Abstract
Low voltage–activated persistent inward calcium currents (Ca PICs) occur in rat motoneurons and are mediated by Cav1.3 L-type calcium channels (L-Ca current). The objectives of this paper were to determine whether this L-Ca current activates a sustained calcium-activated potassium current (SK current) and examine how such SK currents change with spinal injury. For comparison, the SK current that produces the postspike afterhyperpolarization (mAHP) was also quantified. Intracellular recordings were made from motoneurons of adult acute and chronic spinal rats while the whole sacrocaudal spinal cord was maintained in vitro. Spikes/AHPs were evoked with current injection or ventral root stimulation. Application of the SK channel blocker apamin completely eliminated the mAHP, which was not significantly different in chronic and acute spinal rats. The Ca PICs were measured with slow voltage ramps (or steps) with TTX to block sodium currents. In chronic spinal rats, the PICs were activated at −58.6 ± 6.0 mV and were 2.2 ± 1.2 nA in amplitude, significantly larger than in acute spinal rats. Apamin significantly increased the PIC, indicating that there was an SK current activated by L-Ca currents (SKL current), which ultimately reduced the net PIC. This SKL current was not different in acute and chronic spinal rats. The SKAHP and the SKL currents were activated by different calcium currents because the mAHP/SKAHP was blocked by the N, P-type calcium channel blocker ω-conotoxin MVIIC and was resistant to the L-type calcium channel blocker nimodipine, whereas the L-Ca and SKL currents were blocked by nimodipine. Furthermore, the SKAHP current activated within 10 ms of the spike, whereas the SKL current was delayed ~100 ms after the onset of the L-Ca current, suggesting that the SKL currents were not as spatially close to the L-Ca currents. Finally, the SKL and the L-Ca currents were poorly space clamped, with oscillations at their onset and hysteresis in their activation and deactivation voltages, consistent with currents of dendritic origin. The impact of these dendritic currents was especially pronounced in 15% of motoneurons, where apamin led to uncontrollable L-Ca currents that could not be deactivated, even with large hyperpolarizations of the soma. Thus, although the SKL currents are fairly small, they play a critical role in terminating the dendritic L-Ca currents.
INTRODUCTION
Motoneurons have low-threshold persistent inward currents (PICs), composed of persistent sodium and calcium currents (Carlin et al. 2000b; Li and Bennett 2003; Powers and Binder 2003; Svirskis and Hounsgaard 1997). These PICs are facilitated by monoamines, such as serotonin (Hounsgaard and Kiehn 1989; Perrier and Hounsgaard 2003) and norepinephrine (NE) (Lee and Heckman 1999a), which arise mostly, although not exclusively, from the brain stem (Maxwell et al. 1996; Patel et al. 1997). Also, PICs are facilitated by other transmitters, such as glutamate and acetlycholine, acting on metabotropic receptors (Svirskis and Hounsgaard 1998). Functionally, PICs are important for bistable behavior, including production of plateau potentials and self-sustained firing, seen in the normal spinal cord intact animals (Lee and Heckman 1998a,b; Schwindt and Crill 1982) and chronic spinal animals (Bennett et al. 2001). Furthermore, bistable behaviors have been shown to play a major role in motor unit firing in normal and spinal cord–injured humans (Gorassini et al. 1998, 2004; Kiehn and Eken 1997). Because of their dependence on facilitation from monoamines, PICs are dramatically reduced, although not eliminated, by acute spinal transection, which eliminates brain stem monoamine innervation. However, these PICs return with chronic injury, possibly because of a developed supersensitivity to residual monoamines below the transection (Harvey et al. 2005a–c).
The persistent sodium currents (Na PIC) in motoneurons are TTX sensitive, activate subthreshold to firing, and partly inactivate over a few seconds after activation. The persistent calcium currents (Ca PIC) are nimodipine-sensitive, activate near the firing threshold, but persist for longer than the Na PIC (seconds to minutes); thus they play the major role in sustained depolarizations (plateaus) and firing (self-sustained firing) (Bennett et al. 2001; Li and Bennett 2003). The Ca PICs responsible for plateaus in motoneurons have been shown to be mediated by an L-type calcium current (termed L-Ca current, Perrier et al. 2002; Simon et al. 2003), likely acting through the newly identified low-threshold Cav1.3 calcium channels (Xu and Lipscombe 2001).
Besides acting as a charge carrier, the calcium ion is also capable of activating other channels, such as the calcium-activated small conductance potassium channel (SK channel), the calcium-activated big conductance potassium channel (BK channel), and the calcium-activated nonselective cationic (ICAN) channel. Therefore the Ca PIC recorded in motoneurons is likely the direct L-Ca current plus calcium-activated currents. The ICAN current has been shown to not play an important role in the plateau potentials in motoneurons (Perrier and Hounsgaard 1999). Also, BK currents have been found to be responsible for the fast afterhyperpolarization (fAHP) and are activated primarily by the N-type and P/Q-type calcium channels (Umemiya and Berger 1994) but are unlikely to play a major role in plateaus mediated by L-type calcium channels because of their relatively transient activation (McLarnon 1995).
SK channels have been found to be present in most types of neurons, including motoneurons, and are responsible for the medium-duration postspike afterhyperpolarization (mAHP) (McLarnon 1995). Thus the SK currents activated by spikes must hyperpolarize motoneurons by producing mAHPs and must counteract the net Ca PIC that helps sustain firing. However, it is unclear whether the L-Ca current that mediates the Ca PIC (through Cav1.3) directly activates SK currents. SK channels have little voltage dependence, but great Ca2+-dependent sensitivity. The conductance of SK channels increases with accumulation of intracellular Ca2+, and the channels are persistent, inactivating only slowly (McLarnon 1995). Thus in the presence of persistent L-Ca currents, the SK currents could very well produce a persistent outward current (that we term SKL) that opposes this persistent calcium current. Testing this possibility is the purpose of this paper.
Our study showed that the L-type calcium current (L-Ca) does indeed activate an SKL current, which subsequently diminishes the recorded Ca PIC. Using apamin to block the SK channel, the recorded Ca PIC is increased, and the calcium plateau is enlarged and prolonged. For comparison, we also quantified the SK currents that mediated the AHPs during firing (SKAHP) and examined the role of both SKAHP and SKL currents in firing. We recorded PICs and firing in motoneurons in an in vitro preparation (Bennett et al. 1999b) where the sacrocaudal spinal cord was removed either from normal adult rats (acute spinal) or from adult rats after 2 mo of spinal cord injury (chronic spinal). These two preparations allowed us to also examine whether the SK currents were smaller in chronic than acute spinal rats, which would contribute to the large net PICs seen in chronic spinal rats.
METHODS
Intracellular recordings were made from motoneurons in the sacrocaudal spinal cord of adult rats with an acute spinal transection (female Sprague-Dawley; 3–9 mo old) and adult rats with a chronic spinal cord transection (3–8 mo old). The latter chronic spinal rats were transected at the S2 sacral level at 2 mo of age (adult rat), and recordings were made after their affected muscles became spastic, 1–6 mo after this injury (Bennett et al. 1999a, 2004). All recordings were made from the whole sacrocaudal spinal cord that was removed from the rat with an acute S2 sacral transection and maintained in vitro (as detailed below). This transection was made in chronic spinal rats just rostral to the original chronic spinal injury to not further damage the sacrocaudal cord. All experimental procedures were approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee.
In vitro preparation
Details of the experimental procedures have been described in previous publications (Bennett et al. 2001; Li and Bennett 2003; Li et al. 2004b). Briefly, all rats were anesthetized with urethane (0.18 g/100 g; with a maximum dose of 0.45 g), and part of the caudal cord (between the T13 and L6 vertebrae) was exposed and kept wet with modified artificial cerebrospinal fluid (mACSF). The rat was given pure oxygen for 5 min before being transferred to a dissection chamber containing mACSF. All spinal roots were removed, except the S4 and all caudal ventral roots (the latter where taken as a single bundle and collectively referred to as Ca1, because Ca1 is the most prominent caudal root). The cord was secured by gluing its dorsal surface to a small piece of nappy paper. After 1.5 h of incubation in the dissection chamber at room temperature (20–21°C), the cord was transferred to a recording chamber containing normal ACSF (nACSF) maintained at 23–25°C with a flow rate >5 ml/min. The cord was fixed to the bottom of the recording chamber with the ventral side up by pinning the nappy paper to the Sylgard base of the chamber. After a 1-h wash period in the nACSF, to allow residual anesthetic and kynurenic acid to wash out, the nACSF was recycled as follows: it was oxygenated in a 200-ml source bottle, run through the recording chamber, collected, filtered, and finally returned to the source bottle with a pump (Harvey et al. 2005b). Because of the large volume (200 ml) of the source bottle and the small volume of the spinal cord (<0.05 ml), accumulation of metabolic byproducts from the spinal cord was likely negligible.
Intracellular recording
Intracellular recording methods were as described in Li and Bennett (2003) and are briefly summarized here. Sharp intracellular electrodes were filled with 1 M K-acetate and 1 M KCl and beveled to a resistance of 25–30 MΩ using a rotary beveller (Sutter BV-10). A stepper-motor (660, Kopf) was used to advance the electrodes into the ventral horn, and intracellular recordings from motoneurons were made with an Axoclamp 2b intracellular amplifier (Axon Instruments, Union City, CA) running in discontinuous current clamp (DCC; switching rate 5–8 kHz, output bandwidth 3.0 kHz) or discontinuous single-electrode voltage clamp (SEVC; gain 0.8–2.5 nA/mV) modes and sampled at 6.7 kHz with a Clampex system (Axon Instruments). The S4 and Ca1 ventral roots were wrapped around Ag/AgCl electrodes above the recording chamber and sealed with grease (Chemplex 825 silicon compound grease and Dow Corning High Vacuum grease), which allowed for antidromic stimulation identification of motoneurons. Motoneurons with a resting potential below −60 mV and antidromic spike overshoot >0 mV were considered healthy and used for recording.
Drugs and solutions
Two kinds of ACSF were used in these experiments: mACSF in the dissection chamber before recording and nACSF in the recording chamber. The mACSF consisted of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. Normal ACSF consisted of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. Additional drugs were added as required, including 2 µM TTX (Alamone Labs), 0.15 µM apamin (Alamone Labs), 15 µM nimodipine (Sigma), and 5 µM conotoxin G-VII-C (Sigma). The spinal cords were briefly exposed to nACSF solution containing 0.04% pronase E (Helixx Technologies) for 10 s before recording to weaken the pia of the spinal cords and to allow for easier penetration (Buschges 1994).
Persistent inward currents in current- and voltage-clamp recording
Slow triangular current ramps (0.4 nA/s) were applied to the motoneurons to induce firing and associated AHPs, measure basic cell properties, and evoke plateau potentials. The input resistance (R) was measured during the ramp over a 5-mV range near rest and subthreshold to PIC onset. Instantaneous firing frequency (F) was computed from the current ramp recordings using Clampfit 9.0 software. Without TTX present, the plateau potential was seen as a subthreshold rapid change in membrane potential before the firing threshold and a long afterdepolarization after cessation of firing. With TTX present, a full plateau was evoked during a current ramp that produced a sustained depolarization riding on top of the passive triangular ramp response (Li et al. 2004a). Input capacitance (C) was measured from the response to a 0.4-nA hyperpolarizing current step by estimating the time constant, τ, of an exponential fit to the response, using the relation: τ = R × C.
Slow triangular voltage ramps (3.5 mV/s) were applied to measure the PICs in voltage clamp. During the upward portion of the ramp, the current initially increased linearly with voltage in response to the passive leak conductance. A linear relation was fit in this region (5–10 mV below the PIC onset) and extrapolated to the whole range of the ramp. At more depolarized potentials, as the PIC threshold was reached, there was a downward deviation from the extrapolated leak current. The amplitude of the PIC was measured as the peak amplitude of this downward deviation. Large PICs usually caused a negative slope region (NSR) in the current response. The onset voltage for the PIC (Von) was defined as the voltage at which the I–V slope first reached zero (Li and Bennett 2003). The current value corresponding to Von was defined as Ion. The width of the PIC (Vw) was defined as the width of the valley formed by the NSR measured at the current Ion in the I–V plot. That is, Vw = Vjump − Von, where Von and Vjump were the first and second potentials, respectively, at which Ion occurred during the upward current ramp. Previously, it has been shown that the width of the PIC (Vw) corresponds to the amplitude of the plateau potential that is produced by the PIC (Li and Bennett 2003). The changes in conductance caused by the PIC were estimated by the measuring the slope of the I–V relation at rest (resting slope-conductance, S1; same as leak conductance) and comparing this to the slope-conductance well after the PIC was fully activated (after NSR ended), when the I–V slope had again reached a fairly steady state (linear region; termed steady-state S2 slope conductance; see details in results). Steady state was found to occur in the voltages above Vjump (typically −50 to −45 mV), and the S2 slope was thus measured by fitting a line to the segment of data 5 mV above Vjump. The spike voltage threshold (Vth), was averaged from five consecutive spikes, starting with the second spike on the up ramp, and was taken as the voltage at which the rate of change of potential was >10 V/s (Li et al. 2004a).
