further elucidate the relationship between CV risk biomarkers and T levels, future studies are required.
Supplementary Material
Supplemental Table 1 – Spearman Correlation Coefficient.
Acknowledgments
Role of the Funding Source
A.W.P. is a National Institutes of Health K12 Scholar supported by a Male Reproductive Health Research Career Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Program (to Dolores J. Lamb).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Uncategorized References
- 1.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. European journal of endocrinology / European Federation of Endocrine Societies. 2013;169:725–33. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 3.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 4.Scovell J, Ramasamy R, Kovac JR. A critical analysis of testosterone supplementation therapy and cardiovascular risk in elderly men. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2014;8:E356–7. doi: 10.5489/cuaj.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. Jama. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC medicine. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 9.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clinic proceedings. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert opinion on drug safety. 2014;13:1327–51. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 12.Borst SE, Shuster JJ, Zou B, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC medicine. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clinical endocrinology. 2016;85:436–43. doi: 10.1111/cen.13084. [DOI] [PubMed] [Google Scholar]
- 14.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular Risks of Exogenous Testosterone Use Among Men: A Systematic Review and Meta-Analysis. Am J Med. 2017;130:293–305. doi: 10.1016/j.amjmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. The Journal of clinical endocrinology and metabolism. 2012;97:2050–8. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 16.Baillargeon J, Urban RJ, Kuo YF, et al. Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. The Annals of pharmacotherapy. 2014;48:1138–44. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart (British Cardiac Society) 2011;97:870–5. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 18.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2011;96:3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology. 2015;86:283–5. doi: 10.1016/j.urology.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 21.Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clinical chemistry. 2009;55:2098–112. doi: 10.1373/clinchem.2009.130799. [DOI] [PubMed] [Google Scholar]
- 22.Thorsteinsdottir I, Aspelund T, Gudmundsson E, et al. High-Sensitivity Cardiac Troponin I is a Strong Predictor of Cardiovascular Events and Mortality in the AGES-Reykjavik Community Based Cohort of Older Individuals. Clinical chemistry. 2016 doi: 10.1373/clinchem.2015.250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisman EZ, Adler Y, Tenenbaum A. Biomarkers in cardiovascular diabetology: interleukins and matrixins. Advances in cardiology. 2008;45:44–64. doi: 10.1159/000115187. [DOI] [PubMed] [Google Scholar]
- 24.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625–31. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbel C, Dengler TJ, Wangler S, et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic research in cardiology. 2011;106:125–34. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhu F, Wang Q, Guo C, et al. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin Immunol. 2011;141:152–60. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Weil BR, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Elevated endothelin-1 vasoconstrictor tone in prehypertensive adults. The Canadian journal of cardiology. 2012;28:347–53. doi: 10.1016/j.cjca.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Yokoi K, Adachi H, Hirai Y, et al. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population: the Tanushimaru study. Circulation journal : official journal of the Japanese Circulation Society. 2012;76:2779–84. doi: 10.1253/circj.cj-12-0469. [DOI] [PubMed] [Google Scholar]
- 29.Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin I as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: the Minnesota Heart Survey. Clinical chemistry. 2012;58:930–5. doi: 10.1373/clinchem.2011.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spoto B, Mattace-Raso F, Sijbrands E, et al. Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:232–40. doi: 10.2215/CJN.07000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Del Mercado M, Nunez-Atahualpa L, Figueroa-Sanchez M, et al. Serum levels of anticyclic citrullinated peptide antibodies, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein are associated with increased carotid intima-media thickness: a cross-sectional analysis of a cohort of rheumatoid arthritis patients without cardiovascular risk factors. BioMed research international. 2015;2015:342649. doi: 10.1155/2015/342649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X, Yu X, Ding Y-J, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Novo G, Sansone A, Rizzo M, Guarneri FP, Pernice C, Novo S. High plasma levels of endothelin-1 enhance the predictive value of preclinical atherosclerosis for future cerebrovascular and cardiovascular events: a 20-year prospective study. Journal of cardiovascular medicine (Hagerstown, Md) 2014;15:696–701. doi: 10.2459/JCM.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg J, Schou M, Gustafsson F, Badskjaer J, Hildebrandt P. Prognostic threshold levels of NT-proBNP testing in primary care. European heart journal. 2009;30:66–73. doi: 10.1093/eurheartj/ehn525. [DOI] [PubMed] [Google Scholar]
- 35.Despres JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Quebec cardiovascular study. Atherosclerosis. 2000;153:263–72. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 37.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of internal medicine. 2004;141:413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 38.Dhindsa S, Ghanim H, Batra M, et al. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction After Testosterone Replacement in Men With Type 2 Diabetes. Diabetes care. 2016;39:82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. The Journal of clinical endocrinology and metabolism. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes care. 2009;32:1049–51. doi: 10.2337/dc08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gianatti EJ, Hoermann R, Lam Q, Dupuis P, Zajac JD, Grossmann M. Effect of testosterone treatment on cardiac biomarkers in a randomized controlled trial of men with type 2 diabetes. Clinical endocrinology. 2016;84:55–62. doi: 10.1111/cen.12842. [DOI] [PubMed] [Google Scholar]
- 42.Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. The aging male : the official journal of the International Society for the Study of the Aging Male. 2016;19:239–43. doi: 10.1080/13685538.2016.1233960. [DOI] [PubMed] [Google Scholar]
- 43.Maganty A, Kovac JR, Ramasamy R. The putative mechanisms underlying testosterone and cardiovascular risk. F1000Research. 2014;3:87. doi: 10.12688/f1000research.3869.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai W, Ming W, Li Y, et al. Synergistic Effect of a Physiological Ratio of Estradiol and Testosterone in the Treatment of Early-stage Atherosclerosis. Archives of medical research. 2015;46:619–29. doi: 10.1016/j.arcmed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Corona G, Rastrelli G, Maseroli E, et al. Low testosterone syndrome protects subjects with high cardiovascular risk burden from major adverse cardiovascular events. Andrology. 2014;2:741–7. doi: 10.1111/j.2047-2927.2014.00241.x. [DOI] [PubMed] [Google Scholar]
- 46.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. The American journal of cardiology. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 – Spearman Correlation Coefficient.