Analysis of AHP and its conductance and voltage dependence
The postspike AHP was quantified when the membrane potential was held subthreshold to repetitive firing by evoking spikes with antidromic stimulation of the ventral roots. The maximum AHP amplitude was taken as the amplitude of the medium-duration afterhyperpolarization (mAHP), and the duration was quantified as the duration of the mAHP at half its amplitude (half duration). The dependence of the AHP amplitude on potential was measured by systematically varying the potential before the antidromic stimulation with a bias current. As described in results, the AHPs measured in this way had a linear voltage dependence, and we thus fit a linear regression of the AHP versus potential relation and made the following calculations to quantify this voltage dependence. The conductance of the SK channels is known not to have a voltage dependence itself (Lancaster et al. 1991), and so it can be thought of a simple fixed conductance (GAHP). Therefore the SK current (ISK) underlying the AHP can be approximately modeled as the product of this SK conductance and the difference between the potential (V) and the reversal potential for potassium (EK). That is, ISK = GAHP(V − EK). This SK current (ISK) ultimately acts through the leak conductances GL and the SK conductance itself to produce the mAHP
| (1) |
From Eq. 1, the potential V at which the mAHP is zero corresponds to the reversal potential for potassium, EK, which was computed from the regression line fit through the AHP versus potential relation. The slope of the AHP versus potential relation (AHPslope) was measured for each cell from the regression fit. This slope can be seen from Eq. 1 to be equivalent to AHPslope = GAHP/(GAHP + GL), and rearranging this relation gives an estimation of the AHP conductance: GAHP = GLAHPslope/(1 − AHPslope), which was also computed for each cell.
Data analysis
Data were analyzed in Clampfit 8.0 (Axon Instruments), and figures were made in Sigmaplot (Jandel Scientific). Data are shown as mean ± SD. Unless otherwise stated, a paired Student’s t-test was used to test for statistical differences before and after drug applications, with a significance level of P < 0.05. Unpaired t-test were used to compare acute and chronic spinal rats. One motoneuron was recorded per rat for each experimental condition tested.
RESULTS
Motoneurons recorded
Intracellular recordings were made from motoneurons of chronic and acute spinal rats, whereas the whole sacrocaudal spinal cord was maintained in vitro. In the main group of cells, we first examined firing and AHP properties in nACSF (n = 18 chronic and n = 8 acute). Then, in about one half these motoneurons, we applied apamin alone to examine SK current contributions to firing (n = 9 chronic spinal, n = 5 acute spinal). In the remaining motoneurons and some additional motoneurons, we applied TTX followed by apamin to examine SK current involvement in the Ca PIC (n = 22 chronic, n = 8 acute spinal rats). The basic membrane properties (R, C, and Vm) of these motoneurons are summarized in Table 1. For most of the results, we focus on chronic spinal rats because they have larger, more easily quantifiable PICs, although both acute and chronic spinal rats were found to have similar SK currents.
TABLE 1.
Basic properties of motoneurons
| Vm, mV | R, MΩ | Time Constant τ, ms |
Capacitance, µS |
|
|---|---|---|---|---|
| Acute | −69.9 ± 6.0 | 9.1 ± 4.1 | 4.5 ± 2.3 | 0.77 ± 0.23 |
| Chronic | −69.8 ± 8.1 | 8.2 ± 2.9 | 6.1 ± 3.1 | 1.1 ± 0.37 |
mAHP is mediated by an apamin-sensitive SK current
In control nACSF, the sodium spike that was evoked by either antidromic stimulation of the ventral roots (Fig. 1A) or current injection (Fig. 2, A and C) was always followed by a postspike AHP in both chronic (n = 18) and acute (n = 8) spinal rats. This AHP had a classic combination of a small fAHP followed by a pronounced mAHP (Viana et al. 1993). At the end of a series of spikes, there was a final AHP (Fig. 2, A and D), but this was not longer than a typical mAHP, and thus there was not the very slow AHP seen in other neurons (no sAHP) (Lasser-Ross et al. 1997).
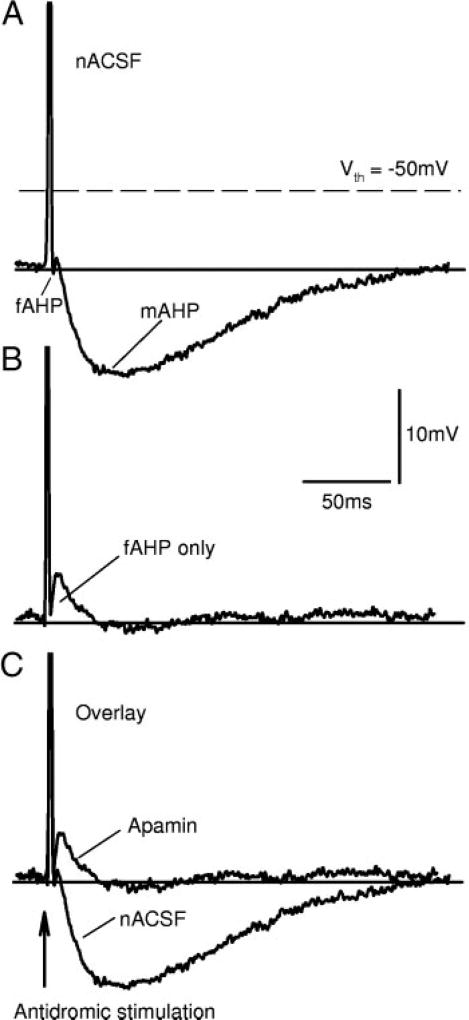
FIG. 1.
Apamin blocks the postspike afterhyperpolarization (mAHP) after an antidromic spike. A: spike induced by antidromic ventral root stimulation, recorded from a motoneuron after chronic injury. Note fast and slow components to postspike AHP, termed fAHP and mAHP, separated by an afterdepolarization. Motoneuron was initially well below spike threshold (Vth) measured during repetitive firing in a current ramp. B: apamin totally blocked mAHP but did not block fAHP. Note that afterdepolarization is now more clear, because it is no longer obscured by mAHP. C: overlay of A and B. Both records from the same cell at the same potential.
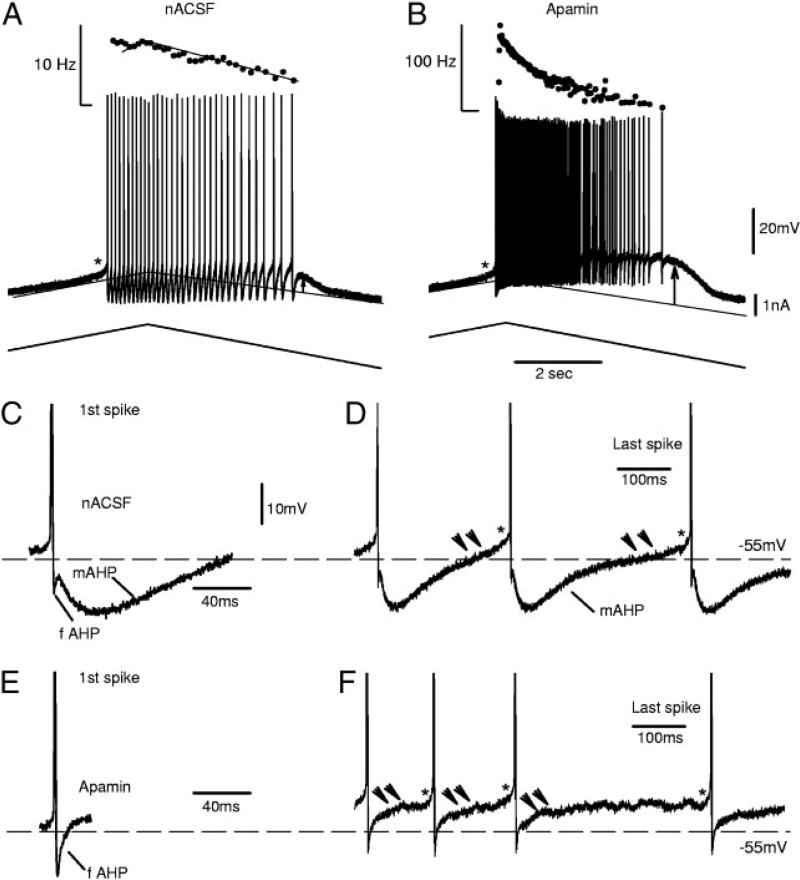
FIG. 2.
Effect of apamin on repetitive firing, recorded from a motoneuron after chronic injury. A: cell fired repetitively on an increasing current ramp with a maximum frequency <10 Hz and a small Ca plateau (afterpotential, arrow) at the end of firing. *Onset of plateau just before a firing started. B: in apamin, the cell fired with an initial frequency >100 Hz, followed by marked firing rate adaptation (slowing). There was also a very large plateau after firing (arrow). C: 1st spike from A enlarged, showing typical AHP, with fAHP and mAHP components. D: last few spikes from firing in A, also showing typical fAHPs and mAHPs. However, after usual concave AHP trajectory, there was a very slow upward ramp in membrane trajectory (double arrows) and a rapid acceleration (*) toward next spike (ramp and acceleration profile), because of onset of a persistent inward sodium current (Na PIC), as previously described during slow firing (Li et al. 2004a). E: mAHP, but not fAHP, after the 1st spike was eliminated by apamin. F: apamin blocked mAHP after all spikes, including last few spikes. However, fAHP again remained, and slow firing persisted with Na PIC–mediated ramp and acceleration profile (double arrow and *).
When the potent and selective SK channel blocker apamin (0.15 µM) was applied, the mAHP was completely blocked, and there only remained the fAHP, which was insensitive to apamin (Fig. 1, B and C; n = 9/9 motoneurons in chronic spinal rats and n = 5/5 in acute spinal rats). This mAHP block with apamin was seen after the spike evoked by antidromic stimulation from potentials subthreshold to repetitive firing (cf. Fig. 1, A and C) or after the first spikes when repetitive firing was evoked by a ramp current injection (cf. Fig. 2, C and E). Thus an apamin-sensitive calcium-activated potassium current (SK current) mediates the entire mAHP in these motoneurons.
mAHP limits the firing rate and PIC activation
When activated by a current injection, motoneurons of chronic (n = 18) or acute (n = 8) spinal rats typically fired at <20 Hz (Fig. 2A), corresponding to intervals greater than the time to peak of the mAHP (36.5 ± 6.5 ms) (Kernell 1965). The firing rate was restricted to this low range in large part because of the mAHP, because when the mAHP was blocked with apamin (0.15 µM), much higher firing rats were achieved, reaching ~100 Hz for the same standard current ramp (Fig. 2B). In chronic spinal rats in apamin, the firing rate jumped extremely rapidly to this high 100-Hz rate (in an all-or-nothing manner; in 8/9 chronic spinal rats tested) because of the activation of a large PIC and associated plateau potential (afterpotential) that could be seen after the termination of firing on the downward current ramp (Fig. 2B, arrow). Before apamin, there was a smaller plateau (Fig. 2A, afterpotential at arrow), because the mAHPs limited the PIC activation (Li et al. 2004a). Both before and after apamin, some firing persisted on the downward current ramps at currents well below the current to recruit the motoneuron (self-sustained firing, 8/9), although this firing was always much faster after apamin (8/8). This self-sustained firing has previously been shown to be largely caused by Ca PICs (nimodipine-sensitive; Li et al. 2004a) and confirms the presence of a Ca PIC.
Motoneurons without a large-enough Ca PIC to produce a plateau (i.e., in acute spinal rats, 5/5; and in 1/9 chronic spinal rats) fired roughly in proportion to the injected current before apamin, with no self-sustained firing (data not shown, but see Bennett et al. 2001 and Li et al. 2004a). However, with the application of apamin, even these cells reached very high rates (near 100 Hz) with standard-size current ramps that normally only produced 10- to 15-Hz firing without apamin, but this firing only reached a peak (100 Hz) at the peak of the current injection rather than at the onset of firing, because there was not a large Ca PIC (data not shown).
Slow firing does not require the mAHP
In nACSF, very slow firing often occurred near the threshold for derecruitment, as previously described for cells with large Na PIC, especially in chronic spinal rats (Harvey et al. 2005b; Li et al. 2004a). This very slow firing was at intervals that well exceeded the mAHP duration (see last few spike intervals in Fig. 2D; <5 Hz; n = 9/9 chronic spinal rats tested), and has previously been shown to result from a near-threshold slow oscillation of the Na PIC, where the Na PIC causes a characteristic slow ramp (at double arrows) and an acceleration in the membrane potential that triggers a spike (*; referred to as ramp and acceleration profile; this profile is blocked by a low dose of TTX that blocks the Na PIC but not the spike; see details in Li et al. 2004a). After each spike, the AHP deactivates the Na PIC, and the Na PIC-dependent ramp and acceleration profile occurs again and triggers another spike (see 2 interspike intervals shown in Fig. 2D). The Na PIC continues to oscillate in this slow manner, producing the slow firing, with one spike per oscillation cycle.
After the block of the mAHP with apamin, this slow firing persisted, again with a characteristic Na PIC–dependent ramp and acceleration profile (at double arrows and * in Fig. 2F), producing long interspike intervals (n = 9/9 chronic spinal rats). This Na PIC–dependent ramp and acceleration profile followed immediately after the fAHP (Fig. 2F), whereas, without apamin, it occurred later, after the mAHP (above dashed line in Fig. 2D). Thus the hyperpolarization from the fAHP alone is apparently sufficient to deactivate the Na PIC, after which, the Na PIC activates again in the characteristic ramp and acceleration profile (see 2 interspike interval at left of Fig. 2F).
Importantly, in apamin, this Na PIC–dependent ramp and acceleration profile also occurred during more rapid firing, with interspike intervals where it would normally be obscured by the mAHP. Thus the Na PIC plays a role at all firing rates, although its actions overlap with that of the AHP at higher rates.
Subthreshold to the Na PIC activation (near −60 mV, which is also subthreshold to repetitive firing; Li et al. 2004a) this ramp and acceleration profile never occurred after a spike evoked by an antidromic ventral root stimulation in apamin (Fig. 1B), consistent with it being mediated by the Na PIC. Instead, the membrane potential came back to rest within 20 ms after the spike (mAHP completely blocked by apamin).
AHP depends on N-, P/Q-type and not L-type calcium currents
When all the calcium currents were blocked with cadmium (400 µM), the mAHP was completely blocked (n = 4/4 cells tested), consistent with the above conclusion that the mAHP is mediated by a calcium-activated potassium current (SK current). A selective block of the high voltage–activated N- and P/Q-type calcium channels with ω-conotoxin MVIIC (5 µM, n = 5) mimicked the effects of cadmium, again completely eliminating the mAHP (fAHP also eliminated, n = 5/5; Fig. 3B). This conotoxin had no effect on the low voltage–activated Ca PIC (see Li and Bennett 2003). In contrast, a block of the L-type calcium channels with nimodipine had no effect on the AHP (mAHP or fAHP, n = 11/11; Fig. 3, C and D), whereas nimodipine completely blocked the Ca PIC (n = 12/12; which is thought to be Cav1.3 L-type calcium channel mediated; see Fig. 5 described below and Li and Bennett 2003). Thus the mAHP in motoneurons is produced by an apamin-sensitive SK current that is activated by calcium that flows through N- and P/Q-type high voltage–activated channels during the spike. Furthermore, the L-type calcium channels do not seem to play a major role in the AHP, in contrast to their major role in producing the low voltage–activated Ca PIC (see details below).
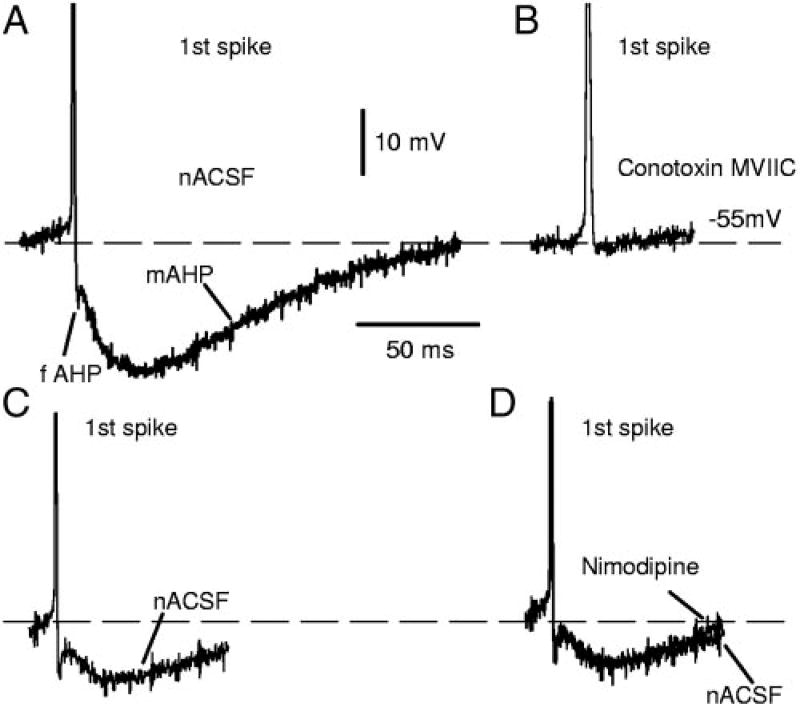
FIG. 3.
Both fAHP and mAHP are blocked by N- P/Q-type calcium channel blocker conotoxin MVIIC but not affected by L-type calcium channel blocker nimodipine, recorded from motoneurons of chronic spinal rats. A: 1st spike on a current ramp followed by fAHP and mAHP (as in Fig. 2C). B: both fAHP and mAHP were eliminated by conotoxin MVIIC. C: 1st spike on a current ramp followed by fAHP and mAHP (different motoneuron from A, with smaller AHP). D: nimodipine had no effect on AHP; overlay of AHPs recorded before and after nimodipine.
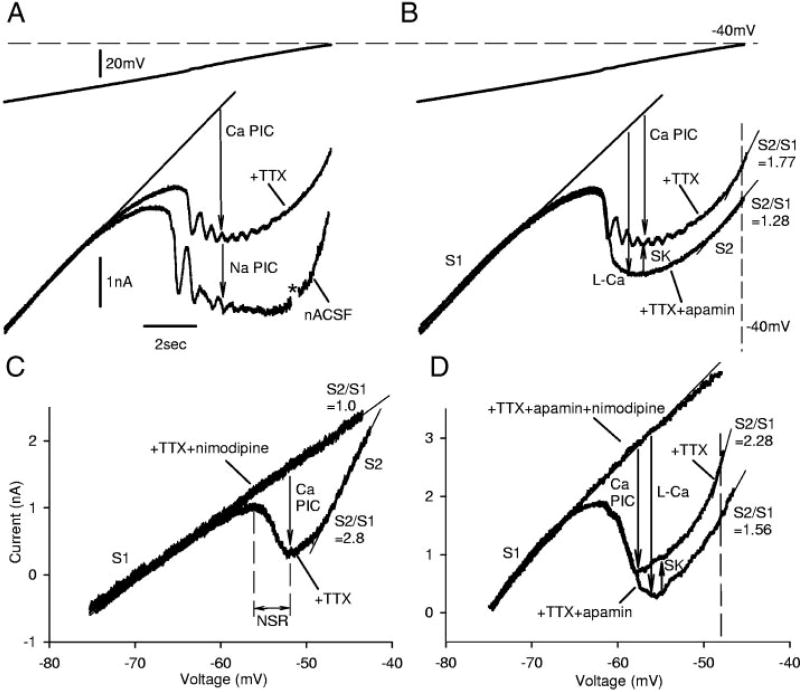
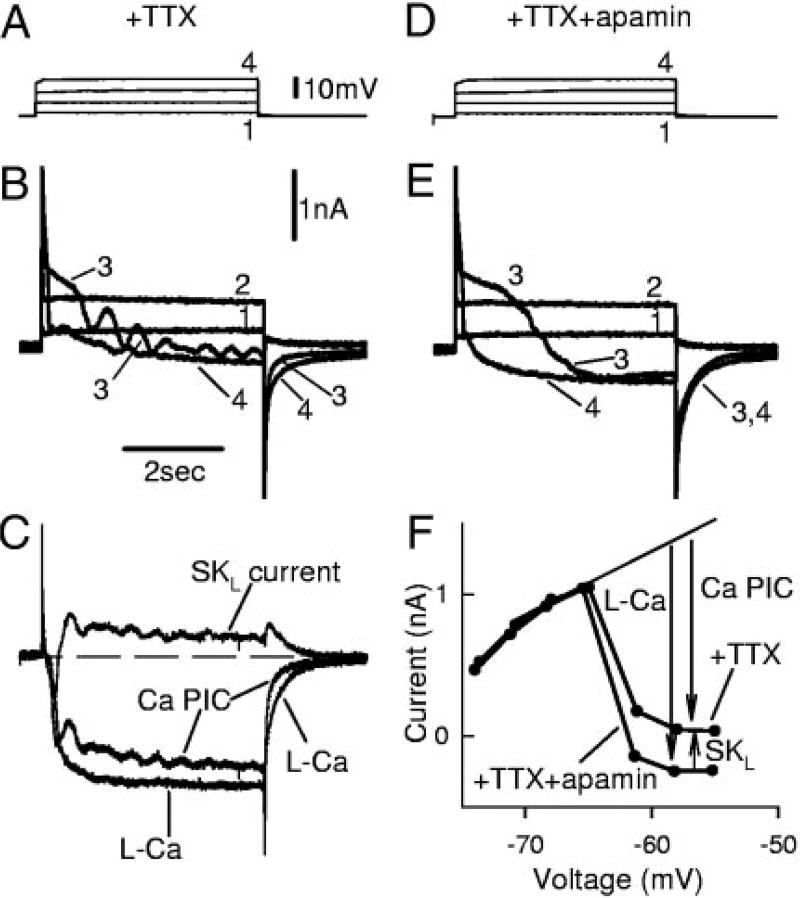
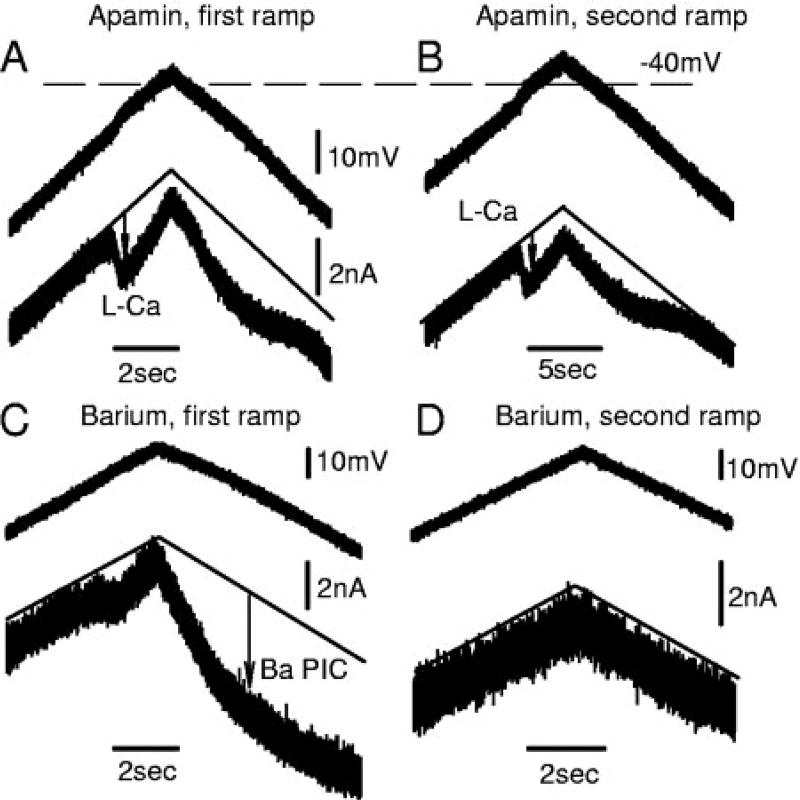
FIG. 5.
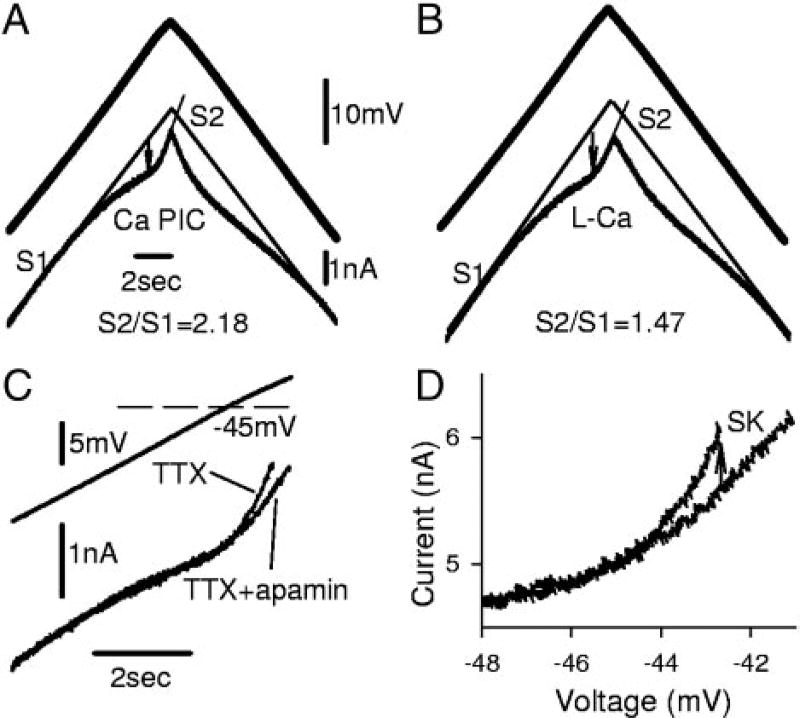
Apamin increases PIC by blocking an sustained calcium-activated potassium (SK) current that is activated by a nimodipine-sensitive L-Ca current, recorded from motoneurons of chronic spinal rats. A: slow ramp in voltage-clamp mode. TTX decreased total PIC by blocking Na PIC, leaving Ca PIC in isolation. B: apamin increased amplitude of Ca PIC by eliminating an outward SK current and revealed a relatively pure L-Ca current (=Ca PIC − SK current), without steep slope conductance (S2) normally seen after full activation of Ca PIC (S2 reduced). However, apamin had no impact on leak conductance (S1) or resting potential. C: nimodipine blocked the Ca PIC (=L-Ca current + SK current) and made S2 equal to S1 (measured in TTX, but without apamin). D: After apamin (and TTX), nimodipine blocked the L-Ca current and made S2 equal to S1. This taken together with C indicates that the SK current is indirectly nimodipine-sensitive (activated by the L-Ca current).
AHP is strongly voltage dependent but does not change with chronic injury
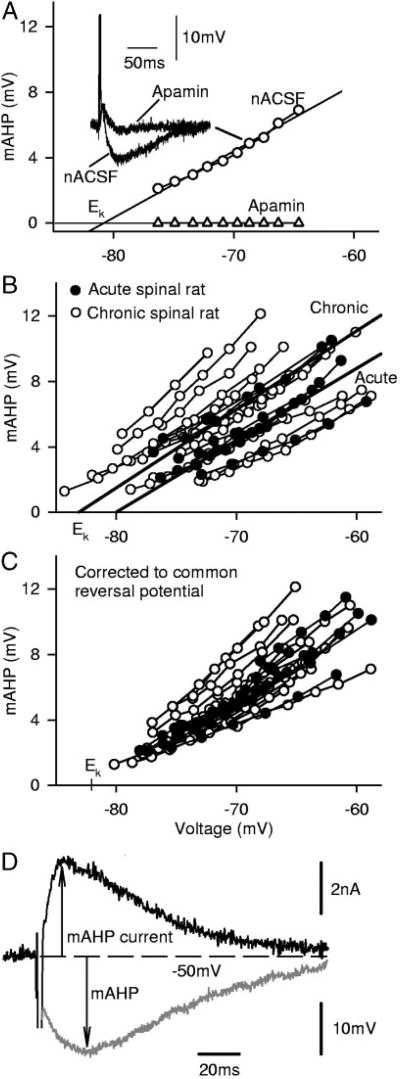
To compare the AHP across cells and between acute and chronic spinal rats, we found it necessary to first quantify the strong voltage dependence of the mAHP, which occurs because the mAHP current is a potassium current close to its reversal potential (EK; see methods; the SK channel conductance GSK itself is voltage-independent; see Introduction). That is, when the mAHP was measured from antidromically evoked spikes while the membrane potential was systematically varied with a bias current (e.g., −70 mV shown in inset of Fig. 4A), the mAHP amplitude varied linearly with the membrane potential [subthreshold to repetitive firing, Fig. 4A; because SK current = GSK(V − EK)]. As expected, apamin blocked the mAHP at all potentials (Fig. 4A, triangles), and thus, regardless of the potential, the mAHP resulted from just an SK potassium current (n = 5 acute and 9 chronic spinal rats tested). At the fixed potential of −70 mV (near rest), the mAHP was not significantly different in acute and chronic spinal rats (n = 7 and n = 15, respectively, tested in nACSF, although not all subsequently tested with apamin). The reversal potential for the mAHP, and thus also EK, was on average −80.1 ± 4.6 and −82.0 ± 2.9 mV in acute and chronic spinal rats (computed where regression lines crossed 0 mV), respectively, and there was no significant difference between these two groups. Because the potassium concentration gradient was not likely to change from cell to cell, the variability in EK was probably just a result of error in recording absolute potential (e.g., tip potential drift). Thus to compensate for this error in Fig. 4C, we corrected the potentials in all cells so that they had a common EK, equal to the overall mean EK (−81 mV). After this correction, there was a very close overlap of the AHP-versus-potential relations, suggesting that, at a given potential, the AHP was very similar in all cells. In particular, the mAHP at the corrected −70 mV potential was again not significantly different in acute and chronic spinal rats (n = 7 and 15, respectively). Of course, the reversal potential for mAHP only represents the reversal potential as seen at the soma, and if the mAHP currents are distal to the soma, it might be expected to be more hyperpolarized. However, because mean reversal potential is only −81 mV and the mAHP varies remarkably linearly with potential, it is likely that the mAHP currents are from channels near the soma.
FIG. 4.
Amplitudes of mAHPs are strongly voltage dependent but do not change significantly after chronic injury. A: amplitudes of mAHPs evoked by antidromic stimulation increased linearly with holding potential, recorded from a motoneuron of chronic spinal rat. Apamin completely blocked mAHPs at any potential. An overlay of mAHPs before and after apamin is also shown. B: linear relationship of mAHP amplitudes vs. voltages, recorded in 15 cells from chronic spinal rats (○) and 7 cells from acute spinal rats (●). Thick straight lines show the average potassium reversal potential for motoneurons from chronic spinal rats and acute spinal rats, respectively, as labeled; there was no significant difference between these 2 groups. C: data from B corrected and replotted so that all cells have a common potassium reversal potential, Ek, corresponding to average Ek. D: mAHP current measured in voltage-clamp mode after a simulated spike (data not shown, 2-ms step of +70 mV). Gray trace shows corresponding mAHP evoked from a spike in current-clamp mode.
The slope of the mAHP versus potential relation (AHPslope; Fig. 4B) was also not significantly different in acute and chronic spinal rats (n = 7 and 15, respectively). Furthermore, as detailed in methods, this AHPslope can be used to compute the effective conductance of the mAHP (GAHP), which is summarized in Table 2 (0.19–0.23 µS) and is not significantly different in acute and chronic spinal rats. The current underlying the mAHP (ISK) was directly measured after a simulated spike (2-ms step to >0 mV) in voltage clamp, as shown in Fig. 4D (n = 5), and this current closely reflected the mAHP shape (gray line). Finally, the mAHP duration (time-to-peak mAHP) was on average not significantly different in chronic spinal rats (85.2 ± 16.1 ms) than in acute spinal rats (77.3 ± 15.9 ms).
TABLE 2.
mAHP properties and leak conductances of motoneurons
| mAHP Amplitude, mV |
mAHP Half Duration, ms |
mAHP Slope |
Leak Conductance, µS |
AHP Reversal Potential, mV |
mAHP Conductance, µS |
|
|---|---|---|---|---|---|---|
| Acute (n = 8) | 5.22 ± 0.73 | 77.3 ± 15.8 | 0.44 ± 0.06 | 0.24 ± 0.10 | 80.0 ± 4.7 | 0.19 ± 0.11 |
| Chronic (n = 18) | 5.71 ± 1.47 | 85.2 ± 16.1 | 0.48 ± 0.12 | 0.22 ± 0.04 | 83.1 ± 2.9 | 0.23 ± 0.13 |
mAHP, postspike afterhyperpolarization.
In summary, the AHP varies dramatically with potential because of the close proximity of the resting potential to the reversal potential for potassium. Despite this, at a given potential, the AHP is not significantly different in amplitude, duration, or conductance in acute and chronic spinal rats but is very similar across all cells.
L-type calcium current of the Ca PIC activates an SK current in chronic spinal rats
In chronic spinal rats, when the membrane potential was slowly increased under voltage-clamp conditions, a large PIC was activated and seen as a downward deviation from the extrapolated subthreshold linear leak current (thin line); this PIC usually produced an outright negative slope region in the I–V relation (NSR; Fig. 5C). Part of this PIC (about one half) was blocked rapidly by TTX, which has previously been attributed to a persistent sodium current in these motoneurons (Na PIC; n = 22/22 chronic spinal; Fig. 5A; Li and Bennett 2003). The remaining current in TTX was blocked by nimodipine (15 µM; leaving only a linear leak current-voltage relation below −40 mV; n = 12/12 tested) and thus was mediated by an L-type Ca PIC (Fig. 5C) (see details in Li and Bennett 2003). This Ca PIC is the focus of the remainder of the Results section. In chronic spinal rats, the Ca PIC was activated with an average onset threshold of −58.6 ± 4.1 mV and reached a peak of 2.2 ± 1.2 nA at −54 ± 3.7 mV (n = 22). With decreasing current ramps (Fig. 7, described later) the Ca PIC was deactivated at a significantly lower voltage than its onset voltage (Voff; 12.5 ± 3.3 mV lower). Acute spinal rats had similar, but much smaller, Ca PICs, which are described later (Fig. 9).
FIG. 7.
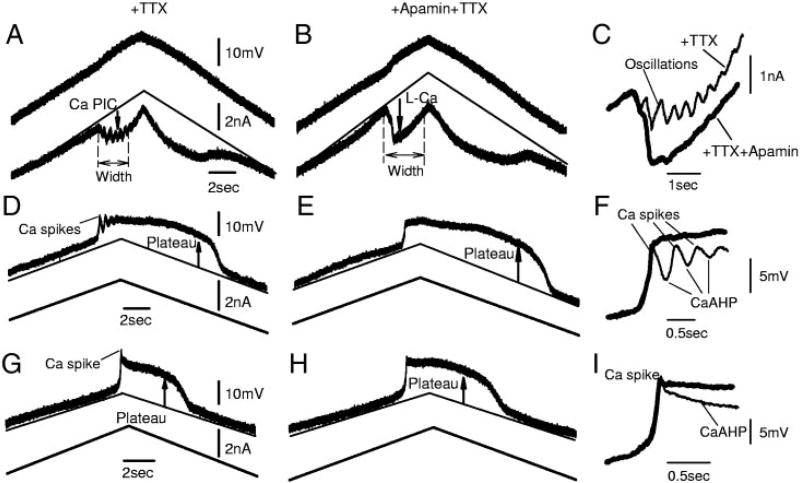
Apamin increases the Ca plateaus associated with the Ca PICs and eliminates Ca spikes at the onset of the plateaus and PICs. A: Ca PIC measured with a voltage ramp in TTX in a motoneuron after chronic injury. Note oscillations during activation of Ca PIC. Two-headed arrow shows width of Ca PIC. B: apamin increased PIC amplitude and width. Also, oscillations were eliminated. C: overlay of peaks of PICs in A and B on a larger scale. D: Ca plateau induced by a upward current ramp, with 3 Ca spikes appearing at onset of Ca plateau, corresponding with oscillations seen in C (same motoneuron). E: apamin blocked Ca spikes at onset of plateau and increased steady-state plateau amplitude and duration. F: overlay of onsets of Ca plateaus in D and E on a larger scale. G: another motoneuron that only had 1 Ca spike at onset of Ca plateau. From an acute spinal rat, with large-enough Ca PIC to produce an negative slope region (NSR; data not shown). H: apamin again blocked Ca spike and increased Ca plateau. I: overlay of onsets of Ca plateaus in G and H.
FIG. 9.
Effects of apamin on Ca PICs in motoneurons of acute spinal rats. A: Ca PIC induced in motoneuron of acute spinal rat with a voltage ramp, seen relative to leak current. Note characteristically steeper S2 slope conductance than S1 slope. No NSR was present because of the small size of PICs, as is common in acute spinal rats. B: apamin increased PIC by blocking SK current (leaving an L-Ca current). Apamin also reduced S2 slope conductance. C: SK current and SK conductance is shown by overlaying current traces before and after apamin during upward ramp. D: in I–V relationships before and after apamin. Data are only shown after onset of PIC, where current deviates in apamin (data from right half of C), and SK current is indicated with arrow.
When apamin was applied to block the outward SK currents (in the presence of TTX), a significantly larger persistent inward current was measured during voltage ramps (n = 22/22 chronic spinal rats tested; Fig. 5, B and D), which we refer to as the L-Ca current to distinguish it from the Ca PIC (before apamin) and to indicate that it is produced by L-type calcium channels. The apamin-sensitive current (Ca PIC minus L-Ca current) we refer to as the SKL current (Fig. 5B). This SKL current must have been activated by the L-Ca current because it was always activated just after the onset of the L-Ca current (Fig. 5, B and D). Furthermore, the SKL current must be indirectly nimodipine-sensitive because both the L-Ca current (in apamin; n = 7/7; Fig. 5D) and the Ca PIC (pre-apamin; n = 12/12; Fig. 5C) were blocked by nimodipine, and their difference is the SKL current. The SKL current at the initial peak of the L-Ca current (Fig. 5B, arrow) was on average 0.59 ± 0.4 nA, which was 26% of the L-Ca current. This initial SKL current was significantly smaller in size and estimated conductance (0.019 ± 0.014 µS; current/[V−EK]) than the SK current underlying the AHP. However, as described below, the SKL current increased substantially as the voltage ramp continued to increase. Finally, the width of the valley formed by the PIC in the I–V relation (as defined in Fig. 7, A and B, and methods; Vjump − Von) was significantly increased, from 11.9 ± 2.7 mV (Ca PIC) in control conditions to 13.9 ± 4.1 mV (L-Ca PIC) in apamin, consistent with the larger plateau formed in apamin.
Neither the resting membrane potential nor the leak conductance (1/R) was significantly affected by apamin (n = 22 chronic and 8 acute spinal rats tested); thus there was not a resting calcium current (different from L-Ca) that activated an SK current.
SK current produces a steep increase in conductance (the wall)
In nACSF, during the increasing voltage ramp, the slope of the I–V response relation was always much steeper after the full activation of the Ca PIC (termed slope-conductance S2 and measured after peak of Ca PIC at Vjump; near −45 mV; Fig. 5D, dashed vertical line) compared with before the Ca PIC activation (slope conductance S1; same as leak conductance; Fig. 5, A–D; n = 22/22 chronic spinal rats). The greater slope conductance after Ca PIC activation (S2/S1 = 2.5 ± 1.2) resulted from 1) the increased conductance arising from the L-Ca current (Li et al. 2004a) and 2) the activation of SKL currents. That is, application of apamin significantly lowered the S2 slope relative to the S1 slope (S2/S1 ratio = 1.6 ± 0.5; n = 22). Characteristically, in apamin, the current during the voltage ramp increased linearly after the initial onset of the L-Ca (after NSR; n = 22/22 chronic spinal), consistent with the onset of a steady L-Ca current that simply increased the overall conductance (by 20% of leak conductance in Fig. 6B). In contrast, before apamin (Fig. 6A; n = 22), the current during the voltage ramp increased much more steeply after the initial onset of the Ca PIC (after NSR), with a nonlinear parabolic shape induced by the SK current, as though intracellular calcium was accumulating. Thus the steep apamin-sensitive S2 slope provides a distinctive feature of the I–V relation that indicates the presence and size of the SKL current. By subtracting the S2/S1 conductance ratios before and after apamin, we get the ratio of the SKL conductance to the leak conductance (S1), which was 0.85 ± 0.8, indicting that the SKL current produced a conductance similar in size to the leak conductance (and AHP conductance; Table 2). This steep apamin-sensitive S2 slope conductance was eliminated by either nimodipine (n = 12/12; Fig. 5C) or apamin (n = 22/22; Fig. 5D), further supporting the idea that the L-Ca current activates an SK current (SKL).
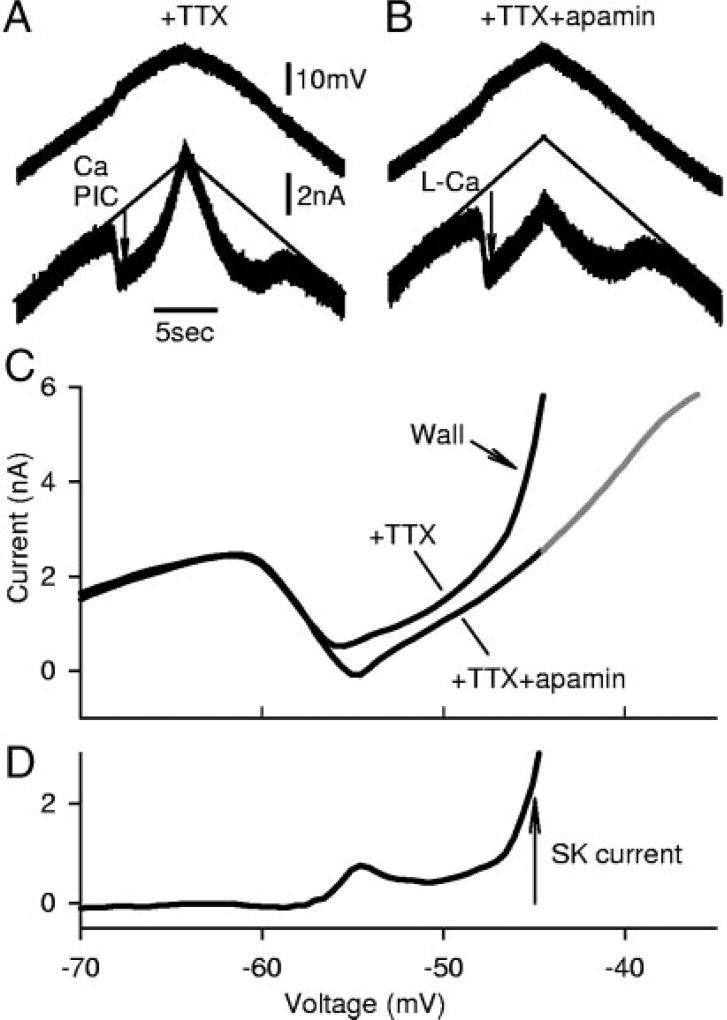
FIG. 6.
SK current increases nonlinearly, generating a very large current (wall) that overcomes L-Ca current as the potential is increased, recorded from a motoneuron of chronic spinal rat. A: after Ca PIC activation, current (and S2 conductance) increased dramatically with upward voltage ramps and made it difficult to reach voltages above −40 mV (because of current-passing limitation of electrode). This steep section of the I–V relation was termed the wall. B: the wall was eliminated by apamin, and there remained a shallow S2 conductance only slightly higher than leak conductance (S1). C: I–V relationship before and after apamin, with potential ramped above −40 mV after apamin (gray trace). D: subtraction of traces in C to obtain SK current. Note initial peak in SK current, followed by a slight reduction and a steep increase at the region of the wall.
The detailed changes in the SK current during the voltage ramp can be seen in Fig. 6D, estimated by subtracting the measured currents before and after apamin (Fig. 6C). As the membrane potential was depolarized to near −40 mV, this outward SKL current always became very large and eventually exceeded the inward L-Ca current, so the net current exceeded the leak current estimate (current above thin leak line in Fig. 6A). At this point (near −40 mV), the SKL current (1.68 ± 0.58 nA) was significantly larger than the SKL current at the initial peak of the PIC (near −50 mV; 0.59 ± 0.4 nA; n = 22), and comparable in size (>1 nA) to the current produced by the AHP (SKAHP, n = 18). This large increase in SK current and steep S2 slope near −40 mV we refer to as the wall, because it corresponds to a near vertical region in the I–V relation. The wall was generally so pronounced that our intracellular electrodes could not depolarize the cell much past −40 mV (electrode current limited to <6–12 nA; Fig. 6C). Once the wall was eliminated by apamin (S2 reduced), we could usually depolarize the cells above −40 mV (Fig. 6C; gray section). In current clamp, the effect of the wall was manifested as a flat (shallow) region, where the membrane potential could not be easily depolarized by current injection (top of plateau described later in Fig. 7).
Apamin-sensitive oscillations at the onset of the Ca PIC
When the Ca PIC was large enough to produce an NSR in the I–V relation (in voltage clamp), a plateau potential was always seen in current clamp during a ramp current injection, induced by the instability of this NSR (Li and Bennett 2003; Schwindt and Crill 1982), which occurred in all chronic spinal rats (n = 22/22). This plateau had a characteristic sharp overshoot at its onset (Fig. 7, D and G), like a spike, although we specifically refer to it as an overshoot spike, to distinguish it from the much larger calcium spikes induced by TEA in motoneurons (Hounsgaard and Mintz 1988). After this calcium overshoot-spike, an afterhyperpolarization (CaAHP) always occurred (n = 22/22), which tended to reduce the plateau. The CaAHP was blocked by apamin (n = 22/22) and thus was mediated by an SKL current. In some cells, this CaAHP was sustained during the plateau and thus served to simply reduce the plateau amplitude (Fig. 7, G–I; n = 10/22 cells). However, in other cells (12/22), the CaAHP reached a peak hyperpolarization in ~200–300 ms and was then turned off, presumably because the CaAHP reduced the L-Ca current underlying the plateau and overshoot-spike. A second calcium overshoot-spike then followed as the L-Ca current reactivated, followed by a second CaAHP. This oscillation of the L-Ca and the SK current continued for a few cycles until a steady-state activation of the L-Ca and the SK currents was reached. This oscillation was always blocked by apamin (n = 12/12), confirming the role of the SKL currents (Fig. 7, D–F). Furthermore, in apamin, the steady-state depolarization was greater than without apamin, showing that the SKL current also produced a steady hyperpolarization throughout the plateau, reducing its size.
Interestingly, during voltage ramps, a similar oscillation was also seen at the onset of the Ca PIC (Fig. 7A), even though this current was measured under voltage clamp, which should at least clamp the potential near the soma, avoiding local oscillations of channels. These oscillations were again blocked by apamin (n = 12/12; Fig. 7B), and thus again were mediated by SKL currents. Likely, these oscillations resulted from calcium overshoot-spikes interacting with CaAHPs, as described above under current clamp, but occurring in the distal unclamped dendrites. Indeed, all cells that had these Ca PIC oscillations under voltage clamp conditions (n = 12) also had calcium overhoot-spikes and CaAHP oscillations under current-clamp conditions (e.g., Fig. 7, A and D, is from same cell). This provides indirect evidence that the SKL currents are of dendritic origin, like the L-Ca currents, and perhaps unlike the SKAHP currents.
SKL current is slow to activate
To examine the time-course of the SKL currents, we used a series of long voltage steps in chronic spinal rats (n = 11 tested). Subthreshold to the Ca PIC, these steps produced a current response proportional to the leak current (trace 1 and 2 in Fig. 8, B and E and left of Fig. 8F). For larger steps above threshold, the Ca PIC was activated and reduced the current below the expected leak current (Fig. 8, B and E, traces 3 and 4, and right of F). As previously reported, with steps just above threshold for the Ca PIC (trace 3), the Ca PIC activated with a substantial delay of 1 s or more (Li and Bennett 2003). Usually, a few of the characteristic SKL-mediated oscillations (apaminsensitive) occurred during this slow Ca PIC activation (like on current ramps; Fig. 7B). With larger voltage steps, Ca PIC activated more rapidly, starting at the onset of the step and reaching 10% of its maximum within 45 ± 7 ms (n = 11), although steady-state activation was not reached for ~1 s. In contrast, the first sign of the SKL current onset, seen as small SK-mediated oscillation (1st inflection), did not occur until 170 ± 19 ms (n = 11). Thus the SK current onset was delayed by ~100 ms, relative to the underlying L-Ca current that activated it, and the L-Ca activation of the SK currents was slower than the sodium spike-mediated activation of the SK currents (AHP onset in <10 ms), suggesting differences in spatial location.
FIG. 8.
Current responses to long voltage steps before and after apamin, recorded in a motoneuron from a chronic spinal rat. A and B: long voltage steps and corresponding current responses. Note that current increases with 1st 2 steps (1 and 2) proportionally to leak current, but after Ca PIC activation (in steps 3 and 4), current is reduced well below leak current (summarized in F). Also, Ca PIC produced a tail current after the end of voltage step (labeled 3 and 4 at right). Note oscillations in near-threshold step response, as L-Ca and SK currents (Ca PIC) were slowly activated (step 3). D and E: after apamin, currents were recorded at matched voltage steps from the same motoneuron; as expected, there was a larger negative deflection resulting from L-Ca current without SK current. Also, tail currents were longer. Near threshold, L-Ca was again slowly activated (step 3), but there were no oscillations. C: Ca PIC and L-Ca currents computed by leak current subtraction, recorded in response to a common voltage step before and after apamin (different from 4 steps in B and E, but from same cell; 2nd point from right in F). Also, SKL current was computed by subtracting L-Ca current from Ca PIC. F: steady-state I–V relationship measured during voltage steps before and after apamin, showing SK current activated with L-Ca current.
Application of apamin increased the overall PIC measured with voltage steps, as expected from the voltage-ramp results and consistent with a block of the outward SK currents, leaving only the underlying L-Ca current (Fig. 8E; n = 11). The L-Ca, like the Ca PIC, was slow to activate with small voltage steps near threshold, but did not have oscillations during activation (Fig. 8E; trace 3). With larger steps, the L-Ca started its activation sooner, just like for the Ca PIC. The net SK current in response to a large step is shown in Fig. 8C, computed by subtraction before and after apamin. Interestingly, after its onset, the SK current was gradually reduced over a few seconds, even though the L-Ca was not reduced (L-Ca computed by leak subtraction; Fig. 8C). This slow reduction of the SK current with time caused the net Ca PIC (before apamin) to reach a steady-state maximum inward current significantly later than the L-Ca current (95% activation occurred at an average time of 2.3 ± 0.9 s for the Ca PIC and 1.1 ± 0.7 s for the L-Ca).
After termination of the voltage steps, the Ca PIC always took ~0.5 s to turn off, producing a characteristic tail current, with a mean duration of 323 ± 372 ms before apamin (time to 50% decay in tail current; n = 11). Associated with this tail current, there was a residual SK current that also decayed off slowly (Fig. 8C). Blocking the SK current with apamin ultimately led to a significantly longer tail current (783 ± 965 ms; n = 11). Thus the outward SK current acted to speed the deactivation of the inward L-Ca current.
SKL currents are relatively larger in acute than chronic spinal rats
Motoneurons of acute spinal rats had small but significant Ca PICs (n = 8 tested), as previously described (Harvey et al. 2005b). These Ca PICs were not large enough to produce an NSR in the current response to a voltage ramp and instead only induced a negative deflection in current relative to the extrapolated leak current (n = 8/8; PIC indicated by arrow in Fig. 9A). Because of the lack of NSR, there were not plateaus seen with current injection in these motoneurons (data not shown). Apamin significantly increased these PICs (arrow larger in Fig. 9B; see also Fig. 10; n = 8), indicating that there was an apamin-sensitive SKL current (Fig. 9D), as in chronic spinal rats. Also, apamin significantly decreased the S2 slope (Fig. 9B), as in chronic spinal rats, indicating a substantial SKL conductance in the region of the wall near −40 mV.
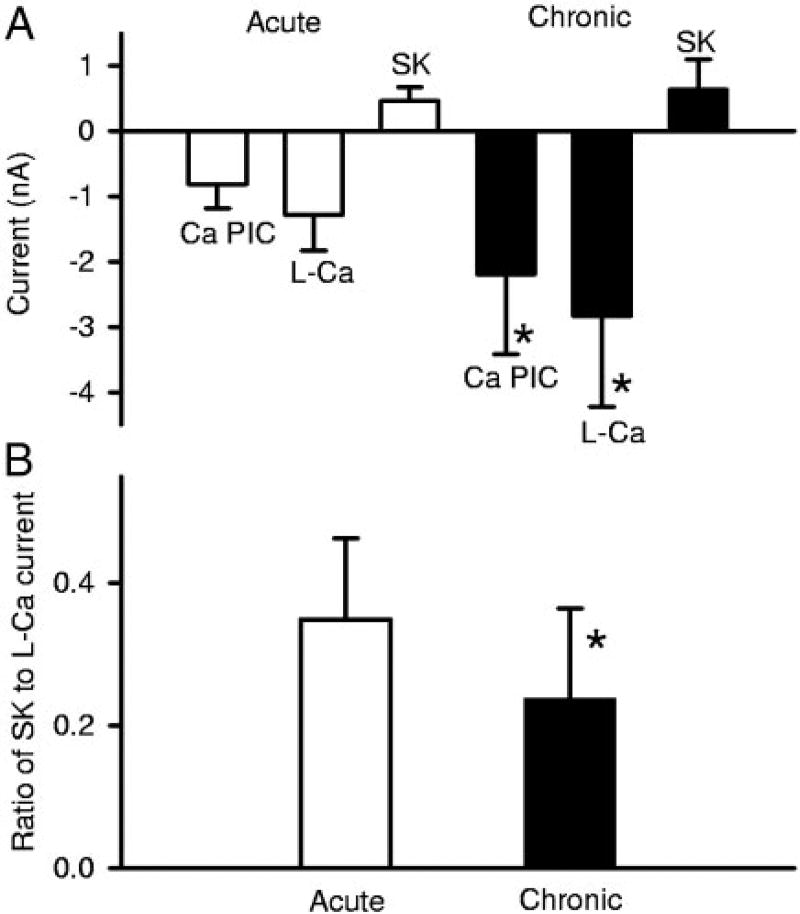
FIG. 10.
Summary of amplitudes of Ca PICs, L-Ca, and SK currents in motoneurons of acute or chronic spinal rats. Amplitudes of Ca PIC and L-Ca currents are significantly larger in motoneurons from chronic spinal rats than from acute spinal rats (*). Mean SK current is also larger in chronic spinal rats, but not significantly. B: ratio of SK current to L-Ca current is significantly higher in normal motoneurons than in motoneurons after chronic injury. Amplitudes of currents were recorded at matched potentials (on average, −48.4 mV for acutes and −49.3mV for chronics).
As expected, the L-Ca current measured in apamin was significantly smaller in acute (n = 8) than chronic (n = 22) spinal rats (Fig. 10A; *significant difference between acute and chronic). However, the SK current itself was unexpectedly not significantly smaller in acute compared with chronic spinal rats. Also, the SK-induced slope conductance (S2) was not significantly different in acute and chronic spinal rats. Taken together, these results suggest that, for a given amount of calcium current (L-Ca), the resulting SKL current activation was relatively greater in acute spinal rats. Indeed, the ratio of the SKL current to the L-Ca current was significantly greater in acute spinal rats (n = 8), by ~40%, compared with in chronic spinal rats (Fig. 10B; n = 22).
Barium also eliminates apamin-sensitive SK currents
To further examine the calcium-activated currents, we substituted barium (Ba) for calcium (Ca) as the charge carrier through the calcium channels (n = 5 chronic spinal rats). This effectively eliminates all calcium-activated currents (including the SK current) because barium is much less effective than calcium in activating these currents (Enomoto et al. 1991). As shown in Fig. 11B, this barium substitution always led to an increase in the PIC (labeled L-Ba) compared with in normal calcium-containing ASCF (Ca PIC), very much as we saw with apamin. Unfortunately, because the barium current through calcium channels is generally known to be larger than the calcium current through the same channels (Hille 2001), part of this increase in the PIC may have been caused by the differing current-carrying capability of barium. However, the shape of the I–V relation in barium was remarkably like that in apamin (Fig. 6B), suggesting that the barium substitution caused an elimination of SK currents that produced the characteristic very steep S2 slope in nACSF. Indeed, this was the case because applying apamin while in barium had no significant effect on the PIC (L-Ba is apamin resistant; n = 5). Also, in barium alone, like in apamin, the S2 slope after the PIC activation was very shallow and close to the leak conductance (S1; Figs. 11A and 6A). On average, the ratio S2/S1 was significantly reduced by barium (n = 5; from ~2.5 ± 0.86 to 1.5 ± 0.17; Fig. 11E), like with apamin. Furthermore, the S2/S1 ratio, at 1.5, was not significantly different in barium and apamin, indicating that both apamin and barium eliminated a similar conductance (barium and apamin sensitive) that was about equal to the leak conductance (2.5 − 1.5 = 1).
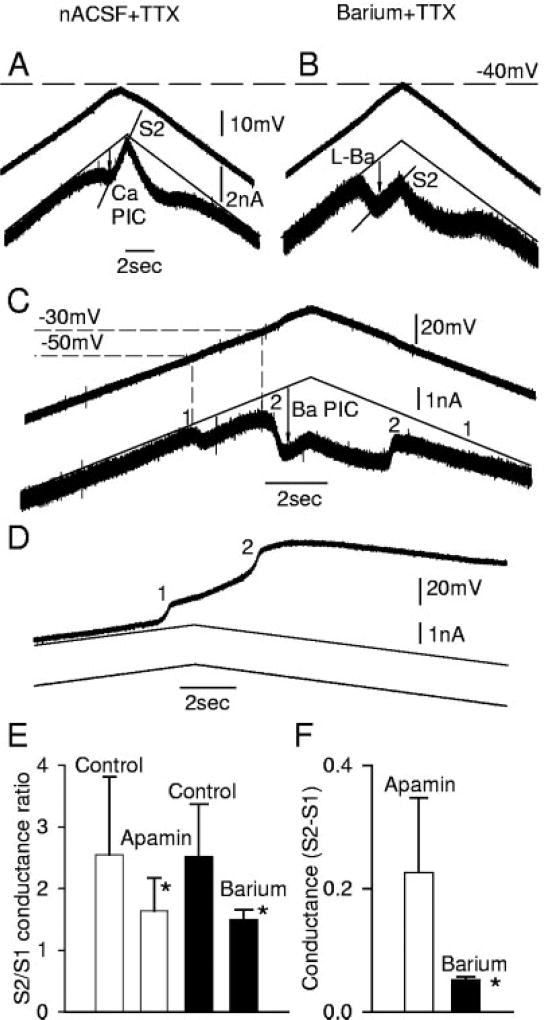
FIG. 11.
SK current is also blocked by replacing Ca with Ba in the normal artificial cerebrospinal fluid (nACSF), recorded from a motoneuron of a chronic spinal rat. A: Ca PIC and a steep S2 slope conductance recorded during a voltage ramp in nACSF and TTX. B: by replacing Ca in the nACSF with Ba, amplitude of PIC is increased, and S2 slope ratio is lowered, like in apamin. C: during a larger-than-standard voltage ramp, 2 Ba PICs are induced: 1 has the usual low threshold of the L-Ca current (near −50 mV, as in A; termed L-Ba current), and the other has a higher activation threshold (near −30 to −35 mV). D: in a corresponding current clamp in barium, 2 plateaus are activated corresponding to the 2 Ba PICs onsets in C (1 and 2). E: both apamin and Ba significantly reduced the S2/S1 slope ratio (*), but they did not have significantly different effects. F: slope conductances activated at potentials above PIC onset (S2 − S1) in apamin and barium.
In barium, however, there was the additional complication that the leak conductance was significantly less than in control conditions (S1 = 0.12 ± 0.03 in barium vs. 0.23 ± 0.16 in nACSF; n = 5), unlike in apamin. Likewise, the activation of conductances at potentials above the PIC onset (S2 − S1) was significantly less in Ba than in apamin (Fig. 11F). This is because barium blocks resting leak currents (S1) and voltage-activated currents (included in S2 − S1), in addition to calcium-activated currents (also included in S2 − S1; Hille 2001). Thus it is difficult to determine whether barium blocks more calcium-activated currents than does apamin.
Barium reveals an unusual high voltage–activated PIC and plateau
Surprisingly, in barium there was always a very large high voltage–activated PIC (high voltage–activated Ba PIC) that was activated above −30 mV (2 in Fig. 11C; n = 5) and not seen in apamin (n = 22). This was activated at a distinctly higher potential than the normal low voltage–activated PIC (1 in Fig. 11C; activated at about − 50 mV, as in nACSF or apamin), and thus remarkably discrete low and high voltage–activated PICs were seen in the same cell (Fig. 11C, 1 and 2, respectively). This high voltage–activated Ba PIC produced a large sustained high voltage–activated Ba plateau potential in current clamp that was very difficult to turn off (Fig. 11D, 2), as described in detail below. This large high voltage–activated Ba PIC may have been in part an artifact of barium application because it was not seen without barium even with the large depolarizations possible in apamin (Fig. 6C). Probably, this high voltage–activated Ba PIC was a result of a loss of inactivation of high voltage–activated calcium channels (N, P/Q-type) resulting from removal of calcium from the bath because calcium channel inactivation is known to have a strong dependence on calcium itself (calcium-dependent calcium inactivation) (Cens et al. 2005; Zong et al. 1994).
During the onset of the high voltage–activated Ba plateau, there was at times a burst of very fast Ba spikes (at ~40 Hz), as shown in the inset in Fig. 12C (n = 3/5). These spikes were much narrower, faster, and larger than the overshoot-spikes seen at the onset of the low voltage–activated Ca PIC, described in Fig. 7. Thus it seems that the channels that mediate the high voltage–activated PIC (likely N,P-type) are more rapid than the L-type calcium channels that mediate the low voltage–activated Ca PIC.
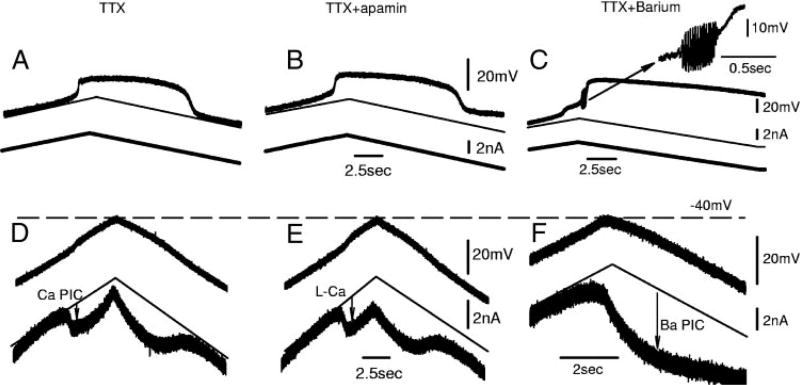
FIG. 12.
Blockade of SK channels can cause a loss of control over PIC, recorded from motoneurons after chronic injury. A and D: in TTX, Ca PIC (D) and Ca plateau (D) is fully deactivated with a downward voltage (D) or current (A) ramp, returning to leak conductance line (thin downward lines). B and E: with addition of apamin, for ~15% motoneurons, PIC cannot be fully deactivated. That is, once L-Ca current (E) or Ca plateau (B) is activated, a downward voltage (E) or current (B) ramp does not fully return recorded current (E) or voltage (B) to leak conductance line (downward thin lines), even with large hyperpolarizations. Note that part of PIC is deactivated, and this portion is likely spatially close enough to electrode to be controlled. C and F: with addition of Ba (and TTX), Ba PIC and Ba plateau cannot be deactivated once it is activated, even with large hyperpolarizations. Note the rapid Ba spikes that occur at onset of high voltage–activated Ba plateau (C), also shown enlarged.
SK currents prevent uncontrollable activation of dendritic Ca PICs
The Ca PICs had a voltage threshold for activation (measured at electrode, in soma) that was significantly higher than the threshold for deactivation (by ~10 mV, hysteresis described above). This has previously been attributed to the dendritic nature of these currents and the inevitable poor space-clamp over these distal dendrites in very large dendritic trees of motoneurons (Hounsgaard and Kiehn 1993; Hultborn 2002). However, in nACSF, this poor space clamp was not a problem because the Ca PICs or Ca plateaus could still be fully deactivated after they were activated (Fig. 12, A and D), and thus quantified, albeit at a distance from their dendritic location. However, in a few cells in apamin (n = 4/26; chronic) and most cells in barium (n = 4/5; chronic), the PIC (L-Ca current or Ba PIC) was uncontrollable, in the sense that, once activated, it could not be completely deactivated, regardless of the hyperpolarization applied. This loss of control over the L-Ca current (or Ba PIC) occurred in voltage clamp, as in Fig. 12E, or in current clamp, where associated plateaus could not be turned off, as in Fig. 12B.
Once we lost control over the L-Ca currents (or Ba PIC) on a voltage ramp (Fig. 13A and C), subsequent voltage ramps evoked smaller L-Ca currents (or Ba PICs) than before (Fig. 13, B and C) because a portion of the PIC was tonically activated (or inactivated). The situation was extreme in barium, where at times the Ba PIC remained fully activated, despite very hyperpolarized holding potentials, and a subsequent voltage ramp evoked no further PIC (Fig. 13C; n = 4/5). When held for many minutes at a hyperpolarized level, some Ba PIC could be evoked (data not shown), presumably because a portion of the Ba PIC eventually inactivated (allowing the dendrites to hyperpolarize and enabling some deactivation of the Ba PIC). Likewise, with a current ramp after the first uncontrolled plateau activation, a second current ramp produced a smaller plateau (data not shown). In these kinds of cells (15% of cells in apamin), we had to be cautious in comparing the pre- and postapamin results because, if the loss of control was not noticed, the L-Ca in apamin appeared smaller than the Ca PIC before apamin, leading to the erroneous conclusion that apamin blocked the calcium current itself. Thus in the results described above, only cell measured without loss of control of the PIC were used (22/26 cells). We interpret the loss of control over the calcium currents after a block of SK currents as indicating that a portion of the dendritic tree distal to the electrode had gone onto an uncontrolled L-Ca-mediated plateau. Why this only occurred in some cells in apamin (15%) is uncertain, although it may relate to how close the electrodes were to the center of L-Ca channel activation. In any event, these results show that the SK currents play a major physiological role of assuring that the L-Ca currents can be turned off after they are activated because such uncontrolled PICs were not seen without apamin or barium present.
FIG. 13.
After loss of control over PIC, only smaller PICs can be evoked, recorded from motoneurons after chronic injury. A: in apamin, during 1st voltage ramp, an L-Ca current is activated, but not fully deactivated because of a loss of control described in Fig. 12. B: on a 2nd voltage ramp, recorded L-Ca was smaller compared with L-Ca PIC in the 1st ramp. C: with Ba, once Ba PIC is activated on the 1st voltage ramp, it cannot be deactivated. D: after this, a 2nd ramp evokes no further Ba PIC, likely because Ba PIC current is already activated or even partly inactivated, Note large negative bias current at start of ramp in D compared with C.
DISCUSSION
Cav1.3 calcium currents activate SK currents
Our results show that the low voltage–activated persistent calcium current in spinal motoneurons activates a calcium-activated potassium current that is directly blocked by apamin and indirectly blocked by nimodipine (SKL current). This outward SK current opposes the inward persistent calcium current, resulting in a smaller net current (Ca PIC, ~20% smaller). Blocking this SK current leads to larger PICs and ultimately larger plateaus, thus explaining the earlier apamin studies of Hounsgaard and Mintz (1988). Previously, it has been suggested that SK currents oppose persistent calcium currents in this manner (Hultborn 1999), but this had not been directly confirmed in motoneurons. Because of its low activation voltage, its block with a relatively high dose of nimodipine (>10 µM; see results and Li and Bennett 2003), and its complete resistance to conotoxins (see results and Li and Bennett 2003), the persistent calcium current that activates these SKL currents is likely mediated by the Cav1.3 channels recently characterized by Xu and Lipscombe (2001). We refer to this calcium current as the Cav1.3 current.
High voltage–activated persistent calcium currents that are, in contrast, sensitive to conotoxins have been reported in motoneurons (N-, P-, Q-type; Carlin et al. 2000a; McCarthy and TanPiengco 1992; Powers and Binder 2003), and they may also activate SK currents. However, the role of these persistent currents is not obvious because, during normal firing, the membrane potential is held well below the spike threshold (−50 mV) by the AHPs, except for the 1-ms depolarization during each spike. Even if these currents were of dendritic origin, their activation at above −30 mV means that they would not be substantially activated, except transiently during the spikes, so they may only play a role during very fast firing. Furthermore, these persistent calcium currents may be partly an artifact of the patch clamp electrodes used to record them, which contained substantial calcium buffers, unlike our sharp electrodes. This may have reduced intracellular calcium sufficiently to stop the usual calcium-activated calcium channel inactivation associated with these calcium currents (N-, P-, and Q-type; Cens et al. 2005; Zong et al. 1994). Indeed, we found similar high voltage–activated persistent currents in barium, high voltage–activated Ba PIC (and not apamin); these are likely an artifact of substituting barium for calcium, which is known to reduce calcium channel inactivation (Cens et al. 2005). Alternatively, because this persistent inward current was only present in barium and not apamin, there may be an apamin-resistant and barium-sensitive potassium current (calcium- or voltage-activated) that normally precisely opposes (masks) this inward high voltage–activated PIC, although this seems unlikely because of the very large size of this barium-induced inward current. The barium-induced high voltage–activated persistent inward current (high voltage–activated Ba PIC) is activated at a distinctly higher potential (> −30 mV) than the usual low-threshold persistent calcium current (Cav1.3; −50-mV threshold) and ultimately causes a peculiar second plateau riding on top of the usual low voltage–activated plateau (Cav1.3 mediated) seen in motoneurons (Fig. 11D).
We also showed that the SKL current is one of the major calcium-activated currents triggered by the Cav1.3 current because a block of all calcium-activated currents produced by substituting barium for calcium has similar effects on the low voltage–activated PIC as application of apamin alone (subthreshold to the high voltage–activated currents just described; < −40 mV). Thus the PIC remaining in apamin, which we refer to as the L-Ca current, is a fairly good approximation of the isolated Cav1.3 current (L-Ca = Cav1.3), and the total persistent inward current is approximately Ca PIC = L-Ca + SKL current (as in Fig. 5). However, the actions of barium are difficult to interpret quantitatively because of the greater charge-carrying capability of barium compared with calcium and additional block of voltage-activated currents by barium (see results and Hille 2001). Other significant calcium-activated currents, such as calcium-activated cation currents (Lee et al. 1996; Wu and Anderson 2000), may be triggered by Cav1.3 currents.
SKL current magnitude and dependence on intracellular calcium
The SKL current is activated just after the L-Ca onset and produces an outward current that is ~25% of the inward L-Ca current at the initial peak of the L-Ca current, making the net inward current (Ca PIC) ~25% smaller than the L-Ca current. With time and greater depolarization (during a slow ramp), the SKL current increases dramatically, even though the L-Ca current does not also increase. Eventually, the SKL current completely overcomes the L-Ca current (magnitude, SKL > L-Ca) so that there is no longer a net persistent inward current. At this time, the high conductance of the SKL current produces a steep increase in I–V relation (with a doubling of the slope conductance), which we refer to as the wall and functionally makes depolarization of the neuron past −40 mV difficult.
The SK channel conductance is known to not have a voltage dependence (McLarnon 1995), but the SKL current should at least vary linearly with voltage, increasing with the difference from the reversal potential for potassium (as for the AHP current, see results). Also, the SKL current should depend on the variations in intracellular Ca (McLarnon 1995), which should in principle be related to the integrated inward calcium current minus the calcium removal through diffusion, buffering, and pumps. Thus the steep, greater-than-linear increase in the SKL current observed during the voltage ramps to −40 mV could reflect an accumulation of intracellular Ca, with calcium buffering not being able to keep pace with the maximal L-Ca current activation. In contrast, the slow decay in the SKL current (over seconds) during a steady submaximal L-Ca current activation during a voltage step (Fig. 8) could represent the calcium buffering overcoming the accumulation of intracellular calcium from the L-Ca current. Sometimes, during a voltage ramp, both of these processes occur together (Fig. 6D): the SKL reaches an initial peak with the L-Ca current peak and slowly decays, but with time increases again to produce the steep SK-mediated wall.
Origin of calcium oscillations at onset of plateau
At the onset of a calcium plateau produced by the Ca PIC, there is usually at least one characteristic calcium spike (overshoot-spike) in motoneurons of cats (Bennett et al. 1998) and rats (Fig. 7). Our results show that this overshoot-spike results from the slightly delayed activation of the SKL current shortly after the L-Ca activation, which allows the L-Ca current to reach a transient peak (calcium spike; apamin-sensitive), after which the SKL current hyperpolarizes the membrane potential, in what we have referred to as a CaAHP after this calcium overshoot-spike. During this hyperpolarization (CaAHP), the L-Ca current must be reduced somewhat, and the SKL current subsides, enabling the L-Ca current to sometimes reactivate and form a second calcium overshoot-spike, and so on. After about one to four such calcium oscillations, the L-Ca and SKL currents reach a steady state and form a steady depolarizing current that produces a plateau potential. Interestingly, these calcium oscillations occur even when the soma is voltage-clamped at a steady voltage, suggesting that the SKL currents are of dendritic origin (see results), which is consistent with a dendritic nature of the L-Ca current (Bennett et al. 1998; Carlin et al. 2000b; Heckman and Lee 1999; Hounsgaard et al. 1988b).
It should be remarked that the present apamin-sensitive oscillations and overshoot-spikes are very different from the calcium spikes seen after an extensive block of potassium currents with tetraethylammonium (TEA) (Hounsgaard and Mintz 1988). Such TEA-induced spikes are much larger (40 mV) and faster than the overshoot-spikes, and thus are likely mediated by the major high voltage–activated calcium currents, like with the Ba spikes described above.
SK current is usually not active at rest
SK currents are not activated subthreshold to the L-Ca currents because apamin has no effect at these membrane potentials. Thus because Ca PICs are not usually activated at the resting membrane potential, the SK currents are also not involved in the resting membrane potential. A few cells do have their Ca PICs activated at rest (Schwindt and Crill 1980), particularly when they are treated with 5-HT (Harvey et al. 2005c; Hounsgaard and Kiehn 1985), and in such cells, the SKL currents would be active at rest, together with the Ca PICs. Most motoneurons do not have SK currents activated at rest (Lape and Nistri 2000; Purvis and Butera 2005), consistent with our findings.
AHP is also mediated by SK currents, but these are activated by different calcium currents
Our results also show that, in rat spinal motoneurons, the classic postspike AHP is almost entirely mediated by an apamin-sensitive SK current (SKAHP), with apamin producing a complete block of the mAHP and only leaving the small transient fAHP (see results). This is to be expected, based on previous results from other neurons and motoneurons, where the mAHP is mediated by SK currents and the fAHP is mediated by apamin-resistant BK currents (Schwindt et al. 1988; Viana et al. 1993). Furthermore, our results showed that these SKAHP currents are activated by the fast transient high voltage–activated calcium currents (conotoxins-sensitive N-, P-, and Q-type) triggered during the action potential, consistent with results of previous studies (Pineda et al. 1998; Viana et al. 1993; Williams et al. 1997). The classic high voltage–activated L-type calcium currents (e.g., Cav1.2 as opposed to Cav1.3) do not contribute substantially to the SKAHP (nimodipine-resistant). Interestingly, these AHP results indicate that the calcium that activates the SKAHP current is completely different from the calcium that activates the SKL current, even though both of these SK currents are apamin sensitive. That is, only low voltage–activated L-type calcium currents (Cav1.3), and not N-, P-, and Q-type calcium currents, are involved in the Ca PIC and associated SKL currents because conotoxins do not affect these, whereas nimodipine blocks the Ca PIC and SKL.
SKAHP stops all-or-nothing PIC activation, whereas SKL does not
When spikes are blocked with TTX, there is a rapid all-or-nothing activation of the L-Ca current that forms a plateau, and, although the SKL currents reduce the net PIC, they do not themselves prevent this all-or-nothing activation of the PIC. In contrast, during firing, the additional SK currents produced by the AHP (SKAHP) do prevent (or at least slow) the rapid all-or-nothing PIC activation seen in TTX. That is, the accumulated hyperpolarization from the AHPs effectively holds the membrane potential below the firing threshold (−50 mV), limiting the full PIC activation. The potential only exceeds this firing threshold for 1 ms or so for each spike, and the L-Ca current does not respond to such rapid transients (Fig. 8 and Li and Bennett 2003). Thus from the standpoint of the slow L-Ca current, the membrane is indeed held below the firing level by the AHP currents. When apamin blocks the AHP currents (SKAHP and SKL), the L-Ca is instead activated in an all-or-nothing manner, and this causes extremely high firing rates, followed by a large plateau potential after firing stops (Fig. 2B), confirming the critical role of the AHP (SK currents) in preventing all-or-nothing PIC activation. Furthermore, these results are consistent with the classic notion that the AHP limits the maximum firing rate (Kernell 1965).
Na PICs regulate the interspike interval in the absence of AHPs
The AHP duration has classically been thought to determine the maximum interspike interval and thus determine the minimum firing rate; indeed, this is the case in motoneurons of pentobarbital anesthetized cats or acutely spinalized rats without large persistent sodium currents (Na PICs; Kernell 1965; Li et al. 2004a). However, recently it has been shown that motoneurons that possess large subthreshold Na PICs are able to fire at much lower rates (and thus longer intervals) than determined by the AHP duration (Harvey et al. 2005b; Li et al. 2004a). This slow firing is often very regular and occurs even in the absence of synaptic noise, unlike the noise-driven slow firing described by Matthews (1996). In particular, when these Na PICs are large enough to produce a negative slope region in the I–V relation, they cause slow intrinsic oscillations (1–4 Hz) in the membrane potential near the firing threshold, and these oscillations ultimately trigger the slow firing. Each cycle of this oscillation takes the form of an initial slow depolarization (ramp; Fig. 2D, double arrow) followed by a faster depolarization (acceleration; Fig. 2D, *), which ultimately triggers a spike. After each spike, an AHP occurs that deactivates the Na PIC transiently, and the Na PIC is reactivated again with the characteristic ramp and acceleration profile. This ramp and acceleration profile is resistant to nimodipine (not L-Ca mediated) and blocked by a low dose of TTX that blocks the Na PIC without affecting the sodium spike, and thus is Na PIC mediated (Li et al. 2004a). Our results indicate that such Na PIC-mediated slow firing can still occur when the mAHP is blocked by apamin. In this case, the fAHP seems to be sufficient to deactivate the Na PIC after each spike, and the same Na PIC-mediated ramp and acceleration depolarization profile follows after each fAHP. Thus slow firing continues with the same underlying Na PIC oscillation but with the mAHP no longer interposed between the spike and the Na PIC activation.
Importantly, in apamin, the firing that is mediated by the Na PIC oscillations is not restricted to very slow rates but occurs at faster rates with interspike intervals much less than the usual mAHP duration (Fig. 2, B and F). Thus Na PIC oscillations likely participate in determining the interspike interval during repetitive firing at all rates. The Na PIC effect is just more obviously separated from the mAHP at very low rates (when the AHP is not blocked with apamin).
SKL channels are likely distal to and distinct from the SKAHP channels
The spatial location of the SK currents underlying the AHP (SKAHP currents) is uncertain in motoneurons, although these are likely very close to the location of the action potential initiation and associated high voltage–activated calcium channels (co-localized with high voltage–activated Ca), because of the speed with which the AHP is initiated after the spike (ms). Thus these SKAHP currents must be located at least in part close to the soma, because spikes are initiated near the soma, in the axon initial segment (IS) and hillock (AH) (Oomura and Maeno 1963; Safronov et al. 2000). Furthermore, the AHP currents and spike are likely located near the intracellular electrode (in the soma), consistent with the very linear AHP versus potential relation, which has a reversal potential at only −81 mV (Fig. 4B).
In contrast, the SKL currents may not be as closely localized/associated to the calcium currents that activate them during plateau potentials (L-Ca current in this case), because SKL initiation is delayed ~100 ms after the onset of the L-type calcium current (results). However, because the space constant for diffusion of calcium is at least two orders of magnitude shorter than the electrical space constant (Zador and Koch 1994), the SKL channels are likely not very distant from the L-Ca currents. In hippocampal neurons, L-Ca channels activate SK channels with substantial delays (≤50 ms), as in motoneurons, and these L-Ca and SK channels are only ~100 nm apart (Marrion and Tavalin 1998). In motoneurons, the L-Ca currents themselves arise from the dendrites, with the major L-Ca activation likely occurring on average ~300 µm distally to the soma (one half of a space constant; Bennett et al. 1998; Elbasiouny et al. 2005). Thus it is likely that the SKL currents are also on dendrites ~300 µm from the soma, fairly near the L-Ca currents (within 100 nm or so). A dendritic origin to SKL currents is also supported by two other findings. 1) SKL oscillations seen in voltage clamp suggest unclamped dendritic SK currents (see results). 2) The SKL current plays a major role in assuring that L-Ca currents can be deactivated (Figs. 12 and 13; similar to results of Cai et al. 2004), and for this it seems especially important to have SKL currents on the most distal, least controllable dendrites where the L-Ca exist. At any rate, it is clear that the population of SK channels that produce the dendritic SKL currents cannot be the same as those that produce the SKAHP currents near the soma. Even if some of the high voltage–activated calcium channels that are activated during a spike are of dendritic origin, the SKL channels are unlikely to be close enough (nm) to participate in the AHP (like in Marrion and Tavalin 1998). It is more likely that the SKAHP and SKL currents are each activated by private microdomains of calcium arising from their two distinct populations of calcium channels (conotoxin-sensitive high voltage–activated channels and conotoxin-resistant L-Ca channels, respectively). In summary, the channels underlying the SKL currents are likely fairly near the Cav1.3 calcium channels of the L-Ca currents in the dendrites, but not immediately co-localized, to explain their activation delay. Furthermore, these SKL channels are likely distal to and distinct from the SK channels that underlie AHP.
Effect of chronic spinal cord injury on the SKL current
Our results indicate that motoneurons of acute and chronic spinal rats have SKL currents activated by the Cav1.3 currents (L-Ca current), and together these two currents form a net persistent inward current (Ca PIC). The whole sacrocaudal spinal cord of normal rats was maintained in vitro in this experiment, and this preparation was termed acute spinal because of the necessary transection needed to remove the spinal cord. Such acute injury has been shown to reduce the motoneuron excitability and, in particular, reduce PICs (Hounsgaard et al. 1988a), and thus it is not surprising that the L-Ca current in acute spinal rats is small (with no negative slope region; Harvey et al. 2005b,c). With chronic spinal cord injury, the motoneurons somehow adapt and recover their excitability and, in particular, exhibit large PICs (Li and Bennett 2003), consistent with the large L-Ca currents seen in the present chronic spinal rats (see results). We do not know about the corresponding SKL current in the intact rat, although it is likely that these are present because there is good evidence that intact animals (and humans) have persistent calcium currents (Ca PICs) that help sustain motor unit firing (Gorassini et al. 1999, 2002). Also, Lee and Heckman (1999b) argue that there may be an outward current, like the SK current, that could contribute to the slow decline of the PIC of motoneurons in spinal cord–intact decerebrate cats. Furthermore, qualitatively the PICs in decerebrate cats (Lee and Heckman 1998a) are similar to those of chronic spinal rats (i.e., both have a pronounced negative slope region that produces plateaus), and so the L-Ca and SKL currents in chronic spinal rats likely most closely approximate the currents seen in the spinal cord–intact animal.
Interestingly, the SKL current is not significantly bigger in chronic spinal than acute spinal rats, despite the much larger L-Ca current in chronic spinal rats. Thus there seems to be less availability of SKL channels in chronic spinal rats than in acute spinal rats because the larger L-Ca current does not activate a larger SKL current, in contrast to what would be expected if the SK channels remained unchanged. Thus the larger net Ca PIC (L-Ca + SKL) seen in chronic spinal, compared with acute spinal, rats may in small part be caused by this reduced SK channel availability. However, similar conclusions cannot be drawn from the SKAHP results. The AHP and associated SKAHP current and conductance is not different in acute and chronic spinal rats (see results); furthermore, the spikes that trigger the AHP are not different in acute and chronic spinal rats (Li et al. 2004a). Therefore there is no change in availability of the SKAHP current with chronic injury. Although the reason for this discrepancy between the changes in SKL and SKAHP currents with chronic injury are uncertain, the difference provides further evidence that these two SK currents are mediated by different populations of SK channels.
Acknowledgments
We thank L. Sanelli for expert technical assistance.
GRANTS
Funding was provided by National Institute of Neurological Disorders and Stroke Grant RO1 NS-47567-01, the Natural Sciences and Engineering Research Council, Canadian Foundation for Innovation, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research.
References
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999a;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini MA, Siu M. In vitro preparation to study spasticity in chronic spinal rats. Soc Neurosci Abstr. 1999b;25:1394. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke C, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Buschges A. The physiology of sensory cells in the ventral scoloparium of the stick insect femoral chordotonal organ. J Exp Biol. 1994;189:285–292. doi: 10.1242/jeb.189.1.285. [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur J Neurosci. 2000a;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000b;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. Voltage- and calcium-dependent inactivation in high voltage-gated Ca(2+) channels. Prog Biophys Mol Biol. 2005;90:104–117. doi: 10.1016/j.pbiomolbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Enomoto K, Furuya K, Maeno T, Edwards C, Oka T. Oscillating activity of a calcium-activated K+ channel in normal and cancerous mammary cells in culture. J Membr Biol. 1991;119:133–139. doi: 10.1007/BF01871412. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–16. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash M, Harvey PJ, Bennett DJ, Yang JF. Role of motoneuron plateau potentials in the generation of involuntary muscle activity after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamines are essential for persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2005a;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adults. J Neurophysiol. 2005b;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal injury. J Neurophysiol. 2007c;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH. The role of voltage-sensitive dendritic conductances in generating bistable firing patterns in motoneurons. J Physiol Paris. 1999;93:97–100. doi: 10.1016/s0928-4257(99)80140-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alphamotoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988a;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988b;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Adv Exp Med Biol. 2002;508:213–218. doi: 10.1007/978-1-4615-0713-0_26. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand. 1965;65:87–100. [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. J Neurophysiol. 2000;83:2987–2995. doi: 10.1152/jn.2000.83.5.2987. [DOI] [PubMed] [Google Scholar]
- Lasser-Ross N, Ross WN, Yarom Y. Activity-dependent [Ca2+]i changes in guinea pig vagal motoneurons: relationship to the slow afterhyperpolarization. J Neurophysiol. 1997;78:825–834. doi: 10.1152/jn.1997.78.2.825. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998a;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998b;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999a;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol. 1999b;82:2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Shaw T, Sandquist M, Partridge LD. Mechanism of action of the non-steroidal anti-inflammatory drug flufenamate on [Ca2+]i and Ca(2+)-activated currents in neurons. Cell Calcium. 1996;19:431–438. doi: 10.1016/s0143-4160(96)90116-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004a;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes in the chronic spinal rat, studied in vitro. J Neurophysiol. 2004b;91:2236–2246. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L, Maxwell DJ, Neilson M, Kerr R. A confocal microscopic survey of serotoninergic axons in the lumbar spinal cord of the rat: co-localization with glutamate decarboxylase and neuropeptides. Neuroscience. 1996;75:471–480. doi: 10.1016/0306-4522(96)00366-1. [DOI] [PubMed] [Google Scholar]
- McCarthy RT, TanPiengco PE. Multiple types of high-threshold calcium channels in rabbit sensory neurons: high-affinity block of neuronal L-type by nimodipine. J Neurosci. 1992;12:2225–2234. doi: 10.1523/JNEUROSCI.12-06-02225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG. Potassium currents in motoneurones. Prog Neurobiol. 1995;47:513–531. doi: 10.1016/0301-0082(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Maeno T. Does the neurone soma actually generate action potentials? Nature. 1963;197:358–359. doi: 10.1038/197358a0. [DOI] [PubMed] [Google Scholar]
- Patel R, Kerr R, Maxwell DJ. Absence of co-localized glutamic acid decarboxylase and neuropeptides in noradrenergic axons of the rat spinal cord. Brain Res. 1997;749:164–169. doi: 10.1016/s0006-8993(96)01399-6. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Ca(2+)-activated nonselective cationic current (I(CAN)) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Pineda JC, Waters RS, Foehring RC. Specificity in the interaction of HVA Ca2+ channel types with Ca2+-dependent AHPs and firing behavior in neocortical pyramidal neurons. J Neurophysiol. 1998;79:2522–2534. doi: 10.1152/jn.1998.79.5.2522. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Purvis LK, Butera RJ. Ionic current model of a hypoglossal motoneuron. J Neurophysiol. 2005;93:723–733. doi: 10.1152/jn.00703.2004. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Wolff M, Vogel W. Excitability of the soma in central nervous system neurons. Biophys J. 2000;78:2998–3010. doi: 10.1016/S0006-3495(00)76838-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988;59:424–449. doi: 10.1152/jn.1988.59.2.424. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol. 1997;78:1740–1742. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- Williams S, Serafin M, Muhlethaler M, Bernheim L. Distinct contributions of high- and low-voltage-activated calcium currents to afterhyperpolarizations in cholinergic nucleus basalis neurons of the guinea pig. J Neurosci. 1997;17:7307–7315. doi: 10.1523/JNEUROSCI.17-19-07307.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Anderson ME. Ca2+-activated non-selective cation current in rabbit ventricular myocytes. J Physiol. 2000;522:51–57. doi: 10.1111/j.1469-7793.2000.0051m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador A, Koch C. Linearized models of calcium dynamics: formal equivalence to the cable equation. J Neurosci. 1994;14:4705–4715. doi: 10.1523/JNEUROSCI.14-08-04705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong S, Zhou J, Tanabe T. Molecular determinants of calcium-dependent inactivation in cardiac L-type calcium channels. Biochem Biophys Res Commun. 1994;201:1117–1123. doi: 10.1006/bbrc.1994.1821. [DOI] [PubMed] [Google Scholar]